Published online Jan 15, 2025. doi: 10.4251/wjgo.v17.i1.98410
Revised: October 13, 2024
Accepted: October 28, 2024
Published online: January 15, 2025
Processing time: 169 Days and 18.1 Hours
Previous cellular studies have demonstrated that elevated expression of Cx43 promotes the degradation of cyclin E1 and inhibits cell proliferation through ubiquitination. Conversely, reduced expression results in a loss of this capacity to facilitate cyclin E degradation. The ubiquitination and degradation of cyclin E1 may be associated with phosphorylation at specific sites on the protein, with Cx43 potentially enhancing this process by facilitating the phosphorylation of these critical residues.
To investigate the correlation between expression of Cx43, SKP1/Cullin1/F-box (SCF)FBXW7, p-cyclin E1 (ser73, thr77, thr395) and clinicopathological indexes in colon cancer.
Expression levels of Cx43, SCFFBXW7, p-cyclin E1 (ser73, thr77, thr395) in 38 clinical colon cancer samples were detected by immunohistochemistry and were analyzed by statistical methods to discuss their correlations.
Positive rate of Cx43, SCFFBXW7, p-cyclin E1(Ser73), p-cyclin E1 (Thr77) and p-cyclin E1 (Thr395) in detected samples were 76.32%, 76.32%, 65.79%, 5.26% and 55.26% respectively. Positive expressions of these proteins were not related to the tissue type, degree of tissue differentiation or lymph node metastasis. Cx43 and SCFFBXW7(r = 0.749), p-cyclin E1 (Ser73) (r = 0.667) and p-cyclin E1 (Thr395) (r = 0.457), SCFFBXW7 and p-cyclin E1 (Ser73) (r = 0.703) and p-cyclin E1 (Thr395) (0.415) were correlated in colon cancer (P < 0.05), and expressions of the above proteins were positively correlated in colon cancer.
Cx43 may facilitate the phosphorylation of cyclin E1 at the Ser73 and Thr195 sites through its interaction with SCFFBXW7, thereby influencing the ubiquitination and degradation of cyclin E1.
Core Tip: In this study, we found that SKP1/Cullin1/F-box (SCF)FBXW7 exhibited a positive correlation with cyclin E1 expression at positions ser73 and thr395 in colon cancer tissues, suggesting that SCFFBXW7 may facilitate phosphorylation-dependent degradation by interacting with cyclin E1 at these specific sites. Furthermore, the expressions of Cx43 and SCFFBXW7 were positively correlated, as well as their association with the levels at positions ser73 and thr395. This indicates that Cx43 may enhance the phosphorylation of these two sites through its interaction with SCFFBXW7, thereby promoting the ubiquitination and degradation of cyclin E1.
- Citation: Luan RG, Liu MD, Deng ZF, Lu CL, Yu ML, Zhang MY, Liu R, An R, Yao YL, Guo DB, Zhang YX, Zhao L. Correlations of the expression of Cx43, SCFFBXW7, p-cyclin E1 (Ser73), p-cyclin E1 (Thr77) and p-cyclin E1 (Thr395) in colon cancer tissues. World J Gastrointest Oncol 2025; 17(1): 98410
- URL: https://www.wjgnet.com/1948-5204/full/v17/i1/98410.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i1.98410
Colon cancer is the third most commonly diagnosed cancer and the second leading cause of cancer-related mortality[1]. Despite significant advancements in recent years with chemotherapeutic agents targeting colon cancer, severe side effects, toxicity, and drug resistance remain major clinical challenges[2]. Therefore, it is imperative to identify additional therapeutic targets for colon cancer treatment to mitigate these adverse effects.
Cx43 is a crucial component of cell gap junctions, regulating cell proliferation and differentiation through intercellular communication via gap junctions[3]. Cx43 plays a significant role in modulating the cell cycle of tumor cells, and its abnormal expression is often associated with tumorigenesis[3,4-6]. Our previous studies have demonstrated that Cx43 expression is reduced in various cancer tissues, including lung and esophageal cancers[7-9].
Our previous study has indicated that AKAP95 and Cx43, functioning as a pair of molecular switches, influence the cell cycle by regulating the expression of cyclin Ds and cyclin Es, as well as the expression and activity of their associated cyclin-dependent kinases[10,11]. In lung cancer, Cx43 down-regulates cyclin E1 expression, thereby inhibiting Rb phosphorylation and E2F activation, which hinders cell cycle progression through competitive bindings to cyclin E1 with AKAP95[10]. Furthermore, Cx43 can negate the protective effect of AKAP95 on cyclin Es[10].
Ubiquitination is a crucial and widespread mechanism of protein degradation within cells. This pathway is catalyzed by ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2), and ubiquitin ligase (E3)[12-14]. Among the family of E3 Ligases, SCF has garnered significant attention in recent years. SCFFBXW7 has been identified as a specific ubiquitin ligase for cyclin E1, mediating its ubiquitination and subsequent degradation[15-17]. The SCF protein complex contains phosphate-binding domains that recognize and bind to phosphorylated sites on substrates, thereby facilitating substrate reactions[18]. We hypothesize that phosphorylation of cyclin E1 is a prerequisite for its ubiquitination and degradation; thus, we examined the expression levels of Cx43, SCFFBXW7, and the phosphorylation status at various sites of cyclin E1 in 38 clinical colon cancer tissues while analyzing correlations among these proteins.
Thirty-eight colon cancer tissue samples were all surgical specimens from colon cancer patients in Air Force Hospital of Eastern Theater during 2015-2020. The age range of the patients was 30-94, with an average of 67.3 years. The pathological diagnosis of patient samples was clear, including 1 case of T1N0M0, 1 case of T1N1aM1a, 5 cases of T2N0M0, 1 case of T2N1aM0, 2 cases of T3N0M0, 16 cases of T4aN0M0, 1 case of T4aN0M1a, 3 cases of T4aN1bM0, 1 case of T4aN1bM1a, 3 cases of T4aN1cM0, 2 cases of T4aN1cM1a, 1 case of T4aN2bM0 and 1 case of TisN0M0 (TNM typing).
General two-step immunohistochemical kit (PV-9000) and DAB chromogenic solution were purchased from Fuzhou Maxim Biotechnology Development Co., Ltd; Hematoxylin dye was purchased from Solarbio Co., Ltd; Anti-rabbit Cx43 (AF0137), FBXW7(DF12400), p-cyclin E1 (Ser73) (AF4413), and p-cyclin E1 (Thr395) (AF3235) were purchased from Affinity Biosciences (Ohio, United States). Anti-rabbit p-cyclin E1 (Thr77) (ET1612-31) was purchased from Hangzhou HuaAn Biotechnoloy Co., Ltd (Hangzhou, China).
The specimens were fixed in 10% neutral formaldehyde and embedded in paraffin, and then 4 µm serial sections were cut. After hydration with xylene and gradient ethanol, the sections were placed in citric acid/ sodium citrate buffer for thermal repair. After blocking endogenous peroxidase, the primary antibody was incubated overnight (8 hours) and then the reaction enhancer and horseradish peroxidase labeled secondary antibody were incubated successively. After incubating DAB reagent, slides were put into hematoxylin staining solution to stain the nucleus, and dehydrate the slides after cleaning.
Brownish yellow in cancer tissues was considered as protein positive expression under the light microscope. Each tissue slide was randomly observed in 10 different visual fields, and 200 tumor cells were counted in each visual field. The ratio of the number of positive cells to the total number of counted cells and the positive intensity were taken as the judgment criteria. Under the light microscope, no brownish yellow in the field of vision or proportion of positive cells was less than 10% would be record as ‘-’; proportion of positive cells was ≥ 10% and < 30% would be recorded as ‘+’; proportion of positive cells was ≥ 30% and < 50% would be recorded as ‘++’; proportion of positive cells was ≥ 50% would be recorded as ‘+++. ‘-’ was regarded as negative results while ‘+’, ‘++’ and ‘+++’ were regarded as positive results. The slides were read by different experimenters for 3 times, and the average results were taken for statistical analysis.
IBM SPSS statistical was used for statistical analysis. χ2 test was used for rate comparison, Spearman rank correlation analysis was used for correlation analysis, and test level α = 0.05.
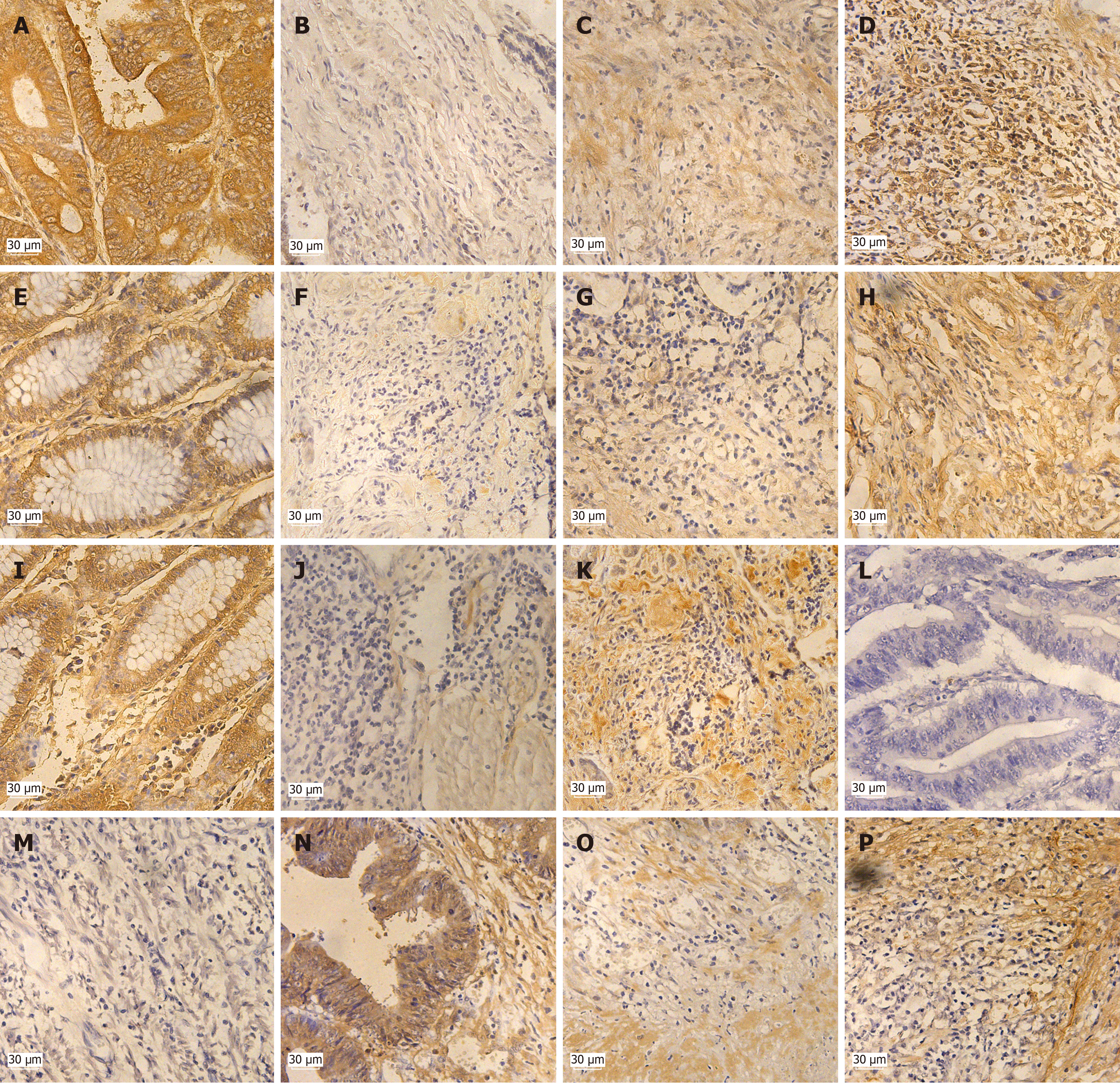
In 38 cases of colon cancer, positive expression of Cx43 and SCFFBXW7 were 29 and 29 cases respectively, and their positive rates were 76.32% and 76.32%; positive expression of phosphorylation site Ser73, Thr77 and Thr395 of cyclin E1 were 25, 2 and 21 cases, and positive rates were 65.79%, 5.26% and 55.26% respectively (Table 1).
| Cx43 | SCFFBW7 | p-cyclin E1 (Ser73) | p-cyclin E1 (Thr77) | p-cyclin E1 (Thr395) | |
| Positive | 29 | 29 | 25 | 2 | 21 |
| Negative | 9 | 9 | 13 | 36 | 17 |
| Positive rate (%) | 76.32 | 76.32 | 65.79 | 5.26 | 55.26 |
Positive rates of expression of Cx43, SCFFBXW7, p-cyclin E1 (Ser73) and p-cyclin E1 (Thr395) were significantly higher than p-cyclin E1 (Thr77). Cx43, SCFFBXW7, p-cyclin E1 (Ser73) and p-cyclin E1 (Thr395) were expressed in both nucleus and cytoplasm (Figure 1).
Tables 2, 3, and 4 showed analysis results of correlation between expression of SCFFBXW7 and p-cyclin E1 (Ser73), SCFFBXW7 and p-cyclin E1 (Thr77), and SCFFBXW7 and p-cyclin E1 (Thr395) in 38 colon cancer tissue cases. The expression of SCFFBXW7 and p-cyclin E1 (ser73), SCFFBXW7 and p-cyclin E1 (Thr395) were statistically significant (P < 0.05) and their spearman correlation coefficient were 0.703 and 0.415 respectively. The results showed that there were positive correlations between the expression of SCFFBXW7 and p-cyclin E1 (ser73), and SCFFBXW7 and p-cyclin E1 (thr395) in colon cancer. No correlation between SCFFBXW7 and p-cyclin E1 (Thr77) was detected this time.
| FBXW7 | Ser73(-) | Ser73(+) | Ser73(++) | Ser73(+++) | r | P value |
| - | 7 | 2 | 0 | 0 | 0.703 | 0.001 |
| + | 5 | 7 | 2 | 2 | ||
| ++ | 1 | 1 | 4 | 2 | ||
| +++ | 0 | 0 | 2 | 3 |
| FBXW7 | Thr77(-) | Thr77(+) | Thr77(++) | Thr77(+++) | r | P value |
| - | 9 | 0 | 0 | 0 | 0.079 | 0.638 |
| + | 15 | 0 | 0 | 0 | ||
| ++ | 9 | 0 | 0 | 0 | ||
| +++ | 5 | 0 | 0 | 0 |
| FBXW7 | Thr395(-) | Thr395(+) | Thr395(++) | Thr395(+++) | r | P value |
| - | 5 | 3 | 0 | 0 | 0.415 | 0.010 |
| + | 8 | 5 | 2 | 1 | ||
| ++ | 4 | 2 | 3 | 0 | ||
| +++ | 0 | 1 | 2 | 2 |
Tables 5, 6, 7, and 8 showed analysis results of correlation between expression of Cx43 and SCFFBXW7, Cx43 and p-cyclin E1 (Ser73), Cx43 and p-cyclin E1 (Thr77), and Cx43 and p-cyclin E1 (Thr395) in 38 colon cancer tissue cases. The expression of Cx43 and SCFFBXW7, Cx43 and p-cyclin E1 (ser73), Cx43 and p-cyclin E1 (Thr395) were statistically significant (P < 0.05) and their spearman correlation coefficient were 0.749, 0.667 and 0.457 respectively. The results showed that there were positive correlations between the expression of Cx43 and SCFFBXW7, Cx43 and p-cyclin E1 (ser73), and Cx43 and p-cyclin E1 (Thr395) in colon cancer while there was no correlation between Cx43 and p-cyclin E1 (Thr77).
| Cx43 | FBXW7(-) | FBXW7(+) | FBXW7(++) | FBXW7(+++) | r | P value |
| - | 6 | 3 | 0 | 0 | 0.749 | 0.001 |
| + | 3 | 6 | 3 | 0 | ||
| ++ | 0 | 6 | 4 | 1 | ||
| +++ | 0 | 0 | 2 | 5 |
| Cx43 | Ser73(-) | Ser73(+) | Ser73(++) | Ser73(+++) | r | P value |
| - | 8 | 1 | 0 | 0 | 0.667 | 0.001 |
| + | 2 | 7 | 2 | 1 | ||
| ++ | 3 | 2 | 3 | 3 | ||
| +++ | 0 | 0 | 3 | 3 |
| Cx43 | Thr77(-) | Thr77(+) | Thr77(++) | Thr77(+++) | r | P value |
| - | 9 | 0 | 0 | 0 | 0.262 | 0.112 |
| + | 12 | 0 | 0 | 0 | ||
| ++ | 11 | 0 | 0 | 0 | ||
| +++ | 6 | 0 | 0 | 0 |
| Cx43 | Thr395(-) | Thr395(+) | Thr395(++) | Thr395(+++) | r | P value |
| - | 5 | 3 | 0 | 1 | 0.457 | 0.004 |
| + | 8 | 3 | 1 | 0 | ||
| ++ | 4 | 4 | 3 | 0 | ||
| +++ | 0 | 1 | 3 | 2 |
Our long-term studies have showed that AKAP95 and Cx43 are a pair of "molecular switches", regulating the progress of cell cycle by affecting the expression of cyclin Es[7-11,19]. AKAP95 and Cx43 could competitively bind to cyclin Ds and cyclin Es, regulating degradation of them: AKAP95 could bind to cyclin Ds and cyclin Es, preventing them from degradation, on the one hand, and inhibiting Cx43 promoting degradation of cyclin Ds and cyclin Es by binding to Cx43; Cx43, on the contrary to AKAP95, could accelerate the degradation of cyclin Ds and cyclin Es[10].
Ubiquitination is an important way of protein hydrolysis in cells. To discuss whether Cx43 affects the degradation of cyclin E1 and its expression level by regulating cyclin E1’s ubiquitination, we further detected the expression of Cx43 and SCFFBXW7 and their correlation in clinical colon cancer tissues this time. Our data showed that there was a positive correlation between expression of Cx43 and SCFFBXW7, which was in line with the relevant report of ‘Cx43 promote the degradation of cyclin E1/2 and hinder the progress of cell cycle’[15-17].
Protein ubiquitination and degradation mediated by SCFs are related to the phosphorylation of substrate molecules[18]. For instance, phosphorylation of Thr286 site of cyclin D1 has been reported to be a necessary condition for cyclin D1’s ubiquitination and degradation[20]. However, there is still a lack of researches on the relationship between ubiquitination and phosphorylation of related site and of cyclin E1. We detected correlation between ubiquitination and three phosphorylation sites of cyclin E1 this time, and our data showed that phosphorylation of sites Ser73 and Thr395 of cyclin E1 was positively correlated with SCFFBXW7 and Cx43 respectively, and Cx43 and SCFFBXW7 were also positively correlated as well, suggesting that ubiquitination and degradation of cyclin E1 might be related to the phosphorylation of sites Ser73 and Thr395 of cyclin E1, and Cx43 might promote the ubiquitination and degradation of cyclin E1 by promoting the phosphorylation of these two sites.
Low positive rate of phosphorylation level of sites Thr77 of cyclin E1 was detected and no correlation between expression levels of Cx43 or SCFFBXW7 showed this time, suggesting that the phosphorylation of site Thr77 might not be related to ubiquitination and degradation of cyclin E1, and Cx43 did not affect its phosphorylation.
In general, our results of 38 clinical colon cancer samples preliminarily showed that ubiquitination and degradation of cyclin E1 were related to phosphorylation of site Ser73 and Thr395, but further experimental verification was still needed. In addition, Cx43 might affect degradation of cyclin E1 by affecting its phosphorylation and the expression of SCFFBXW7.
It is postulated that Cx43 might facilitate the phosphorylation of cyclin E1 at the Ser73 and Thr395 sites via its interaction with SCFFBXW7, thereby exerting an impact on the ubiquitination and degradation of cyclin E1.
| 1. | Torp SH, Solheim O, Skjulsvik AJ. The WHO 2021 Classification of Central Nervous System tumours: a practical update on what neurosurgeons need to know-a minireview. Acta Neurochir (Wien). 2022;164:2453-2464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 158] [Article Influence: 39.5] [Reference Citation Analysis (1)] |
| 2. | Hammond WA, Swaika A, Mody K. Pharmacologic resistance in colorectal cancer: a review. Ther Adv Med Oncol. 2016;8:57-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 396] [Article Influence: 39.6] [Reference Citation Analysis (1)] |
| 3. | Lezcano V, Bellido T, Plotkin LI, Boland R, Morelli S. Role of connexin 43 in the mechanism of action of alendronate: dissociation of anti-apoptotic and proliferative signaling pathways. Arch Biochem Biophys. 2012;518:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 4. | Decrock E, De Vuyst E, Vinken M, Van Moorhem M, Vranckx K, Wang N, Van Laeken L, De Bock M, D'Herde K, Lai CP, Rogiers V, Evans WH, Naus CC, Leybaert L. Connexin 43 hemichannels contribute to the propagation of apoptotic cell death in a rat C6 glioma cell model. Cell Death Differ. 2009;16:151-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 151] [Article Influence: 8.4] [Reference Citation Analysis (1)] |
| 5. | Bruzzone R. Learning the language of cell-cell communication through connexin channels. Genome Biol. 2001;2:REPORTS4027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 6. | Johnstone SR, Best AK, Wright CS, Isakson BE, Errington RJ, Martin PE. Enhanced connexin 43 expression delays intra-mitotic duration and cell cycle traverse independently of gap junction channel function. J Cell Biochem. 2010;110:772-782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 7. | Liu W, Hua S, Dai Y, Yuan Y, Yang J, Deng J, Huo Y, Chen X, Teng B, Yu X, Zhang Y. Roles of Cx43 and AKAP95 in ovarian cancer tissues in G1/S phase. Int J Clin Exp Pathol. 2015;8:14315-14324. [PubMed] |
| 8. | Wang S, Wang K, Deng Z, Jiang Z, Wang D, Yao Y, Guo D, Kong X, Guan Z, Zhang Y. Correlation between the protein expression levels of A-kinase anchor protein95, p-retinoblastoma (Ser780), cyclin D2/3, and cyclin E2 in esophageal cancer tissues. Asia Pac J Clin Oncol. 2019;15:e162-e166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 9. | Guan Z, Zhuang W, Lei H, Wang D, Yao Y, Guo D, Sun Q, Chen Y, Chen X, Lin H, Teng B, Zhang Y. Epac1, PDE4, and PKC protein expression and their correlation with AKAP95 and Cx43 in esophagus cancer tissues. Thorac Cancer. 2017;8:572-576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 10. | Chen R, Chen Y, Yuan Y, Zou X, Sun Q, Lin H, Chen X, Liu M, Deng Z, Yao Y, Guo D, Zhang Y. Cx43 and AKAP95 regulate G1/S conversion by competitively binding to cyclin E1/E2 in lung cancer cells. Thorac Cancer. 2020;11:1594-1602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 11. | Chen X, Kong X, Zhuang W, Teng B, Yu X, Hua S, Wang S, Liang F, Ma D, Zhang S, Zou X, Dai Y, Yang W, Zhang Y. Dynamic changes in protein interaction between AKAP95 and Cx43 during cell cycle progression of A549 cells. Sci Rep. 2016;6:21224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 12. | Hochstrasser M. Biochemistry. All in the ubiquitin family. Science. 2000;289:563-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 13. | Cardozo T, Pagano M. The SCF ubiquitin ligase: insights into a molecular machine. Nat Rev Mol Cell Biol. 2004;5:739-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 819] [Cited by in RCA: 887] [Article Influence: 40.3] [Reference Citation Analysis (1)] |
| 14. | Li Y, Jin K, Bunker E, Zhang X, Luo X, Liu X, Hao B. Structural basis of the phosphorylation-independent recognition of cyclin D1 by the SCF(FBXO31) ubiquitin ligase. Proc Natl Acad Sci U S A. 2018;115:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 15. | Reiterer V, Figueras-Puig C, Le Guerroue F, Confalonieri S, Vecchi M, Jalapothu D, Kanse SM, Deshaies RJ, Di Fiore PP, Behrends C, Farhan H. The pseudophosphatase STYX targets the F-box of FBXW7 and inhibits SCFFBXW7 function. EMBO J. 2017;36:260-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 16. | Shiba-Ishii A, Hong J, Hirokawa T, Kim Y, Nakagawa T, Sakashita S, Sakamoto N, Kozuma Y, Sato Y, Noguchi M. Stratifin Inhibits SCF(FBW7) Formation and Blocks Ubiquitination of Oncoproteins during the Course of Lung Adenocarcinogenesis. Clin Cancer Res. 2019;25:2809-2820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 17. | Sailo BL, Banik K, Girisa S, Bordoloi D, Fan L, Halim CE, Wang H, Kumar AP, Zheng D, Mao X, Sethi G, Kunnumakkara AB. FBXW7 in Cancer: What Has Been Unraveled Thus Far? Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (1)] |
| 18. | Cenciarelli C, Chiaur DS, Guardavaccaro D, Parks W, Vidal M, Pagano M. Identification of a family of human F-box proteins. Curr Biol. 1999;9:1177-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 293] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 19. | Kong XY, Zhang DC, Zhuang WX, Hua SH, Dai Y, Yuan YY, Feng LL, Huang Q, Teng BG, Yu XY, Liu WZ, Zhang YX. AKAP95 promotes cell cycle progression via interactions with cyclin E and low molecular weight cyclin E. Am J Transl Res. 2016;8:811-826. [PubMed] |
| 20. | Jia L, Sun Y. F-box proteins FBXO31 and FBX4 in regulation of cyclin D1 degradation upon DNA damage. Pigment Cell Melanoma Res. 2009;22:518-519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/