Published online Jan 15, 2025. doi: 10.4251/wjgo.v17.i1.97831
Revised: September 2, 2024
Accepted: October 28, 2024
Published online: January 15, 2025
Processing time: 184 Days and 20.5 Hours
Hepatocellular carcinoma (HCC) is an inflammation-associated tumor with a dismal prognosis. Immunotherapy has become an important treatment strategy for HCC, as immunity is closely related to inflammation in the tumor microenvironment. Inflammation regulates the expression of programmed death ligand-1 (PD-L1) in the immunosuppressive tumor microenvironment and affects im
To investigate the effect and mechanism of action of IL-17A on PD-L1 expression and to identify attractive candidates for the treatment of HCC.
The upregulation of PD-L1 expression in HCC cells by IL-17A was assessed by reverse transcription PCR, western blotting, and flow cytometry. Mechanistic studies were conducted with gene knockout models and pathway inhibitors. The function of IL-17A in immune evasion was explored through coculture of T cells and HCC cells. The effects of IL-17A on the malignant biological behaviors of HCC cells were evaluated in vitro, and the antitumor effects of an IL-17A inhibitor and its synergistic effects with a PD-L1 inhibitor were studied in vivo.
IL-17A upregulated PD-L1 expression in HCC cells in a dose-dependent manner, whereas IL-17A receptor knockout or treatment with a small mothers against decapentaplegic 2 inhibitor diminished the PD-L1 expression induced by IL-17A. IL-17A enhanced the survival of HCC cells in the coculture system. IL-17A increased the viability, G2/M ratio, and migration of HCC cells and decreased the apoptotic index. Cyclin D1, VEGF, MMP9, and Bcl-1 expression increased after IL-17A treatment, whereas BAX expression decreased. The combination of IL-17A and PD-L1 inhibitors showed synergistic antitumor efficacy and increased cluster of differentiation 8 + T lymphocyte infiltration in an HCC mouse model.
IL-17A upregulates PD-L1 expression via the IL-17A receptor/phosphorylation-small mothers against decapentaplegic 2 signaling pathway in HCC cells. Blocking IL-17A enhances the therapeutic efficacy of PD-L1 antibodies in HCC in vivo.
Core Tip: Overexpression of programmed death ligand-1 (PD-L1) on tumor cells promotes cancer immune escape through inhibiting T cell function. Interleukin-17A (IL-17A) can increase the expression of PD-L1 in tumor cells and promote tumor progression. However, related research in hepatocellular carcinoma (HCC) is scarce. We clarified a novel mechanism by which IL-17A upregulated PD-L1 expression in HCC cells by the IL-17A receptor/phosphorylation-small mothers against decapentaplegic 2 axis. IL-17A could drive immune escape and promote proliferation, migration, and angiogenesis of HCC cells while inhibiting the apoptosis of HCC cells. IL-17A inhibition enhanced the therapeutic efficacy of the PD-L1 antibody in HCC in vivo.
- Citation: Yang ZX, Zhang LT, Liu XJ, Peng XB, Mao XR. Interleukin-17A facilitates tumor progression via upregulating programmed death ligand-1 expression in hepatocellular carcinoma. World J Gastrointest Oncol 2025; 17(1): 97831
- URL: https://www.wjgnet.com/1948-5204/full/v17/i1/97831.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i1.97831
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide[1]. Most patients are diagnosed at an advanced stage, which is associated with a higher mortality rate[1]. The major HCC guidelines recommend immune checkpoint blockade (ICB) as a first- or second-line therapy for the treatment of patients with cancer. However, the effective rates of ICB monotherapy vary only from 15% to 23% and increase to approximately 30% after combined treatment in patients with advanced HCC[2]. The tumor immune microenvironment (TME) aggressively promotes the progression of HCC and adversely affects the efficacy of antitumor therapies. Inflammatory factors and immune che
Programmed death ligand-1 (PD-L1) is typically overexpressed on cancer cells and is correlated with a poor prognosis in patients with HCC[5]. The overexpression of PD-L1 on tumor cells not only promotes cancer cell immune escape by inhibiting T cell function[6] but also promotes tumor development signals[7]. The expression of PD-L1 on HCC cells can be upregulated by inflammatory factors[8], oncogenic pathways[9], and transcriptional regulators[10].
Interleukin-17A (IL-17A) plays a protumor or antitumor role via different signaling pathways in various types of TMEs, as shown in previous studies[11-14]. IL-17A is a double-edged sword in the tumor microenvironment. Increasing evi
Growing evidence has shown that the limited efficacy of immunotherapy is closely related to tumor-associated chronic inflammation. Targeting inflammation, including IL-17A, as an adjuvant therapy to increase the therapeutic effectiveness of ICBs is one potential treatment approach. Patients with colorectal cancer could benefit from cancer immunotherapy consisting of anti-IL-17 monoclonal antibodies as an adjuvant therapy[18]. However, Liao et al[16] reported conflicting results that targeting IL-17A and PD-L1 diminishes the therapeutic effect of PD-L1 inhibitors in lung cancer. Other studies reported that IL-17A itself could increase the efficacy of immunotherapy in gastric cancer[19], and breast adenocarcinoma[20]. The results of multiple studies are inconsistent and might be related to the heterogeneity of the TME. IL-17A plays a protumor role in alcohol-induced HCC[21], but its implications for ICB in HCC and the underlying mechanisms remain elusive.
Our previous study revealed a positive correlation between the levels of IL-17A and soluble PD-L1 in the plasma of HCC patients. IL-17A was also associated with tumor load and tumor invasiveness in that study. The specific role of IL-17A in HCC development and the mechanism by which PD-L1 expression is upregulated require further exploration. Given the possible effect of IL-17A on PD-L1, we hypothesized that blocking IL-17A might sensitize HCC cells to ICB. To investigate this hypothesis, we examined the phenotypic changes in HCC cells after IL-17A treatment and the potential signaling pathway by which IL-17A regulates PD-L1 expression. Our study also evaluated the therapeutic efficacy of an IL-17A inhibitor combined with a PD-L1 inhibitor in a syngeneic model.
The human HCC cell lines HCCLM3, Huh7, and Hep3B and the mouse HCC cell line Hepa1-6 were purchased from the American Type Culture Collection (Manassas, VA, United States). Cells were cultured in Dulbecco’s Modified Eagle’s Medium (HyClone, Cytiva, United States) supplemented with 10% fetal bovine serum (HyClone, Cytiva, United States) and 100 U/mL penicillin/streptomycin. All cells were cultured at 37 °C with 5% carbon dioxide in a humidified incubator.
Recombinant human IL-17A (No. 7955-IL-025) was obtained from R and D Systems. Transforming growth factor (TGF)-beta signal pathway inhibitor (ITD-1) (S6713), an IL-17A inhibitor (a2120), and a PD-L1 inhibitor (a2115) were obtained from Selleck Chemicals. Anti-PD-L1 (ab213480) and anti-IL-17A receptor (IL-17RA) (ab263908) antibodies were purchased from Abcam. Anti-GAPDH was purchased from Bioworld, and anti-small mothers against decapentaplegic 2 (SMAD2) (AF1300), anti-phosphorylation-SMAD2 (AF2545), anti-Bcl-2 (AF0060), anti-cyclinD1 (AF1516), anti-Ki67 (AF1738), and 5, 6-carboxyfluorescein diacetate, succinimidyl ester (C1031) were purchased from Beyotime Biotechnology. Anti-BAX (No. 9664) was obtained from Cell Signaling Technology, and anti-VEGF (ET1604-28) and anti-MMP9 (ET1704-69) were purchased from HUABIO. Anti-PD-L1 (mouse, 66248-1), anti-IL-17A (mouse 26163-1-AP), and PD-L1 ELISA kits were obtained from Protein tech. A cell counting kit 8 kit (K1018) was purchased from APExBIO. Annexin V-FITC/PI apoptosis detection kits (E-CK-A211) and anti-PD-L1 (FCM), anti-PD-1 (FCM), anti-cluster of differentiation (CD) 8 (FCM), and IL-17A ELISA kits were purchased from Elabscience Biotechnology. Recombinant human IL-2, anti-human CD3 (317325), and anti-human CD28 (302933) antibodies were obtained from BioLegend.
C57BL/6J mice (male, 4-5 weeks) were purchased from Cavens, Changzhou, China. The mice were housed in a specific pathogen-free environment at the Animal Research Center of Gansu University of Chinese Medicine and had access to adequate food and water. All mouse studies were performed according to protocols approved by the Animal Research Committee of Gansu University of Chinese Medicine.
The cells were cultured in 6-well plates with or without IL-17A. After 24 h, proteins were extracted in radioimmunoprecipitation assay lysis buffer containing protease and phosphatase inhibitors. Protein concentrations were assayed with a bicinchoninic acid protein assay kit. The proteins were boiled in loading buffer for 10 minutes after which proteins were separated by 10% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred from the gel to polyvinylidene difluoride membranes, and blocked with QuickBlock™ Western for 30 min. The membranes were subsequently incubated with primary antibodies overnight at 4 °C and then incubated with secondary antibodies for 1 hour at room temperature. Protein expression was visualized via enhanced chemiluminescence detection reagents and an Amersham Imager 600 system (GE Healthcare, Boston, MA, United States). The results were analyzed using ImageJ2x software.
The IL-17RA knockout virus was constructed by Genechem Shanghai, and the sequences of the single guide RNAs against the IL-17RA knockout virus were as follows: (1) 5′-CCACAGTTGCTTTGAGCACAT-3′; (2) 5′-GCTGAACACCAAtgAACGTTT-3′; and (3) 5′-AGGAGAtgGTGGAGAGCAACT-3′. The viruses were subsequently used to infect Huh7 cells, and the cultured medium was replaced after 12 h. After 48 h, 1 μg/mL puromycin was added to select infected cells. IL-17RA knockout clones were identified by western blotting and quantitative reverse transcription PCR.
The cells were cultured in 6-well plates with or without IL-17A. RNAiso Plus reagent was used to extract total RNA. Then, 1 μg of total RNA was converted into complementary DNA via a reverse transcription kit. Quantitative PCR was performed on a CFX96 deep well real-time PCR system (Bio-Rad) with a reaction volume of 35 μL containing 7.7 μL of RNase-free water, 7 μL of complementary DNA, 1.4 μL of forward primer, 1.4 μL of reverse primer, and 17.5 μL of TB green premix ex-Taq II. The 2-∆∆ct method was used to determine the RNA expression level. PCR primers were purchased from Tsingke Biological Technology (Beijing, China). The sequences of primers used were as follows: (1) PD-L1, 5′-GGTGCCGACTACAAGCGAATTAC-3′ (forward), 5′-GGAATTGGTGGTGGTGGTCTTAC-3′ (reverse); (2) IL-17RA, 5′-GAAAtgGCATCCAGGTCCATC-3′ (forward), 5′-CCTAAATGGACAGGGCGAGAG-3′ (reverse); and (3) GAPDH, 5′-TGTGTCCGTCGTGGATCTGA-3′ (forward), 5′-TTGCTGTTGAAGTCGCAGGAG-3′ (reverse).
Peripheral blood was obtained from healthy volunteers after which peripheral blood mononuclear cells were extracted according to the manufacturer’s instructions (TBD Sciences, Tianjin, China). Peripheral blood mononuclear cells were placed in RPMI-1640 medium and incubated for 2 h. Then, the suspended cells were transferred to culture bottles and stimulated with IL-2 (1000 U/mL). Moreover, antibodies against CD3 (1 μg/mL) and CD28 (2 μg/mL) were added to the medium to stimulate T cell activation. The culture medium containing IL-2 (1000 U/mL) was replaced every 3 days. HCCLM3, Huh7, and Hep3B cells were labeled with carboxy fluorescein diacetate succinimidyl ester. Next, activated T cells were seeded into medium containing tumor cells (T cell to tumor cell ratio was 10:1) and cocultured with or without IL-17A (100 ng/mL) for 24 h. Finally, live tumor cells and CD8+ T cells were analyzed by flow cytometry.
HCCLM3, Huh7, and Hep3B cells were seeded in 96-well plates (5 × 103 cells/well). IL-17A was diluted to gradient concentrations in serum-free Dulbecco’s modified eagle medium. After the cells adhered to the wall, the medium was substituted with the prepared solution, and the cells were cultured for 24 h. Cell viability was tested via a cell counting kit 8 assay kit. The absorbance was measured at 450 nm using a microplate reader.
Scratch wound and Transwell assays were used to measure the migration ability of the cells. Different cells were cultured in 6-well plates (5 × 105 cells/well) for the scratch test. The monolayer was scratched, and the detached cells were removed. The cells were cultured with or without IL-17A (100 ng/mL). The cells in each well were photographed at 0 h, 24 h, and 48 h after the scratch was generated. The area of wound healing was calculated according to the formula (migration area (%) = (A0 - An)/A0 × 100%, where A0 represents the initial wound area and An represents the remaining area of the wound at the metering point).
For the Transwell assay, 10 × 105 cells suspended in medium (200 μL) without fetal bovine serum were incubated in the upper chamber, while complete medium (600 μL) with or without IL-17A (100 ng/mL) was added to the lower chamber. After 24 h, the cells were fixed in 4% paraformaldehyde for 20 min and stained with 0.1% crystal violet for 30 min. Nonmigrating cells in the upper chamber were removed with a cotton swab. The number of migrating cells was calculated using ImageJ2x software.
For the detection of apoptotic cells, HCC cells were cultured in 6-well plates with or without IL-17A (100 ng/mL). After 24 h, the cells were collected and resuspended in 500 μL of staining buffer containing 5 μL of Annexin VFITC and 5 μL of propidium iodide. The cells were then incubated in the dark for 20 min after which apoptotic cells were detected by flow cytometry (Agilent, United States). The results were evaluated using NovoExpress software.
For cell cycle distribution analysis, HCC cells were collected and washed with PBS. The cells were then incubated with DNA staining solution and permeabilization solution for 30 min in the dark. The cell cycle distribution was determined by flow cytometry, and the data were analyzed using NovoExpress software.
For the detection of PD-L1, HCC cells were cultured in 24-well plates with or without IL-17A (100 ng/mL). After 24 h, the cells were collected and washed with PBS. The cells were then incubated with staining buffer and an anti-PD-L1 antibody for 30 min in the dark. Flow cytometry was used to detect PD-L1 expression on the cells.
For the detection of PD-1, T cells were collected after coculture with tumor cells and resuspended in staining buffer. An anti-PD-1 antibody was added to the buffer after which T cells were incubated for 30 min in the dark. Flow cytometry was used to detect PD-1 expression on the cells.
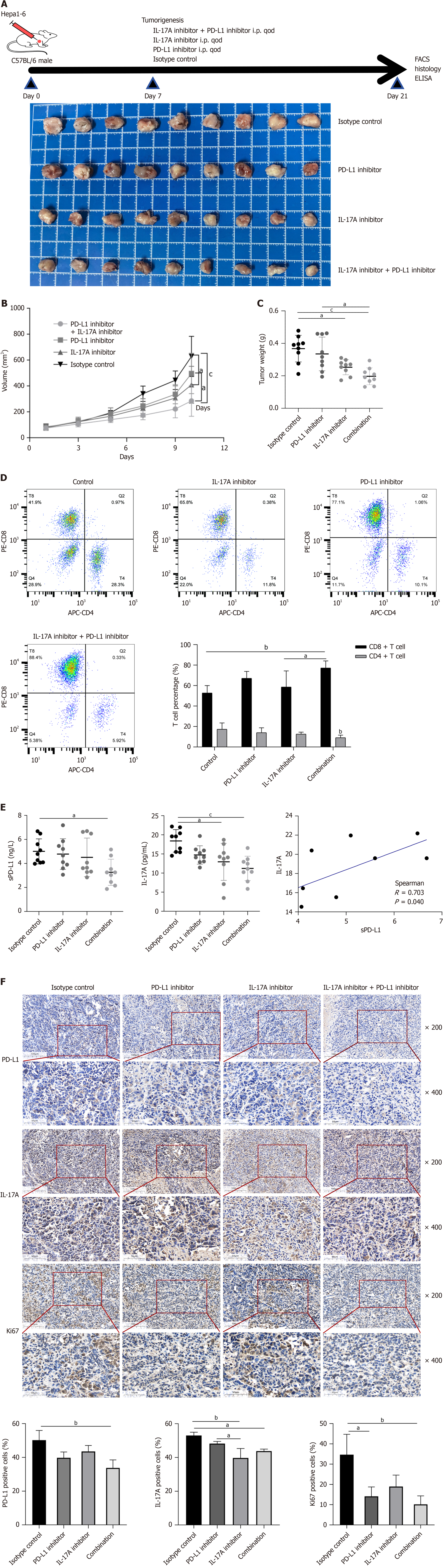
A total of 1 × 106 Hepa1-6 tumor cells were injected subcutaneously into C57BL/6J mice (n = 9/group). When the tumors grew to 50 mm3 to 100 mm3 in size, the mice were randomly divided into the following groups: isotype control; the anti-IL-17A; the anti-PD-L1; and the combined groups. The antibodies (100 μg/mouse) were intraperitoneally injected into the mice once every other day for 10 days. The length and width of the tumors were measured using electronic calipers every 2 days. The tumor volume was calculated as length × width2/2. The mice were sacrificed 24 h after the last treatment. The serum was collected by centrifugation at 3000 rpm for 10 min and prepared for ELISA. Tumor tissues were collected for flow cytometry and immunohistochemistry.
Serums PD-L1 and IL-17A levels were tested using ELISA kits according to the manufacturer’s instructions. The standards and test samples were pipetted into 96-well plates and incubated at 37 °C. Biotinylated detection antibodies were added to each well, after which the plates were incubated, and unbound biotinylated antibodies were washed with washing buffer. The horseradish peroxidase-conjugated working solution was pipetted into each well. The plates were incubated, after which the wells were washed, and the substrate stop solution was added. The optical absorbance was measured at 450 nm in a microplate reader (Thermo Scientific Varioskan Flash, MA, United States). The protein concentration was calculated according to the standard curves.
Paraffin-embedded tumor tissue was sectioned, and immunohistochemical staining was performed to detect PD-L1, IL-17A, and Ki67. The experimental procedures included deparaffinization, rehydration, antigen retrieval, endogenous peroxidase inactivation, nonspecific protein blocking, incubation with primary antibodies, and incubation with secondary antibodies. The slides were subsequently incubated with horseradish enzyme-labeled streptavidin working solution. Finally, the slides were successively stained with diaminobenzidine solution and hematoxylin. The negative control was performed by omitting the primary antibodies. The positive rate was evaluated ImageJ2x software.
RNA-seq data with standard annotations were downloaded from The Cancer Genome Atlas (TCGA), (https://portal.gdc.cancer.gov/). The data were analyzed using TCGA biolinks.
Statistical analysis was conducted using statistical product and service solutions (v29.0, Chicago, IL, United States) and Graphpad Prism8.0.2. Comparisons between groups were performed via t tests or one-way analysis of variance. Correlation analysis was performed via Pearson’s test or Spearman’s test. P < 0.05 indicated statistical significance.
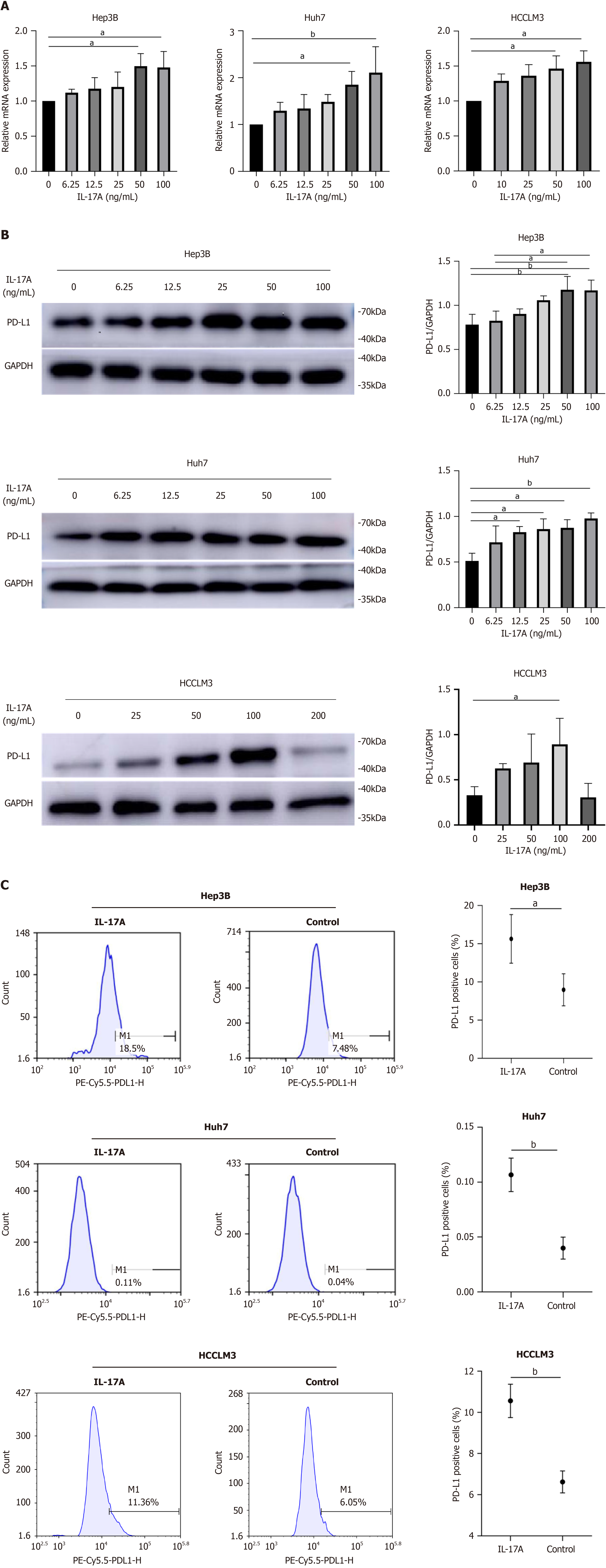
IL-17A promoted the expression of PD-L1 mRNA and PD-L1 protein in Hep3B, Huh7, and HCCLM3 cells in a dose-dependent manner (Figure 1A and B). Compared with the 0 ng/mL treatment, the 100 ng/mL treatment for 24 h significantly increased the PD-L1 level. The low doses of IL-17A were insufficient to regulate the transcript and protein levels of PD-L1 in HCC cells. Further verification of this upregulation effect was performed. Flow cytometric analysis also revealed that the expression of PD-L1 was greater in the IL-17A-100 ng/mL group than in the control group (Figure 1C). Therefore, we treated HCC cells with 100 ng/mL IL-17A for 24 h in this study.
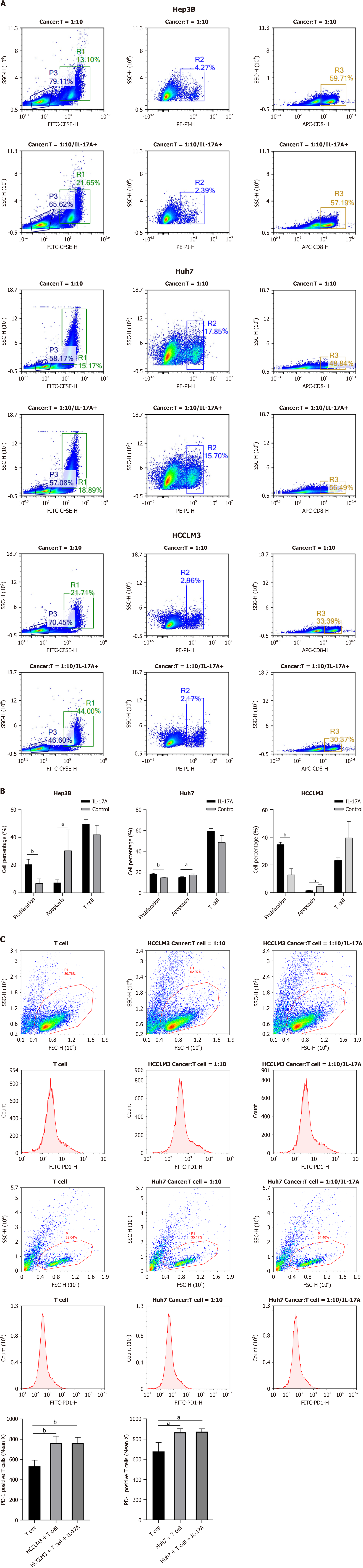
It is generally acknowledged that the overexpression of PD-L1 promotes the immune escape of tumor cells. To evaluate the PD-L1 activity induced by IL-17A, we performed a coculture assay, which consistently revealed that IL-17A increased the proliferation ability and decreased the death of tumor cells in the T cell and HCC cell coculture system (Figure 2A). Bar graphs revealed a significant difference between the two groups with or without IL-17A treatment (Figure 2B). The level of PD-1 on T cells was greater in the coculture system than on T cells cultured alone (Figure 2C). IL-17A had no effect on PD-1 levels in cocultured cells (Figure 2C).
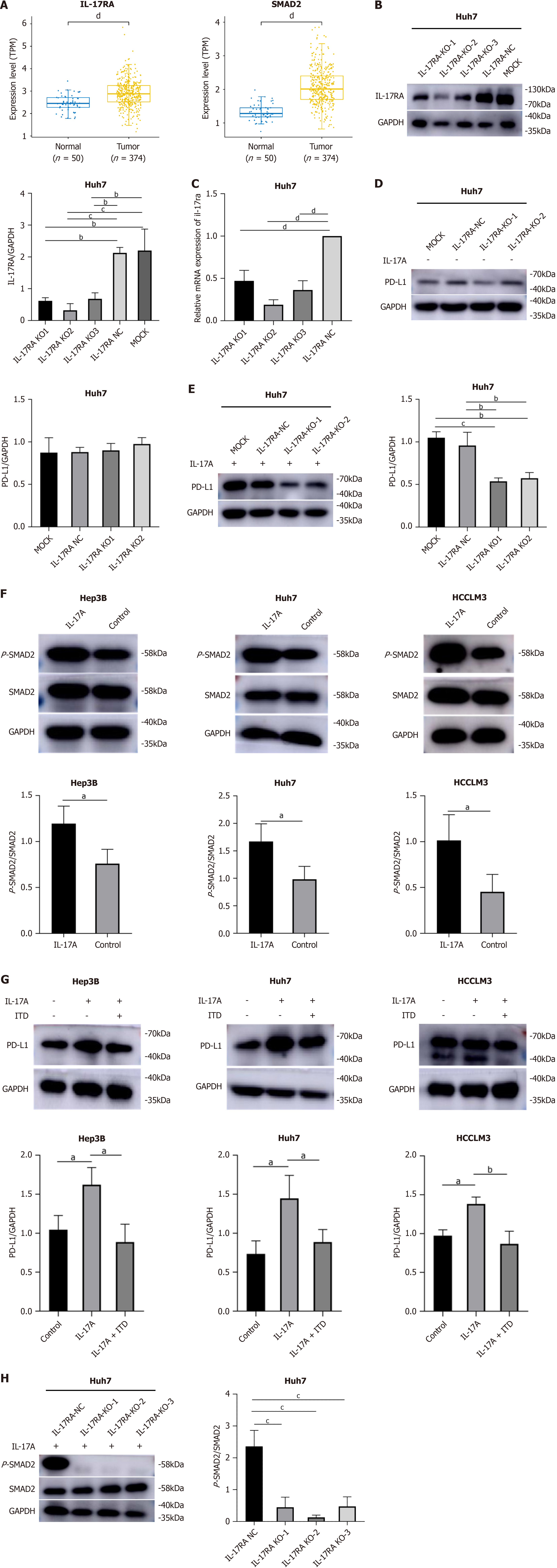
To further explore the possible mechanism by which IL-17A regulates the expression of PD-L1, blocking experiments were performed, and RNA-seq data from liver HCC samples (n = 374) and nontumor samples (n = 50) from the genomic data common were analyzed. The results of the TCGA database analysis revealed that IL-17RA and SMAD2 expression increased in liver HCC samples compared with normal samples (Figure 3A). To determine the possible effect of IL-17RA on IL-17A-induced PD-L1 expression, we introduced lentivirus-mediated stable silencing of IL-17RA in Huh7 cells. The efficiency of IL-17RA knockdown is shown in Figure 3B and C. We investigated the effect of IL-17RA knockdown on PD-L1 expression. As shown in Figure 3D, the level of PD-L1 was not altered in the IL-17RA-knockdown group compared with the negative control (NC) group. Then, cells in which IL-17RA was stably silenced and control cells were treated with 100 ng/mL IL-17A for 24 h. Western blot analysis demonstrated that IL-17A-induced PD-L1 expression was inhibited by IL-17RA knockdown (Figure 3E). Therefore, the expression of PD-L1 in HCC cells may be regulated by the IL-17A/IL-17RA pathway.
Western blotting revealed that IL-17A notably increased only the level of p-SMAD2 protein but did not affect the level of SMAD2 protein (Figure 3F). The ratio of p-SMAD2 to SMAD2 in the IL-17A-100 ng/mL group was greater than that in the control group (Figure 3F). Subsequent analysis revealed that the IL-17A-induced increase in PD-L1 expression was inhibited by pretreatment with ITD-1 (an inhibitor of Smad2 phosphorylation) (Figure 3G). The dose of ITD was determined according to the cell viability inhibitory concentrations (IC50 values) established by the cell counting kit-8 assay (Supplementary Figure 1). However, further studies are needed to verify whether IL-17A binds to IL-17RA to directly or indirectly regulate p-SMAD2 expression. The cells in the IL-17RA-knockdown and NC groups were treated with IL-17A. The protein levels of SMAD2 and p-SMAD2 were tested via western blotting. The ratio of p-SMAD2 to SMAD2 in the IL-17RA-knockdown group was lower than that in the NC group (Figure 3H). Together, these findings suggest that the upregulation of PD-L1 expression by IL-17A may be mediated through the IL-17RA/p-SMAD2 signaling pathway.
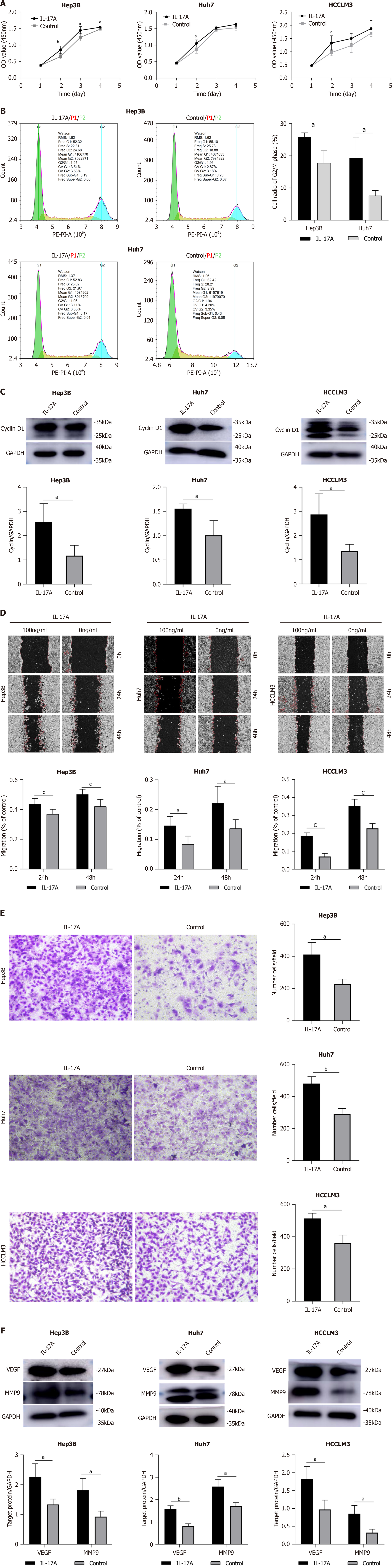
The proliferation abilities of the HCC cells were analyzed, and growth curves were generated. The viability of HCC cells increased significantly after IL-17A treatment for 24 h (Figure 4A). Notably, even with prolonged incubation, IL-17A did not exert a sustained effect on the proliferation of HCC cells (Figure 4A). As expected, further experiments confirmed that IL-17A could promote HCC cell proliferation. Flow cytometry analysis revealed that, compared with the control, IL-17A greatly increased the proportion of HCC cells in G2/M phase (Figure 4B). The effect of IL-17A on the protein expression of CyclinD1 in HCCLM3, Huh7, and Hep3B cells was subsequently observed by western blotting. The results revealed that IL-17A increased the level of CyclinD1 in these cells (Figure 4C).
The migration abilities of Hep3B, Huh7, and HCCLM3 cells after IL-17A treatment for 24 h were observed by wound healing and Transwell assays, which showed that HCC cell migration was promoted by IL-17A (Figure 4D and E). VEGF plays a crucial role in angiogenesis, and MMP9 promotes angiogenesis indirectly by interacting with VEGF[22]. We also evaluated the effects of IL-17A on VEGF and MMP9 protein expression in HCC cells. The results revealed that the protein levels of VEGF and MMP9 were increased in HCC cells treated with IL-17A compared with those in the control group of HCC cells (Figure 4F). These results indicate that IL-17A can stimulate the proliferation, migration, and angiogenesis of HCC cells.
To assess the impact of IL-17A on the apoptosis of Hep3B cells, we cultured the cells with IL-17A for 24 h and tested apoptosis via flow cytometry. The results demonstrated that IL-17A reduced the percentage of apoptotic Hep3B cells (Figure 5A). Moreover, we performed western blotting to investigate the role of IL-17A in the expression of BAX and Bcl-2 in Hep3B cells. IL-17A downregulated the protein expression of BAX and upregulated the protein expression of Bcl-2 in the treatment group compared with the control group (Figure 5B). Similar results were observed in Huh7 and HCCLM3 cells (Figure 5A and B). Collectively, these data suggest that IL-17A inhibits HCC cell apoptosis.
According to the regulation of PD-L1 expression and the role of IL-17A in malignant cell behavior, we hypothesized that blocking IL-17A might suppress tumor progression and enhance the therapeutic effect of anti-PD-L1 therapy. To test this hypothesis, we subsequently performed a preclinical study using a mouse model. These results indicated that anti-IL-17A antibodies combined with anti-PD-L1 antibodies and anti-IL-17A antibodies alone could inhibit tumor growth. Moreover, the efficacy of this combination was better than that of anti-IL-17A antibody or anti-PD-L1 antibody monotherapy (Figure 6A-C). The effects of different treatments on immune cell recruitment, serum parameters, and pathological parameters were also assessed. The number of infiltrating CD8+ T cells in the combined therapy group was greater than that in the control group and the anti-IL-17A monotherapy group (Figure 6D). The number of infiltrating CD4+ T cells in the combined therapy group was lower than that in the control group (Figure 6D). The serum expression of soluble PD-L1 and IL-17A was lower in the combined therapy group than in the control group (Figure 6E). A positive correlation was observed between the serum expression of soluble PD-L1 and that of IL-17A in the control group (Figure 6E). The expression of Ki67, IL-17A, and PD-L1 in tumor tissues in the combined therapy group was obviously lower than that in the control group (Figure 6F).
Several studies have shown that IL-17A can increase the expression of PD-L1 in tumor cells and promote the progression of tumors, including colorectal cancer[23], ovarian cancer[24], pancreatic carcinoma[25], and lung cancer[16]. However, related research in HCC is scarce. To our knowledge, only one study has reported the regulatory effect of IL-17A on PD-L1 in hepatoma stem cells[26]. However, the significance of IL-17A in the regulation of PD-L1 in HCC cells has not been clearly defined. Our previous study indicated that the plasma level of IL-17A was positively correlated with the expression of soluble PD-L1 in HCC patients. Similar results were observed in the mouse model in this study. Fur
It is important to explore how IL-17A enhances PD-L1 expression in HCC cells. However, the specific mechanism of IL-17A in HCC is unclear. To some extent, this study revealed a novel mechanism by which IL-17A regulates PD-L1 expression in HCC cells. Liver fibrosis, particularly cirrhosis, is closely correlated with the occurrence of HCC. The SMAD pathway is crucial for the origin and development of liver fibrosis. Therefore, many studies on the mechanism of liver cancer progression and invasion have focused on the SMAD pathway. SOX18 expression is increased by TGF-β1 via stimulation of the SMAD2/3 complex, which promotes tumor associated macrophage and regulatory T cell accumulation and HCC development[28]. KIN17 accelerates the migration ability and invasiveness of HCC cells by activating the TGF-β/SMAD2 pathway[29].
Golgi protein 73 promotes epithelial-mesenchymal transition and invasiveness of HCC cells by increasing p-SMAD2 and p-SMAD3 levels[29]. MiR-148a inhibits the development of HCC by decreasing SMAD2 expression[30]. Moreover, the relationship between SMADs and immune checkpoint molecules has been studied in other solid tumors. For example, one study revealed that p-SMAD2 expression was greater in PD-L1-positive vs PD-L1-negative non-small cell lung cancer patients[31]. However, whether IL-17A mediates PD-L1 expression through p-SMAD2 has not been reported in HCC. In the present study, we found that SMAD2 expression was increased in HCC samples compared with normal samples according to the TCGA database analysis. Further experiments revealed that IL-17A increased p-SMAD2 levels in HCC cells. Inhibition of p-SMAD2 with the pharmacological inhibitor ITD-1 markedly reduced IL-17A-related PD-L1 expression. Taken together, these results confirm that IL-17A induces PD-L1 expression through the SMAD2 pathway.
Cytokines in the IL-17A family exert their biological effects via IL-17 receptors. IL-17A/IL-17RA signaling plays a critical role in tumorigenesis and tumor development[13]. To further verify whether IL-17A regulates PD-L1 expression through IL-17RA in HCC, we silenced IL-17RA via gene knockout. The results indicated that specific deletion of IL-17RA in HCC cell lines had the opposite effect on IL-17A-induced PD-L1 expression. These findings demonstrated that IL-17A positively regulates PD-L1 protein expression by acting on IL-17RA in HCC. Another study revealed that IL-17RA could regulate the SMAD pathway in the formation of hypertrophic scars[23]. To the best of our knowledge, no study has reported the relationship between IL-17RA and the SMAD2 signaling pathway in tumors. Here, we verified that IL-17A-induced p-SMAD2 expression was reversed by IL-17RA knockout, whereas SMAD2 expression was unchanged. Collectively, these data emphasize that IL-17A induces PD-L1 protein expression in HCC cells via the IL-17RA/p-SMAD2 axis.
Atezolizumab in combination with bevacizumab has become the standard first-line therapy for advanced HCC. However, advanced HCC develops primarily from cirrhosis and is always accompanied by esophageal and gastric varices. Therefore, VEGF-neutralizing antibodies may increase the risk of bleeding in these patients. A new methodology is therefore needed to improve the therapeutic sensitivity to anti-PD-L1 antibodies beyond the VEGF-neutralizing antibody. In one study, the combination of an IL-17A inhibitor and a PD-1 inhibitor exhibited better antitumor efficacy than monotherapy in patients with colorectal cancer[32].
Research has shown that IL-17A contributes to the immune evasion of HCC cells, possibly by upregulating PD-L1 expression. IL-17A also promotes HCC cell proliferation, migration, and angiogenesis and inhibits cell apoptosis. On the basis of these results, it was necessary to assess the therapeutic efficacy of an IL-17A inhibitor in combination with an anti-PD-L1 antibody for the treatment of HCC in vivo. We expected that targeting IL-17A could delay carcinoma growth and significantly enhance immune therapeutic efficacy in an HCC mouse model. However, anti-PD-L1 monotherapy was not sufficient to exert a strong antitumor effect. These results are consistent with the idea that combining an IL-17A inhibitor with an anti-PD-L1 antibody has a synergistic antitumor effect on HCC[33], but the dosage and frequency of drug administration in other studies were different from those in our study. Therefore, targeting IL-17A is a potential treatment strategy for optimizing PD-L1 antibodies for the treatment of HCC.
The combination of an IL-17A inhibitor and PD-1 blockade stimulates the infiltration of CD8+, interferon-γ+, CD3+ T cells into tumors and demonstrates significant efficacy in colorectal cancer models[23]. The prognosis of tumor patients is associated with CD8+ T cell enrichment in tumors[25]. IL-17A can recruit CD8+ T cells to infiltrate tumors in cancer models[14]. Clinical studies have shown that CD8+ T cell infiltration is predictive of whether patients with tumors will benefit from ICB treatment[34]. Consistent with previous work, our study also confirmed that the infiltration of CD8+ T cells in the combined treatment group was significantly greater than that in the other groups in HCC models. In addition, the data indicated that the levels of IL-17A and PD-L1 in tumor tissue and serum were lower in the combined treatment group than in the control group. This might partly explain why combination therapy is preferable for HCC.
These results provide a foundation for exploring the role of IL-17A in accelerating HCC progression in a PD-L1-dependent manner. Further clinical studies are needed to estimate the therapeutic efficacy and safety of anti-IL-17A combined with PD-L1 blockade in patients with HCC.
This study demonstrated a potential mechanism by which IL-17A upregulates PD-L1 expression through the IL-17RA/p-SMAD2 signaling pathway in HCC cells. Blockade of IL-17A enhances the therapeutic efficacy of PD-L1 antibodies for the treatment of HCC.
The authors would like to thank the staff of the Medical Frontier Innovation Research Center of the First Hospital of Lanzhou University. The authors thank two anonymous reviewers who provided helpful and constructive comments that improved the manuscript substantially.
| 1. | Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400:1345-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1284] [Cited by in RCA: 1556] [Article Influence: 389.0] [Reference Citation Analysis (41)] |
| 2. | Pinter M, Jain RK, Duda DG. The Current Landscape of Immune Checkpoint Blockade in Hepatocellular Carcinoma: A Review. JAMA Oncol. 2021;7:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 272] [Article Influence: 54.4] [Reference Citation Analysis (1)] |
| 3. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10123] [Cited by in RCA: 11498] [Article Influence: 479.1] [Reference Citation Analysis (2)] |
| 4. | Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, Kadel EE III, Koeppen H, Astarita JL, Cubas R, Jhunjhunwala S, Banchereau R, Yang Y, Guan Y, Chalouni C, Ziai J, Şenbabaoğlu Y, Santoro S, Sheinson D, Hung J, Giltnane JM, Pierce AA, Mesh K, Lianoglou S, Riegler J, Carano RAD, Eriksson P, Höglund M, Somarriba L, Halligan DL, van der Heijden MS, Loriot Y, Rosenberg JE, Fong L, Mellman I, Chen DS, Green M, Derleth C, Fine GD, Hegde PS, Bourgon R, Powles T. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3113] [Cited by in RCA: 3967] [Article Influence: 495.9] [Reference Citation Analysis (1)] |
| 5. | Liao H, Chen W, Dai Y, Richardson JJ, Guo J, Yuan K, Zeng Y, Xie K. Expression of Programmed Cell Death-Ligands in Hepatocellular Carcinoma: Correlation With Immune Microenvironment and Survival Outcomes. Front Oncol. 2019;9:883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 6. | Fang W, Zhou T, Shi H, Yao M, Zhang D, Qian H, Zeng Q, Wang Y, Jin F, Chai C, Chen T. Progranulin induces immune escape in breast cancer via up-regulating PD-L1 expression on tumor-associated macrophages (TAMs) and promoting CD8(+) T cell exclusion. J Exp Clin Cancer Res. 2021;40:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 177] [Article Influence: 35.4] [Reference Citation Analysis (1)] |
| 7. | Clark CA, Gupta HB, Sareddy G, Pandeswara S, Lao S, Yuan B, Drerup JM, Padron A, Conejo-Garcia J, Murthy K, Liu Y, Turk MJ, Thedieck K, Hurez V, Li R, Vadlamudi R, Curiel TJ. Tumor-Intrinsic PD-L1 Signals Regulate Cell Growth, Pathogenesis, and Autophagy in Ovarian Cancer and Melanoma. Cancer Res. 2016;76:6964-6974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 321] [Article Influence: 32.1] [Reference Citation Analysis (1)] |
| 8. | Zong Z, Zou J, Mao R, Ma C, Li N, Wang J, Wang X, Zhou H, Zhang L, Shi Y. M1 Macrophages Induce PD-L1 Expression in Hepatocellular Carcinoma Cells Through IL-1β Signaling. Front Immunol. 2019;10:1643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 158] [Article Influence: 22.6] [Reference Citation Analysis (1)] |
| 9. | Ploeger C, Schreck J, Huth T, Fraas A, Albrecht T, Charbel A, Ji J, Singer S, Breuhahn K, Pusch S, Köhler BC, Springfeld C, Schirmacher P, Mehrabi A, Goeppert B, Roessler S. STAT1 and STAT3 Exhibit a Crosstalk and Are Associated with Increased Inflammation in Hepatocellular Carcinoma. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 10. | Yan Y, Zheng L, Du Q, Yan B, Geller DA. Interferon regulatory factor 1 (IRF-1) and IRF-2 regulate PD-L1 expression in hepatocellular carcinoma (HCC) cells. Cancer Immunol Immunother. 2020;69:1891-1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (1)] |
| 11. | Bian Z, Wu X, Chen Q, Gao Q, Xue X, Wang Y. Oct4 activates IL-17A to orchestrate M2 macrophage polarization and cervical cancer metastasis. Cancer Immunol Immunother. 2024;73:73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (1)] |
| 12. | Cui G, Li Z, Florholmen J, Goll R. Dynamic stromal cellular reaction throughout human colorectal adenoma-carcinoma sequence: A role of TH17/IL-17A. Biomed Pharmacother. 2021;140:111761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 13. | Wang X, Wu S, Wu W, Zhang W, Li L, Liu Q, Yan Z. Candida albicans Promotes Oral Cancer via IL-17A/IL-17RA-Macrophage Axis. mBio. 2023;14:e0044723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (1)] |
| 14. | Feng WQ, Zhang YC, Xu ZQ, Yu SY, Huo JT, Tuersun A, Zheng MH, Zhao JK, Zong YP, Lu AG. IL-17A-mediated mitochondrial dysfunction induces pyroptosis in colorectal cancer cells and promotes CD8 + T-cell tumour infiltration. J Transl Med. 2023;21:335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 62] [Reference Citation Analysis (1)] |
| 15. | Wang X, Yang L, Huang F, Zhang Q, Liu S, Ma L, You Z. Inflammatory cytokines IL-17 and TNF-α up-regulate PD-L1 expression in human prostate and colon cancer cells. Immunol Lett. 2017;184:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 266] [Article Influence: 29.6] [Reference Citation Analysis (1)] |
| 16. | Liao H, Chang X, Gao L, Ye C, Qiao Y, Xie L, Lin J, Cai S, Dong H. IL-17A promotes tumorigenesis and upregulates PD-L1 expression in non-small cell lung cancer. J Transl Med. 2023;21:828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (1)] |
| 17. | Wang S, Wang G, Zhang L, Li F, Liu K, Wang Y, Shi Y, Cao K. Interleukin-17 promotes nitric oxide-dependent expression of PD-L1 in mesenchymal stem cells. Cell Biosci. 2020;10:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 18. | Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, Li Y. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. 2021;6:263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 1738] [Article Influence: 347.6] [Reference Citation Analysis (2)] |
| 19. | Wang JT, Li H, Zhang H, Chen YF, Cao YF, Li RC, Lin C, Wei YC, Xiang XN, Fang HJ, Zhang HY, Gu Y, Liu X, Zhou RJ, Liu H, He HY, Zhang WJ, Shen ZB, Qin J, Xu JJ. Intratumoral IL17-producing cells infiltration correlate with antitumor immune contexture and improved response to adjuvant chemotherapy in gastric cancer. Ann Oncol. 2019;30:266-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 20. | Mattarollo SR, Loi S, Duret H, Ma Y, Zitvogel L, Smyth MJ. Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors. Cancer Res. 2011;71:4809-4820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 268] [Article Influence: 17.9] [Reference Citation Analysis (1)] |
| 21. | Ma HY, Yamamoto G, Xu J, Liu X, Karin D, Kim JY, Alexandrov LB, Koyama Y, Nishio T, Benner C, Heinz S, Rosenthal SB, Liang S, Sun M, Karin G, Zhao P, Brodt P, Mckillop IH, Quehenberger O, Dennis E, Saltiel A, Tsukamoto H, Gao B, Karin M, Brenner DA, Kisseleva T. IL-17 signaling in steatotic hepatocytes and macrophages promotes hepatocellular carcinoma in alcohol-related liver disease. J Hepatol. 2020;72:946-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 169] [Article Influence: 28.2] [Reference Citation Analysis (2)] |
| 22. | Bausch D, Pausch T, Krauss T, Hopt UT, Fernandez-del-Castillo C, Warshaw AL, Thayer SP, Keck T. Neutrophil granulocyte derived MMP-9 is a VEGF independent functional component of the angiogenic switch in pancreatic ductal adenocarcinoma. Angiogenesis. 2011;14:235-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (1)] |
| 23. | Liu C, Liu R, Wang B, Lian J, Yao Y, Sun H, Zhang C, Fang L, Guan X, Shi J, Han S, Zhan F, Luo S, Yao Y, Zheng T, Zhang Y. Blocking IL-17A enhances tumor response to anti-PD-1 immunotherapy in microsatellite stable colorectal cancer. J Immunother Cancer. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 186] [Article Influence: 37.2] [Reference Citation Analysis (1)] |
| 24. | Aotsuka A, Matsumoto Y, Arimoto T, Kawata A, Ogishima J, Taguchi A, Tanikawa M, Sone K, Mori-Uchino M, Tsuruga T, Oda K, Kawana K, Osuga Y, Fujii T. Interleukin-17 is associated with expression of programmed cell death 1 ligand 1 in ovarian carcinoma. Cancer Sci. 2019;110:3068-3078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 25. | Picard FSR, Lutz V, Brichkina A, Neuhaus F, Ruckenbrod T, Hupfer A, Raifer H, Klein M, Bopp T, Pfefferle PI, Savai R, Prinz I, Waisman A, Moos S, Chang HD, Heinrich S, Bartsch DK, Buchholz M, Singh S, Tu M, Klein L, Bauer C, Liefke R, Burchert A, Chung HR, Mayer P, Gress TM, Lauth M, Gaida M, Huber M. IL-17A-producing CD8(+) T cells promote PDAC via induction of inflammatory cancer-associated fibroblasts. Gut. 2023;72:1510-1522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (1)] |
| 26. | Wei Y, Shi D, Liang Z, Liu Y, Li Y, Xing Y, Liu W, Ai Z, Zhuang J, Chen X, Gao Q, Jiang J. IL-17A secreted from lymphatic endothelial cells promotes tumorigenesis by upregulation of PD-L1 in hepatoma stem cells. J Hepatol. 2019;71:1206-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 27. | Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, Xu Y, Fan J. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 666] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 28. | Chen J, Feng W, Sun M, Huang W, Wang G, Chen X, Yin Y, Chen X, Zhang B, Nie Y, Fan D, Wu K, Xia L. TGF-β1-Induced SOX18 Elevation Promotes Hepatocellular Carcinoma Progression and Metastasis Through Transcriptionally Upregulating PD-L1 and CXCL12. Gastroenterology. 2024;167:264-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 66] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 29. | Dai Z, Huang Q, Huang X, Zhu C, Zahid KR, Liu T, Li Q, Wu C, Peng M, Xiao X, Raza U, Yu N, Zeng T. KIN17 promotes cell migration and invasion through stimulating the TGF-β/Smad2 pathway in hepatocellular carcinoma. Mol Carcinog. 2023;62:369-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 30. | Huang Z, Wen J, Yu J, Liao J, Liu S, Cai N, Liang H, Chen X, Ding Z, Zhang B. MicroRNA-148a-3p inhibits progression of hepatocelluar carcimoma by repressing SMAD2 expression in an Ago2 dependent manner. J Exp Clin Cancer Res. 2020;39:150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 31. | David JM, Dominguez C, McCampbell KK, Gulley JL, Schlom J, Palena C. A novel bifunctional anti-PD-L1/TGF-β Trap fusion protein (M7824) efficiently reverts mesenchymalization of human lung cancer cells. Oncoimmunology. 2017;6:e1349589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 151] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 32. | Li S, Na R, Li X, Zhang Y, Zheng T. Targeting interleukin-17 enhances tumor response to immune checkpoint inhibitors in colorectal cancer. Biochim Biophys Acta Rev Cancer. 2022;1877:188758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 33. | Song M, Wang L, Jiang S, Liang J, Li W, Rao W, Du Q, Liu G, Meng H, Tang L, Li Z, Yang Y, Zhang L, Zhang B. Pathogenic Th17 cell-mediated liver fibrosis contributes to resistance to PD-L1 antibody immunotherapy in hepatocellular carcinoma. Int Immunopharmacol. 2024;129:111601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 34. | Chalabi M, Fanchi LF, Dijkstra KK, Van den Berg JG, Aalbers AG, Sikorska K, Lopez-Yurda M, Grootscholten C, Beets GL, Snaebjornsson P, Maas M, Mertz M, Veninga V, Bounova G, Broeks A, Beets-Tan RG, de Wijkerslooth TR, van Lent AU, Marsman HA, Nuijten E, Kok NF, Kuiper M, Verbeek WH, Kok M, Van Leerdam ME, Schumacher TN, Voest EE, Haanen JB. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. 2020;26:566-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 1006] [Article Influence: 167.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/