Published online Jan 15, 2025. doi: 10.4251/wjgo.v17.i1.100757
Revised: September 26, 2024
Accepted: October 30, 2024
Published online: January 15, 2025
Processing time: 108 Days and 21.9 Hours
Gallbladder neuroendocrine carcinoma (NEC) represents a subtype of gallbladder malignancies characterized by a low incidence, aggressive nature, and poor prognosis. Despite its clinical severity, the genetic alterations, mechanisms, and signaling pathways underlying gallbladder NEC remain unclear.
This case study presents a rare instance of primary gallbladder NEC in a 73-year-old female patient, who underwent a radical cholecystectomy with hepatic hilar lymphadenectomy and resection of liver segments IV-B and V. Targeted gene sequencing and bioinformatics analysis tools, including STRING, GeneMANIA, Metascape, TRRUST, Sangerbox, cBioPortal and GSCA, were used to analyze the biological functions and features of mutated genes in gallbladder NEC. Twelve mutations (APC, ARID2, IFNA6, KEAP1, RB1, SMAD4, TP53, BTK, GATA1, GNAS, and PRDM3) were identified, and the tumor mutation burden was determined to be 9.52 muts/Mb via targeted gene sequencing. A protein-protein interaction network showed significant interactions among the twelve mutated genes. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes analyses were used to assess mutation functions and pathways. The results revealed 40 tumor-related pathways. A key regulatory factor for gallbladder NEC-related genes was identified, and its biological functions and features were compared with those of gallbladder carcinoma.
Gallbladder NEC requires standardized treatment. Comparisons with other gallbladder carcinomas revealed clinical phenotypes, molecular alterations, functional characteristics, and enriched pathways.
Core Tip: Gallbladder neuroendocrine carcinoma (NEC) is a rare and aggressive malignancy with unclear genetic alterations and mechanisms. Our case study of a 73-year-old female with gallbladder NEC revealed 12 mutated genes (APC, ARID2, IFNA6, KEAP1, RB1, SMAD4, TP53, BTK, GATA1, GNAS, JAK2, PRDM3) and a high tumor mutation burden. Bioin
- Citation: Yang YC, Chen ZT, Wan DL, Tang H, Liu ML. Targeted gene sequencing and bioinformatics analysis of patients with gallbladder neuroendocrine carcinoma: A case report. World J Gastrointest Oncol 2025; 17(1): 100757
- URL: https://www.wjgnet.com/1948-5204/full/v17/i1/100757.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i1.100757
Neuroendocrine carcinoma (NEC) predominantly affects the digestive and respiratory tracts, including the stomach, intestines, pancreas, and lung, accounting for approximately 97% of all NEC cases[1]. However, the occurrence of gallbladder NEC is rare in clinical practice[2]. Owing to a lack of specific guidelines or consensus, treatment for gallbladder NEC often follows protocols for gallbladder adenocarcinoma[3]. Notably, gallbladder NEC exhibits ag
This case report describes an instance of gallbladder NEC in a 73-year-old female patient, incorporating targeted gene sequencing with standard histopathological evaluation. Notably, the mutational spectrum, regulatory factors, functional interactions, and enriched pathways of mutated genes in gallbladder NEC have not been studied thoroughly. This study aimed to explore the clinical profiles, genetic features, and clinical management of gallbladder NEC, while comparing its biological characteristics to those of gallbladder adenocarcinoma to obtain crucial insights into this disease. The report follows the CARE checklist for clinical case reporting.
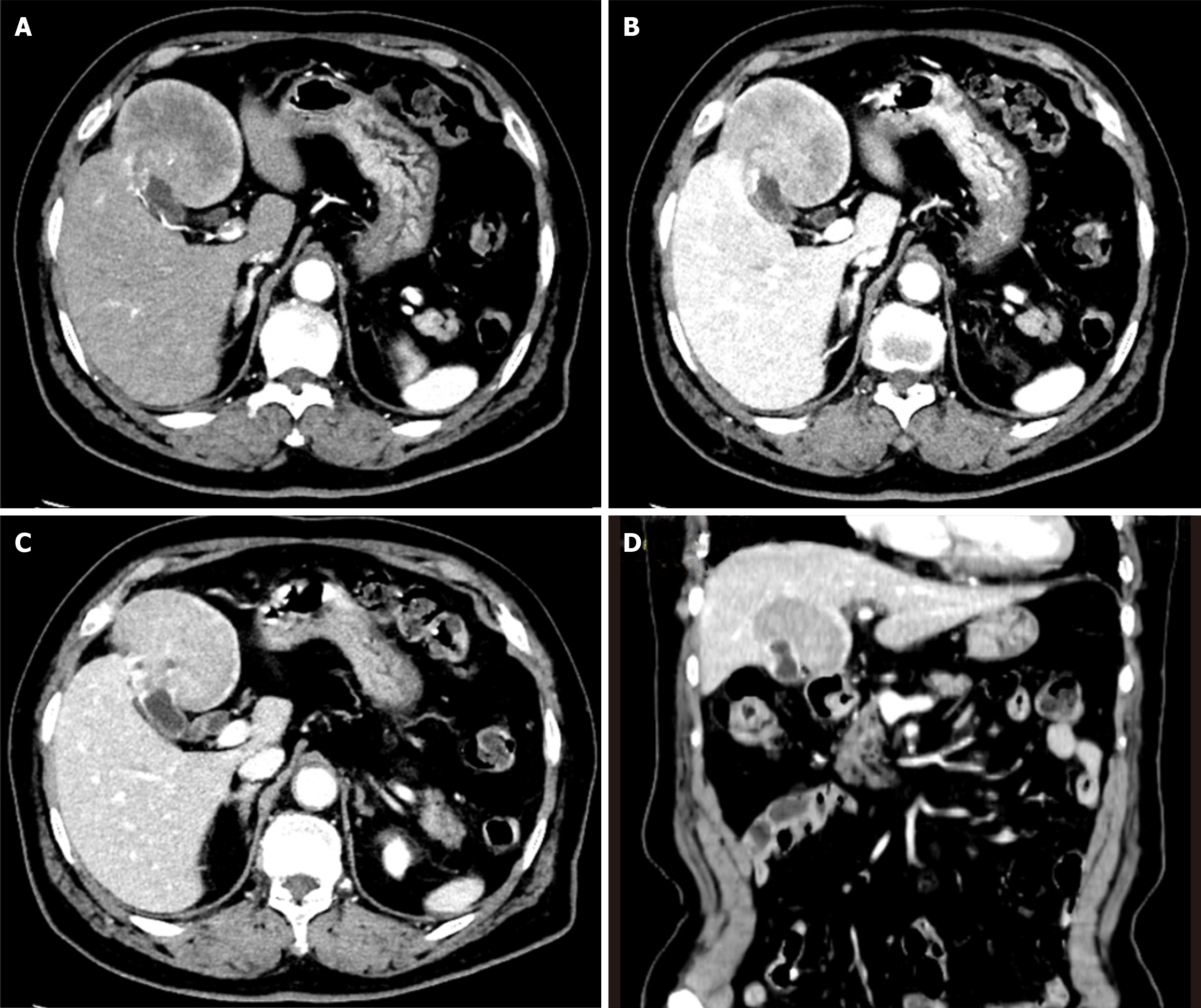
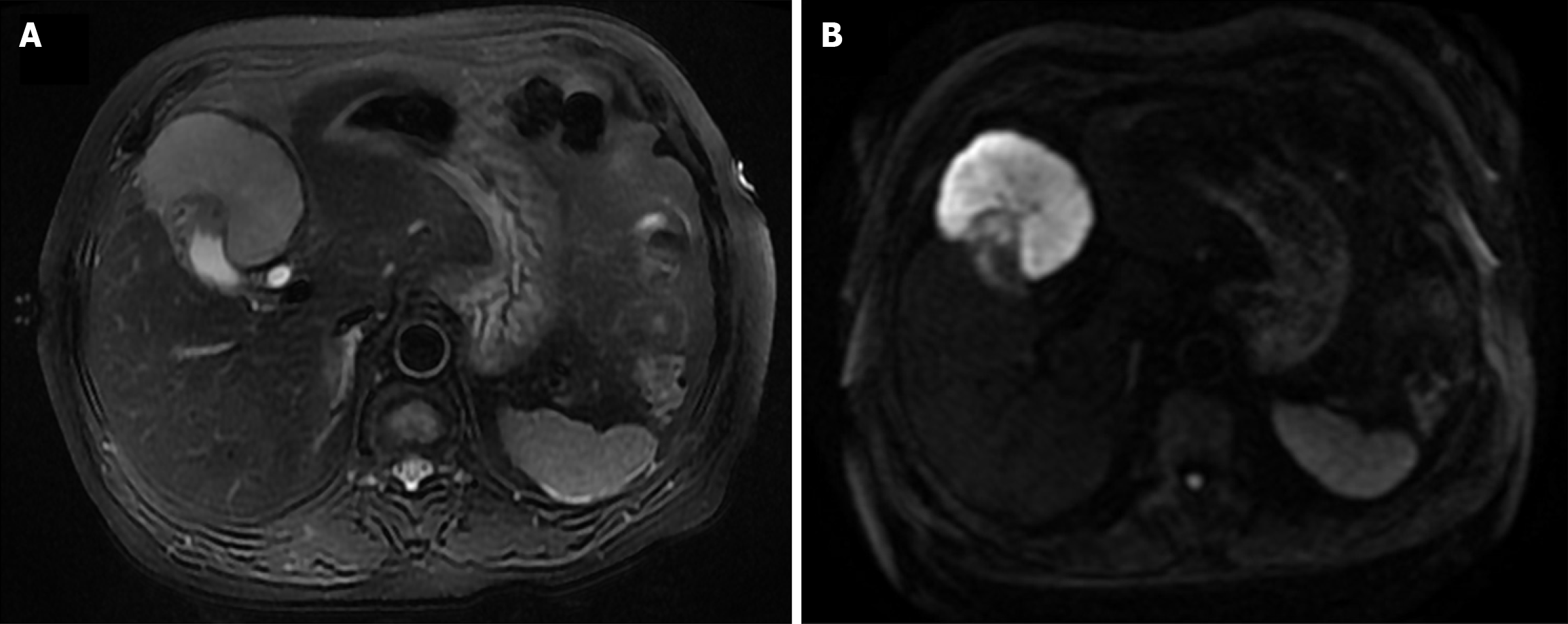
A 73-year-old Chinese female presented with upper abdominal discomfort lasting for one week on December 30, 2019. An initial abdominal ultrasonography revealed a 7 cm hyperechoic focus with ill-defined margins and multiple gallbladder stones in the context of cholecystitis. No edema, anemia, jaundice, hepatomegaly, or splenomegaly were observed on admission. The patient did not experience discomfort, weight loss, fever, or night sweats. A history of hypertension for 5 years and cholelithiasis for 10 years was noted. Levels of tumor markers, including carcinoembryonic antigen, carbohydrate antigen 19-9, and alpha-fetoprotein, were normal. Biochemical tests for aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, and bilirubin were also within the normal range. Abdominal contrast-enhanced computed tomography (CT) demonstrated a 7 cm mass in liver segments IVb and V, accompanied by thickening of the gallbladder fundus wall with early and prolonged enhancement. Multiple gallbladder stones were also identified. No pancreatic enlargement or bile duct dilatation was observed (Figure 1). Dynamic contrast-enhanced magnetic resonance imaging (MRI) showed a 6.7 cm mass with low T1 and high T2 signal intensities, along with diffusion-weighted imaging findings (Figure 2). Given the imaging features, gallbladder carcinoma with liver invasion and cholelithiasis was initially suspected. After discussions with a multidisciplinary team, a diagnosis of extensive gallbladder carcinoma with liver invasion and cholelithiasis was confirmed. Surgical resection was planned prior to chemotherapy, considering the potential complications of cholelithiasis and the patient's refusal to undergo chemotherapy.
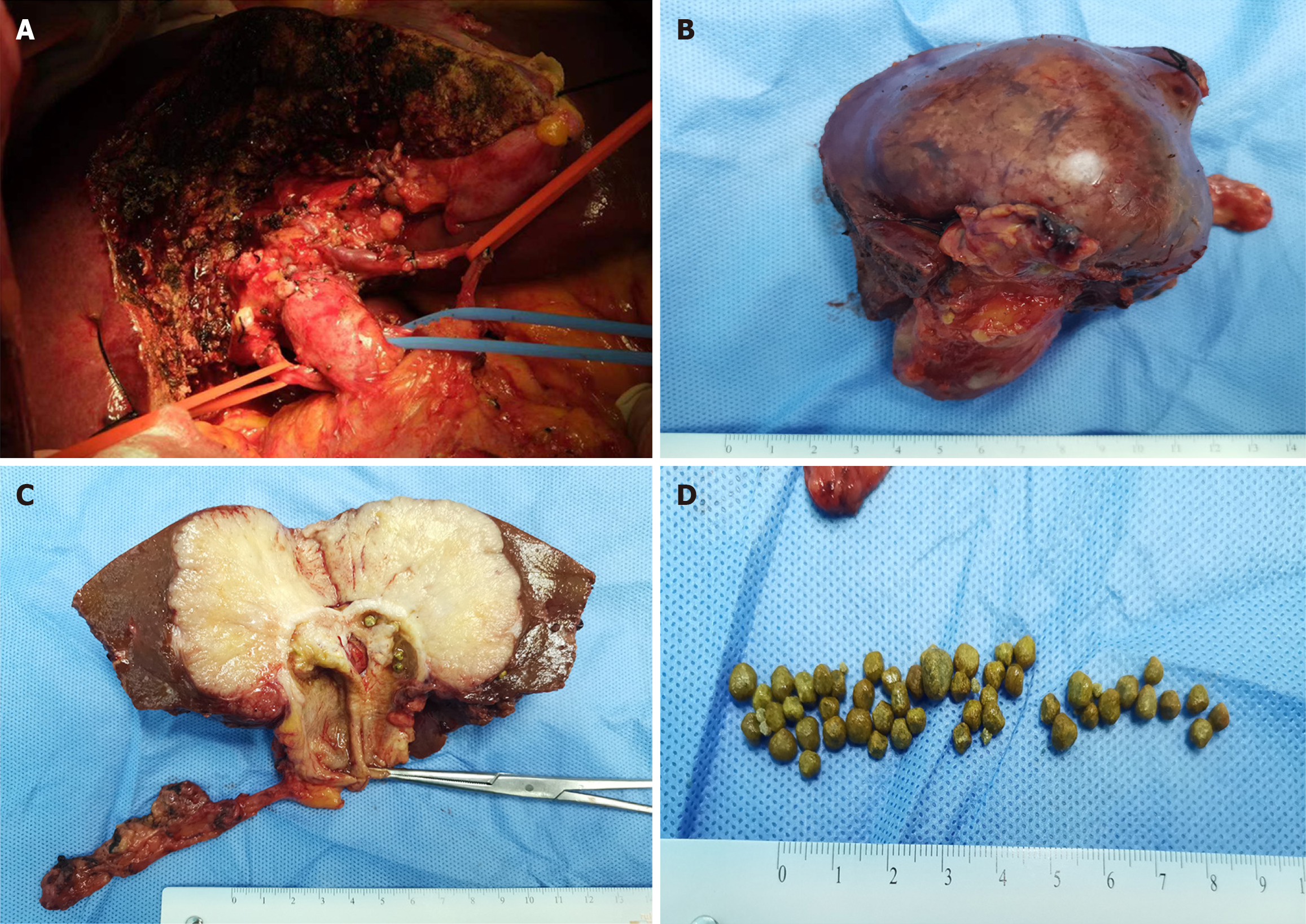
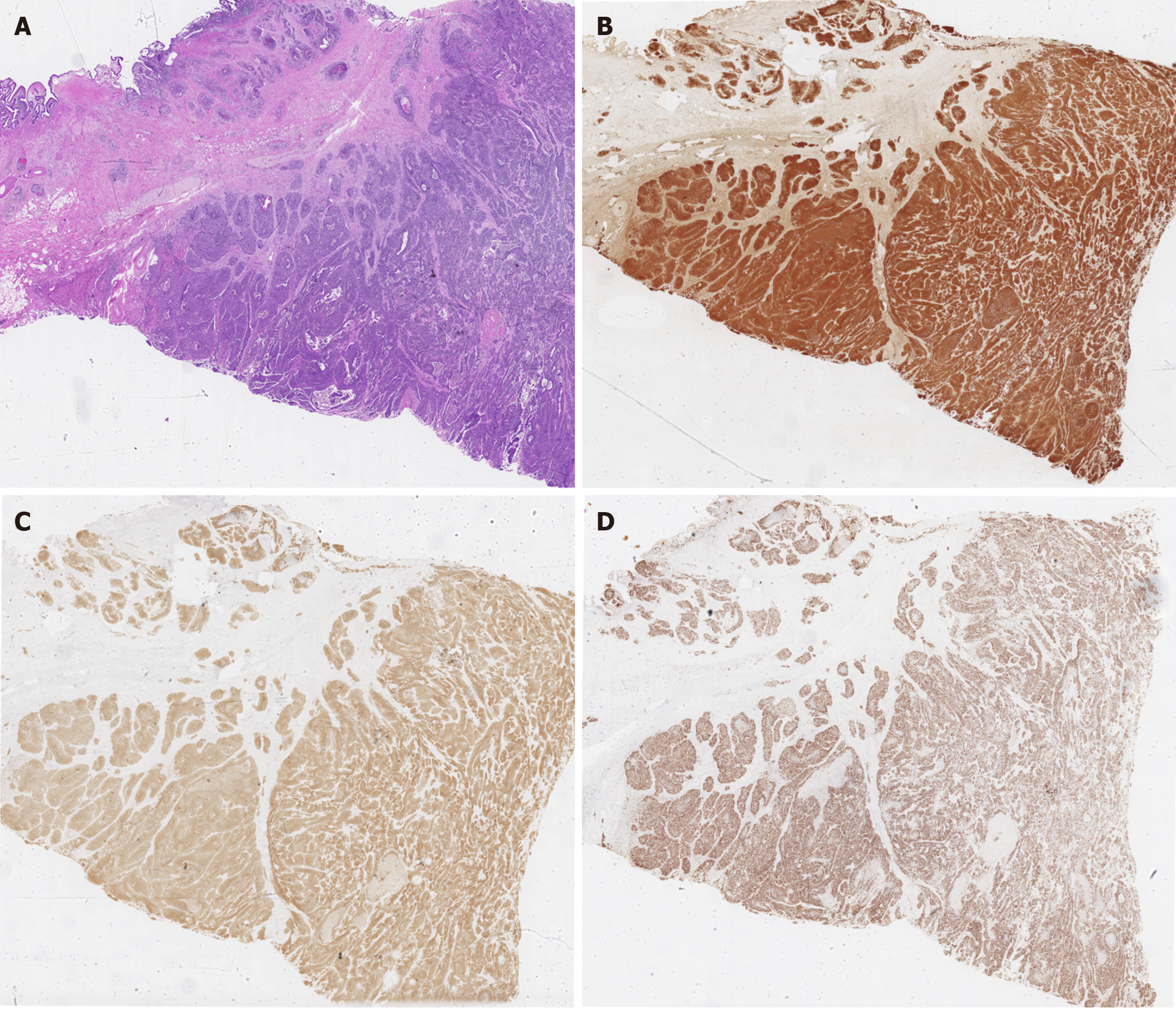
A radical cholecystectomy with hepatic hilar lymphadenectomy and resection of liver segments IV-B and V was performed. A 6 cm × 7 cm greyish-yellow globular lesion, largely occupied by multiple yellow stones, was macroscopically identified at the gallbladder fundus (Figure 3). Immunohistochemical analysis revealed a nest-like pattern of heterogeneous tumor cells undergoing necrosis infiltrating the gallbladder and liver. These cells were positive for CK7, CK19, CK (pan), chromogranin A (CgA), and synaptophysin (Syn), while negative results were obtained for CD56, CK5/6, P40, and hepatocytes. Notably, the mitotic count was 24 per 10 high-power fields, along with a Ki-67 index of 70%, indicating a poorly differentiated NEC (Figure 4).
Comprehensive genetic testing was performed on tumor DNA extracted from the gallbladder NEC, encompassing selected introns of 448 cancer-related genes and all exons. As summarized in Table 1, twelve somatic mutations were identified. The tumor mutation burden (TMB) was determined to be 9.52 muts/Mb. An immunohistochemical assay for PD-L1 was performed to predict the response to PD-1/PD-L1 inhibitors, and the results revealed a lack of expression in the gallbladder NEC. Additionally, the tumor proportion score and combined positivity score were calculated to assess the immunohistochemical expression of PD-L1, as depicted in Figure 5.
| Genes | Original name | Cytoband | Exon count | Variant type | Abundance variation |
| APC | APC regulator of WNT signaling pathway | 5q22.2 | 20 | Copy number variation | CN: 1.2 |
| ARID2 | AT-rich interaction domain 2 | 12q12 | 22 | c.3382C>T (p.Q1128) | 77.3% |
| IFNA6 | interferon alpha 6 | 9p21.3 | 1 | c.53C>G (p.S18) | 42.8% |
| KEAP1 | Kelch like ECH associated protein 1 | 19p13.2 | 7 | c.1408C>T (p.R470C) | 87.9% |
| RB1 | RB transcriptional corepressor 1 | 13q14.2 | 27 | c.772-776del (p.N258Efs1) | 76.1% |
| SMAD4 | SMAD family member 4 | 18q21.2 | 12 | Copy number variation | CN: 1.1 |
| TP53 | tumor protein p53 | 17p13.1 | 11 | Copy number variation | CN: 1.1 |
| c.574C>T (p.Q192) | 81.3% | ||||
| BTK | Bruton tyrosine kinase | Xq22.1 | 21 | c.574C>T (p.D232V) | 48.6% |
| GATA1 | GATA binding protein 1 | Xp11.23 | 6 | c.173C>A (p.A58E) | 42.5% |
| GNAS | GNAS complex locus | 20q13.32 | 22 | c.1048G>C (p.E350Q) | 42.9% |
| MECOM | MDS1 and EVI1 complex locus | 3q26.2 | 24 | c.1161G>T (p.Q387H) | 2.4% |
The patient recovered smoothly and was discharged on the 8th day post-surgery. One month later, six cycles of chemotherapy, consisting of cisplatin (area under the curve of 5 on day 1, repeated every 21 days) and etoposide (80 mg/m2 on days 1-3, repeated every 21 days), were administered. However, at two months post-discharge, an MRI scan revealed a recurrence in segment VI of the liver. Subsequently, second-line oral chemotherapy was initiated with capecitabine (3 tablets BID, days 1-14) and temozolomide (200 mg, days 10-14), repeated every 3 weeks. After 1 month, multiple recurrences were detected in the liver, leading to disease progression and the patient's demise 15 months post-surgery.
A 73-year-old Chinese female presented with upper abdominal discomfort lasting for one week on December 30, 2019. An initial abdominal ultrasonography revealed a 7 cm hyperechoic focus with ill-defined margins and multiple gallbladder stones in the context of cholecystitis. No edema, anemia, jaundice, hepatomegaly, or splenomegaly were observed on admission. The patient did not experience discomfort, weight loss, fever, or night sweats.
A history of hypertension for 5 years and cholelithiasis for 10 years was noted.
Levels of tumor markers, including carcinoembryonic antigen, carbohydrate antigen 19-9, and alpha-fetoprotein, were normal. Biochemical tests for AST, ALT, albumin, and bilirubin were also within the normal range.
Abdominal contrast-enhanced CT demonstrated a 7 cm mass in liver segments IVb and V, accompanied by thickening of the gallbladder fundus wall with early and prolonged enhancement. Multiple gallbladder stones were also identified. No pancreatic enlargement or bile duct dilatation was observed (Figure 1). Dynamic contrast-enhanced MRI showed a 6.7 cm mass with low T1 and high T2 signal intensities, along with diffusion-weighted imaging findings (Figure 2).
After discussions with a multidisciplinary team, a diagnosis of extensive gallbladder carcinoma with liver invasion and cholelithiasis was confirmed. Surgical resection was planned prior to chemotherapy, considering the potential complications of cholelithiasis and the patient's refusal to undergo chemotherapy.
Given the imaging features, gallbladder carcinoma with liver invasion and cholelithiasis was initially suspected.
A radical cholecystectomy with hepatic hilar lymphadenectomy and resection of liver segments IV-B and V was performed. A 6 cm × 7 cm greyish-yellow globular lesion, largely occupied by multiple yellow stones, was macroscopically identified at the gallbladder fundus (Figure 3).
The patient recovered smoothly and was discharged on the 8th day post-surgery. One month later, six cycles of chemotherapy, consisting of cisplatin (area under the curve of 5 on day 1, repeated every 21 days) and etoposide (80 mg/m2 on days 1-3, repeated every 21 days), were administered. However, at two months post-discharge, an MRI scan revealed a recurrence in segment VI of the liver. Subsequently, second-line oral chemotherapy was initiated with capecitabine (3 tablets BID, days 1-14) and temozolomide (200 mg, days 10-14), repeated every 3 weeks. After 1 month, multiple recurrences were detected in the liver, leading to disease progression and the patient's demise 15 months post-surgery.
It has been hypothesized that gallbladder NEC evolves from adenocarcinoma, and the interconversion between NET tumors and adenocarcinomas in the gastrointestinal tract has been reported previously[5]. However, our understanding of the differences between gallbladder NEC and gallbladder carcinoma remains limited. This study reviews existing literature and uses bioinformatics analysis to compare the clinicopathologic and genetic characteristics of gallbladder NECs and gallbladder carcinomas.
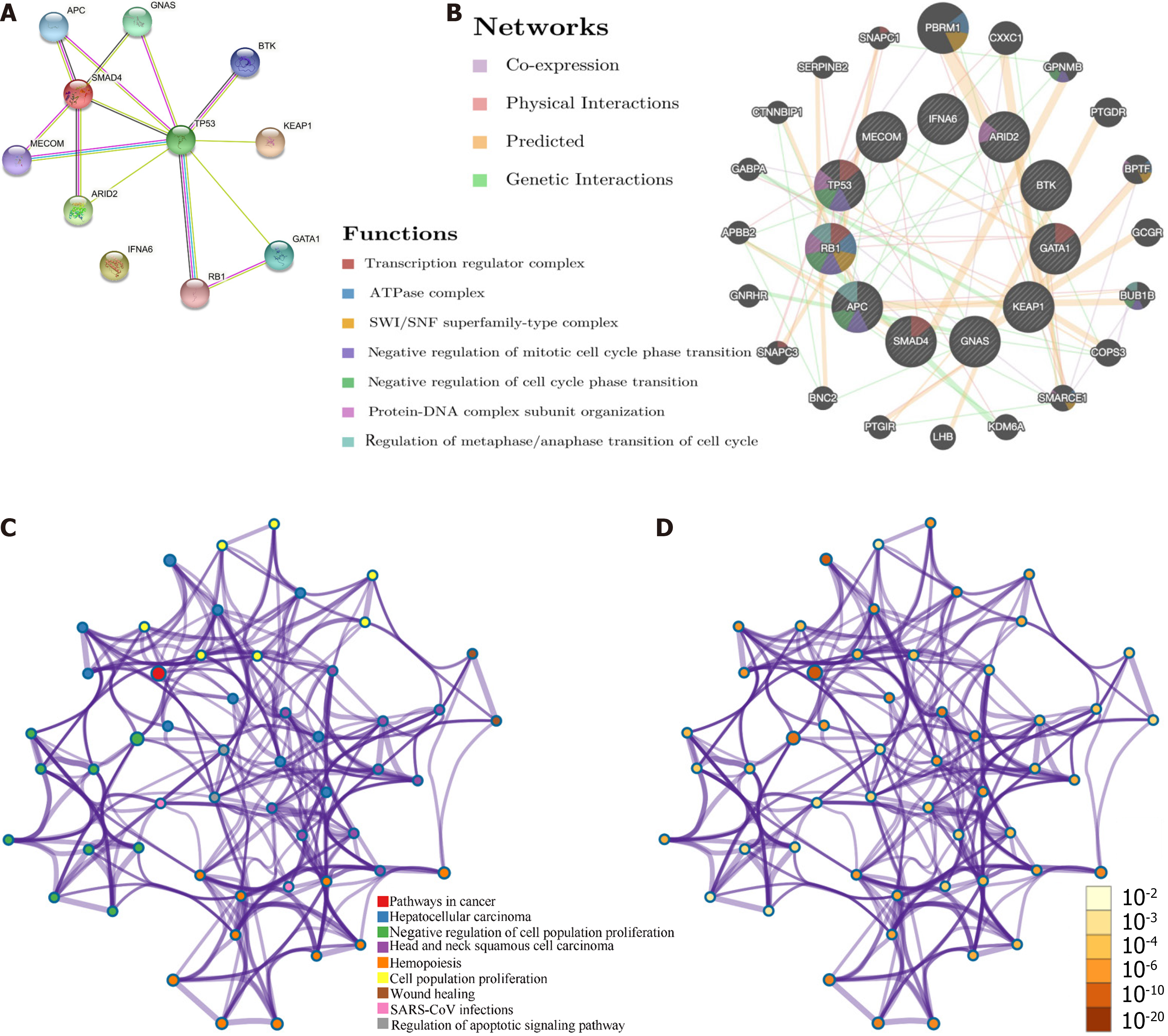
Targeted gene sequencing helped identify 12 mutations unique to the specimen (Table 1). To understand the biological properties and potential therapeutic targets of this rare tumor more effectively, bioinformatics databases were utilized to explore oncogenic mechanisms. Protein-protein interaction networks (PPIs) were analyzed and visualized using the STRING[6] (version: 11.5, https://string-db.org/) database. The resulting STRING network consisted of 11 nodes and 14 edges, with an average local clustering coefficient of 0.776 (PPI enrichment P value < 0.0001; Figure 6A). GeneMANIA (http://genemania.org/) was used to explore the potential biological mechanisms, and our results[7] revealed that the mutated genes were associated with the transcription regulator complex, ATPase complex, SWI/SNF superfamily-type complex, negative regulation of mitotic cell cycle phase transition, negative regulation of cell cycle phase transition, protein-DNA complex subunit organization, and regulation of metaphase/anaphase transition of cell cycle (Figure 6B). In addition, protein-protein interaction enrichment analysis was performed using the Metascape (https://metascape.org/) database (Figure 6C and D).
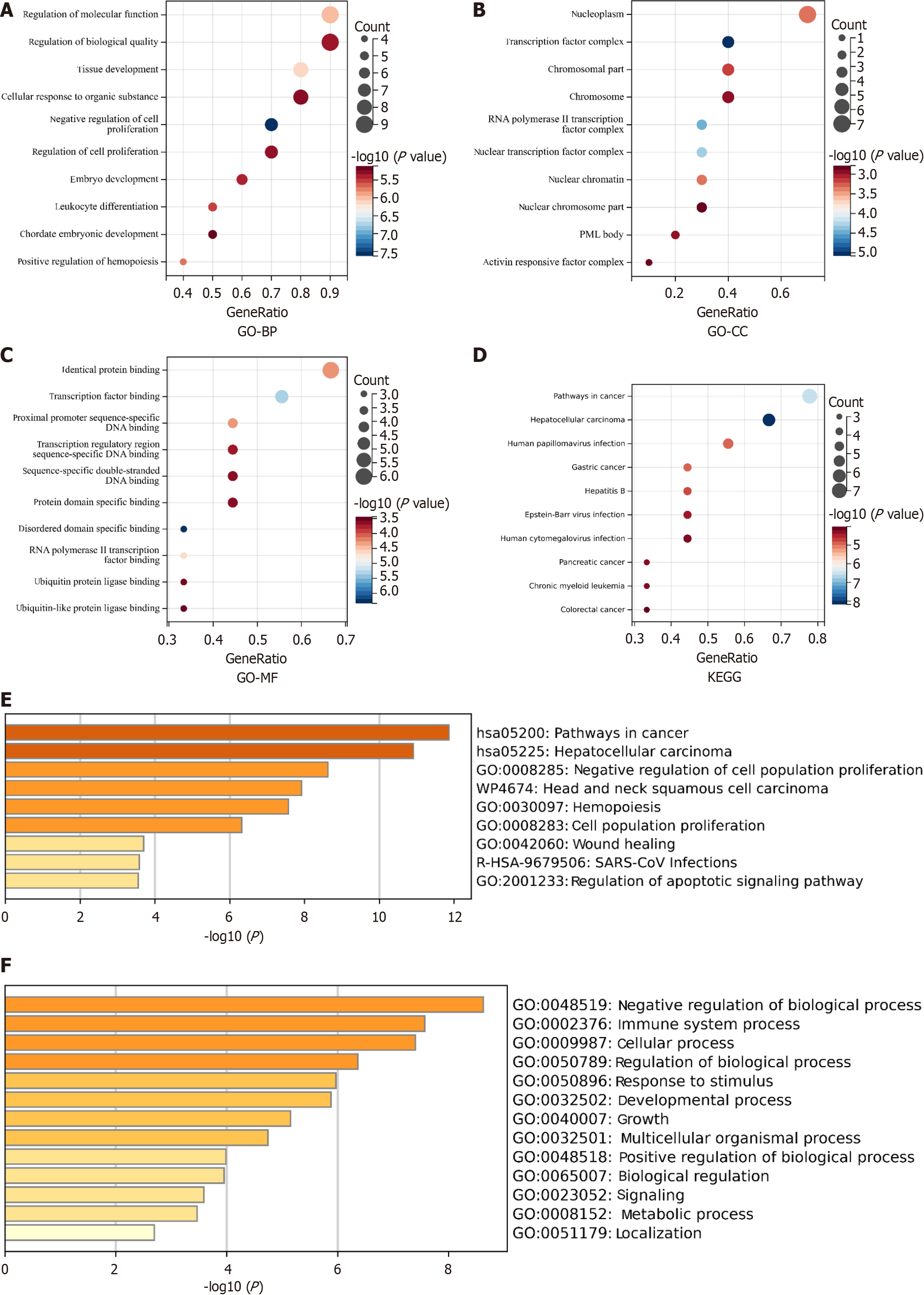
Transcription factors are important regulators of gene expression that play a crucial role in tumor development. The TRRUST[8] (version 2, https://www.grnpedia.org/trrust/) database was used to identify the transcription factors associated with these mutated genes in humans. YY1, KAT2B, PAX5, DNMT1, EZH2, FOS, GATA1, SPI1, MYC, E2F1, and TP53 were identified as pivotal transcription factors linked to these mutations (Table 2). Using the Sangerbox 3.0 (http://vip.sangerbox.com) database, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses were performed to explore the potential biological functions of these genes. GO analysis of biological processes suggested that these genes might be involved in regulating molecular function (MF) and biological quality, as well as tissue development (Figure 7A). GO analysis of cellular components indicated that these genes were mainly located in the nucleoplasm, transcription factor complex, and chromosomes (Figure 7B). GO analysis of the MF showed enrichment in identical protein binding, transcription factor binding, and proximal promoter sequence-specific DNA binding (Figure 7C). KEGG enrichment analysis revealed potential involvement in cancer pathways, hepatocellular carcinomas, and human papillomavirus infections (Figure 7D). These results are consistent with the findings from the Metascape database (Figure 2E and F).
| Key TF | Description | Overlapped genes | P value | FDR | |
| 1 | YY1 | YY1 transcription factor | APC, GNAS, TP53 | 1.74E-05 | 1.32E-04 |
| 2 | KAT2B | K(lysine) acetyltransferase 2B | RB1, SMAD4 | 2.40E-05 | 1.32E-04 |
| 3 | PAX5 | Paired box 5 | RB1, TP53 | 6.45E-05 | 2.36E-04 |
| 4 | DNMT1 | DNA (cytosine-5-)-methyltransferase 1 | RB1, TP53 | 1.42E-04 | 3.91E-04 |
| 5 | EZH2 | Enhancer of zeste homolog 2 (Drosophila) | APC, TP53 | 2.38E-04 | 5.24E-04 |
| 6 | FOS | FBJ murine osteosarcoma viral oncogene homolog | SMAD4, TP53 | 4.85E-04 | 7.61E-04 |
| 7 | GATA1 | GATA binding protein 1 (globin transcription factor 1) | GATA1, GNAS | 4.85E-04 | 7.61E-04 |
| 8 | SPI1 | SFFV proviral integration oncogene spi1 | BTK, GATA1 | 5.73E-04 | 7.88E-04 |
| 9 | MYC | V-myc myelocytomatosis viral oncogene homolog (avian) | GATA1, TP53 | 1.48E-03 | 1.81E-03 |
| 10 | E2F1 | E2F transcription factor 1 | RB1, TP53 | 2.64E-03 | 2.90E-03 |
| 11 | TP53 | Tumor protein p53 | RB1, TP53 | 3.92E-03 | 3.92E-03 |
Sakaki et al[9] proposed that gallbladder NEC arises from the metamorphosis of gallbladder adenocarcinoma. A recent study involving the whole exome sequencing analysis of 151 gallbladder cancer patients identified the most common mutated genes as TP53 (27%), KMT2C (11%), SMAD4 (11%), PER3 (8%), ERBB3 (8%), ARID2 (7%), ARID1A (7%), and ERBB2 (7%), with the ErbB signaling pathway being the most commonly altered pathway[10]. Our findings indicate that the mutational profiles of gallbladder NEC partially overlap with those of gallbladder adenocarcinoma. Therefore, we further analyzed the relationship between these two tumor types.
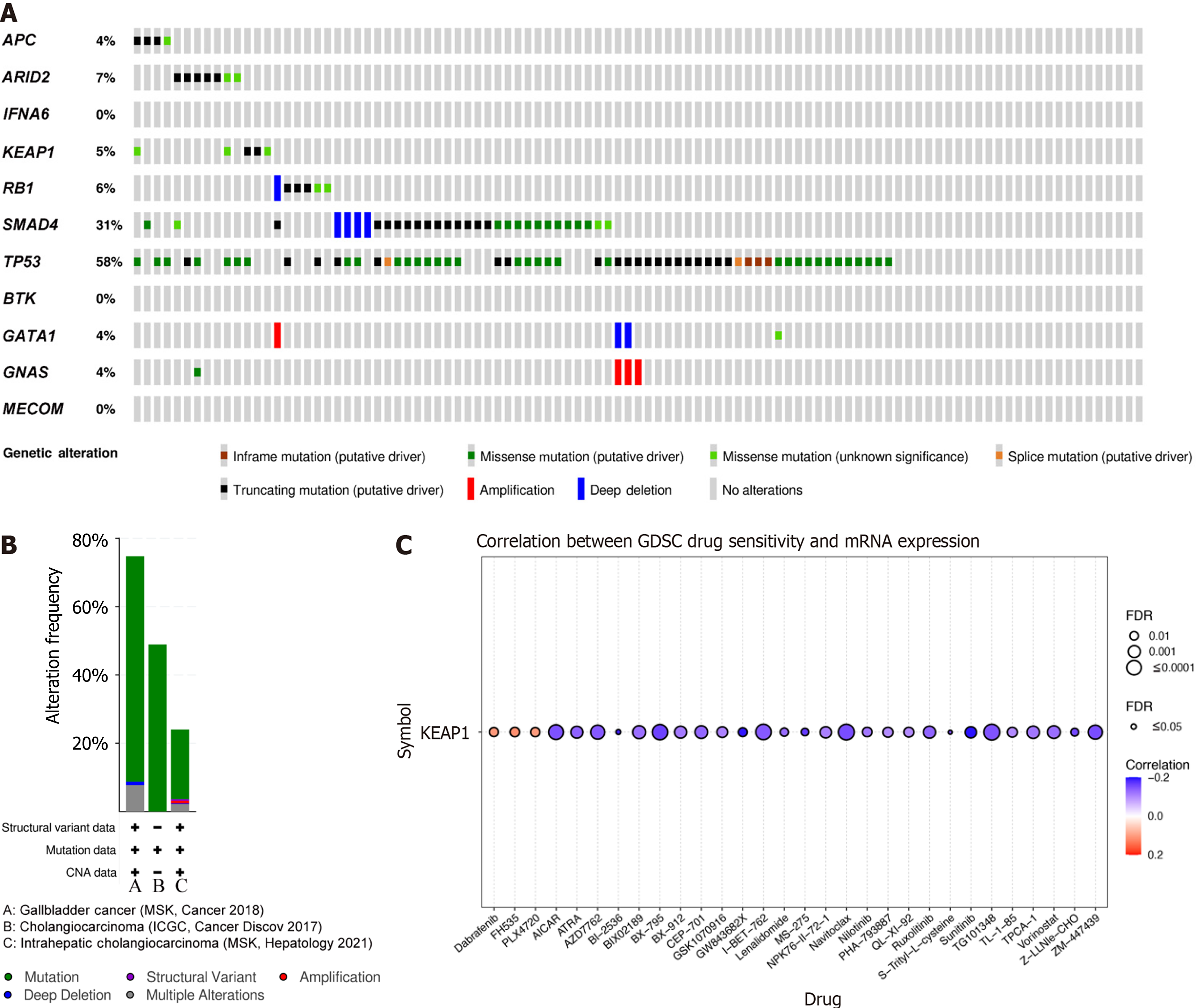
Tumorigenesis is driven by genetic mutations, making the nucleus a key target for tumor suppression. The cBioPortal (https://www.cbioportal.org/) database was used to explore gene mutation information for gallbladder carcinoma (MSK, Cancer 2018). As shown in Figure 8A, a high mutation rate was observed for SMAD4 and TP53 in gallbladder carcinoma patients. Among the 101 sequenced cases, genetic alterations were found in 31% and 58% of 3159 gallbladder carcinoma patients. Meanwhile, these genes showed the highest mutation rates in gallbladder carcinoma (74.76% of 103 cases), followed by cholangiocarcinomas (48.92% of 417 cases) and intrahepatic cholangiocarcinomas (24.03% of 412 cases; Figure 8B). Taken together, these results suggest that the identified mutations may play a crucial role in gallbladder carcinoma development.
KEAP1 is an important tumor suppressor gene. Mutations in KEAP1 reduce its affinity for Nrf2 in the cytoplasm, resulting in Nrf2 accumulation in the nucleus, which promotes tumor occurrence and development[11]. Importantly, KEAP1 mutations are found in many types of cancer, including gallbladder cancer[12-14]. Genetic mutations serve as targets for precision therapy in cancer treatment. In the present sample, KEAP1 was the most frequently mutated gene, with a mutation frequency of 87.9%. To identify potential drugs targeting KEAP1 mutations, the GSCA (http://bioinfo.life.hust.edu.cn/GSCA/) web server was used to explore drug–gene interactions. Figure 8C shows the correlation between KEAP1 expression and the sensitivity of the top 30 GDSC drugs during pan-cancer analysis.
The genetic and molecular characteristics of gallbladder NECs are poorly understood, and no molecular targeted therapies for gallbladder NEC have been developed for clinical use[15]. There is an urgent clinical need to identify molecular markers that contribute to its pathological progression and develop new therapeutic modalities. While gallbladder NECs and gallbladder adenocarcinomas are distinct entities, they are closely related to each other. However, treatment strategies for gallbladder adenocarcinoma are not entirely applicable to gallbladder neuroendocrine tumors.
The available literature on gallbladder NECs is limited to case reports and case series. Common symptoms include epigastric pain, weight loss, and anorexia, often affecting females over 60[16]. Gallbladder stones and cholecystitis are thought to promote NEC development, as in our case, where gallbladder NECs co-occurred with gallbladder stones. Although imaging modalities, including ultrasound, enhanced CT, and MRI aid in diagnosis, distinguishing NECs from other gallbladder cancers remains challenging. Gallbladder NECs often originate from the deeper layers of the lamina propria or submucosa, which might explain the partial preservation of the gallbladder mucosal epithelium and the linear enhancement seen on CT and MRI. Similar findings have been reported in previous studies on NECs in the gastrointestinal tract[17,18]. In addition, metastatic lymph nodes in gallbladder NECs tend to be larger than those in adenocarcinomas[19], complicating accurate preoperative diagnosis.
A definitive diagnosis of gallbladder NEC necessitates an integrative approach combining pathological and immunohistochemical analyses. Among the array of immunohistochemical markers, CgA, Syn, and neuro-specific enolase (NSE) are widely recognized to be essential for differentiating NECs from other gallbladder pathologies[20]. CgA is a secretory protein found in neural and neuroendocrine tissues, including the adrenal medulla, thyroid C-cells, and certain endocrine tumors. In the context of gallbladder NEC, the detection of CgA through immunohistochemistry analysis indicates the presence of neuroendocrine elements or neuroendocrine differentiation in the necrotic process. Syn, another important biomarker, is a synaptic vesicle protein expressed in neurons and neuroendocrine cells. Its presence in gallbladder NEC tissues is suggestive of neuronal or neuroendocrine differentiation, which further supports disease diagnosis. NSE, also known as gamma-enolase, is an enzyme found primarily in neurons and neuroendocrine cells. Elevated levels of NSE in the context of gallbladder NEC can signify neuronal damage or the presence of neuroendocrine components within necrotic tissues. The combined use of these biomarkers, along with standard pathological examination, significantly enhances the accuracy of diagnosis of gallbladder NEC. Healthcare professionals can make more informed treatment decisions by identifying the presence and distribution of these specific proteins.
In a previous study involving 21 patients with gallbladder NECs, over 80% showed positive staining for CgA and Syn[21]. Another study involving 15 patients with gallbladder NECs reported positive rates of 92.3% and 100% for CgA and Syn, respectively[22]. Consistent with these findings, immunohistochemical results of the patients showed positivity for CgA and Syn. However, the occurrence of NSE was not verified through immunohistochemistry analysis. Additionally, a previous study has reported that an elevated Ki-67 index and high mitotic rate are strongly associated with poor prognosis[21]. Unfortunately, the patients in this study exhibited high Ki-67 index and mitotic indices. Despite postoperative adjuvant chemotherapy, the tumor recurred rapidly and the patient succumbed to the disease 15 months after surgery.
Globally, there are no definitive guidelines or consensus on optimal treatment strategies for gallbladder NEC. Treatment options for gallbladder NEC are typically guided by recommendations for gallbladder cancer. In early-stage gallbladder NEC, radical surgery can improve long-term survival, while chemotherapy serves as a palliative treatment modality for advanced cases, though its efficacy remains debatable. Wang et al[3] assessed 62 patients with gallbladder NECs and found no significant impact of postoperative adjuvant chemotherapy on overall survival. Similarly, a large multicenter study in China reported no improvement in long-term survival using platinum-based chemotherapy as an adjuvant therapy[16]. However, a Japanese case report enumerated upon a complete response to a combination of cisplatin, irinotecan, and radiotherapy in advanced gallbladder NEC, with no recurrence after 3 years of follow-up[23]. Ayabe et al[24] analyzed survival outcomes for patients with gastrointestinal NETs and gallbladder adenocarcinomas using a national database, revealing that gallbladder NECs are associated with the poorest survival among gas
The dependence of the study on a single case report, albeit justified given the rarity of gallbladder NEC, inherently limits the generalizability of the findings. Although it provides valuable insights into the unique clinical, histopathological, and molecular characteristics of this malignancy, a single case cannot fully reflect the heterogeneity and complexity of the disease across patients. The identified genetic mutations, TMB, and molecular pathways may not be representative of all cases, given the potential for significant variation in the genetic and biological behavior of gallbladder NEC. To address this limitation, future research should aim to expand the sample size by including multiple cases of gallbladder NEC, potentially through multicenter collaborations between different institutions that pool resources and data to conduct larger-scale studies. Such efforts would increase the statistical accuracy of the findings and provide a broader understanding of the commonalities and differences among cases, thereby enhancing the generalizability of the results.
In summary, a rare case of rare gallbladder NEC was presented, and clinicopathologic and genetic characteristics were discussed. The prognosis of gallbladder NEC was unsatisfactory. Gene sequencing tests may improve clinical management and disease prognosis. Studies involving a higher number of cases are needed to investigate more effective treatments for gallbladder NEC.
| 1. | Zhu J, Xiao W, Li Y. Management of Primary Hepatopancreatobiliary and Ampulla Large Cell Neuroendocrine Carcinoma. J Laparoendosc Adv Surg Tech A. 2022;32:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (2)] |
| 2. | Chu H, Zhang C, Shi Y, Wu W, Hu Z, Zhang J, Huang D. Gallbladder neuroendocrine carcinoma: A single center experience. Medicine (Baltimore). 2020;99:e21912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 3. | Wang W, Yang CX, Yu XZ, Zhang SL, Wang J, Wang J. Clinicopathological characteristics and prognostic factors of patients with primary gallbladder neuroendocrine carcinomas. J Dig Dis. 2022;23:166-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 4. | Li M, Liu F, Zhang Y, Wu X, Wu W, Wang XA, Zhao S, Liu S, Liang H, Zhang F, Ma Q, Xiang S, Li H, Jiang L, Hu Y, Gong W, Zhang Y, Ma T, Zhang K, Liu Y, Liu Y. Whole-genome sequencing reveals the mutational landscape of metastatic small-cell gallbladder neuroendocrine carcinoma (GB-SCNEC). Cancer Lett. 2017;391:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 5. | Vortmeyer AO, Lubensky IA, Merino MJ, Wang CY, Pham T, Furth EE, Zhuang Z. Concordance of genetic alterations in poorly differentiated colorectal neuroendocrine carcinomas and associated adenocarcinomas. J Natl Cancer Inst. 1997;89:1448-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 112] [Article Influence: 3.9] [Reference Citation Analysis (2)] |
| 6. | Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, Jensen LJ, Mering CV. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607-D613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10161] [Cited by in RCA: 12521] [Article Influence: 1788.7] [Reference Citation Analysis (1)] |
| 7. | Franz M, Rodriguez H, Lopes C, Zuberi K, Montojo J, Bader GD, Morris Q. GeneMANIA update 2018. Nucleic Acids Res. 2018;46:W60-W64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 341] [Cited by in RCA: 1028] [Article Influence: 146.9] [Reference Citation Analysis (1)] |
| 8. | Han H, Cho JW, Lee S, Yun A, Kim H, Bae D, Yang S, Kim CY, Lee M, Kim E, Lee S, Kang B, Jeong D, Kim Y, Jeon HN, Jung H, Nam S, Chung M, Kim JH, Lee I. TRRUST v2: an expanded reference database of human and mouse transcriptional regulatory interactions. Nucleic Acids Res. 2018;46:D380-D386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1155] [Cited by in RCA: 1423] [Article Influence: 177.9] [Reference Citation Analysis (1)] |
| 9. | Sakaki M, Hirokawa M, Sano T, Horiguchi H, Wakatsuki S, Ogata S. Gallbladder Adenocarcinoma with Florid Neuroendocrine Cell Nests and Extensive Paneth Cell Metaplasia. Endocr Pathol. 2000;11:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 10. | Li M, Liu F, Zhang F, Zhou W, Jiang X, Yang Y, Qu K, Wang Y, Ma Q, Wang T, Bai L, Wang Z, Song X, Zhu Y, Yuan R, Gao Y, Liu Y, Jin Y, Li H, Xiang S, Ye Y, Zhang Y, Jiang L, Hu Y, Hao Y, Lu W, Chen S, Gu J, Zhou J, Gong W, Zhang Y, Wang X, Liu X, Liu C, Liu H, Liu Y, Liu Y. Genomic ERBB2/ERBB3 mutations promote PD-L1-mediated immune escape in gallbladder cancer: a whole-exome sequencing analysis. Gut. 2019;68:1024-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (1)] |
| 11. | Yamamoto M, Kensler TW, Motohashi H. The KEAP1-NRF2 System: a Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol Rev. 2018;98:1169-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 696] [Cited by in RCA: 1473] [Article Influence: 184.1] [Reference Citation Analysis (1)] |
| 12. | Takahashi T, Sonobe M, Menju T, Nakayama E, Mino N, Iwakiri S, Nagai S, Sato K, Miyahara R, Okubo K, Hirata T, Date H, Wada H. Mutations in Keap1 are a potential prognostic factor in resected non-small cell lung cancer. J Surg Oncol. 2010;101:500-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 13. | Tian L, Lu Y, Yang T, Deng Z, Xu L, Yao W, Ma C, Li X, Zhang J, Liu Y, Wang J. aPKCι promotes gallbladder cancer tumorigenesis and gemcitabine resistance by competing with Nrf2 for binding to Keap1. Redox Biol. 2019;22:101149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 14. | Shibata T, Kokubu A, Gotoh M, Ojima H, Ohta T, Yamamoto M, Hirohashi S. Genetic alteration of Keap1 confers constitutive Nrf2 activation and resistance to chemotherapy in gallbladder cancer. Gastroenterology. 2008;135:1358-1368, 1368.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 399] [Article Influence: 22.2] [Reference Citation Analysis (1)] |
| 15. | Liu F, Li Y, Ying D, Qiu S, He Y, Li M, Liu Y, Zhang Y, Zhu Q, Hu Y, Liu L, Li G, Pan W, Jin W, Mu J, Cao Y, Liu Y. Whole-exome mutational landscape of neuroendocrine carcinomas of the gallbladder. Signal Transduct Target Ther. 2021;6:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 16. | Wang Y, Huang B, Fu Q, Wang J, Ye M, Hu M, Qu K, Liu K, Hu X, Wei S, Sun K, Xiao W, Zhang B, Li H, Li J, Zhang Q, Liang T. Comprehensive Clinical Analysis of Gallbladder Neuroendocrine Neoplasms: A Large-Volume Multicenter Study During One Decade. Ann Surg Oncol. 2022;29:7619-7630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 17. | Kim SH, Kim SH, Kim MA, Shin CI, Han JK, Choi BI. CT differentiation of poorly-differentiated gastric neuroendocrine tumours from well-differentiated neuroendocrine tumours and gastric adenocarcinomas. Eur Radiol. 2015;25:1946-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 18. | Bae JS, Kim SH, Yoo J, Kim H, Han JK. Differential and prognostic MRI features of gallbladder neuroendocrine tumors and adenocarcinomas. Eur Radiol. 2020;30:2890-2901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 19. | Faraoun SA, Guerrache Y, Dautry R, Boudiaf M, Dohan A, Barral M, Hoeffel C, Rousset P, Fohlen A, Soyer P. Computed Tomographic Features of Primary Small Cell Neuroendocrine Tumors of the Gallbladder. J Comput Assist Tomogr. 2018;42:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 20. | Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 758] [Article Influence: 47.4] [Reference Citation Analysis (2)] |
| 21. | Do MY, Jang SI, Kang HP, Kim EJ, Lee KJ, Park GE, Lee SJ, Lee DK, Woo SM, Cho JH. Comparison of the Clinical Features and Outcomes of Gallbladder Neuroendocrine Carcinoma with Those of Adenocarcinoma: A Propensity Score-Matched Analysis. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Yan S, Wang Y, Chen X, Zhang Y, Huang Z, Zhao J, Zhou J, Li Z, Bi X, Luo Z, Cai J, Zhao H. Clinical Analysis of 15 Cases of Gallbladder Neuroendocrine Carcinoma and Comparison with Gallbladder Adenocarcinoma Using a Propensity Score Matching. Cancer Manag Res. 2020;12:1437-1446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Takeda Y, Kobayashi N, Kessoku T, Okubo N, Suzuki A, Tokuhisa M, Miwa H, Udaka N, Ichikawa Y. Case reports: chemoradiotherapy for locally advanced neuroendocrine carcinoma of the gallbladder. Clin J Gastroenterol. 2022;15:803-808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Ayabe RI, Wach M, Ruff S, Martin S, Diggs L, Wiemken T, Hinyard L, Davis JL, Luu C, Hernandez JM. Primary Gallbladder Neuroendocrine Tumors: Insights into a Rare Histology Using a Large National Database. Ann Surg Oncol. 2019;26:3577-3585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/