Published online Sep 15, 2024. doi: 10.4251/wjgo.v16.i9.4006
Revised: June 29, 2024
Accepted: July 26, 2024
Published online: September 15, 2024
Processing time: 106 Days and 22 Hours
Pancreatic cancer remains one of the most lethal malignancies, and has limited effective treatment. Gemcitabine (GEM), a chemotherapeutic agent, is commonly used for clinical treatment of pancreatic cancer, but it has characteristics of low drug delivery efficiency and significant side effects. The study tested the hypo
To investigate the cytotoxicity of MSC-derived Exo-GEM against pancreatic cancer cells in vitro.
Exosomes were isolated from MSCs and characterized by transmission electron microscopy and nanoparticle tracking analysis. Exo-GEM through electroporation, sonication, or incubation, and the loading efficiency was evaluated. The cytotoxicity of Exo-GEM or GEM alone against human pancreatic cancer Panc-1 and MiaPaca-2 cells was assessed by MTT and flow cytometry assays.
The isolated exosomes had an average size of 76.7 nm. The encapsulation efficacy and loading efficiency of GEM by electroporation and sonication were similar and significantly better than incubation. The cytotoxicity of Exo-GEM against pancreatic cancer cells was stronger than free GEM and treatment with 0.02 μM Exo-GEM significantly reduced the viability of both Panc-1 and MiaPaca-2 cells. Moreover, Exo-GEM enhanced the frequency of GEM-induced apoptosis in both cell lines.
Human bone marrow MSC-derived Exo-GEM have a potent cytotoxicity against human pancreatic cancer cells by enhancing their apoptosis, offering a promising drug delivery system for improving therapeutic outcomes.
Core Tip: This study investigates the utilization of exosomes derived from human bone marrow mesenchymal stem cells as a novel system to deliver gemcitabine (GEM) for the treatment of pancreatic cancer. Through the optimization of GEM loading into exosomes, the results indicate that exosomes loaded with GEM (Exo-GEM) have a potent cytotoxicity against pancreatic cancer cells by enhancing their apoptosis in vitro. These findings underscore the potential of Exo-GEM as a more efficient and targeted therapeutic approach for enhancing therapeutic efficacy in patients with pancreatic cancer.
- Citation: Tang ZG, Chen TM, Lu Y, Wang Z, Wang XC, Kong Y. Human bone marrow mesenchymal stem cell-derived exosomes loaded with gemcitabine inhibit pancreatic cancer cell proliferation by enhancing apoptosis. World J Gastrointest Oncol 2024; 16(9): 4006-4013
- URL: https://www.wjgnet.com/1948-5204/full/v16/i9/4006.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i9.4006
Pancreatic ductal adenocarcinoma is one of the most lethal malignant tumors within the pancreatic duct, and its diagnosis and treatment remain highly challenging in the world. Despite notable progressions in therapeutic approaches, pancreatic cancer continues to be a leading cause of cancer-related death, and as projected, pancreatic cancer will ascend to the second-ranking cause by 2030[1]. Additionally, more research has focused on the potential of targeted therapy and immunotherapy for pancreatic cancer. However, the therapeutic efficacy of targeted and immunotherapies for pancreatic cancer is limited, due to its aggressiveness and the intricate nature of establishing efficacious therapeutic strategies[2]. Gemcitabine (GEM) has been commonly used as a monotherapy or in conjunction with other pharmaceutical agents for the primary treatment of pancreatic cancer patients[3]. However, the efficacy of monotherapy with GEM is limited because of its rapid metabolism, ineffectively delivering the drug to tumors with dense stromal tissues, and the eventual development of resistance to chemotherapy[4,5]. Hence, the discovery of an effective drug delivery system for administering GEM is crucial for the management of pancreatic cancer.
Exosomes are extracellular vesicles and have been demonstrated to be an effective drug delivery system. Exosomes have characteristics involving intercellular communication, high biocompatibility permeability and retention effects, remarkable stability in the circulation[6]. Apparently, loading of exosomes with GEM may effectively deliver the drug into the tumor cell and environment and can be clinically translatable. Actually, recent studies have shown that exosomes from tumors, M1 macrophages and mesenchymal stem cells (MSCs), when loaded with GEM, exhibit remarkably overcome drug resistance in pancreatic cancer[7-10].
MSCs can secrete a substantial quantity of exosomes[11] and have the capability to navigate selectively towards inflammatory regions and tumor microenvironments. However, the efficiency of GEM-loaded exosomes (Exo-GEM) in specifically targeting pancreatic cancer cells has not been clarified. The current methods for loading GEM include electroporation, sonication, and incubation. Nevertheless, the loading efficiency of these methods remains controversial. Experiment evidence is needed to substantiate the hypothesis that exosomes derived from MSCs can enhance the cytotoxicity of GEM against pancreatic cancer cells. If demonstrated, the Exo-GEM may provide a new therapeutic strategy for safe drug delivery.
This study compared the loading and encapsulation efficiency of GEM within exosomes derived from human bone MSCs by electroporation, ultrasonication, and co-incubation. Additionally, the study tested the cytotoxicity of Exo-GEM and free GEM against human pancreatic cancer Panc-1 and MiaPaca-2 cells in vitro and their apoptosis. This study aimed to demonstrate the stronger cytotoxicity of Exo-GEM against pancreatic cancer cells and that Exo-GEM was a potential therapeutic agent for the treatment of pancreatic cancer.
Human bone marrow MSCs (HU-BMSCs), pancreatic cancer Panc-1 and MiaPaca-2 cells were obtained from Saiye Biotechnology, Guangzhou, ATCC, and the Institute of Cell Biology, Chinese Academy of Sciences, Shanghai, China, respectively.
GEM (Item No: HY-17026) was purchased from MedChemExpress, Monmouth Junction, United States. Fetal bovine serum and DMEM were obtained from Gibco, New York, United States. Trypsin-EDTA solution was bought from Kaiji Biology, Zigong, China, and exosome-free serum was acquired from Shandong Jiekai Biology, Jinan, China.
The equipment used included a Beckman ultracentrifuge, an Eppendorf electroporator, and a Ningbo Licheng ultrasonic cell disruptor.
HU-BMSC, Panc-1, and MiaPaca-2 cells were cultured in DMEM containing 10% fetal bovine serum and 1% penicillin-streptomycin at 37 °C in an incubator of 5% CO2, and their media were changed every other day.
Exosomes were isolated using a widely used ultracentrifugation method. HU-BMSC cells were grown up to > 90% confluence, and the cells were continually cultured in DMEM with 10% exosome-free serum and 1% penicillin-streptomycin solution for 48 hours. Their supernatants were harvested and centrifuged at 2000 × g for 15 minutes, followed by centrifuging at 10000 × g for 30 minutes. Finally, the suspensions were centrifuged at 120000 × g for 70 minutes. The pellets containing exosomes were resuspended in phosphate buffered saline for storage.
Electroporation method: GEM was dissolved in DMSO at a concentration of 1 mg/mL. GEM solution (100 μL) was mixed with 900 μL of exosomes (0.2 mg/mL). This mixture was transferred to a pre-cooled 2 mm cuvette and subjected to three pulses at 400 V, 125 μF, and 5 ms, followed by cooling on ice for 10 minutes.
Ultrasonication method: The above mixture of GEM and exosomes was sonicated with 20% amplitude for 30 seconds for three cycles with an interval of 90 seconds of ice cooling. After sonication, it was incubated at 37 °C for 2 hours.
Co-incubation method: MSC cells (5 × 105 cells/dish) were cultured overnight in 100 mm cell culture dishes, washed twice with phosphate buffered saline and cultured with 10 mL of exosome-free complete medium containing 10 μM GEM for 96 hours. The supernatants (200 mL/sample) were harvested for exosome isolation and high-performance liquid chromatography (HPLC) analysis.
The contents of GEM in exosomes were quantified by HPLC. The supernatants containing unencapsulated drug were subjected to ultracentrifugation and filtered through a 0.22 μm filter (Millipore, United States) for HPLC analysis using a column of C18 at a temperature of 40 °C; with the acetonitrile-0.1% trifluoroacetic acid solution (3:97, V/V) as a mobile phase, a flow rate of 1.0 mL/min, detection at 268 nm wavelength and 10 μL injection volume.
The encapsulation efficacy of GEM in exosomes was calculated using the formula of encapsulation efficacy (%) = (total GEM - GEM in supernatant)/total GEM × 100%.
Drug loading efficiency (%) = (total GEM - GEM in supernatant)/weight of exosome × 100%.
The cytotoxicity of free GEM, Exo-GEM or control exosomes against Panc-1 and MiaPaca-2 cells was tested by the MTT assay. The cells were treated in triplicate with various concentrations of GEM, Exo-GEM or exosomes in 96-well plates for varying time points. The absorbance of individual wells was measured at 570 nm and the IC50 values of individual drugs were calculated using the GraphPad Prism 9.0 software.
Panc-1 and MiaPaca-2 cells were treated in triplicate with different concentrations of GEM, Exo-GEM or exosomes for 72 hours. The cells were stained with Annexin V-EGFP and PI and the frequency of apoptotic cells in individual groups was determined by flow cytometry.
Data are presented as mean ± SD. The difference was analyzed by one-way ANOVA and post hoc LSD-t tests using SPSS 22.0 software (SPSS Inc., Chicago, United States). A P-value of < 0.05 was defined as statistically significant.
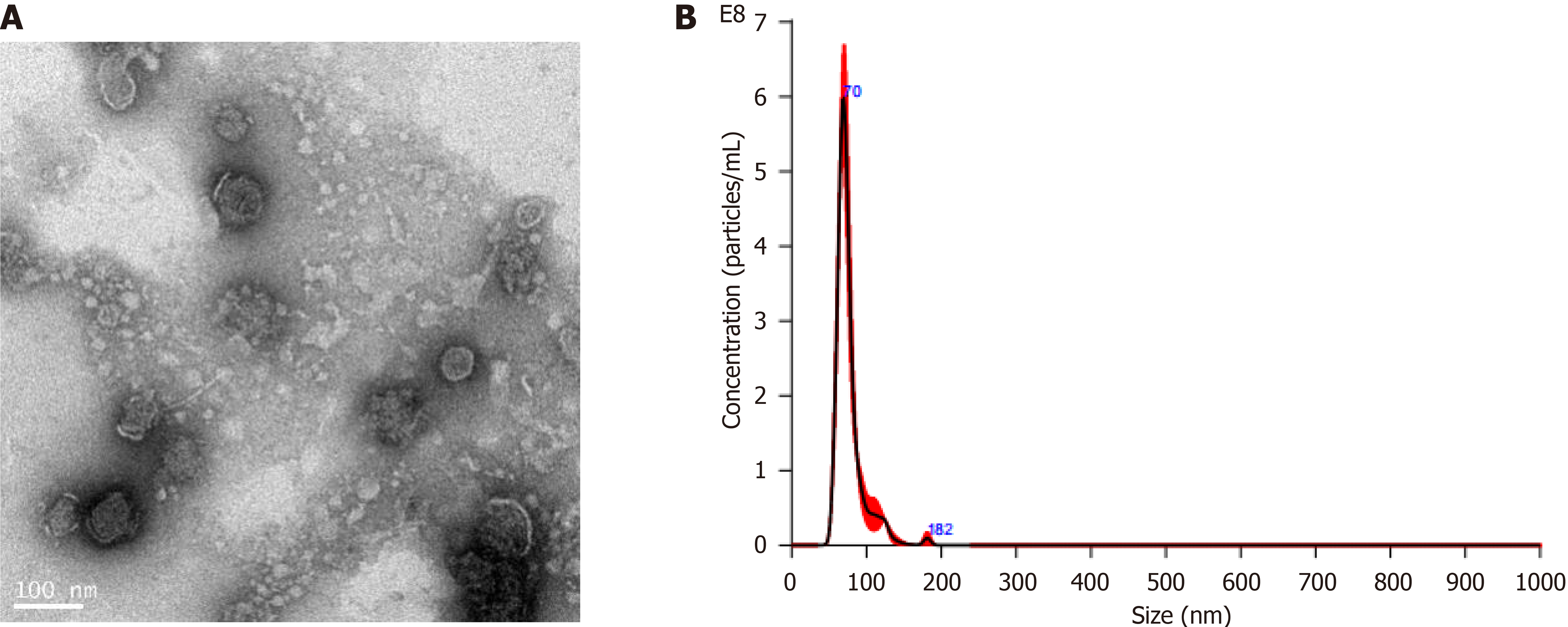
Exosomes were isolated from the supernatants of cultured HU-BMSCs by sequential centrifugations. The isolated exosomes were examined and characterized by transmission electron microscopy. The results displayed that the isolated exosomes had a diameter of 60-100 nm (Figure 1A). The average particle size was 76.7 nm, and the contents were (1.4 × 1010) ± (7.8 × 108) particles/mL in the supernatants of cultured MSCs, determined by nanoparticle tracking analysis (Figure 1B).
Quantitative analyses of the encapsulation and drug loading efficiency of electroporation, sonication, and incubation methods indicated that both electroporation and sonication methods had similar GEM encapsulation and loading efficacies, which were significantly higher than that of incubation (P < 0.001, Table 1). Hence, electroporation was selected for the subsequent loading of GEM.
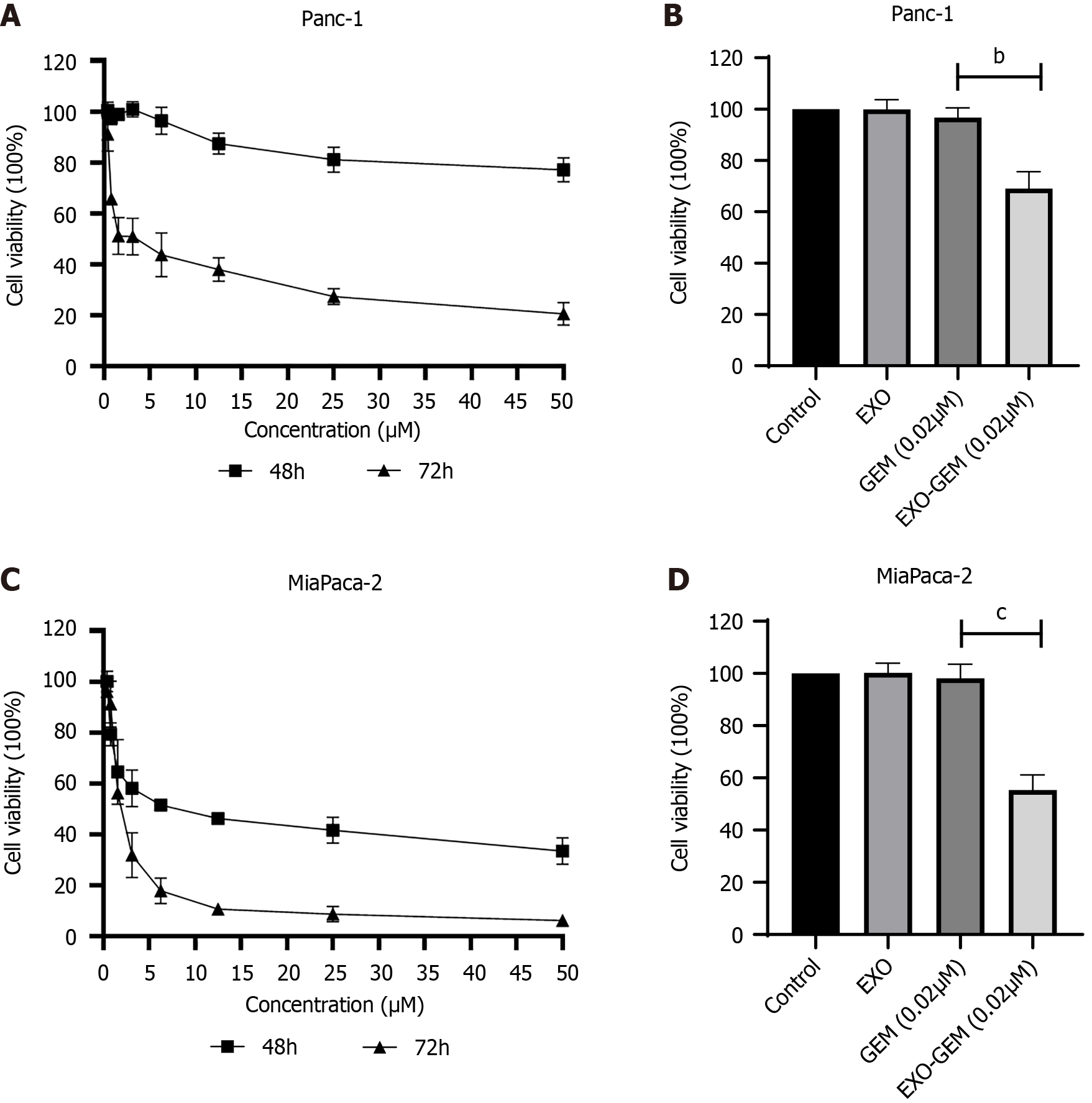
Next, the cytotoxicity of different concentrations of GEM, Exo-GEM or exosomes against Panc-1 and MiaPaca-2 cells was tested by the MTT assay. As shown in Figure 2A, the cytotoxicity of GEM increased from 48 to 72 hours and the IC50 value of GEM was 3.82 ± 1.04 μM at 72 hours post treatment, indicating that the cytotoxicity of GEM was time-dependent in Panc-1 cells. While treatment with 0.02 μM GEM resulted in 96.70% ± 3.81% viability in Panc-1 cells treatment with the same concentration of Exo-GEM significantly decreased the viability of Panc-1 cells to 69.02% ± 6.58% (P < 0.001, Figure 2B). These suggest that Exo-GEM treatment enhanced the cytotoxicity of GEM against Panc-1 cell in vitro. Similarly, treatment with GEM decreased the viability of MiaPaca-2 cells in a time-dependent manner, with an IC50 value of 2.13 ± 0.22 μM at 72 hours of treatment in MiaPaca-2 cells (Figure 2C). Treatment with Exo-GEM significantly reduced the viability of MiaPaca-2 cells to 55.30% ± 5.83 %, which was significantly lower than that of treatment with the same dose of GEM (98.18% ± 5.34%, P < 0.001, Figure 2D). In contrast, treatment with the same dose of exosomes alone did not significantly change the viability of both Panc-1 and MiaPaca-2 cells. Thus, Exo-GEM enhanced the cytotoxicity against pancreatic cancer cells in vitro.
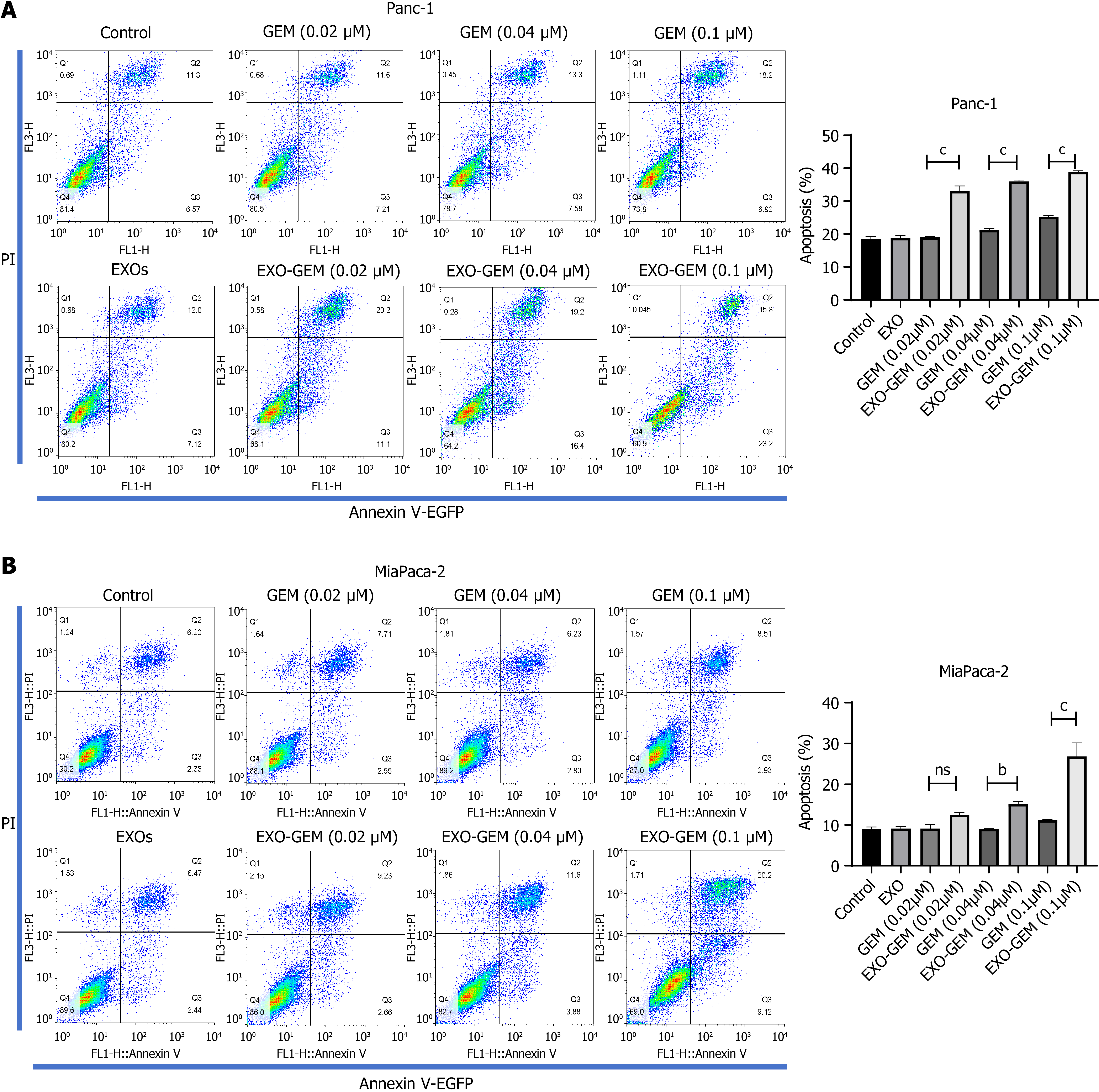
To understand the cytotoxicity of Exo-GEM, Panc-1 and MiaPaca-2 cells were treated with different concentrations (0.02, 0.04, and 0.1 μM) of GEM, Exo-GEM or exosomes for 72 hours. The frequency of apoptotic cells was quantified by flow cytometry. The data indicated that treatment with Exo-GEM notably increased the frequency of apoptotic cells, related to that of treatment with free GEM in Panc-1 (Figure 3A) and Miapaca-2 cells (Figure 3B).
The present study effectively isolated exosomes from HU-BMSCs, transmission electron microscopy and nanoparticle tracking analysis revealed that the isolated exosomes had a reasonable size and were suitable for drug delivery applications. Comparison of various drug loading methods unveiled that both electroporation and sonication exhibited similar efficacies, which were superior to incubation. Our findings were consistent with a previous report that electroporation and sonication are superior methods for disrupting the exosomal membrane, enhancing drug loading capabilities without compromising the integrity of the exosomes[12]. More importantly, treatment with Exo-GEM significantly enhanced the cytotoxicity of GEM against both Panc-1 and MiaPaca-2 cells by increasing their apoptosis.
The potent cytotoxicity of Exo-GEM may stem from the specific transportation and little severe adverse effect of GEM in the exosomes[13]. Potentially, the Exo-GEM may surmount the constraints of conventional chemotherapy, including inadequate solubility and drug resistance. We observed that treatment with Exo-GEM enhanced the GEM-induced apoptosis of pancreatic cancer cells in vitro. Our findings were in agreement with previous observations[14,15] and support the notion that exosomes prevent the development of drug resistance in cancer cells. Therefore, our findings underscore the significance of exosomes in improving drug effectiveness in cancer therapies.
This study represents a notable progression in the field of drug delivery and pancreatic cancer therapy through the elucidation of the innovative utilization of Exo-GEM. The results indicate a potential alteration in the treatment of pancreatic cancer, presenting a more efficient and focused approach to chemotherapy. Our study had limitations. This study was conducted in vitro and any finding was not validated in vivo. Thus, the therapeutic potential of Exo-GEM to treat pancreatic cancer remains to be determined. Further investigations are necessary to determine the safety and effectiveness of Exo-GEM in vivo, expend exosome isolation and drug loading scares, and evaluate the suitability of this delivery system for other forms of drugs and cancers.
In summary, exosomes were successfully isolated from HU-BMSCs and loaded with GEM and the Exo-GEM exhibited a reasonable size. Functionally, the Exo-GEM had potent cytotoxicity against pancreatic cancer cells by enhancing the GEM-triggered apoptosis. This innovative delivery system holds the potential to enhance the drug’s effectiveness while mitigating its adverse effects, improving therapeutic outcomes of pancreatic cancer patients. It is imperative to address the aforementioned limitations and expand our investigations to fully exploit the capabilities of exosome-mediated drug delivery for cancer therapy.
I wish to express my appreciation to Prof. Chen You-Hai, a distinguished and knowledgeable scholar, for his invaluable guidance throughout the writing process of this manuscript.
| 1. | Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5379] [Cited by in RCA: 5340] [Article Influence: 445.0] [Reference Citation Analysis (0)] |
| 2. | Liu X, Li Z, Wang Y. Advances in Targeted Therapy and Immunotherapy for Pancreatic Cancer. Adv Biol (Weinh). 2021;5:e1900236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Philip PA, Lacy J, Portales F, Sobrero A, Pazo-Cid R, Manzano Mozo JL, Kim EJ, Dowden S, Zakari A, Borg C, Terrebonne E, Rivera F, Sastre J, Bathini V, López-Trabada D, Asselah J, Saif MW, Shiansong Li J, Ong TJ, Nydam T, Hammel P. Nab-paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): a multicentre, open-label phase 2 study. Lancet Gastroenterol Hepatol. 2020;5:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 170] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 4. | Kattel K, Mondal G, Lin F, Kumar V, Mahato RI. Biodistribution of Self-Assembling Polymer-Gemcitabine Conjugate after Systemic Administration into Orthotopic Pancreatic Tumor Bearing Mice. Mol Pharm. 2017;14:1365-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Liang C, Shi S, Meng Q, Liang D, Ji S, Zhang B, Qin Y, Xu J, Ni Q, Yu X. Complex roles of the stroma in the intrinsic resistance to gemcitabine in pancreatic cancer: where we are and where we are going. Exp Mol Med. 2017;49:e406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 126] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 6. | Sun D, Zhuang X, Zhang S, Deng ZB, Grizzle W, Miller D, Zhang HG. Exosomes are endogenous nanoparticles that can deliver biological information between cells. Adv Drug Deliv Rev. 2013;65:342-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 7. | Li YJ, Wu JY, Wang JM, Hu XB, Cai JX, Xiang DX. Gemcitabine loaded autologous exosomes for effective and safe chemotherapy of pancreatic cancer. Acta Biomater. 2020;101:519-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 228] [Article Influence: 38.0] [Reference Citation Analysis (1)] |
| 8. | Zhao Y, Zheng Y, Zhu Y, Zhang Y, Zhu H, Liu T. M1 Macrophage-Derived Exosomes Loaded with Gemcitabine and Deferasirox against Chemoresistant Pancreatic Cancer. Pharmaceutics. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (1)] |
| 9. | Klimova D, Jakubechova J, Altanerova U, Nicodemou A, Styk J, Szemes T, Repiska V, Altaner C. Extracellular vesicles derived from dental mesenchymal stem/stromal cells with gemcitabine as a cargo have an inhibitory effect on the growth of pancreatic carcinoma cell lines in vitro. Mol Cell Probes. 2023;67:101894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (1)] |
| 10. | Zhou Y, Zhou W, Chen X, Wang Q, Li C, Chen Q, Zhang Y, Lu Y, Ding X, Jiang C. Bone marrow mesenchymal stem cells-derived exosomes for penetrating and targeted chemotherapy of pancreatic cancer. Acta Pharm Sin B. 2020;10:1563-1575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (1)] |
| 11. | Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, Lim SK. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65:336-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 655] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 12. | Kooijmans SA, Vader P, van Dommelen SM, van Solinge WW, Schiffelers RM. Exosome mimetics: a novel class of drug delivery systems. Int J Nanomedicine. 2012;7:1525-1541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 243] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 13. | Saari H, Lázaro-Ibáñez E, Viitala T, Vuorimaa-Laukkanen E, Siljander P, Yliperttula M. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J Control Release. 2015;220:727-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 490] [Article Influence: 44.5] [Reference Citation Analysis (1)] |
| 14. | Johnsen KB, Gudbergsson JM, Skov MN, Pilgaard L, Moos T, Duroux M. A comprehensive overview of exosomes as drug delivery vehicles - endogenous nanocarriers for targeted cancer therapy. Biochim Biophys Acta. 2014;1846:75-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 398] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 15. | Vader P, Breakefield XO, Wood MJ. Extracellular vesicles: emerging targets for cancer therapy. Trends Mol Med. 2014;20:385-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 339] [Article Influence: 28.3] [Reference Citation Analysis (0)] |