Published online Aug 15, 2024. doi: 10.4251/wjgo.v16.i8.3529
Revised: May 26, 2024
Accepted: June 18, 2024
Published online: August 15, 2024
Processing time: 105 Days and 14 Hours
Minute gastric cancers (MGCs) have a favorable prognosis, but they are too small to be detected by endoscopy, with a maximum diameter ≤ 5 mm.
To explore endoscopic detection and diagnostic strategies for MGCs.
This was a real-world observational study. The endoscopic and clinicopathological parameters of 191 MGCs between January 2015 and December 2022 were retrospectively analyzed. Endoscopic discoverable opportunity and typical neoplastic features were emphatically reviewed.
All MGCs in our study were of a single pathological type, 97.38% (186/191) of which were differentiated-type tumors. White light endoscopy (WLE) detected 84.29% (161/191) of MGCs, and the most common morphology of MGCs found by WLE was protruding. Narrow-band imaging (NBI) secondary observation detected 14.14% (27/191) of MGCs, and the most common morphology of MGCs found by NBI was flat. Another three MGCs were detected by indigo carmine third observation. If a well-demarcated border lesion exhibited a typical neo
WLE combined with NBI and indigo carmine are helpful for detection of MGCs. A clear demarcation line combined with a typical neoplastic color using nonmagnifying observation is sufficient for diagnosis of MGCs. ME-NBI improves the endoscopic diagnostic confidence of MGCs.
Core Tip: Minute gastric cancers (MGCs) represent the incipient stage of GC; therefore, they may be missed during endoscopy because the maximum diameter is ≤ 5 mm. Narrow-band imaging secondary observation combined with indigo carmine third observation could detect MGCs missed by white light endoscopy. A lesion with a clear demarcation line and a typical neoplastic color using nonmagnifying observation can be diagnosed as MGCs.
- Citation: Ji XW, Lin J, Wang YT, Ruan JJ, Xu JH, Song K, Mao JS. Endoscopic detection and diagnostic strategies for minute gastric cancer: A real-world observational study. World J Gastrointest Oncol 2024; 16(8): 3529-3538
- URL: https://www.wjgnet.com/1948-5204/full/v16/i8/3529.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i8.3529
Gastric cancer (GC) is the fifth most common cancer and the third leading cause of cancer death worldwide, with > 1 million new cases and an estimated 783000 deaths in 2018[1]. The Japanese Gastric Cancer Association (JGCA) reported that the 5-year overall survival rates of patients with GC who underwent surgical resection with pathological stage IA, IB, II, IIIA, IIIB, and IV disease were 91.5%, 83.6%, 70.6%, 53.6%, 34.8%, and 16.4%, respectively[2]. The 5-year overall survival rates of early GC (EGC) treated via endoscopy with absolute and extended indications were 91.6% and 90.3%, and the 5-year disease-specific survival rates were 99.9% and 99.7%, respectively[3]. In 2015, the Japan Gastroenterological Endoscopy Society in collaboration with JGCA created guidelines for endoscopic submucosal dissection (ESD) and endoscopic mucosal resection for EGC[4]. For patients with EGC conforming to the absolute endoscopic indication, endoscopic treatment is preferable to traditional surgery for organ retention and similar therapeutic efficacy, so endoscopic treatment has gradually become the standard for EGC[5,6].
Unlike colorectal cancer, GC does not have a well-defined focal precancerous lesion. As the incipient phase of GC, minute GC (MGC) maintains the initial state of neogenesis. MGCs are too small to be detected by endoscopy. We retrospectively analyzed the endoscopic and clinicopathological features of MGCs to evaluate endoscopic detection and diagnostic strategies.
The study included consecutive MGC patients who underwent ESD or surgery at the Second Affiliated Hospital of Zhejiang University School of Medicine from January 2015 to December 2022. The flowchart of patient enrollment is shown in Figure 1. Of the enrolled patients, 184 GC patients, including 191 MGC lesions, were retrospectively analyzed. The Japanese criteria were used to diagnose GC in our study. The study was approved by the Research Ethics Committee of The Second Affiliated Hospital of Zhejiang University School of Medicine (Protocol No. 2022/0502).
According to the JGCA, the stomach is divided into the upper, middle, and lower thirds. Macroscopic classifications were made in accordance with the Paris classification system. Based on the JGCA classification, lesions were classified as well differentiated tubular adenocarcinoma (Tub1), moderately differentiated tubular adenocarcinoma (Tub2), poorly differentiated adenocarcinoma (Por1/2), signet ring cell carcinoma (Sig), papillary adenocarcinoma (Pap), and mucinous adenocarcinoma (Muc). In addition, rare subtypes such as gastric adenocarcinoma of fundic gland type (GA-FG), oxyntic gland adenoma (OGA), and gastric adenocarcinoma of fundic gland mucosa type (GA-FGM) were also analyzed. The lesions were classified as differentiated or undifferentiated according to the Nakamura classification.
All procedures were performed using GIF-H260, GIF-H290, GIF-HQ290, GIF-H260Z or GIF-H290Z endoscope (Olympus Medical Systems, Tokyo, Japan).
We reviewed all endoscopic images of MGCs. According to the sequence of photography, the first endoscopic image of a suspicious lesion was defined as discoverable opportunity. White light endoscopy (WLE) provided information on morphological changes, color changes, covering with thin white moss, and spontaneous hemorrhage. Narrow band imaging (NBI) endoscopy was used to detect brownish areas. According to the diagnostic system based on microvascular and microsurface patterns (the VS classification), magnifying endoscopy (ME)-NBI was used to detect irregular superficial microstructures and/or microvessels to evaluate the nature of lesions[7]. Chromoendoscopy using indigo carmine spraying was used to visualize surface unevenness and boundaries.
According to the images obtained from MGCs, the lesions were divided into three types as follows: Type 1, only visible but indefinite nature by endoscopy; type 2, visible and suspected to be a neoplastic lesion by endoscopy, classified as low confidence in diagnosis; and type 3, visible and diagnosed as a neoplastic lesion by endoscopy, classified as high confidence in diagnosis. The endoscopic differences in the diagnosis of MGCs between the high confidence group and the low confidence group were analyzed. The typical endoscopic features that enable diagnosis of neoplastic lesions were summarized.
Statistical analysis was performed using IBM SPSS software (version 23.0). Categorical variables were described as frequencies and/or percentages. The χ2 test or Fisher’s exact test was used to compare between groups. P < 0.05 was considered indicative of statistical significance.
A total of 184 eligible GC patients, including 191 MGC lesions, were enrolled in our study. There were 107 males and 77 females with a median age of 59 years (range: 38–85 years). Among them, 131 lesions were examined using ME-NBI and 60 using conventional WLE or WLE combined with NBI. MGCs were mostly located in the lower part of the stomach (157/191, 82.2%). All MGCs were of a single pathological type, and the most common pathological type was the differentiated type (186/191, 97.38%). There were 10 lesions invading the submucosa, including eight GA-FG, one GA-FGM, and one moderately differentiated adenocarcinoma. This moderately differentiated adenocarcinoma was a surgical resection specimen. There were 56 multiple cancers, including 40 synchronous multiple cancers and 16 heterochronous multiple cancers. Morphological change was seen in 84.82% (162/191) of MGCs, and the most common morphology was protruding. Color changes were observed by WLE in 76.44% (146/191) of MGCs, and the most common colors were yellowish-red and reddish (Table 1).
| Item | Value |
| Patients, n | 184 |
| Sex (male/female) | 107/77 |
| Age, range (median), yr | 38-85 (59) |
| Lesions | 191 |
| Endoscopy (ME-NBI/WLE or WLE-NBI) | 131/60 |
| Size, range (median), mm | 1.0-5.0 (4.0) |
| Location (upper/middle/lower) | 12/22/157 |
| JGCA classification1 (Tub1/Tub2/Sig/GA-FG/GA-FGM/OGA) | 65/10/5/8/2/1 |
| Nakamura classification (differentiated/undifferentiated) | 186/5 |
| Therapy (ESD/surgery) | 179/12 |
| Depth of invasion (mucosa/submucosa) | 180/11 |
| Multiple cancer (absent/synchronous/heterochronous) | 135/40/16 |
| Morphology change (protruding/flat/depressed) | 95/28/68 |
| Colour change (reddish/yellowish-red/no colour change/whitish) | 65/55/45/25 |
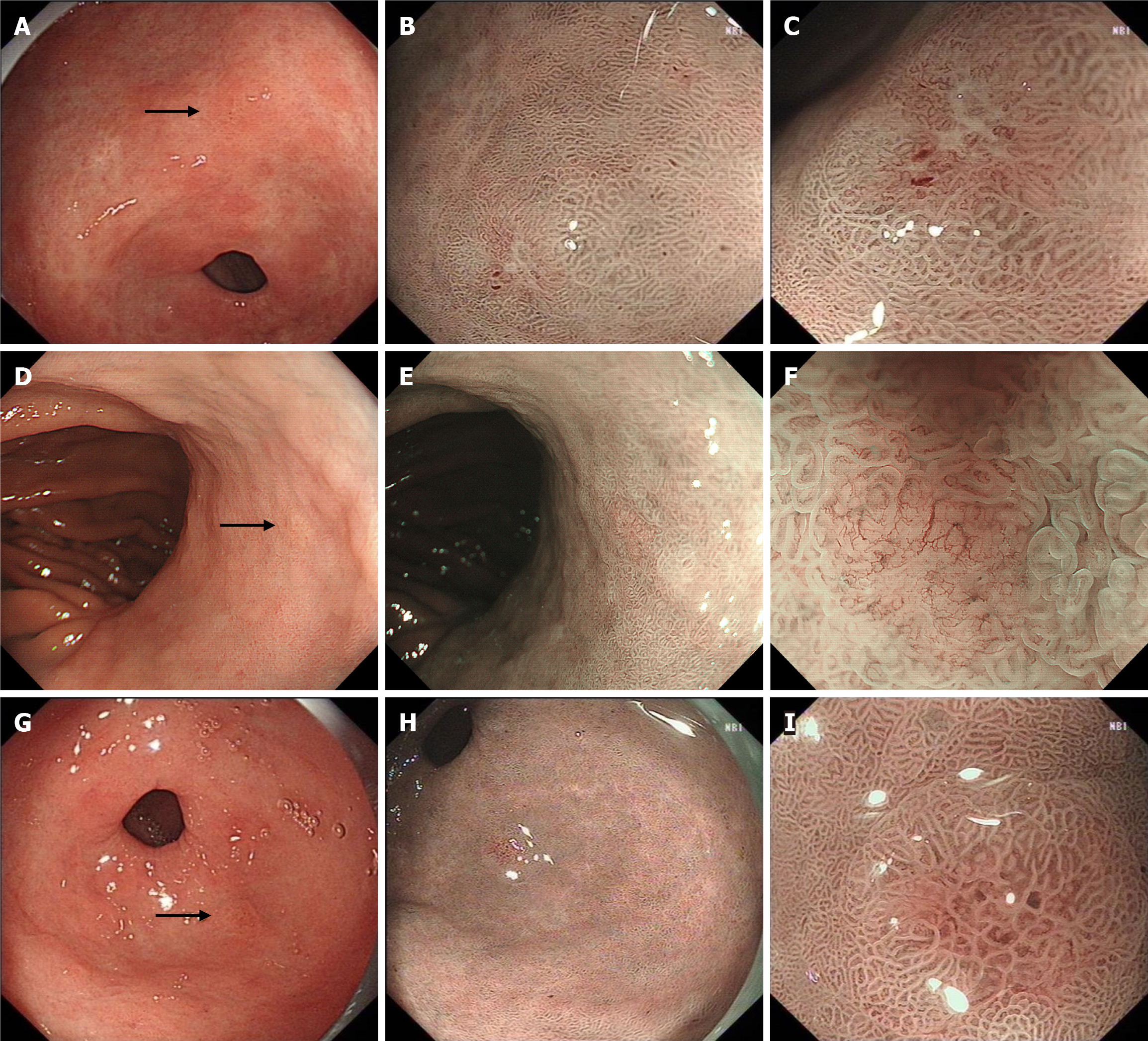
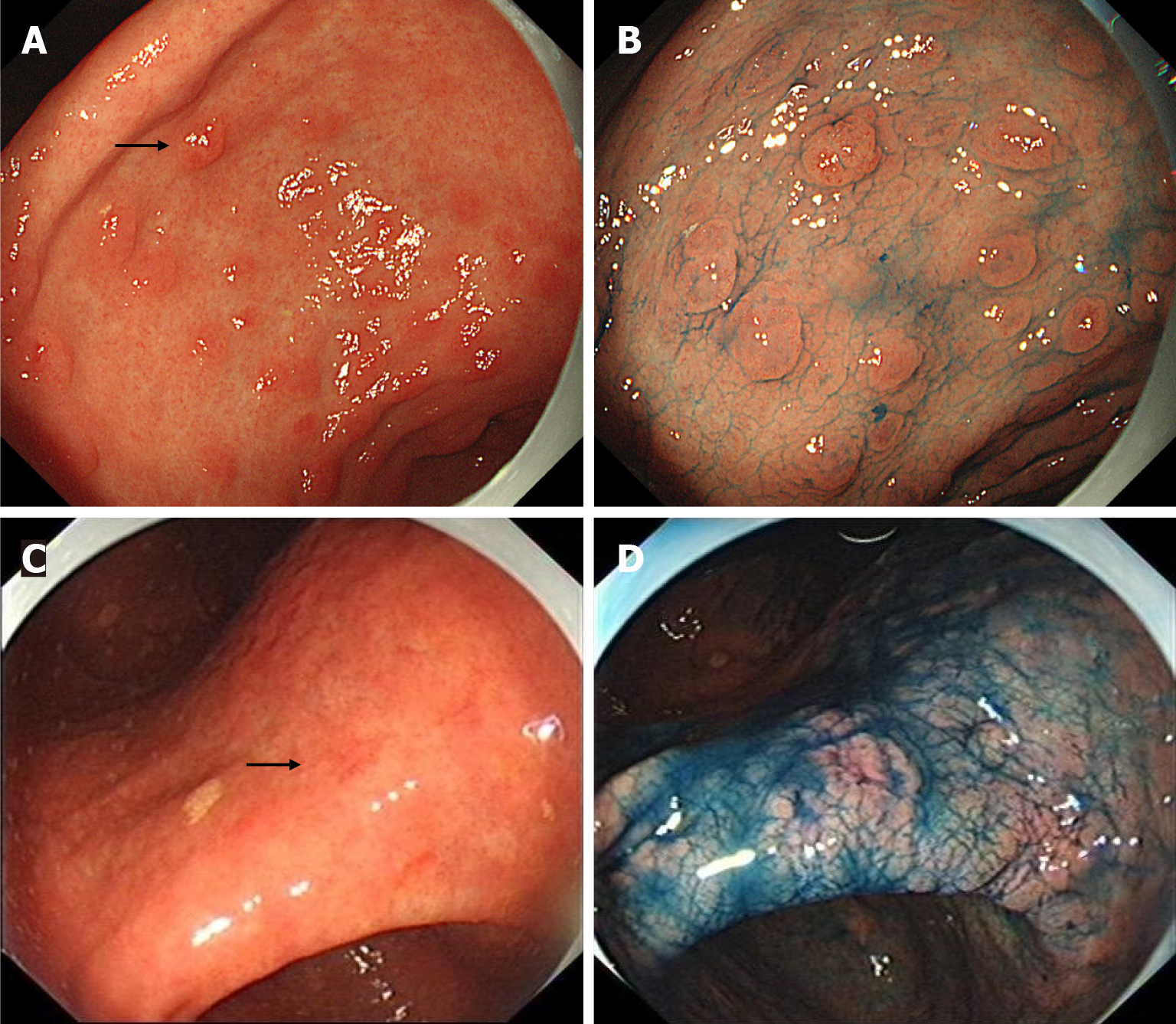
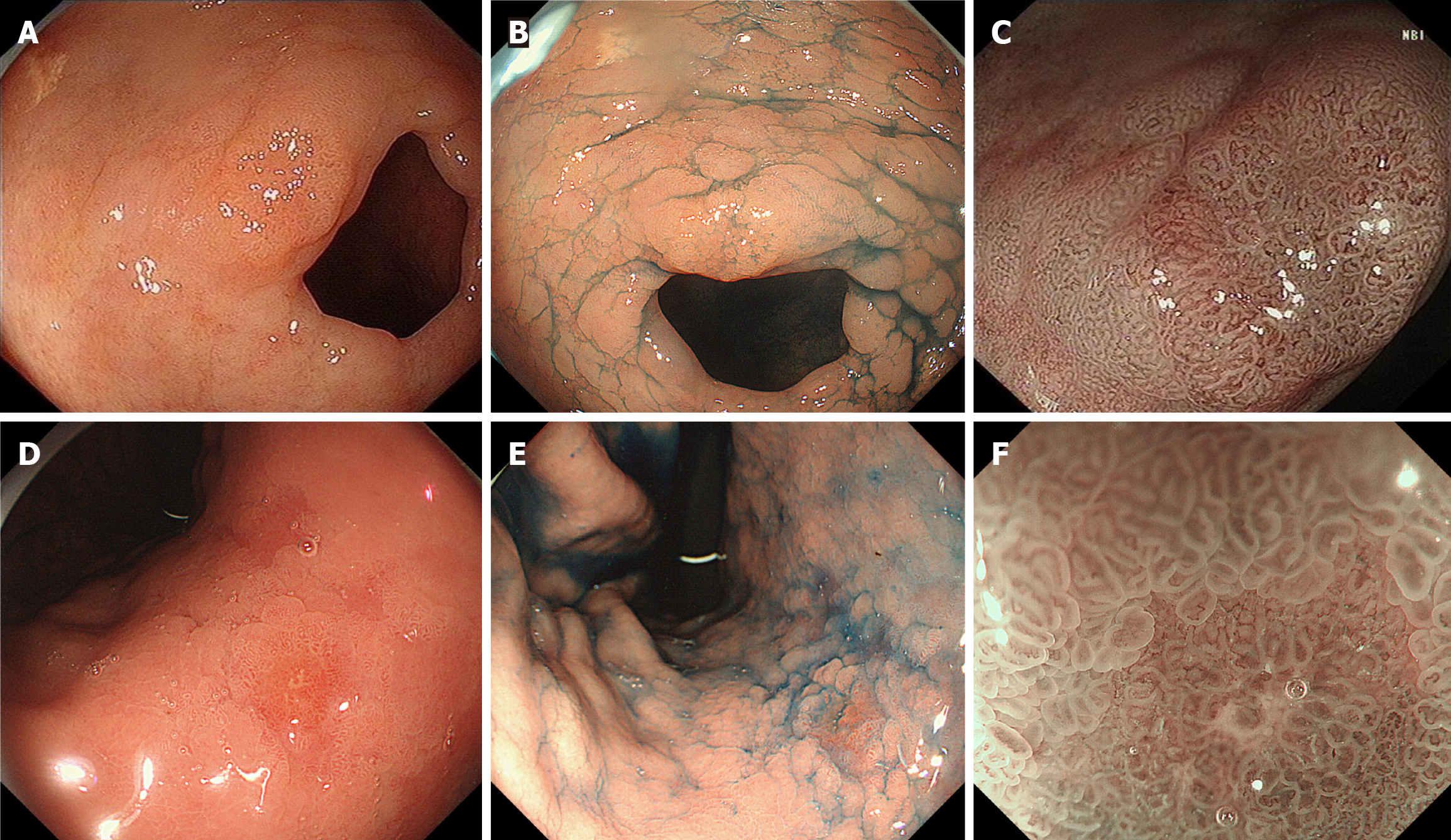
We retrospectively analyzed all endoscopic images of MGCs, and found that all MGCs were initially observed by WLE. A total of 161 lesions (84.29%, 161/191) were detected by WLE. Twenty-seven lesions (14.14%) were found by secondary NBI observation but missed during the initial WLE screening. Three lesions were not found by WLE initial screening and NBI secondary observation, but found by chromoendoscopy third observation using indigo carmine. The endoscopic differences between MGCs found by WLE and NBI are shown in Table 2. The most common morphology of MGCs found by WLE was protruding, while the most common morphology of MGCs found by NBI was flat. The surface covered with thin white moss and spontaneous bleeding were helpful for the detection of MGCs by WLE. The typical MGCs found by NBI are shown in Figure 2, and the typical MGCs found by indigo carmine in Figure 3.
| Found by WLE (n = 161) | Found by NBI (n = 27) | P value | |
| Color change | P = 0.444 | ||
| Reddish | 58 | 6 | |
| Yellowish red | 44 | 10 | |
| No color change | 36 | 8 | |
| Whitish | 22 | 3 | |
| Morphological change | P < 0.001 | ||
| Protruding | 84 | 6 | |
| Flat | 15 | 14 | |
| Depressed | 62 | 7 | |
| Covering with thin white moss | P = 0.130 | ||
| Present | 60 | 6 | |
| Absent | 101 | 21 | |
| Spontaneous bleeding | 1 | ||
| Present | 11 | 0 | |
| Absent | 150 | 27 |
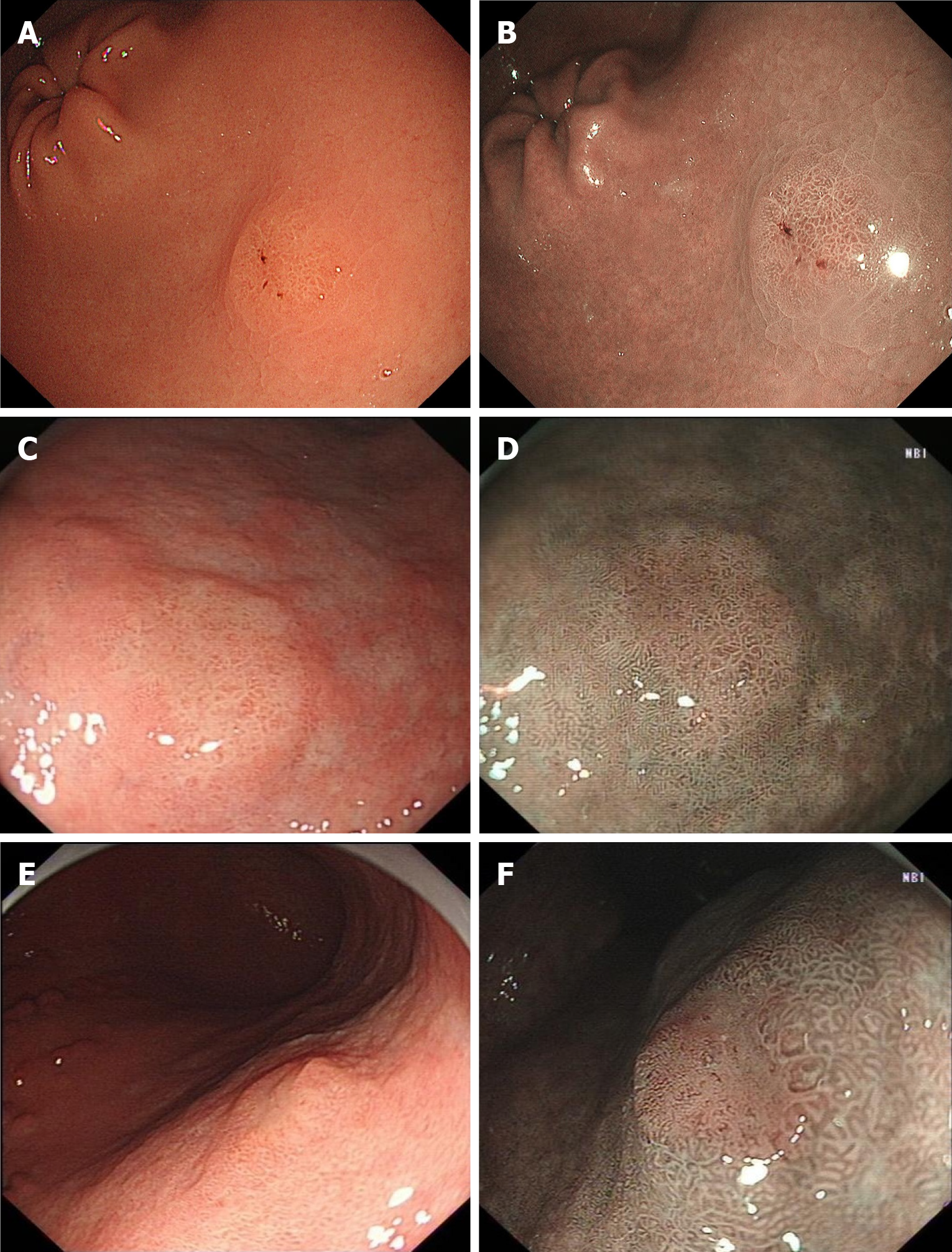
We found that some lesions were diagnosed as neoplasms only using WLE combined with NBI before obtaining pathological results (Figure 4). A clear demarcation line with a typical neoplastic color was sufficient to diagnose MGC. The typical neoplastic color was yellowish-red or whitish under WLE and brownish under NBI.
There were 131 MGCs examined by ME-NBI and 124 by indigo carmine. A total of 114 MGCs were observed using ME-NBI and indigo carmine. Eighty-six lesions were diagnosed as MGCs before obtaining pathological results, and classified as the high diagnostic confidence group. Sixty-five lesions were suspected as MGCs, and classified as the low diagnostic confidence group. Forty lesions were only visible under endoscopy, but the nature could not be determined via endoscopy. For MGCs diagnosed using ME-NBI, the proportion with high diagnostic confidence was significantly higher than the proportion with low diagnostic confidence and the only visible groups (94.19% > 56.92% > 32.50%). For MGCs diagnosed using indigo carmine, the proportion with high diagnostic confidence was significantly higher than the proportion with low diagnostic confidence and the only visible groups (89.53% > 50.77% > 35.00%; Table 3). Figure 5 showed the diagnostic differences between the high- and low-confidence groups using ME-NBI.
| Only visible (n = 40) | Low confidence (n = 65) | High confidence (n = 86) | P value | |
| Endoscopy | P < 0.001 | |||
| ME-NBI (n = 131) | 13 (32.50) | 37 (56.92) | 81 (94.19) | |
| WLE/WLE-NBI (n = 60) | 27 (67.50) | 28 (43.08) | 5 (5.81) | |
| Indigo carmine | P < 0.001 | |||
| Used (n = 124) | 14 (35.00) | 33 (50.77) | 77 (89.53) | |
| Unused (n = 67) | 26 (65.00) | 32 (49.23) | 9 (10.47) |
Conventional WLE is the standard for screening of EGC, but its sensitivity is unsatisfactory[8,9]. NBI is an innovative optical technique that enhances the visibility of the mucosal surface structure and the microvascular structure[10,11]. NBI could be the standard for early detection of superficial squamous cell carcinoma in the head, neck, and esophagus[12,13]. However, NBI endoscopy is unsuitable for large-scale screening of EGCs, because it is often too dark in the gastric cavity. Type 0-I polypoid gastric cancer and type 0-III ulcerative gastric cancer can be easily detected via endoscopy[14]. However, the morphological and color changes in MGCs are often subtle, so they may be missed during endoscopy. All MGCs in our study were of the superficial type and 84.29% were detected by WLE. Many differentiated minute and small human gastric adenocarcinomas have a depression accompanied by a circumferential elevation of the surrounding noncancerous mucosa[15]. In our study, 84.82% of MGCs exhibited morphological change, and the most common morphology was protruding. Color change was observed by WLE in 76.44% of MGCs, and the most common colors were yellowish-red and reddish. Thin white moss was observed on the surface in 34.55% of lesions, and 5.76% had spontaneous hemorrhages. Therefore, MGCs should be detected by careful WLE examination of morphology and color change.
Second-generation NBI (2G-NBI) images are significantly brighter, enabling the screening of EGCs. Although 2G-NBI is no better than WLE for real-time EGC detection in high-risk individuals, the positive predictive value of 2G-NBI is higher than WLE[16]. There were 27 MGCs missed by initial WLE screening but detected by secondary NBI observation. These lesions were brownish under NBI, and most of them were flat. Therefore, if no lesion is found by WLE in patients at high risk for GC, NBI endoscopy is recommended for a second screening. Compared with standard WLE, chromoendoscopy has a high diagnostic efficacy of EGCs and premalignant gastric lesions[17]. In our study, there were three MGCs missed by WLE and NBI, but found after spraying indigo carmine, suggesting that indigo carmine could be used for endoscopic screening of high-risk GC.
If a well-demarcated border lesion has an irregular color or surface pattern as viewed via WLE, an endoscopic diagnosis of EGC should be made[14]. We found that if a clear demarcation line lesion has a typical neoplastic color via WLE combined with NBI, an endoscopic diagnosis could be made. Yellowish-red or whitish under WLE and brownish under NBI were typical neoplastic colors. ME requires special endoscopic systems that are too high a threshold for many endoscopists to operate, and NBI enhances the visualization of mucosal surface features. If a lesion has a clear demarcation line and a typical neoplastic color by nonmagnifying observation, an endoscopic diagnosis of MGC should be made.
ME based on microvascular architecture enables differentiation of flat EGC from gastritis[18]. Yao et al[7] proposed a diagnostic system (the VS classification) based on an irregular microstructure and/or microvascular pattern with a clear demarcation, which enabled accurate diagnosis of superficial cancer[7]. Using the VS classification, ME-NBI has greater diagnostic accuracy than WLE for EGC[19,20]. Our findings agreed with these reports; ME-NBI increased the endoscopic diagnostic confidence of neoplastic lesions. Therefore, if a non-neoplastic lesion is diagnosed by ME-NBI, biopsy may not be necessary. If a neoplastic lesion is diagnosed by ME-NBI and meets the criteria of ESD, endoscopic resection could be performed directly without biopsy. However, if the nature of the lesion could not be determined by ME-NBI, a biopsy should be obtained for pathological confirmation.
Atrophy caused by Helicobacter pylori infection generally starts from the gastric antrum and progresses to the gastric body along the lesser curvature, so tumors are located, in order of frequency, in the lower > middle > upper stomach[21,22]. In our study, MGCs were located in order in the lower > middle > upper stomach. Ikeda et al[23] found that the predominant histological type changed from differentiated to undifferentiated with progression of the tumor[23]. In our study, all MGCs were only one pathological type, and the main pathological type of MGCs was differentiated. Perri et al[24] found that 18% of MGCs invaded the submucosa[24]. In our study, except for eight GA-FG and one GA-FGM, only one moderately differentiated adenocarcinoma invaded the submucosa. This may be because surgery was the only therapy in the study of Perri et al[24]. Inadequate stretching of surgical specimens may result in underestimation of tumor size. Therefore, we speculate that the proportion of MGCs invading the submucosa is low, and even MGCs have no possibility of submucosal invasion. The Osaka University ESD study group reported that the incidence of synchronous or metachronous multiple GCs was 13.9% during a mean follow-up of 26.8 mo[25]. However, ~30% of multiple GCs were seen in our study. It may be because the research group in our study focused on MGCs, and the accompanying tumors were larger in diameter and easy to be detected.
Our study emphasized the diagnostic strategies for MGCs, which was novel and particularly challenging due to their small size (≤ 5 mm diameter). It was a real-world observational study that included 191 MGCs over an 8-year period. The study highlighted the effectiveness of combining WLE, NBI, and indigo carmine to improve detection of MGC. The robust data offered valuable insights into the detection and diagnosis of MGC, making the results relevant for everyday clinical practice.
Our study had several limitations. First, it was a retrospective study conducted at a single institution with a small sample size, which may have led to selection bias. Second, only experienced endoscopists used NBI and indigo carmine for endoscopy, which may have resulted in observer bias. Third, some lesions were initially found in other hospitals and transferred to our hospital for further treatment. The previous gastroscopic biopsy affected the endoscopic observation of MGCs. Fourth, the Japanese criteria were used to diagnose MGCs in our study. If the World Health Organization classification was used , some MGCs would be diagnosed as high-grade intraepithelial neoplasia.
Most MGCs could be detected by careful WLE screening. NBI and indigo carmine are helpful to detect the lesions missed by WLE. A lesion with a clear demarcation line and a typical neoplastic color should be diagnosed as MGC. ME-NBI could improve diagnostic confidence and play the role of optical biopsy.
The authors would like to thank Wen-Ren Xing for her contribution in refining the English language of this manuscript.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56596] [Article Influence: 7074.5] [Reference Citation Analysis (134)] |
| 2. | Katai H, Ishikawa T, Akazawa K, Isobe Y, Miyashiro I, Oda I, Tsujitani S, Ono H, Tanabe S, Fukagawa T, Nunobe S, Kakeji Y, Nashimoto A; Registration Committee of the Japanese Gastric Cancer Association. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001-2007). Gastric Cancer. 2018;21:144-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 375] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 3. | Tanabe S, Hirabayashi S, Oda I, Ono H, Nashimoto A, Isobe Y, Miyashiro I, Tsujitani S, Seto Y, Fukagawa T, Nunobe S, Furukawa H, Kodera Y, Kaminishi M, Katai H. Gastric cancer treated by endoscopic submucosal dissection or endoscopic mucosal resection in Japan from 2004 through 2006: JGCA nationwide registry conducted in 2013. Gastric Cancer. 2017;20:834-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 4. | Ono H, Yao K, Fujishiro M, Oda I, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Ichinose M, Matsui T. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc. 2016;28:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 413] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 5. | Hatta W, Gotoda T, Koike T, Masamune A. History and future perspectives in Japanese guidelines for endoscopic resection of early gastric cancer. Dig Endosc. 2020;32:180-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 6. | Ono H, Yao K, Fujishiro M, Oda I, Uedo N, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Fujimoto K. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig Endosc. 2021;33:4-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 359] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 7. | Yao K, Anagnostopoulos GK, Ragunath K. Magnifying endoscopy for diagnosing and delineating early gastric cancer. Endoscopy. 2009;41:462-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 344] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 8. | Choi KS, Jun JK, Park EC, Park S, Jung KW, Han MA, Choi IJ, Lee HY. Performance of different gastric cancer screening methods in Korea: a population-based study. PLoS One. 2012;7:e50041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 9. | Pasechnikov V, Chukov S, Fedorov E, Kikuste I, Leja M. Gastric cancer: prevention, screening and early diagnosis. World J Gastroenterol. 2014;20:13842-13862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 247] [Cited by in RCA: 308] [Article Influence: 25.7] [Reference Citation Analysis (2)] |
| 10. | Gono K, Obi T, Yamaguchi M, Ohyama N, Machida H, Sano Y, Yoshida S, Hamamoto Y, Endo T. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9:568-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 620] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 11. | Kikuchi O, Ezoe Y, Morita S, Horimatsu T, Muto M. Narrow-band imaging for the head and neck region and the upper gastrointestinal tract. Jpn J Clin Oncol. 2013;43:458-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Muto M, Minashi K, Yano T, Saito Y, Oda I, Nonaka S, Omori T, Sugiura H, Goda K, Kaise M, Inoue H, Ishikawa H, Ochiai A, Shimoda T, Watanabe H, Tajiri H, Saito D. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010;28:1566-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 545] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 13. | Goda K, Dobashi A, Tajiri H. Perspectives on narrow-band imaging endoscopy for superficial squamous neoplasms of the orohypopharynx and esophagus. Dig Endosc. 2014;26 Suppl 1:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Yao K, Uedo N, Muto M, Ishikawa H. Development of an e-learning system for teaching endoscopists how to diagnose early gastric cancer: basic principles for improving early detection. Gastric Cancer. 2017;20:28-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Mori M, Enjoji M, Sugimachi K. Histopathologic features of minute and small human gastric adenocarcinomas. Arch Pathol Lab Med. 1989;113:926-931. [PubMed] |
| 16. | Yoshida N, Doyama H, Yano T, Horimatsu T, Uedo N, Yamamoto Y, Kakushima N, Kanzaki H, Hori S, Yao K, Oda I, Katada C, Yokoi C, Ohata K, Yoshimura K, Ishikawa H, Muto M. Early gastric cancer detection in high-risk patients: a multicentre randomised controlled trial on the effect of second-generation narrow band imaging. Gut. 2021;70:67-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 17. | Zhao Z, Yin Z, Wang S, Wang J, Bai B, Qiu Z, Zhao Q. Meta-analysis: The diagnostic efficacy of chromoendoscopy for early gastric cancer and premalignant gastric lesions. J Gastroenterol Hepatol. 2016;31:1539-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Yao K, Iwashita A, Tanabe H, Nagahama T, Matsui T, Ueki T, Sou S, Kikuchi Y, Yorioka M. Novel zoom endoscopy technique for diagnosis of small flat gastric cancer: a prospective, blind study. Clin Gastroenterol Hepatol. 2007;5:869-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Maki S, Yao K, Nagahama T, Beppu T, Hisabe T, Takaki Y, Hirai F, Matsui T, Tanabe H, Iwashita A. Magnifying endoscopy with narrow-band imaging is useful in the differential diagnosis between low-grade adenoma and early cancer of superficial elevated gastric lesions. Gastric Cancer. 2013;16:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 20. | Yu H, Yang AM, Lu XH, Zhou WX, Yao F, Fei GJ, Guo T, Yao LQ, He LP, Wang BM. Magnifying narrow-band imaging endoscopy is superior in diagnosis of early gastric cancer. World J Gastroenterol. 2015;21:9156-9162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (2)] |
| 21. | Shin KY, Jeon SW, Cho KB, Park KS, Kim ES, Park CK, Chung YJ, Kwon JG, Jung JT, Kim EY, Kim KO, Jang BI, Lee SH, Park JB, Yang CH. Clinical outcomes of the endoscopic submucosal dissection of early gastric cancer are comparable between absolute and new expanded criteria. Gut Liver. 2015;9:181-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Hahn KY, Park CH, Lee YK, Chung H, Park JC, Shin SK, Lee YC, Kim HI, Cheong JH, Hyung WJ, Noh SH, Lee SK. Comparative study between endoscopic submucosal dissection and surgery in patients with early gastric cancer. Surg Endosc. 2018;32:73-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 23. | Ikeda Y, Mori M, Kamakura T, Haraguchi Y, Saku M, Sugimachi K. Increased incidence of undifferentiated type of gastric cancer with tumor progression in 912 patients with early gastric cancer and 1245 with advanced gastric cancer. Cancer. 1994;73:2459-2463. [PubMed] [DOI] [Full Text] |
| 24. | Perri F, Iuliano R, Valente G, Angelillo IF, Arrigoni A, Campra D, Recchia S, Andriulli A. Minute and small early gastric cancers in a Western population: a clinicopathologic study. Gastrointest Endosc. 1995;41:475-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Kato M, Nishida T, Yamamoto K, Hayashi S, Kitamura S, Yabuta T, Yoshio T, Nakamura T, Komori M, Kawai N, Nishihara A, Nakanishi F, Nakahara M, Ogiyama H, Kinoshita K, Yamada T, Iijima H, Tsujii M, Takehara T. Scheduled endoscopic surveillance controls secondary cancer after curative endoscopic resection for early gastric cancer: a multicentre retrospective cohort study by Osaka University ESD study group. Gut. 2013;62:1425-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 212] [Article Influence: 16.3] [Reference Citation Analysis (0)] |