Published online Aug 15, 2024. doi: 10.4251/wjgo.v16.i8.3471
Revised: March 8, 2024
Accepted: June 3, 2024
Published online: August 15, 2024
Processing time: 195 Days and 13.3 Hours
The intrapapillary capillary loop (IPCL) characteristics, visualized using mag
To investigate differences in pathological microvascular structures of ESCC, which correspond to the deepest parts of the lesions' infiltration.
Patients with ESCC and precancerous lesions diagnosed at Peking University Third Hospital were enrolled between January 2019 and April 2023. Patients first underwent magnified endoscopic examination, followed by endoscopic submucosal dissection or surgical treatment. Pathological images were scanned using a three-dimensional slice scanner, and the pathological structural differences in different types, according to the JES classification, were analyzed using nonparametric tests and t-tests.
The 35 lesions were divided into four groups according to the JES classification: A, B1, B2, and B3. Statistical analyses revealed significant differences (aP < 0.05) in the short and long calibers, area, location, and density between types A and B. Notably, there were no significant differences in these parameters between types B1 and B2 and between types B2 and B3 (P > 0.05). However, significant differences in the short calibers, long calibers, and area of IPCL were observed between types B1 and B3 (aP < 0.05); no significant differences were found in the den
Pathological structures of IPCLs in the deepest infiltrating regions differ among various IPCL types classified by the JES classification under magnifying endoscopy, especially between the types A and B.
Core Tip: Based on the Japan Esophageal Society (JES) classification, this study first clarified the differences in pathological microvascular structures of esophageal squamous cell carcinoma, which correspond to the deepest parts of the lesions’ infiltration. The results show significant differences in the pathological structures of the intrapapillary capillary loops, which correspond to the deepest parts of the lesions’ infiltration, between the different types. This finding might help optimize the JES classification and potentially advance the accuracy of endoscopic biopsy diagnosis.
- Citation: Shu WY, Shi YY, Huang JT, Meng LM, Zhang HJ, Cui RL, Li Y, Ding SG. Microvascular structural changes in esophageal squamous cell carcinoma pathology according to intrapapillary capillary loop types under magnifying endoscopy. World J Gastrointest Oncol 2024; 16(8): 3471-3480
- URL: https://www.wjgnet.com/1948-5204/full/v16/i8/3471.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i8.3471
Esophageal cancer is the seventh most prevalent cancer and sixth most common cause of cancer-related mortality worldwide[1]. China contributes significantly to global esophageal cancer statistics by consistently maintaining its leading position in both incidence and mortality rates of this disease. More than 90% of esophageal cancer cases are esophageal squamous cell carcinomas (ESCCs)[2]. The overall survival rate correlates with the tumor stage at diagnosis, with an overall 5-year survival rate below 30%[3]. The overall 5-year survival rate can reach above 90% in patients diagnosed early with appropriate treatment[4]. Thus, early ESCC detection and precise assessment of its infiltration depth are crucial.
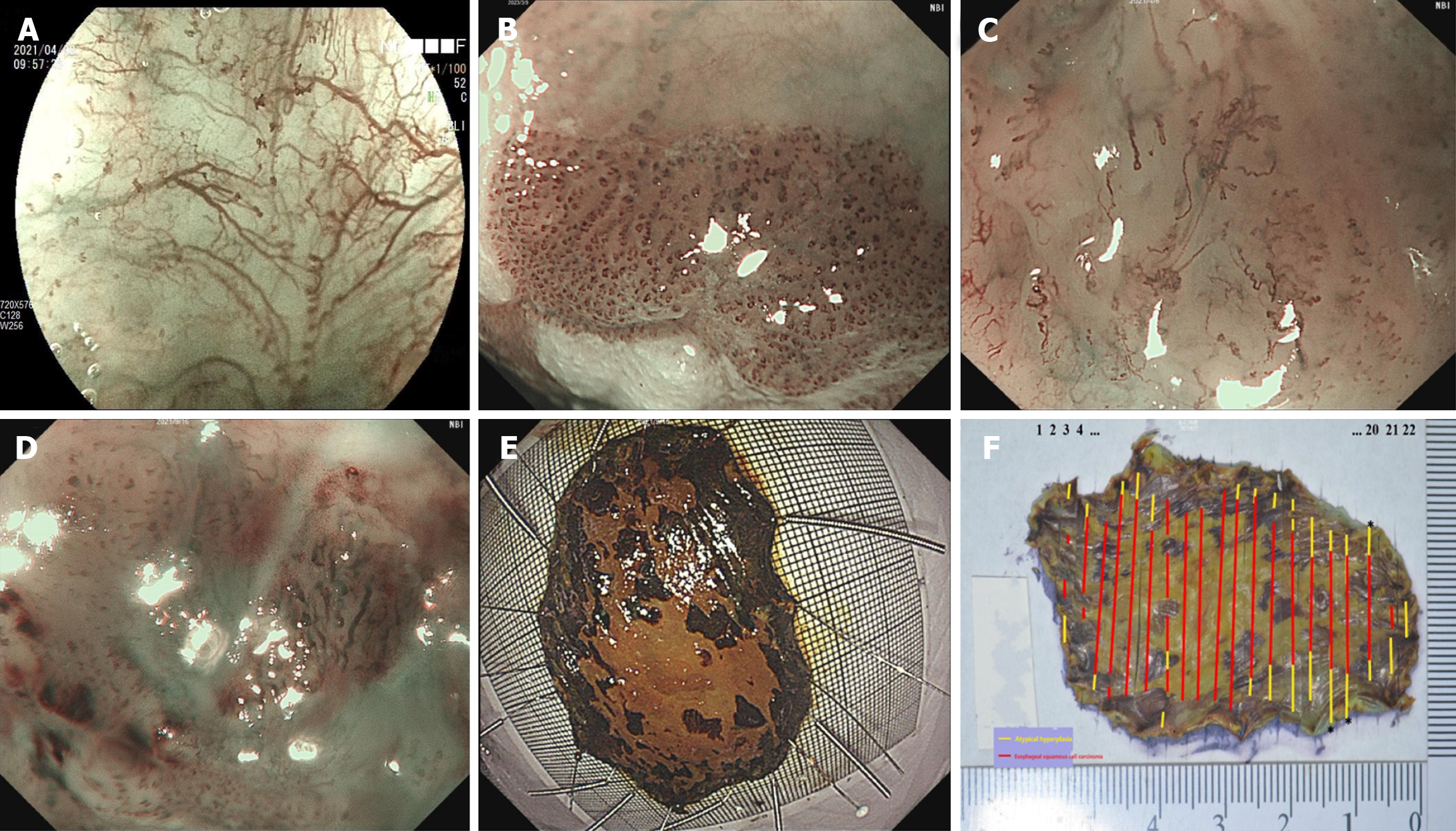
In recent years, diverse diagnostic modalities, including computed tomography, esophagography, and endoscopy, have been utilized to detect ESCC. Various endoscopic technologies have been used in this process, including conventional white-light endoscopy, chromoendoscopy, endoscopic ultrasound, and magnifying endoscopy. Magnifying endoscopy is commonly used for diagnosing ESCC and assessing the depth of invasion[5]. This technique allows visualization of the esophageal surface microvascular structures and enables the determination of infiltration depth based on morphological changes in the intrapapillary capillary loop (IPCL). Alterations in the IPCL primarily involve dilation, distortion, caliber, and shape changes. Three main classifications based on IPCL changes have been proposed: Inoue's IPCL classification[6], Arima's classification[7], and the classification of the Japan Esophageal Society (JES)[8]. The JES classification integrates several aspects of the first two classifications and is the most widely used in clinical practice. The JES classification mainly categorizes IPCL into types A, B1, B2, and B3 (Figure 1A-D), which correspond to noncancerous lesions, T1a-EP or T1a-LPM, T1a-MM or T1b-SM1 (submucosal tumor invasion limited to within 200 μm), and T1b-SM2 (submucosal tumor invasion at more than 200 μm) tumors, respectively. Previous studies have indicated that the diagnostic accuracy rates for types B1, B2, and B3 vessels are 86.0%-97.5%, 38%-86%, and 55%-100%, respectively[9-11]. Notably, the diagnostic efficiency for type B2 vessels was significantly lower than that for type B1 and B3 vessels, and the diagnostic sensitivity of type B3 vessels was notably low. Furthermore, recent studies have suggested that the diagnostic efficiency of the JES classification may not be entirely satisfactory, particularly regarding the accuracy for type B2 vessels and sensitivity for type B3 vessels[9,12-15]. Consequently, there is room for modification of the JES classification.
Therefore, this study initially categorized ESCC and precancerous lesions using the JES classification. Subsequently, we explored the pathological alterations within the IPCL located in the deepest infiltrating regions of the lesions among the various IPCL types. We aimed to clarify the pathological distinctions between IPCLs at varying infiltration depths in ESCC, which might help optimize the JES classification and potentially advance the precise endoscopic biopsy diagnosis.
This was a retrospective study. All experimental protocols were approved by the Medical Ethics Committee of Peking University Third Hospital (Approval No: IRB00006761-M2023341).
We included the following: (1) From January 2019 to May 2023, patients diagnosed with ESCC or precancerous lesions who underwent narrow-band imaging or blue laser imaging combined with magnifying endoscopy (ME- NBI/BLI) at Peking University Third Hospital; and (2) Patients with complete data on endoscopy and pathology findings and basic information.
The exclusion criteria were as follows: (1) Exhibiting recurrence of ESCC and having undergone chemotherapy or radiotherapy treatments; (2) Being diagnosed with metastatic esophageal cancer or esophageal adenocarcinoma; and (3) Lacking essential images and fundamental information, as well as having uncertain diagnosis due to obscured images.
All objective endoscopic images were retrospectively reviewed by two experts proficient in ME-NBI (GIF-H290Z endoscope; Olympus Medical Co., Ltd., Tokyo, Japan) or ME-BLI (EG-760Z endoscope; Fujifilm Co., Tokyo, Japan) and with substantial experience in the field, based on the JES classification[8]. The two experts were blinded to the clinical details of the patients and histological outcomes of the lesions. When discrepancies arose, a third experienced endoscopist was consulted for a final assessment.
The lesion area was delineated using Lugol's solution before resection[16]. To facilitate the procedure, 0.009% saline solution infused with melphalan was injected to elevate the edge of the lesion. The lesion was alternately incised using a dual knife (KD-650L; Olympus Medical Co., Ltd.) and an insulation-tipped diathermic knife (KD-611L; Olympus Medical Co., Ltd.) to obtain the specimen. To ensure accuracy, the entire mucosal and mucosal muscle layers were meticulously pulled outward and affixed onto a foam or rubber plate using a fine needle. This process allowed for clear visualization of the lesion on the mucosal surface. Measurements were conducted using a ruler, and photographs were captured for documentation (Figure 1E). Subsequently, the specimens were promptly immersed in a 10% formaldehyde solution (Solarbio, Beijing, China) for preservation.
The resected esophagus was cut and opened along the longitudinal line on the side opposite the lesion. The opened esophagus was then gently stretched longitudinally and fixed such that the length of the specimen was similar to that in vivo. Finally, the specimens were treated with an iodine solution after fixation to describe the macroscopic findings accurately.
Before cutting the resected esophagus, formalin-fixed specimens were treated with an iodine solution to confirm the unstained area. Rinsing the samples with tap water for at least one hour resulted in good staining. The samples were treated with a relatively low concentration (0.1%-0.5%) of iodine solution to increase the contrast between the stained and unstained areas. Finally, the dissected specimens were cut parallel to the long axis of the esophagus[17].
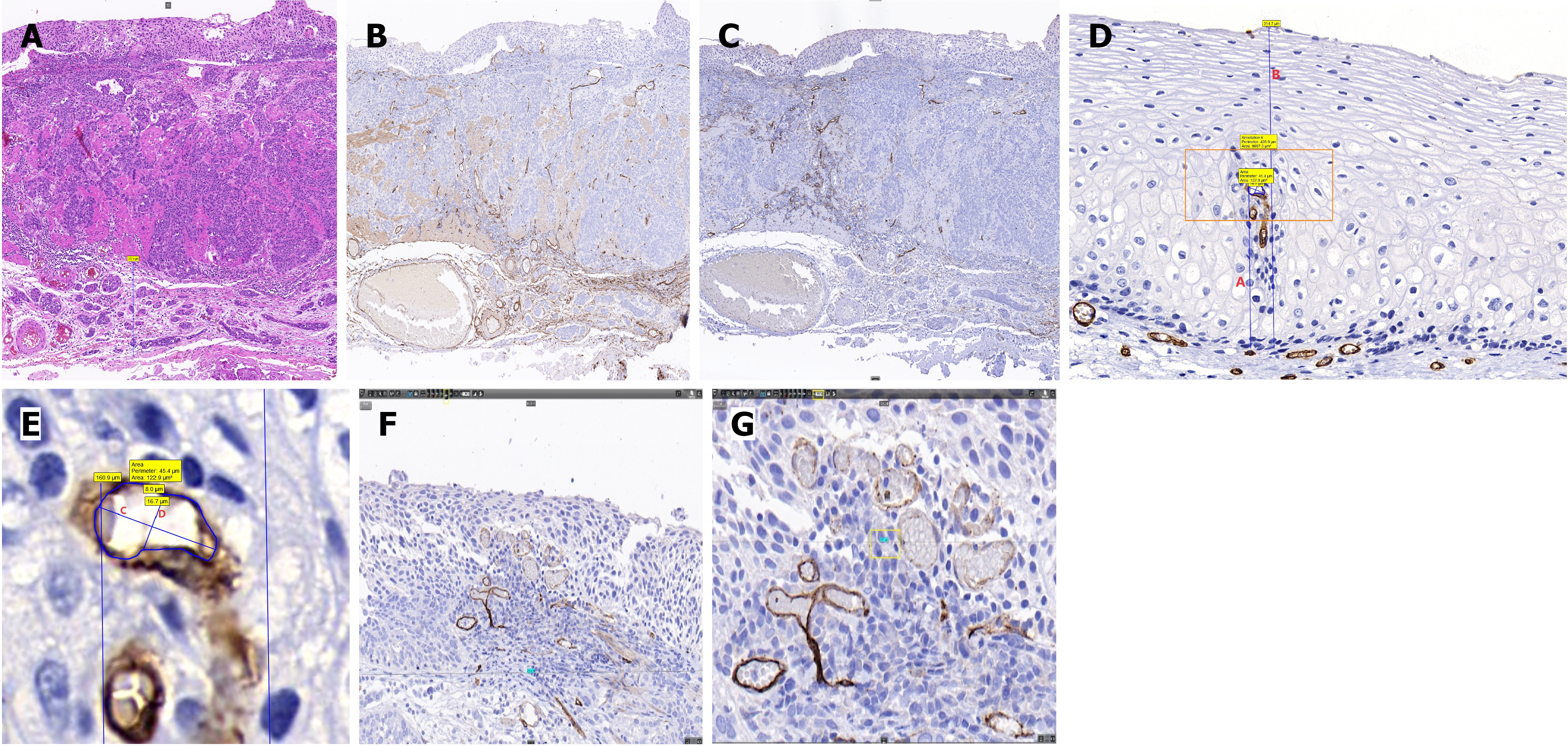
The 10% formaldehyde-fixed specimens were photographed before and after tissue sampling. The closest mucosal margins were identified upon identifying the lesion type, location, and extent. Tissue samples were obtained by making perpendicular cuts (Leica RM2125 RTS; Leica Biosystems, Wetzlar, Germany) 2-3 mm from the nearest lesion margin, starting 1 mm from the edge of the lesion. All collected tissues were meticulously sampled and examined. The subsequent steps involved dehydration, embedding, and slicing the tissue into 4-µm sections. Hematoxylin and eosin (H&E) staining was performed, and the sections exhibiting the deepest infiltration were stained for cluster of differentiation 34 (CD34) (clone 10C9, anti-human, catalogue number ZM-0046; Zsbio, Beijing, China) and D2-40 (clone D2-40, anti-human, catalogue number ZM-0465; Zsbio) markers. Each H&E-stained section was examined individually under a light microscope (Eclipse 80i; Nikon, Tokyo, Japan) to assess the overall lesion. Additionally, the H&E-stained section displaying the deepest infiltration depth, and its corresponding CD34 and D2-40-stained sections, were scanned using a three-dimensional (3D) section scanning instrument (Midi, PANNORAMIC; 3DHISTECH, Budapest, Hungary) at a magnification of × 20. Finally, the scanned images were analyzed for IPCL features using Case Viewer software, an observation tool in the 3D section scanner. Photographs were acquired for fresh and fixed specimens (Figure 1E and F).
Data were collected from three distinct fields of view with the deepest lesion infiltration for each lesion. In each CD34-stained specimen, comparisons were made based on H&E and D2-40 staining, which were utilized to identify cancerous areas and vascular distribution based on the JES classification[18-20]. In the Case Viewer software, each field of view was set to × 40, translating to an actual magnification of × 800. The transverse diameter of each field of view was 719.8 μm. Within every field, specific measurements were obtained, including the short caliber, long caliber, and area of the three IPCL vessels, with each IPCL vessel being the one closest to the luminal side of its intrapapillary position, apical distance from the vertex of the IPCLs to the basement membrane, which was defined as X; and thickness of the epithelial layer at the corresponding position of the tallest vessel within each of the three capillary papillae, which was defined as Y. Location was defined as the ratio of X to Y. In cases where the basement membrane was damaged or unidentifiable, an approximate outline of the basement membrane was created based on recognizable areas from neighboring regions, employing the 'measurement annotation tool' for measurement. The values obtained from three vessels in each field of view were averaged to obtain the measurement value for that specific field of view. Density calculations were performed by selecting the largest area within each of the initially chosen × 40 fields of view. At a magnification of × 100 (equivalent to an actual magnification of × 2000, with a fixed width of 287.9 μm), the sum of the areas of all blood vessels in the field of view was measured to determine the density of this field of view. Pathological data were collected as described in previous studies[21,22] (Figure 2).
The sample size was determined based on the evaluation indices of each group's caliber, density, and area of IPCLs. Reference data from a previous study[21], including the mean and standard deviation (SD) of the caliber, density, and area of IPCLs, were used for this calculation. PASS 2021 (v 2021.0.3; NCSS Statistical Software, Kaysville, UT, United States) software was used. Multisample mean comparisons were employed for sample size calculation. Four groups, namely types A, B1, B2, and B3, based on the JES classification, were included in the study. The type I error (α) was set at 0.05, and the type II error (β) was set at 0.1. The mean multiplier was set to 1. The sample size was the same in each group. The sample size in each group was calculated separately, based on the caliber, area, and density data, resulting in eight participants per group.
All statistical analyses were performed using SPSS 27.0 (IBM Corp., Armonk, NY, United States). Patients were initially categorized into four groups according to the JES classification: Types A, B1, B2, and B3. The analysis encompassed the short caliber, long caliber, area, location, and density of IPCLs. The results indicated that the data characteristics within each group followed a normal distribution. However, apart from the location, the variance in the data was not uniform, necessitating the application of nonparametric tests. Upon integrating types B1 and B2 into one group, the data characteristics of types A, B1/B2, and B3 mirrored those of the aforementioned four groups. Data are expressed as mean ± SD. P < 0.05 was considered statistically significant.
We selected 35 specimens from 26 patients diagnosed with ESCC and precancerous lesions who underwent endoscopic submucosal dissection or surgery at Peking University Third Hospital (Beijing, China) between January 2019 and May 2023. Table 1 shows the clinicopathological characteristics of the 26 patients (20 males and 6 females). The average age of the patients was 62 years (range: 49-77). The specimens were classified into types A, B1, B2, and B3, with 9, 8, 10, and 8 specimens, respectively. These specimens corresponded to 9, 12, 7, and 7 cases with pathological infiltration depths classified as normal lesion/intraepithelial neoplasia (N/IN), T1a-EP/LPM, T1a-MM/T1b-SM1, and T1b-SM2, respectively, based the 11th Edition of JES[17].
| Parameters | Value |
| Total number of patients | 26 |
| Sex as male/female | 20/6 |
| Mean age in yr (range) | 62 (49-77) |
| Total number of lesions | 35 |
| Tumor invasion depth cases, N/IN, T1a-EP/LPM, T1a-MM/T1b-SM1, T1b-SM2 | 9, 12, 7, 7 |
| JES classification types A, B1, B2, B3 | 9, 8, 10, 8 |
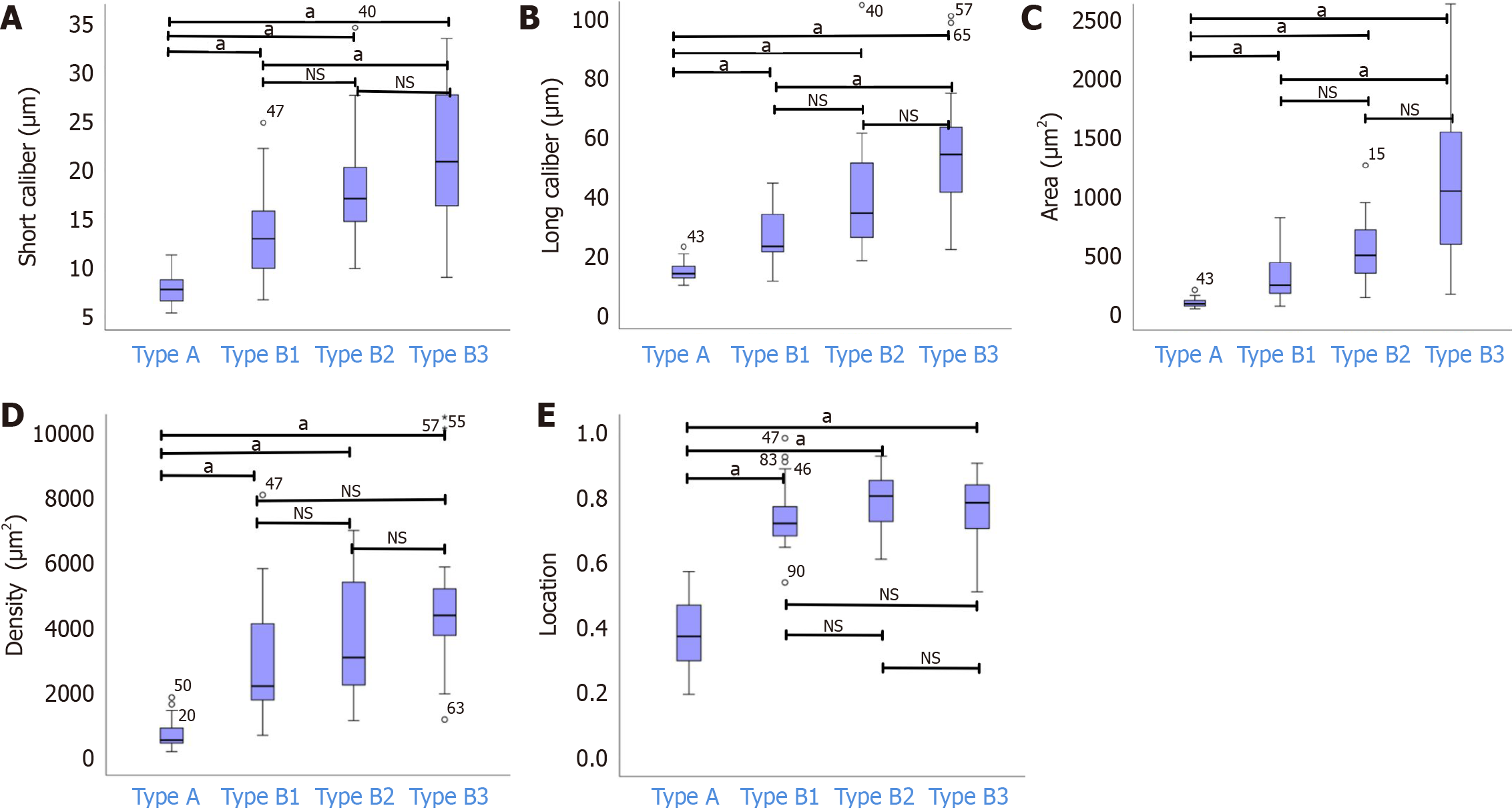
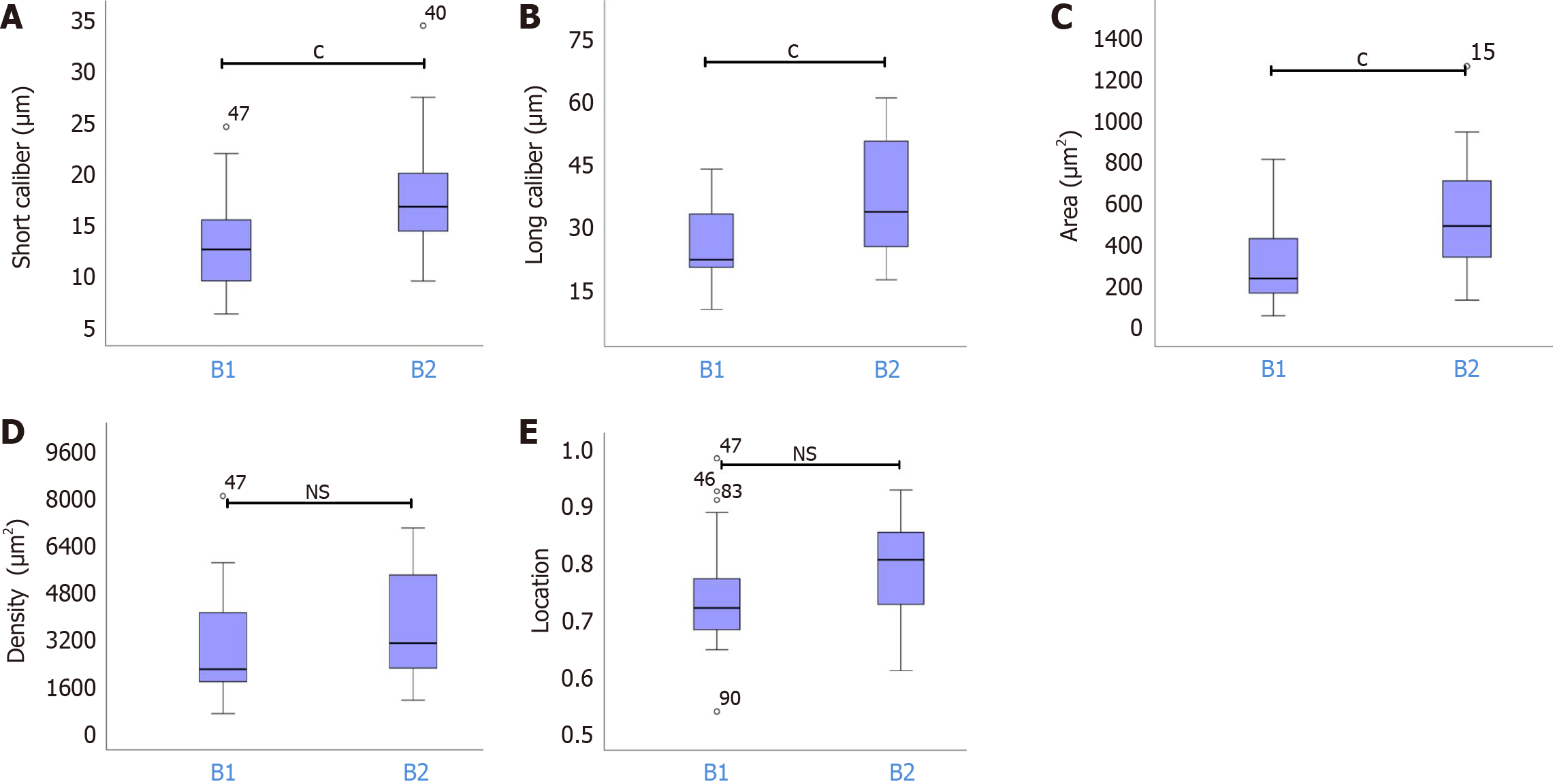
The mean ± SD short calibers of IPCLs for types A, B1, B2, and B3 were 7.49 ± 1.43 μm, 13.31 ± 4.65 μm, 17.52 ± 5.03 μm, and 21.35 ±7.55 μm, respectively. Similarly, the mean ± SD long calibers for types A, B1, B2, and B3 were 13.87 ± 3.18 μm, 26.50 ± 9.54 μm, 38.76 ± 17.72 μm, and 53.38 ± 20.46 μm, respectively. The corresponding mean ± SD areas were 92.48 ± 34.80 μm², 333.05 ± 224.94 μm², 601.59 ± 489.61 μm², and 1088.11 ± 625.45 μm², and the mean ± SD densities were 693.84 ± 424.44 μm², 3183.89 ± 2527.81 μm², 4194.07 ± 2922.58 μm², and 5220.32 ± 3509.80 μm². Additionally, the mean ± SD locations for types A, B1, B2, and B3 were 0.38 ± 0.11, 0.75 ± 0.10, 0.79 ± 0.08, and 0.77 ± 0.10, respectively. Statistical analysis revealed significant differences (aP < 0.05) in the short and long calibers, area, location, and density between types A and B. Notably, there were no significant variations in these parameters between types B1 and B2 and between types B2 and B3 (P > 0.05). However, significant differences in the short and long calibers and area were observed between types B1 and B3 (aP < 0.05); no significant differences were found in the density or location (P > 0.05) (Figure 3).
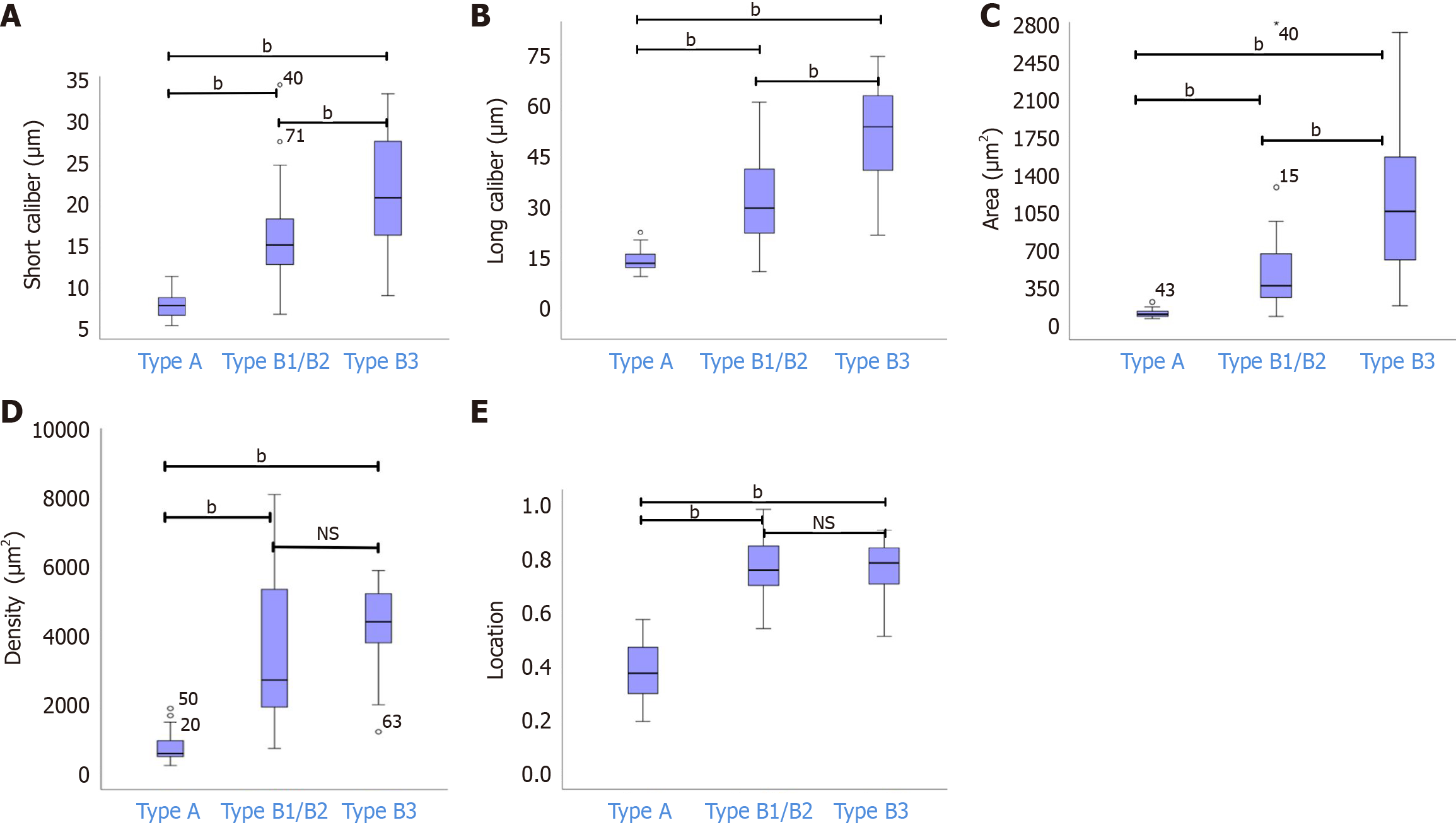
After combining types B1 and B2 into one group, the means ± SD for the short caliber, long caliber, area, density, and location of type B1/B2 were 15.65 ± 5.26 μm, 33.31 ± 15.78 μm, 482.24 ± 413.84 μm², 3745.10 ± 2775.48 μm², and 0.77 ± 0.10, respectively. Statistical analyses indicated significant differences in the short and long calibers, and area among types A, B1/B2, and B3 (bP < 0.05). Additionally, there were significant disparities in location and density between type A and both types B1/B2 and B3 (bP < 0.05). However, the differences in the location and density between types B1/B2 and B3 were not statistically significant (P > 0.05) (Figure 4).
Subgroup analyses for types B1 and B2 revealed significant differences in the short caliber, long caliber, and area (cP < 0.05), with no significant disparities in location and density between the groups (Figure 5). The results are summarized in Table 2.
| Parameter | Type A | Type B1 | Type B2 | Type B1/B2 | Type B3 | P value |
| Short caliber of IPCL in μm | 7.4 ± 1.43 | 13.31 ± 4.65 | 17.52 ± 5.03 | 15.65 ± 5.26 | 21.35 ± 7.55 | < 0.001 |
| Long caliber of IPCL in μm | 13.87 ± 3.18 | 26.50 ± 9.54 | 38.76 ± 17.72 | 33.31 ± 15.78 | 53.38 ± 20.46 | < 0.001 |
| Area of IPCL in μm² | 92.48 ± 34.80 | 333.05 ± 224.94 | 601.59 ± 489.61 | 482.24 ± 413.84 | 1088.11 ± 625.45 | < 0.001 |
| Density in μm² | 693.84 ± 424.44 | 3183.89 ± 2527.81 | 4194.07 ± 2922.58 | 3745.10 ± 2775.48 | 5220.32 ± 3509.80 | < 0.001 |
| X in μm | 122.30 ± 12.74 | 274.73 ± 25.90 | 295.15 ± 17.71 | 286.07 ± 110.61 | 256.55 ± 17.23 | < 0.001 |
| Y in μm | 317.22 ± 24.81 | 363.52 ± 30.71 | 373.56 ± 21.13 | 369.10 ± 131.05 | 331.18 ± 18.80 | > 0.05 |
| Location on X/Y | 0.38 ± 0.11 | 0.75 ± 0.10 | 0.79 ± 0.08 | 0.77 ± 0.10 | 0.77 ± 0.10 | < 0.001 |
This study explored the pathological characteristics of IPCLs among the different types based on the JES classification. The findings of this study may provide a pathological basis for modifying the JES classification. The results revealed variations in the short and long diameters, area, density, and location of the IPCLs at the deepest infiltration associated with different IPCL types. In type B1 and B2 vessels, significant differences in vessel caliber and area were observed, despite the absence of an explicit mention in the JES classification that type B2 vessels have a larger caliber than type B1 vessels. Hence, caliber could serve as a distinguishing factor between type B1 and B2 vessels that may be further refined. Moreover, as indicated by previous findings, notable disparities in the density and location exist between type A and type B vessels. Thus, Kuwano et al[23] observed a significant difference in the number of blood vessels between in situ or minimally invasive cancer lesions and adjacent normal tissues, in early esophageal cancer. This finding suggests a gradual change in density and location from type A to type B vessels[24], underscoring the value of these characteristics in subdividing type A vessels. Moreover, Kikuchi et al[25] found that ESCC lesions observed by ME-NBI had increased vascular density in the cancerous areas compared with that in the noncancerous areas. Furthermore, the results of our study demonstrated that cancerous IPCLs were generally located within a distance of less than 200 µm from the top of the epithelial layer. Currently, laser confocal endomicroscopy enables magnification of up to × 1000 and a scanning depth ranging from the mucosal surface to 250 µm below the mucosa[26-28]. Consequently, laser confocal endomicroscopy could offer a theoretically feasible approach for precise biopsy during endoscopy.
The findings of this study varied slightly from those of previous studies in several respects. Previous studies classified IPCLs into three groups, namely types A, B1/B2, and B3, based on the JES classification[21]. However, previous studies individually examined the characteristics of IPCLs, taking either a pathological or endoscopic perspective, without effectively integrating the two[25]. In contrast, this study further subdivided these groups into types A, B1, B2, and B3. We initially observed the endoscopic manifestations and subsequently investigated the characteristics of the IPCLs at the deepest infiltration level, thus establishing a close link between the endoscopic results and the infiltration depth. Moreover, the present study's findings suggest that the density differences among types B1, B2, and B3 vessels may not be significantly pronounced. At the same time, there are significant differences in the density between type A and type B vessels[25]. In contrast, a previous study has indicated that the density of B3 vessels is higher than that of B1/B2 vessels[21]. Therefore, a larger sample size is required to ascertain the presence of actual density differences between type B vessels, along with comparisons of endoscopic manifestations. Finally, the results of this study diverged from those of previous research[29] concerning the location of IPCLs. Thus, the main distinction lies in the spatial positioning of type A and type B vessels; however, the differences in the location of type B1, B2, and B3 vessels may not be significant. This discrepancy can be attributed to the growth pattern observed in most ESCC, which typically progresses from the basal to the luminal side. This growth pattern can result in vessels' upward displacement or compression towards the luminal side, thereby nourishing the tumor cells situated closer to the lumen. From an endoscopic perspective, this phenomenon may explain why IPCLs in most cancerous lesions appear clearer and closer to the mucosal side than normal IPCLs.
The present study had some limitations, including its single-center retrospective design and the measurement of the caliber of IPCLs, which is only based on a vertical cross-section. Thus, the evaluation may be carried out at an angle to another direction of the maximum diameter in the future. In addition, the classification of type A vessels does not allow a distinction between normal, inflammatory, and intraepithelial neoplasia. Future research could address these limitations by grouping type A vessels into finer subcategories. This would enable a more detailed exploration of the progression of pathological features from normal to precancerous lesions and, ultimately, cancer, facilitating timely detection and appropriate interventions throughout the process.
This study first clarified the microvascular structural changes in the deepest parts of the lesions' infiltration of ESCC corresponding to the different types of IPCLs, classified by the JES classification. The results showed significant differences in the pathological structures of the IPCLs, which corresponded to the deepest parts of the lesions' infiltration, between the different types. This finding might help optimize the JES classification and potentially improve the accuracy of endoscopic biopsy diagnosis.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68390] [Article Influence: 13678.0] [Reference Citation Analysis (201)] |
| 2. | Arnold M, Ferlay J, van Berge Henegouwen MI, Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. 2020;69:1564-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 419] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 3. | Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, Xia C, Sun K, Yang Z, Li H, Wang N, Han R, Liu S, Li H, Mu H, He Y, Xu Y, Fu Z, Zhou Y, Jiang J, Yang Y, Chen J, Wei K, Fan D, Wang J, Fu F, Zhao D, Song G, Chen J, Jiang C, Zhou X, Gu X, Jin F, Li Q, Li Y, Wu T, Yan C, Dong J, Hua Z, Baade P, Bray F, Jemal A, Yu XQ, He J. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6:e555-e567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 1083] [Article Influence: 135.4] [Reference Citation Analysis (3)] |
| 4. | Duggan MA, Anderson WF, Altekruse S, Penberthy L, Sherman ME. The Surveillance, Epidemiology, and End Results (SEER) Program and Pathology: Toward Strengthening the Critical Relationship. Am J Surg Pathol. 2016;40:e94-e102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 354] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 5. | Ishihara R, Muto M. Current status of endoscopic detection, characterization and staging of superficial esophageal squamous cell carcinoma. Jpn J Clin Oncol. 2022;52:799-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | Inoue H. Magnification endoscopy in the esophagus and stomach. Dig Endosc. 2001;13:S0-S41. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 7. | Arima M, Tada M, Arima H. Evaluation of microvascular patterns of superficial esophageal cancers by magnifying endoscopy. Esophagus. 2005;2:191-197. [DOI] [Full Text] |
| 8. | Oyama T, Inoue H, Arima M, Momma K, Omori T, Ishihara R, Hirasawa D, Takeuchi M, Tomori A, Goda K. Prediction of the invasion depth of superficial squamous cell carcinoma based on microvessel morphology: magnifying endoscopic classification of the Japan Esophageal Society. Esophagus. 2017;14:105-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 243] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 9. | Zeng YT, Sun YY, Tan WC, Luo SA, Zou BH, Luo GY, Huang CY. Study of preoperative diagnostic modalities in Chinese patients with superficial esophageal squamous cell carcinoma. World J Gastrointest Surg. 2022;14:986-996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Mizumoto T, Hiyama T, Oka S, Yorita N, Kuroki K, Kurihara M, Yoshifuku Y, Sanomura Y, Urabe Y, Arihiro K, Tanaka S, Chayama K. Diagnosis of superficial esophageal squamous cell carcinoma invasion depth before endoscopic submucosal dissection. Dis Esophagus. 2018;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Sudou K, Wakatsuki T, Sakaki T, Nagahara H, Sakabayashi Y, Fukumoto Y, Furutachi S, Mannami T. Validation of magnifying endoscopic classification of the Japan esophageal society for esophageal squamous cell carcinoma. United Eur Gastroenterol J. 2019;7:720. |
| 12. | Hatta W, Koike T, Ogata Y, Kondo Y, Ara N, Uno K, Asano N, Imatani A, Masamune A. Comparison of Magnifying Endoscopy with Blue Light Imaging and Narrow Band Imaging for Determining the Invasion Depth of Superficial Esophageal Squamous Cell Carcinoma by the Japanese Esophageal Society's Intrapapillary Capillary Loop Classification. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Ueda T, Dohi O, Naito Y, Yoshida T, Azuma Y, Ishida T, Matsumura S, Kitae H, Takayama S, Mizuno N, Nakano T, Iwai N, Hirose R, Inoue K, Yoshida N, Kamada K, Uchiyama K, Ishikawa T, Takagi T, Konishi H, Nishimura A, Kishimoto M, Itoh Y. Diagnostic performance of magnifying blue laser imaging versus magnifying narrow-band imaging for identifying the depth of invasion of superficial esophageal squamous cell carcinoma. Dis Esophagus. 2021;34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Tanaka I, Hirasawa D, Saito H, Matsuda T, Nakahori M, Maeda Y, Okuzono T, Suzuki K, Igarashi K, Nawata Y, Ito S, Unno S, Chonan A. The sub-classification of type B2 vessels according to the magnifying endoscopic classification of the Japan Esophageal Society. Dig Endosc. 2020;32:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Gotoda T, Hori K, Nakagawa M, Kobayashi S, Toyokawa T, Ishiyama S, Imagawa A, Abe M, Kono Y, Kanzaki H, Iwamuro M, Kawano S, Kawahara Y, Okada H. A prospective multicenter study of the magnifying endoscopic evaluation of the invasion depth of superficial esophageal cancers. Surg Endosc. 2022;36:3451-3459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Inoue H, Rey JF, Lightdale C. Lugol chromoendoscopy for esophageal squamous cell cancer. Endoscopy. 2001;33:75-79. [PubMed] |
| 17. | Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus. 2017;14:1-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 479] [Cited by in RCA: 739] [Article Influence: 82.1] [Reference Citation Analysis (2)] |
| 18. | Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part II and III. Esophagus. 2017;14:37-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 355] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 19. | Möbius C, Demuth C, Aigner T, Wiedmann M, Wittekind C, Mössner J, Hauss J, Witzigmann H. Evaluation of VEGF A expression and microvascular density as prognostic factors in extrahepatic cholangiocarcinoma. Eur J Surg Oncol. 2007;33:1025-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Qu W, Fu JD, Yang F, Yang GL, Zhang YL, Wang XY, Gu HX, Zhang HY, Wang L. Clinical implications of PTEN and VEGF expression status, as well as microvessel density in esophageal squamous cell carcinoma. Oncol Lett. 2015;10:1409-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Wu HL, Guan BX, Liu B, Wang HJ, Zhang MB, Li GC, Zhu KX, Zhou CJ, Guo JQ. The intrapapillary capillary loop (IPCL) changes in superficial esophageal lesions. Dis Esophagus. 2017;30:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Kumagai Y, Sobajima J, Higashi M, Ishiguro T, Fukuchi M, Ishibashi K, Baba H, Mochiki E, Yakabi K, Kawano T, Tamaru J, Ishida H. Angiogenesis in superficial esophageal squamous cell carcinoma: assessment of microvessel density based on immunostaining for CD34 and CD105. Jpn J Clin Oncol. 2014;44:526-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Kuwano H, Sonoda K, Yasuda M, Sumiyoshi K, Nozoe T, Sugimachi K. Tumor invasion and angiogenesis in early esophageal squamous cell carcinoma. J Surg Oncol. 1997;65:188-193. [PubMed] [DOI] [Full Text] |
| 24. | Kubota Y, Kaneko K, Konishi K, Ito H, Yamamoto T, Katagiri A, Muramoto T, Yano Y, Kobayashi Y, Oyama T, Kushima M, Imawari M. The onset of angiogenesis in a multistep process of esophageal squamous cell carcinoma. Front Biosci (Landmark Ed). 2009;14:3872-3878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Kikuchi D, Iizuka T, Hoteya S, Nomura K, Kuribayashi Y, Toba T, Tanaka M, Yamashita S, Furuhata T, Matsui A, Mitani T, Inoshita N, Kaise M. Vascular density of superficial esophageal squamous cell carcinoma determined by direct observation of resected specimen using narrow band imaging with magnifying endoscopy. Dis Esophagus. 2017;30:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Lee SK. Usefulness of Probe-Based Confocal Laser Endomicroscopy for Esophageal Squamous Cell Neoplasm. Clin Endosc. 2019;52:91-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Mashimo H, Gordon SR, Singh SK. Advanced endoscopic imaging for detecting and guiding therapy of early neoplasias of the esophagus. Ann N Y Acad Sci. 2020;1482:61-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Shimamura Y, Inoue H, Rodriguez de Santiago E, Abad MRA, Fujiyoshi Y, Toshimori A, Tanabe M, Sumi K, Iwaya Y, Ikeda H, Onimaru M, Kushima M, Goda K. Diagnostic yield of fourth-generation endocytoscopy for esophageal squamous lesions using a modified endocytoscopic classification. Dig Endosc. 2021;33:1093-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Kaga M, Inoue H, Kudo SE, Hamatani S. Microvascular architecture of early esophageal neoplasia. Oncol Rep. 2011;26:1063-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/