Published online Aug 15, 2024. doi: 10.4251/wjgo.v16.i8.3428
Revised: May 1, 2024
Accepted: May 24, 2024
Published online: August 15, 2024
Processing time: 158 Days and 6.3 Hours
Colorectal cancer is a term used to describe colon and rectal cancer, which is the third most common type of cancer. A MEDLINE and PubMed search resulted in the inclusion of manuscripts written in the last 10 years, using keywords relevant to the topic of the manuscript. By analyzing the aim of the searched studies and manuscripts, adequate articles were included that described the stated problem. The frequency of colorectal cancer varies with climate, nutrition, and many other factors, primarily endogenous, hereditary, intestinal microbiome, as well as external factors, such as exposure of the individual to stress, and bad eating habits. Colon cancer and rectal cancer or colorectal cancer in general in the early stages of the disease, may not show symptoms or are barely noticeable. Colorectal cancer symptoms will most often not develop until the disease has progressed to stage 2 or beyond. Regular screening tests for colon or rectal cancer, especially colonoscopy, are recommended as part of a regular checkup for people aged 50 years or younger who are at high risk due to a family history of the disease or other cancers. Diet and colonoscopy as an early screening method play an im
Core Tip: Consuming a smaller amount of fatty, fried food, salted and smoked foods with the intake of more fruits and vegetables, as well as foods rich in folic acid (beans, wheat germ, asparagus) and calcium changes the intestinal microbiome, reducing the inflammatory process at the level of the intestinal mucosa, and thus the occurrence of colorectal carcinoma. Undoubtedly, colonoscopy is important in the diagnosis and prevention of colorectal cancer.
- Citation: Jovandaric MZ. Importance of diet and intestinal microbiota in the prevention of colorectal cancer - colonoscopy early screening diagnosis. World J Gastrointest Oncol 2024; 16(8): 3428-3435
- URL: https://www.wjgnet.com/1948-5204/full/v16/i8/3428.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i8.3428
Colorectal cancer, i.e., colon and rectal cancer, is the third most common type of cancer affecting men and women, with almost 50000 deaths per year[1]. The manifestation of symptoms of colorectal cancer depends primarily on the location of the cancer, its stage, as well as the involvement of surrounding organs[2]. Colon and rectal cancer is a rare disease in Africa, while it is very common in Western Europe and America. According to the American Cancer Society, in 1990, 155000 new cases of colon and rectal cancer were diagnosed in the United States, of which approximately 61000 resulted in death. The incidence of colorectal cancer is about 30% higher in men than in women, while the mortality is about 40% higher. This difference in incidence is thought to be due to differences in exposure to risk factors (alcohol consumption, cigarette smoking, sex hormones, etc.) and interactions between risk factors. Differences in the localization of colorectal cancer with respect to gender have been observed. In women, the ratio of colon cancer to rectal cancer is 3:1, and in men, it is 2:1. It was also observed that right-sided colorectal cancer is more common in women. One of the most significant risk factors for the development of colorectal cancer is the patient’s age. It has been proven that the risk of developing this tumor increases with age. About 91% of new cases and about 94% of deaths are older than 50 years, while the incidence of colorectal cancer is 15 times higher in people older than 50 years compared to people aged 20 to 49 years. It is believed that with age there is a decline in the immunobiological resistance of the organism, which causes the accumulation of mutations that manage to avoid the reporter mechanisms and become the focus of carcinogenesis. However, in recent decades, an increased incidence of this tumor has been registered in the younger population. It has been observed that colorectal cancers in younger people are most often the result of persistent ulcerative colitis or familial adenomatous polyposis. It has also been noted that younger people tend to have a right-sided localization of this tumor, and the incidence of rectal cancer and rectosigmoid cancer seems to increase in patients under 40 years of age. Although the incidence of colorectal cancer in the general population in the United States has been observed to be decreasing due to an increase in colonoscopy screening, the rate of colorectal cancer continues to increase among adolescents and young adults. Although in some of these cases, the hereditary factor seems to play an important role, it can still be attributed to only 8% of the total number of cancer cases of all forms. Moreover, many experts believe that the main culprit could be eating habit[3].
Sometimes the symptoms of colorectal cancer do not appear until the cancer has developed. Given that symptoms may go unnoticed during the early stages of the disease, clinical practice recommends screening for colon cancer from the age of 50. Stool testing for occult (hidden) bleeding is very important in the prevention of colon cancer. The occult bleeding test refers to proving occult, invisible bleeding from the upper part of the digestive tract or bleeding in the lower part of the digestive tract. This test is recommended for all people over the age of 50, and those with a confirmed family history of colon cancer should have this test done annually after reaching the age of 40. The fecal occult blood test is a diagnostic test for evaluating occult blood in the stool that is not 100% certain, while a method called the fecal immunochemical test that uses antibodies to recognize blood in the stool is much better due to its increased specificity, sensitivity and reduced costs. Statistics show that regular control can prevent more than 60% of deaths caused by colon cancer. To reduce the risk of cancer, a good knowledge of risk factors as well as protective factors is necessary[4].
Risk factors are factors whose action can influence an increase in the risk of developing cancer, while protective factors have the opposite effect, so their action could influence the reduction of the risk of developing carcinoma[5]. Some risk factors such as smoking can be avoided, while some other factors cannot (genetic predisposition). Factors such as regular physical activity and a healthy diet can act favorably in the prevention of some types of cancer, as well as various other problems[6].
Smoking is a risk factor for many different diseases, not only cancer. Therefore, it is advised to avoid exposure to tobacco smoke whenever possible. When it comes to cancer of the anus, other factors that can increase the risk include human papillomavirus infection, immunosuppression, and the presence of chronic inflammatory bowel diseases[7]. Mere exposure to risk factors does not mean the sure occurrence of cancer, just as avoiding risk factors and increasing the action of protective factors does not mean that the disease will certainly not occur, but in this way, the possibility of occurrence is reduced. The occurrence of cancer is mainly determined by the joint action of various factors[8].
The author searched available data on colorectal carcinoma, the influence of diet, and intestinal microbiota on the development of colorectal cancer. PubMed, Scopus, The Cochrane Library, and Web of Science were searched for all articles available in their entirety written in English in the last 10 years. In the literature search, the terms “colorectal carcinoma”, nutrition, “intestinal microbiota”, outcome, treatment, and diagnosis were used. Studies and manuscripts that were not written in their entirety, or were related to carcinoma of another location, as well as manuscripts that were not written in English, were excluded from the study. Manuscripts that correspond to the current topic were selected for writing this narrative review. The results of the literature search are presented in section 3, with an accompanying discussion of nutrition in the prevention of colorectal carcinoma, as well as the impact of changes in the intestinal microbiota on the development of colorectal carcinoma.
Several epidemiological studies have shown that a diet high in fat appears to increase the risk of this type of cancer. It has been shown that people who consumed high amounts of saturated fat and the least amount of fiber were four times more likely to develop colon polyps (which are often a prelude to cancer), compared to men whose diet contained a lot of fiber and little fat[9]. Fat intake stimulates the release of bile acids from the gallbladder. Fat and bile pass through the intestinal system to the large intestine, where bile acids are converted into secondary bile acids by the action of bacteria. Secondary bile acids are cocarcinogens, which means that they cause changes in the cells of the intestinal walls, which can initiate the cancerous process[10].
Eating more insoluble fiber (from bran, fruits and vegetables, and legumes) can also help prevent cancer. Fiber shortens the time food spends in the digestive tract, thus reducing the duration of exposure to natural carcinogens. Fiber also binds to bile acids, preventing them from irritating the walls of the colon[11]. The results of several studies show that people whose diet contains a lot of fruits and vegetables have a much lower risk of developing colon and rectal cancer. These foods are not only good sources of fiber but are also rich in beta-carotene and other protective phytochemicals[12].
The researchers followed the diet of people suffering from rectal cancer as well as the diet of healthy people. They noted that those without rectal cancer ate more folate-rich foods, including leafy green vegetables such as spinach and asparagus. It is generally known that vegetables rich in folic acid can protect not only from colorectal cancer but also cancer in other locations[13].
Calcium also appears to play a role in protecting against colon and rectal cancer. Increased calcium intake dramatically lowers the rate of cell turnover in the colon. Laboratory tests show that calcium (similar to fiber) binds to bile acids. A good source of calcium is kale. Salted, pickled, or smoked foods can form carcinogenic compounds in the body that trigger the development of tumors in the digestive system[14].
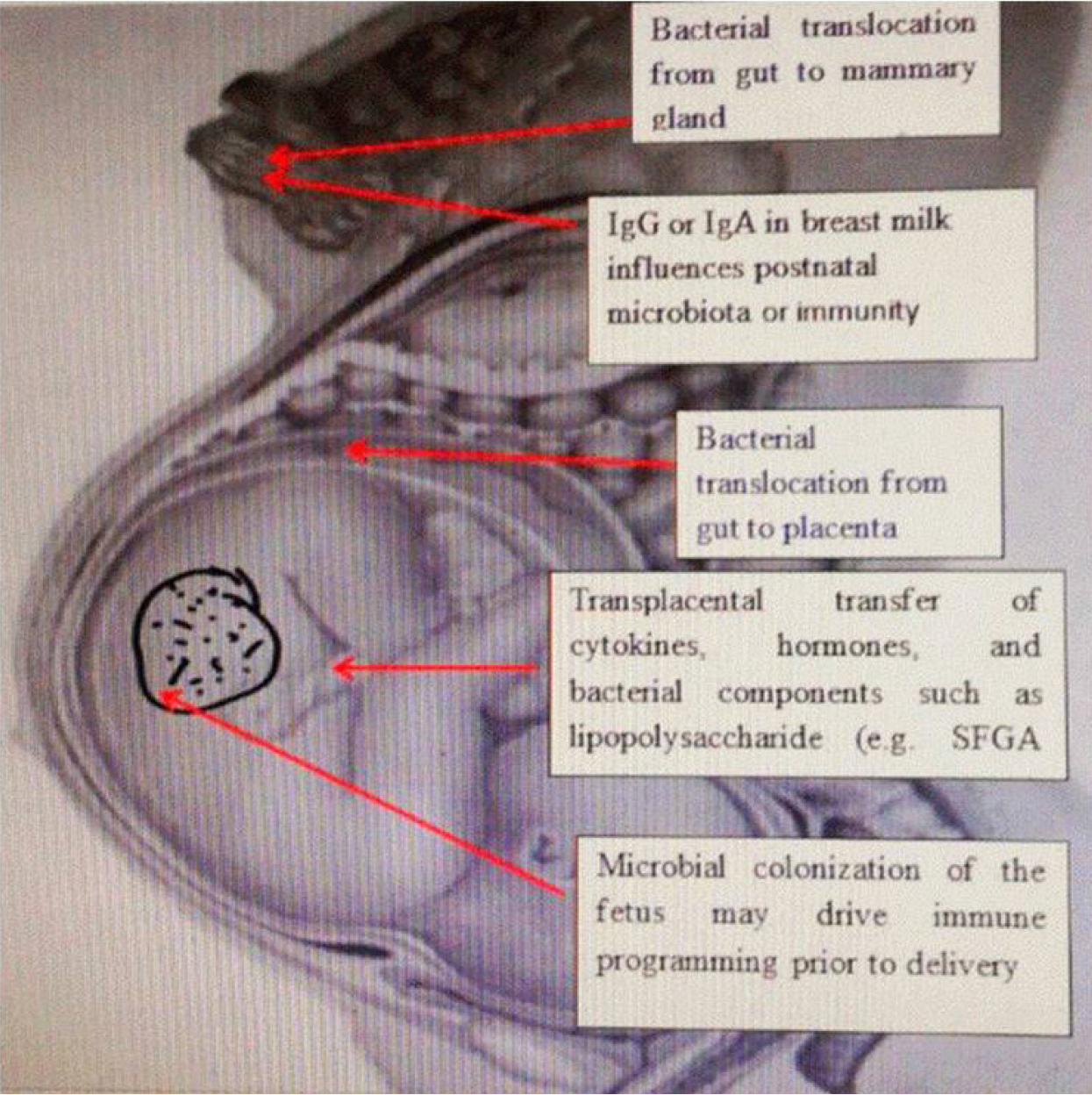
Microbiota is a common name for all microorganisms that live in the human body. Microbiota includes bacteria, archaea, protozoa, fungi and viruses. There is a microbiota of the stomach, small intestine, large intestine, as well as microbiota of the skin, vagina, etc. The largest number of microorganisms found in the human body live in the large intestine[15]. The microbiome is an integral part of microorganisms and is necessary for the proper function of organs. At birth, our digestive system is underdeveloped and unstable. When the child reaches 2-3 years old, relative stability is reached in the form of the composition of the microbiota in the large intestine. The richness and diversity of the intestinal microbiota formed in the early years of life characterize a healthy intestinal microbiome. However, the composition of the optimal, healthy microbiota is different for each individual. The composition of a person’s microbiome is highly variable and can change depending on age, ethnic origin, geographic location, diet, lifestyle, medications, and environmental factors[16,17] (Figure 1).
The microbiome of healthy adults mainly consists of four groups of bacteria: Firmicutes, Bacteriodetes, Proteobacteria, and Actinobacteria. It is known that the ratio of Firmicutes vs Bacteriodetes indicates a predisposition to the development of colorectal cancer[18]. The composition of the intestinal flora is relatively constant, although due to the various influences of the external environment, it can change in composition in all parts of the gastrointestinal tract, both in the variety of bacteria and in their quantity, starting from the oral cavity, through the esophagus, the intestine, and ending with the rectum[19].
It is known that the microbiota has a strong connection with the development of cancer. Studies have shown significant differences in the gastric microbiota between patients with gastric cancer (GC) and patients without cancer, suggesting that the microbiota may play a role in the development of GC. Although Helicobacter pylori (H. pylori) infection is widely recognized as a primary risk factor for GC, recent studies based on microbiota sequencing technology have revealed that Streptococcus anginosus is more common in the gastric mucosa of GC patients. Its infection can spontaneously cause chronic gastritis, atrophy of mural cells, mucoid chemotaxis, and heterotrophic hyperplasia, which promotes the development of precancerous lesions of GC. Streptococcus anginosus also disrupts gastric barrier function, promotes GC cell proliferation, and inhibits apoptosis[20,21].
The main function of the intestinal microbiota is to maintain a symbiotic relationship with the intestinal mucosa, and as such it has a significant metabolic, immunological, and intestinal-protective function in a healthy person. Gut microbiota participates in the creation of short-chain fatty acids (SCFA), the synthesis of vitamin K and folate, the metabolism of drugs, and the deconjugation of bile acids, preventing the colonization of pathogens in the intestinal tract[22].
The intestinal microbiota largely produces nutrients from carbohydrates that it receives from its host. Fermentation of undigested carbohydrates and indigestible oligosaccharides is carried out with the help of bacteria such as Bacteroides, Roseburia, Bifidobacterium, Fecalibacterium, and Enterobacteria. As a product, SCFA, such as butyrate, propionate, and acetate, are rich sources of energy for the host[23]. It has also been proven that the intestinal microbiota has a positive effect on fat metabolism by suppressing the inhibition of lipoprotein lipase activity in adipocytes, and Bacteroides promote lipid digestion[24].
Similarly, it effectively participates in protein metabolism using its enzymes that break down proteins. In the cell wall of bacteria, there are transporters for amino acids that facilitate the passage of amino acids from the intestinal lumen into the bacteria, where the bacteria further convert amino acids into small antimicrobial molecules called bacteriocins[25]. Intestinal microorganisms participate in the synthesis of vitamin K, but also vitamin B components[26]. It is known that bacteria of the genus Bacteroides participate in the synthesis of conjugated linoleic acid, which has immunomodulatory effects in the body, reduces the level of fat and glucose, and thus has an antiatherogenic effect[27].
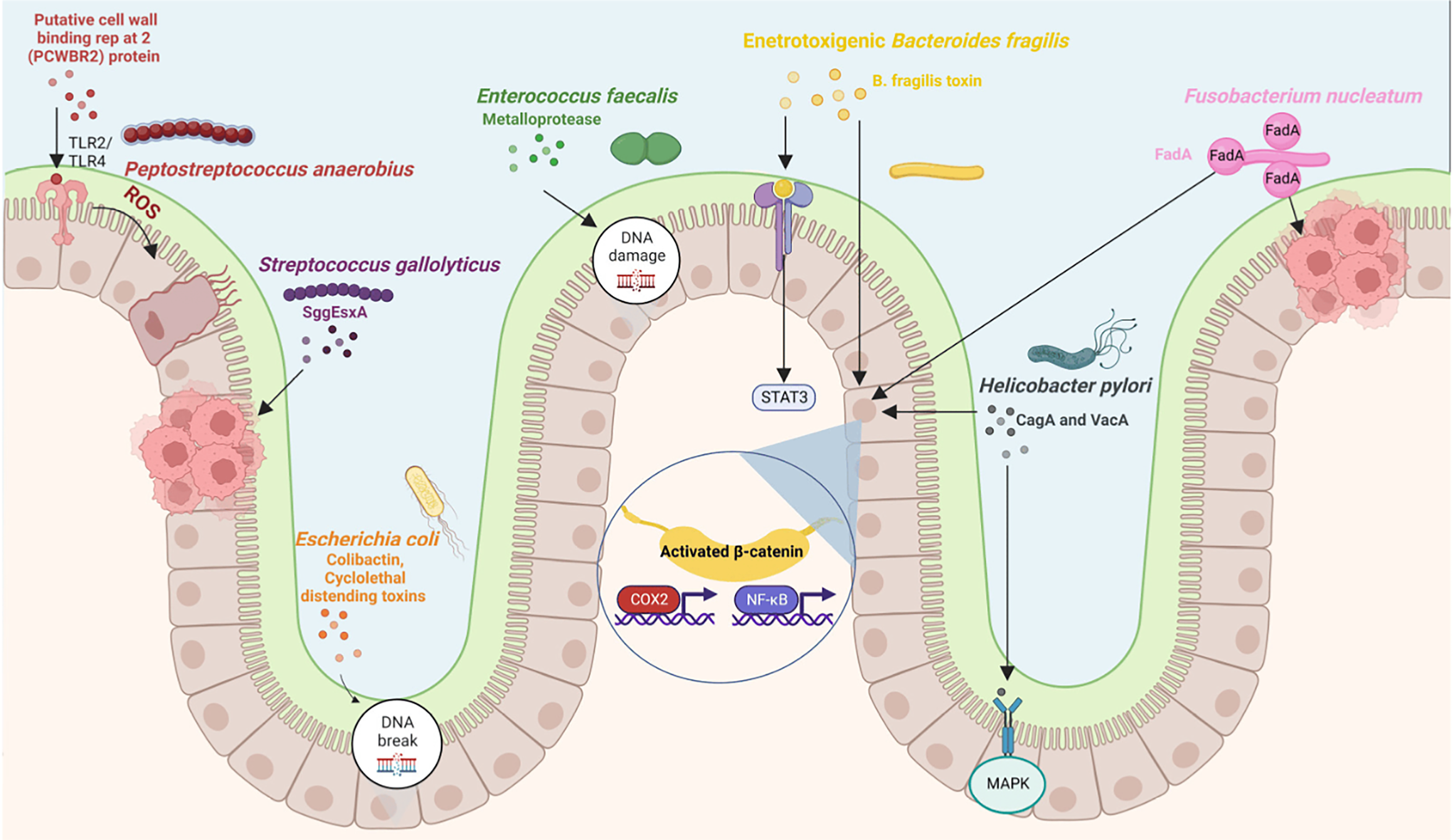
Gut microbiota, especially Bacteroides intestinalis, and to some extent both Bacteroides fragilis and Escherichia coli, have the ability to deconjugate and dehydrate primary bile acids and convert them into secondary bile acids in the large intestine[28] (Figure 2). Also, the normal gut microbiota has a positive effect on the energy metabolism of cells and thus contributes to the overall health of a person[29]. A condition for keeping the gastrointestinal tract healthy is maintaining intestinal bacteria in balance, preventing the colonization of pathogenic microorganisms. How the above is achieved is due to the presence of a double layer of mucus that prevents the penetration of harmful bacteria from the lumen into the epithelial cells of the intestinal tract[30,31].
Gut microbiota contributes to immunomodulation together with the innate and adaptive immune system. Beneficial bacteria in the gut play a key role in the development of innate immune cells, especially macrophages, monocytes, and neutrophils that form the first line of defense against invading pathogens[32]. Intestinal dysbiosis can increase intestinal permeability and disrupt the tight junction between intestinal epithelial cells. Microbe-derived metabolites such as β-glucan, endotoxins (lipopolysaccharide), and bacterial and viral RNAs, are termed pathogen-associated molecular patterns (PAMPs). PAMPs can disrupt the gut-liver axis, causing gut dysfunction and gut dysbiosis, which will cause increased gut permeability in liver cirrhosis and small intestinal bacterial overgrowth. Through the portal vein, blood flows from the intestine to the liver, PAMP activates Toll-like receptor 4 (TLR4) on liver macrophages and liver immune cells. The TLR4 signal in macrophages resident in the liver initiates the inflammatory process by activating tumor necrosis factor-α and interleukin-8, which are important factors in liver damage, causing an inflammatory process in liver cells with consequent necrosis of hepatocytes. Pancreatic inflammation also plays a key role in the development of pancreatic cancer (PC). The most common cause is intestinal dysbiosis with a predominance of harmful bacteria from the oral cavity to the end of the large intestine, i.e., the rectum, so that pathogenic bacteria pass through the mucous layer of epithelial cells, reach the pancreas, and cause an inflammatory process that contributes to the development of PC. Chronic inflammation of pancreatic tissue creates favorable conditions for oncogenic mutation in insulin-positive endocrine cells and induces differentiation of epithelial cells, resulting in the development of pancreatic ductal adenocarcinoma (PDAC). The number of microbes in PC tissue is up to a thousand times higher than in healthy pancreatic tissue. The first pathogen to be discovered in pancreatic tumor tissue and associated with pancreatic carcinoma was H. pylori. In addition, Fusobacterium spp., an oral pathogen, was found to be significantly increased in PDAC. There was also an increase in the amount of the fungus Malassezia spp. in PDAC tumor tissue. It can be said that H. pylori infection of the upper parts of the gastrointestinal tract is closely related to the risk of developing PDAC as the presence of H. pylori, by stimulating cell proliferation, simultaneously creates the conditions for the development of cancer. A significant increase in the number of bacteria belonging to the Phila bacteria, Firmicutes, Proteobacteria, Actinobacteria, Fusobacteria, Verrucomicrobia, genera Porphyromonas, Prevotella, Bifidobacterium and synergists of the archaeal type Euryarchaeota was reported compared to healthy controls. Other intestinal microbiota changes found in PC are characterized by a decrease in the number of Firmicutes, Proteobacteria, and Lactobacillus[33].
Dysbiosis is a term for microbial imbalance, including intestinal flora. The term “dysbiosis” is often used to describe a disturbed balance of microflora compared to a healthy and normal population. Others define dysbiosis as a change in the composition of the microbiota as a link to a disease. Dysbiosis and the pattern of commensal bacteria can influence and cause many clinical conditions[34]. It has been proven that intestinal dysbiosis is associated with diseases such as inflammatory bowel disease, irritable bowel syndrome, chronic fatigue syndrome, obesity, cancer, colitis, diabetes, various psychological and neurological conditions, and autism[35].
Once synonymous with infections and diseases of the upper respiratory tract, Klebsiella has now emerged as one of the pathogens most often isolated from patients with gastrointestinal diseases. Klebsiella is a gram-negative anaerobic bacterium, belonging to the Enterobacteriaceae family and normally lives both in the mouth and in the intestines. Among the various microorganisms that colonize the human gut, gram-negative bacteria are most famously associated with gastrointestinal diseases, such as inflammatory bowel disease, including Crohn’s disease, ulcerative colitis, and colorectal cancer[36].
Colorectal cancer can lead to unexplained weight loss in a variety of ways. Cancer cells use a lot of energy, and the immune system also uses energy as it works to fight disease. Cancer cells can release substances into the body that change the way food is converted into energy, which can cause weight loss[37]. Similar to unexplained weight loss, malignant cells consume the host’s energy for their survival, so a person can have general exhaustion for no apparent reason. However, colon cancer often causes fatigue due to occult bleeding[38].
Symptoms of carcinoma are often very non-specific. They can be accompanied by abdominal pain, a feeling of bloating, a change in bowel function that manifests itself in the form of cramps or a change in stool consistency, alternating constipation, and diarrhea[39]. The presence of blood in the stool is very often the first sign of colon or rectal cancer. In some situations, the stool can only be dark or black, and in the advanced stage of the disease, it is not so rare to see clotted blood[40]. However, very often, rectal bleeding is attributed to hemorrhoids, which can prevent early diagnosis of cancer[41].
As with all cancers, treatment is most successful when the diagnosis of colorectal cancer is made in the early stages of the disease. Colorectal cancer, is the third most common cause of cancer in both sexes, the percentage of which has been decreasing in the last ten years due to the increase in the number of colonoscopies and colon screening[42]. A colonoscopy of the mucosa of the colon can detect colonic diverticulosis and colonic polyps, which are common findings in colonoscopy. Diverticulosis of the large intestine means herniation of the wall of the large intestine, which occurs very often, most often in people over 60 years old. The frequency in the elderly population is over 40%. It is most often asymptomatic, although complications may occur in the form of inflammation, diverticulitis, or manifest as gastrointestinal bleeding. Polyps of the colorectal tract are growths that can be benign, such as hyperplastic polyps, to altered adenomatous cells or the appearance of villous polyps, which are considered precancerous lesions. Like diverticulosis, the incidence of colorectal polyps increases in adulthood and in developed countries that consume fast food, low in nuts and plant-based fiber. To prevent the formation of polyps, it is necessary to change the way of life, more physical activity, hiking, mountaineering, spending time in nature, especially over the age of 60, as well as changing the type of diet. It is considered that the Mediterranean type of diet is the healthiest because it contains an optimal amount of fruit, gluten-free whole grain cereals, and vegetables with a lot of fiber that accelerates the passage of food through the digestive tract, enabling regular bowel movements, which at the same time prevents the formation of polyps in the intestinal tract[43].
Eating less fatty, fried, salty, and smoked food with the consumption of more fruit and vegetables, as well as foods rich in folic acid (beans, wheat germ, asparagus) and calcium changes the intestinal microbiome, reducing the inflammatory processes of the intestinal mucosa, and thus the possible occurrence of colorectal cancer.
| 1. | Wang H, Yuan Z, Wang S, Pang W, Wang W, Liu X, Yi B, Han Q, Yao Y, Zhang Q, Zhang X, Zhang C. The comparison of risk factors for colorectal neoplasms at different anatomical sites. Int J Colorectal Dis. 2023;38:26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 2. | Xia C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N, Chen W. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135:584-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1790] [Cited by in RCA: 2486] [Article Influence: 621.5] [Reference Citation Analysis (2)] |
| 3. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68343] [Article Influence: 13668.6] [Reference Citation Analysis (201)] |
| 4. | Jayasinghe M, Prathiraja O, Caldera D, Jena R, Coffie-Pierre JA, Silva MS, Siddiqui OS. Colon Cancer Screening Methods: 2023 Update. Cureus. 2023;15:e37509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 5. | Wu S, Zhu W, Thompson P, Hannun YA. Evaluating intrinsic and non-intrinsic cancer risk factors. Nat Commun. 2018;9:3490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 254] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 6. | Sammallahti H, Kokkola A, Rezasoltani S, Ghanbari R, Asadzadeh Aghdaei H, Knuutila S, Puolakkainen P, Sarhadi VK. Microbiota Alterations and Their Association with Oncogenomic Changes in Pancreatic Cancer Patients. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Ng R, Sutradhar R, Yao Z, Wodchis WP, Rosella LC. Smoking, drinking, diet and physical activity-modifiable lifestyle risk factors and their associations with age to first chronic disease. Int J Epidemiol. 2020;49:113-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 266] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 8. | Zeng H, Umar S, Rust B, Lazarova D, Bordonaro M. Secondary Bile Acids and Short Chain Fatty Acids in the Colon: A Focus on Colonic Microbiome, Cell Proliferation, Inflammation, and Cancer. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 363] [Article Influence: 51.9] [Reference Citation Analysis (1)] |
| 9. | Kim M, Park K. Dietary Fat Intake and Risk of Colorectal Cancer: A Systematic Review and Meta-Analysis of Prospective Studies. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | Wong CC, Yu J. Gut microbiota in colorectal cancer development and therapy. Nat Rev Clin Oncol. 2023;20:429-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 397] [Reference Citation Analysis (0)] |
| 11. | Vernia F, Longo S, Stefanelli G, Viscido A, Latella G. Dietary Factors Modulating Colorectal Carcinogenesis. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 12. | Kumar A, Chinnathambi S, Kumar M, Pandian GN. Food Intake and Colorectal Cancer. Nutr Cancer. 2023;75:1710-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 13. | Fu H, He J, Li C, Deng Z, Chang H. Folate intake and risk of colorectal cancer: a systematic review and up-to-date meta-analysis of prospective studies. Eur J Cancer Prev. 2023;32:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 14. | Kim SH, Moon JY, Lim YJ. Dietary Intervention for Preventing Colorectal Cancer: A Practical Guide for Physicians. J Cancer Prev. 2022;27:139-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 15. | Guan ZW, Yu EZ, Feng Q. Soluble Dietary Fiber, One of the Most Important Nutrients for the Gut Microbiota. Molecules. 2021;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 16. | Jovandaric MZ, Dugalic S, Babic S, Babovic IR, Milicevic S, Mihajlovic D, Culjic M, Zivanovic T, Trklja A, Markovic B, Plesinac V, Jestrovic Z, Medjo B, Raus M, Dugalic MG. Programming Factors of Neonatal Intestinal Dysbiosis as a Cause of Disease. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Reference Citation Analysis (0)] |
| 17. | Nishida A, Ando Y, Kimura I, Miyamoto J. Involvement of Gut Microbial Metabolites Derived from Diet on Host Energy Homeostasis. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Van Hul M, Cani PD. The gut microbiota in obesity and weight management: microbes as friends or foe? Nat Rev Endocrinol. 2023;19:258-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 178] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 19. | Huang Z, Li Y, Park H, Ho M, Bhardwaj K, Sugimura N, Lee HW, Meng H, Ebert MP, Chao K, Burgermeister E, Bhatt AP, Shetty SA, Li K, Wen W, Zuo T. Unveiling and harnessing the human gut microbiome in the rising burden of non-communicable diseases during urbanization. Gut Microbes. 2023;15:2237645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 20. | Mima K, Hamada T, Inamura K, Baba H, Ugai T, Ogino S. The microbiome and rise of early-onset cancers: knowledge gaps and research opportunities. Gut Microbes. 2023;15:2269623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 21. | Palamides P, Jolaiya T, Idowu A, Loell E, Onyekwere C, Ugiagbe R, Agbo I, Lesi O, Ndububa D, Adekanle O, Carranza M, Ally R, Njom H, Adeleye IA, Harrison U, Clarke A, Fischer W, Smith S, Haas R. Helicobacter pylori patient isolates from South Africa and Nigeria differ in virulence factor pathogenicity profile and associated gastric disease outcome. Sci Rep. 2020;10:11409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Quaglio AEV, Grillo TG, De Oliveira ECS, Di Stasi LC, Sassaki LY. Gut microbiota, inflammatory bowel disease and colorectal cancer. World J Gastroenterol. 2022;28:4053-4060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 219] [Cited by in RCA: 282] [Article Influence: 70.5] [Reference Citation Analysis (116)] |
| 23. | Markowiak-Kopeć P, Śliżewska K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 747] [Article Influence: 124.5] [Reference Citation Analysis (0)] |
| 24. | Joseph N, Vasodavan K, Saipudin NA, Yusof BNM, Kumar S, Nordin SA. Gut microbiota and short-chain fatty acids (SCFAs) profiles of normal and overweight school children in Selangor after probiotics administration. J Funct Foods. 2019;57:103-111. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Wu L, Tang Z, Chen H, Ren Z, Ding Q, Liang K, Sun Z. Mutual interaction between gut microbiota and protein/amino acid metabolism for host mucosal immunity and health. Anim Nutr. 2021;7:11-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 26. | Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, Tuohy K. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57:1-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 993] [Cited by in RCA: 1741] [Article Influence: 193.4] [Reference Citation Analysis (1)] |
| 27. | Chen C, Niu M, Pan J, Du N, Liu S, Li H, He Q, Mao J, Duan Y, Du Y. Bacteroides, butyric acid and t10,c12-CLA changes in colorectal adenomatous polyp patients. Gut Pathog. 2021;13:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 28. | Pandey H, Tang DWT, Wong SH, Lal D. Gut Microbiota in Colorectal Cancer: Biological Role and Therapeutic Opportunities. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 92] [Reference Citation Analysis (0)] |
| 29. | Moszak M, Szulińska M, Bogdański P. You Are What You Eat-The Relationship between Diet, Microbiota, and Metabolic Disorders-A Review. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 250] [Article Influence: 41.7] [Reference Citation Analysis (1)] |
| 30. | Gustafsson JK, Johansson MEV. The role of goblet cells and mucus in intestinal homeostasis. Nat Rev Gastroenterol Hepatol. 2022;19:785-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 389] [Reference Citation Analysis (0)] |
| 31. | Luis AS, Hansson GC. Intestinal mucus and their glycans: A habitat for thriving microbiota. Cell Host Microbe. 2023;31:1087-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 164] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 32. | Campbell C, Kandalgaonkar MR, Golonka RM, Yeoh BS, Vijay-Kumar M, Saha P. Crosstalk between Gut Microbiota and Host Immunity: Impact on Inflammation and Immunotherapy. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 140] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 33. | Papa V, Schepis T, Coppola G, Chiappetta MF, Del Vecchio LE, Rozera T, Quero G, Gasbarrini A, Alfieri S, Papa A. The Role of Microbiota in Pancreatic Cancer. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 34. | Wei S, Bahl MI, Baunwall SMD, Hvas CL, Licht TR. Determining Gut Microbial Dysbiosis: a Review of Applied Indexes for Assessment of Intestinal Microbiota Imbalances. Appl Environ Microbiol. 2021;87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 35. | Kim HS, Son J, Lee D, Tsai J, Wang D, Chocron ES, Jeong S, Kittrell P, Murchison CF, Kennedy RE, Tobon A, Jackson CE, Pickering AM. Gut- and oral-dysbiosis differentially impact spinal- and bulbar-onset ALS, predicting ALS severity and potentially determining the location of disease onset. BMC Neurol. 2022;22:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 36. | Lucas C, Barnich N, Nguyen HTT. Microbiota, Inflammation and Colorectal Cancer. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 241] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 37. | Fabregas JC, Ramnaraign B, George TJ. Clinical Updates for Colon Cancer Care in 2022. Clin Colorectal Cancer. 2022;21:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 83] [Reference Citation Analysis (1)] |
| 38. | Whitaker K. Earlier diagnosis: the importance of cancer symptoms. Lancet Oncol. 2020;21:6-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 39. | Holtedahl K, Borgquist L, Donker GA, Buntinx F, Weller D, Campbell C, Månsson J, Hammersley V, Braaten T, Parajuli R. Symptoms and signs of colorectal cancer, with differences between proximal and distal colon cancer: a prospective cohort study of diagnostic accuracy in primary care. BMC Fam Pract. 2021;22:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 40. | Haneishi Y, Furuya Y, Hasegawa M, Picarelli A, Rossi M, Miyamoto J. Inflammatory Bowel Diseases and Gut Microbiota. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 135] [Reference Citation Analysis (0)] |
| 41. | Schwandner O, Pech O. [Rectal bleeding: easy to overcome or still a challenge in proctology?]. Chirurg. 2019;90:640-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 42. | Di Leo M, Iannone A, Arena M, Losurdo G, Palamara MA, Iabichino G, Consolo P, Rendina M, Luigiano C, Di Leo A. Novel frontiers of agents for bowel cleansing for colonoscopy. World J Gastroenterol. 2021;27:7748-7770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 43. | Ray J, Zidong Z, Yuan J, Quan M, Hachem C. The relationship between colon polyps and colonic diverticulosis: a retrospective review. Ann Gastroenterol. 2023;36:314-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/