Published online Jul 15, 2024. doi: 10.4251/wjgo.v16.i7.3082
Revised: May 23, 2024
Accepted: June 13, 2024
Published online: July 15, 2024
Processing time: 95 Days and 1.9 Hours
Hepatocellular carcinoma (HCC) is a malignant tumor that has a high incidence and mortality worldwide. Despite extensive studies, the detailed molecular mechanism of HCC development remains unclear. Studies have shown that the occurrence and development of HCC are closely related to abnormal gene expression. BCAR3 has been shown to be overexpressed in a variety of malignant tumors. However, the role of BCAR3 in HCC remains unclear.
To investigate the expression of BCAR3 and BCAR3-related competing endo
The data of HCC were obtained from the Cancer Genome Atlas database and The Genotype Tissue Expression, including transcriptome data and clinical information. Multiple common databases, including UALCAN, Timer 2.0, cBioPortal, LinkedOmics, starBase, Gene Ontology and Kyoto Encyclopedia of Genes and Genomes, were used to analyse the expression of BCAR3, prognostic value, genetic alteration, co-expressed genes, differentially expressed genes, BCAR3 gene-related ceRNAs and functional enrichment analysis in HCC patients. Kaplan-Meier analysis, univariate and multivariate Cox regression analysis were used to analyze survival prognosis and the Spearman test was used to measure correlations between BCAR3 and immune functions. And R language package was used to analyze the correlation between BCAR3 and immune invasion of HCC.
Our study indicated that BCAR3 was differentially expressed in various tumor tissues. The over-expression of BCAR3 gene was an unfavorable prognostic indicator for HCC patients, and associated with unfavorable cytogenetic risk and gene mutations. Moreover, most immune cells were positively correlated with BCAR3 (P < 0.05). According to the results of functional enrichment analysis, BCAR3 was involved in the positive regulation of epidermal growth factor receptor signaling pathway and ERBB signaling pathway, and was related to DNA replication and GTPase regulator activity. Finally, our study found that based on RAB30-DT and miR-19b-3p pathways, targeting BCAR3 might promote the occurrence and development of HCC.
Collectively, this study indicated that the BCAR3 gene was involved in the occurrence and development of HCC, and it might be a new biomarker and therapeutic target for HCC, but the specific mechanism remains to be further verified.
Core Tip:BCAR3 gene was over-expressed in a variety of malignant tumors, and its high expression was significantly associated with poor prognosis of cancer patients. In hepatocellular carcinoma (HCC), the expression of BCAR3 was observably higher in cancer tissues than in normal tissues, and the expression level was higher in HCC tissues with TP53 mutation compared to those without. BCAR3 was positively correlated with a variety of immune cells in HCC tissues, and might play an important role in the immune micro-environment of tumors. In addition, the BCAR3/miR-19b-3p/RAB30-DT competing endogenous RNA regulatory axis may be involved in the development of HCC.
- Citation: Shi HQ, Huang S, Ma XY, Tan ZJ, Luo R, Luo B, Zhang W, Shi L, Zhong XL, Lü MH, Chen X, Tang XW. BCAR3 and BCAR3-related competing endogenous RNA expression in hepatocellular carcinoma and their prognostic value. World J Gastrointest Oncol 2024; 16(7): 3082-3096
- URL: https://www.wjgnet.com/1948-5204/full/v16/i7/3082.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i7.3082
Primary hepatocellular carcinoma (HCC) is one of the most prevalent malignant tumors with the fourth highest mortality rate globally, among which HCC is the most predominant, accounting for approximately 80% of primary HCC[1,2]. Chronic infection with hepatitis B virus or hepatitis C virus, high alcohol consumption, excess body weight, and smoking are the major pathogenic factors of HCC[3]. HCC lacks effective early diagnosis methods compared with other malignant tumors, and advanced HCC demonstrates a very poor prognosis, with mortality approaches the worldwide incidence. A large number of studies have revealed that HCC occurrence and development are closely associated with abnormal gene expression[4]. Therefore, effective biomarkers related to HCC diagnosis and prognosis were searched from the molecular level.
BCAR3, namely NSP2 or AND-34, is a member of the novel Src homology 2 (SH2) containing protein family[5]. Studies revealed that BCAR3 expression was associated with the occurrence and development of various cancers. BCAR3 played an important role in the metastasis and invasion of breast cancer cells and promoted the proliferation and migration of triple-negative breast cancer by regulating MET signaling[6,7]. The experiment by Hou et al[8] confirmed that BCAR3 knockout significantly inhibited cell migration and colorectal cancer cell invasion, but did not affect tumor cell proliferation. In contrast, high BCAR3 expression was strongly associated with a better prognosis in multiple myeloma[9]. However, the association between BCAR3 expression and HCC prognosis, as well as the role of BCAR3 in HCC remains unclear.
Competing endogenous RNAs (ceRNAs) are a class of non-coding RNAs (ncRNAs) that competitively bind to shared miRNAs and play a regulatory role at the post-transcriptional level[10]. The ceRNA hypothesis suggested that ceRNAs act as miRNA sponges via miRNA response elements and thus play a role in proliferation, metastasis, and drug resistance in several common tumors, such as gastric cancer, gallbladder cancer, and HCC[11-13]. Therefore, we aimed to investigate the association of BCAR3 and its related ceRNAs with HCC and the prognosis of patients with HCC through bioinformatics analysis, to provide new ideas for HCC diagnosis and treatment.
RNA-sequencing expression profiles for 33 tumors were downloaded from The Cancer Genome Altas (TCGA) database (https://portal.gdc.com), including 9807 tumor tissues. RNA-seq data of normal tissues were downloaded from TCGA and The Genotype Tissue Expression (GTEx). Meanwhile, BCAR3 gene expression data were extracted from GTEx and TCGA datasets for subsequent analyses. Furthermore, 424 RNA-seq data and corresponding clinical information of HCC from the TCGA database were used to investigate the associations between BCAR3 expression and clinicopathological factors of patients with HCC.
The UALCAN database (http://ualcan.path.uab.edu/), containing RNA-seq and clinical data of 33 cancer types from the TCGA data set, was used for BCAR3 expression analysis in different tumor sample types and downloaded the box plots. The GTEx (https://www.gtexportal.org/home/), containing RNA-seq data from 31 normal tissues, was utilized for BCAR3 expression analysis in different normal tissues and downloaded the box plots.
Two-group data performed by the Mann-Whitney U test were used to investigate the differential expression of BCAR3 between tumors and normal tissues across different cancer types from GTEx and TCGA datasets. P-value of < 0.05 indicated statistical significance (aP < 0.05).
Timer 2.0 (http://timer.comp-genomics.org) was usually applied to investigate the prognostic significance of genes in different cancer types. We investigated the prognostic values of BCAR3 expression for overall survivals (OS) in pancancers using these two databases. Kaplan-Meier survival analysis and the log-rank test were conducted to calculate the P-value.
Pan-cancer analysis of BCAR3 genetic alterations was performed with cBioPortal web (https://www.cbioportal.org/), including genetic alteration frequency, mutation type, and copy number alteration among different tumors.
The R software pheatmap package was used to investigate the correlation analyses between BCAR3 and various immune cells. Spearman’s correlation analysis was utilized to describe the correlation between quantitative variables without a normal distribution. P-values of < 0.05 were considered statistically significant.
The LinkedOmics database (http://www.linkedomics.orglogin.php) was applied to identify the co-expressed genes correlated with BCAR3 expression in the RNA-seq data of patients with HCC from the TCGA database. The Pearson correlation analysis was utilized to calculate the correlation, and the volcano map of the co-expressed genes was plotted from the LinkedOmics website. The Limma package in R 4.2.1 was used to collect the differently expressed genes (DEGs) of HCC, with P adj of < 0.05 and LogFC of ≥ 1 as the screening conditions. Finally, the co-expressed genes and the DEGs were intersected to obtain overlapping genes, which were visualized to obtain the heat map.
Functional enrichment analyses screened the above-mentioned overlapping genes, including Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways, and tissue enrichment analysis, performed using the Metascape database.
The BCAR3-related miRNA and long-stranded ncRNA (lncRNA) data were downloaded from the starBase database (http://starbase.sysu.edu.cn/), with a program number of ≥ 2 as the miRNA screening criteria. The corresponding lncRNA was then screened out based on the miRNA obtained under the above conditions.
Wilcox-on rank-sum test was used to compare the difference between the two groups. “DESeq2” and “survival” R software were used for differential expression data analysis. Kaplan-Meier survival analysis was used for receiver operating characteristic (ROC) curve analysis and univariate and multivariate Cox regression analysis. The Spearman test was utilized to measure correlations between BCAR3 and immune functions. A P-value of < 0.05 indicated a significant threshold.
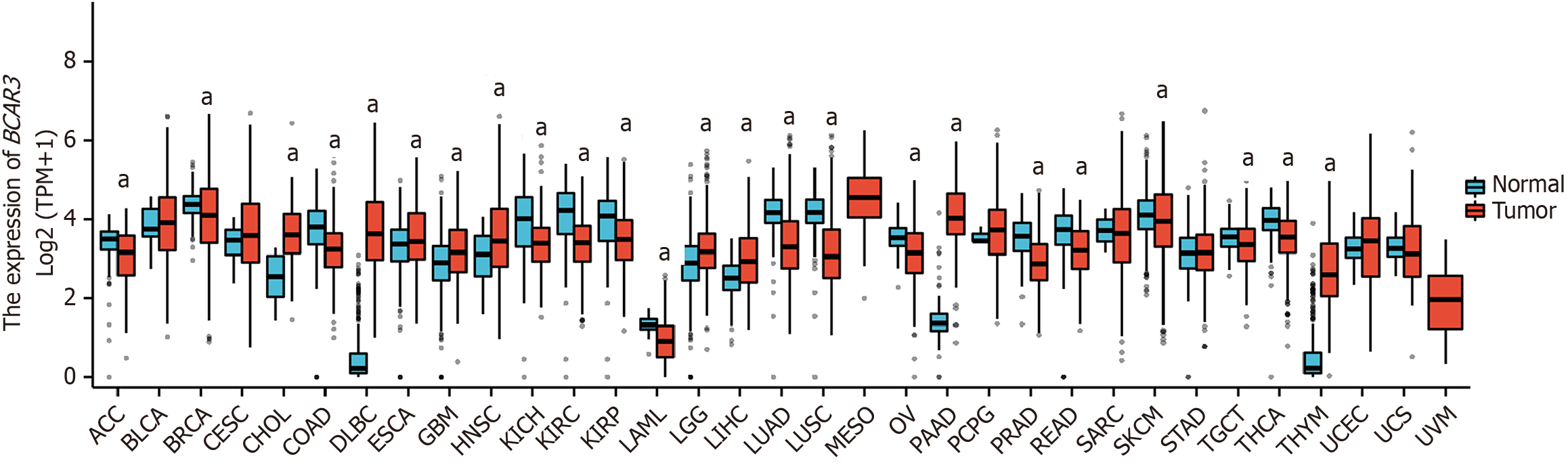
Based on the TCGA and GTEx databases, BCAR3 was expressed in 33 kinds of malignant tumor tissue samples, among which 24 demonstrated significant differences in BCAR3 expression level (Figure 1). BCAR3 was down-regulated in cholangial carcinoma, diffuse large B-cell lymphoma, esophageal carcinoma, glioblastoma multiforme, neck squamous cell carcinoma, brain lower-grade glioma, liver HCC, pancreatic adenocarcinoma, and thymoma compared with para-cancer normal tissues. On the contrary, BCAR3 was under-expressed in adrenocortical carcinoma, breast invasive carcinoma, colon adenocarcinoma, kidney chromophobe, kidney renal clear cell carcinoma, kidney renal papillary cell carcinoma, acute myeloid leukemia, lung adenocarcinoma, lung squamous cell carcinoma, ovarian serous cystadenocarcinoma, prostate adenocarcinoma, rectum adenocarcinoma, skin cutaneous melanoma, testicular germ cell tumor, and thyroid carcinoma. Whereas, no statistical difference in BCAR3 expression level was found in bladder urothelial carcinoma, cervical squamous cell carcinoma and endocervical adenocarcinoma, pheochromocytoma and paraganglioma, sarcoma, stomach adenocarcinoma, uterine corpus endometrial carcinoma (UCEC), uterine carcinosarcoma, and their corresponding normal tissues. Additionally, mesothelioma and uveal melanoma were excluded due to a lack of normal tissue samples.
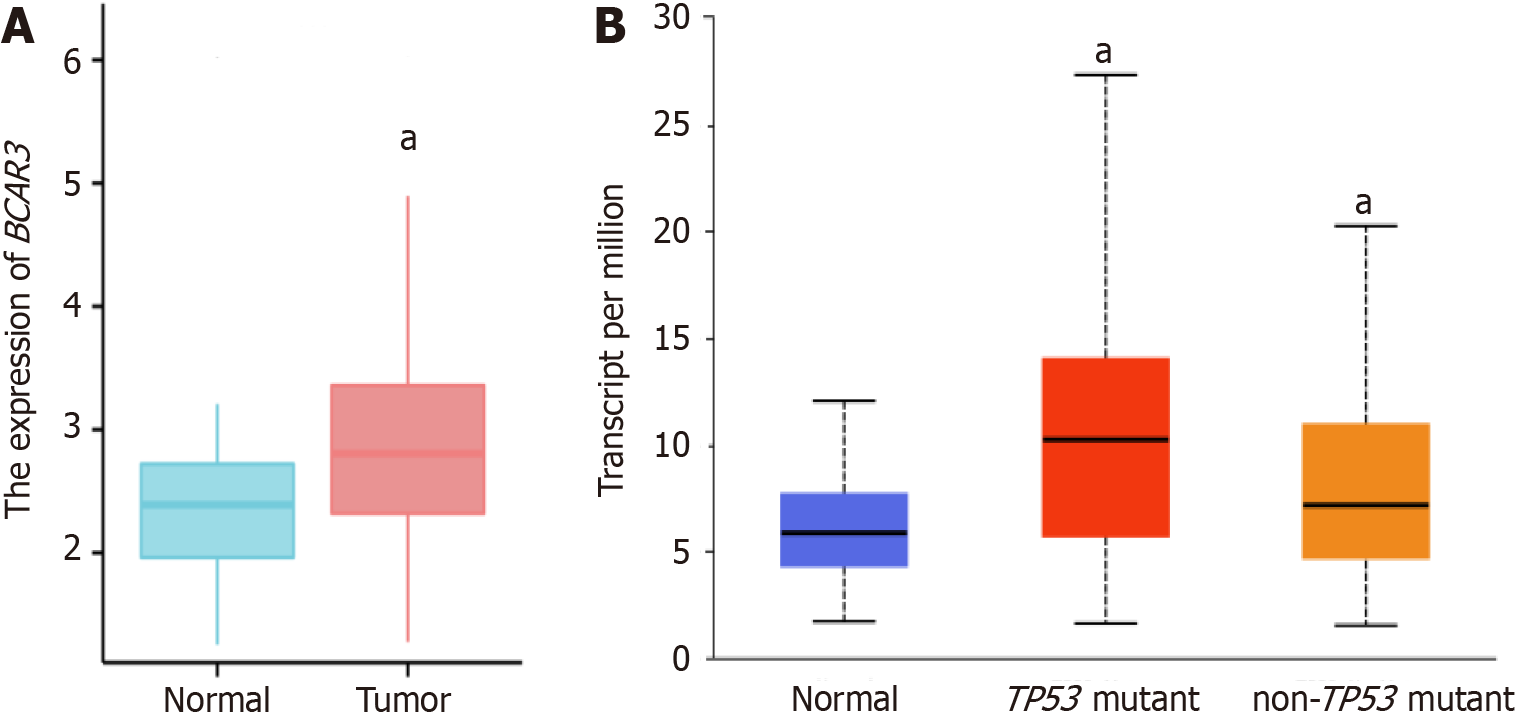
BCAR3 was significantly more expressed in HCC tissue compared with para-cancer normal tissues (P < 0.01; Figure 2A). Moreover, BCAR3 expression level in HCC was correlated with the mutation status of TP53. BCAR3 expression was higher in the TP53-mutated HCC tissue samples (P < 0.001; Figure 2B).
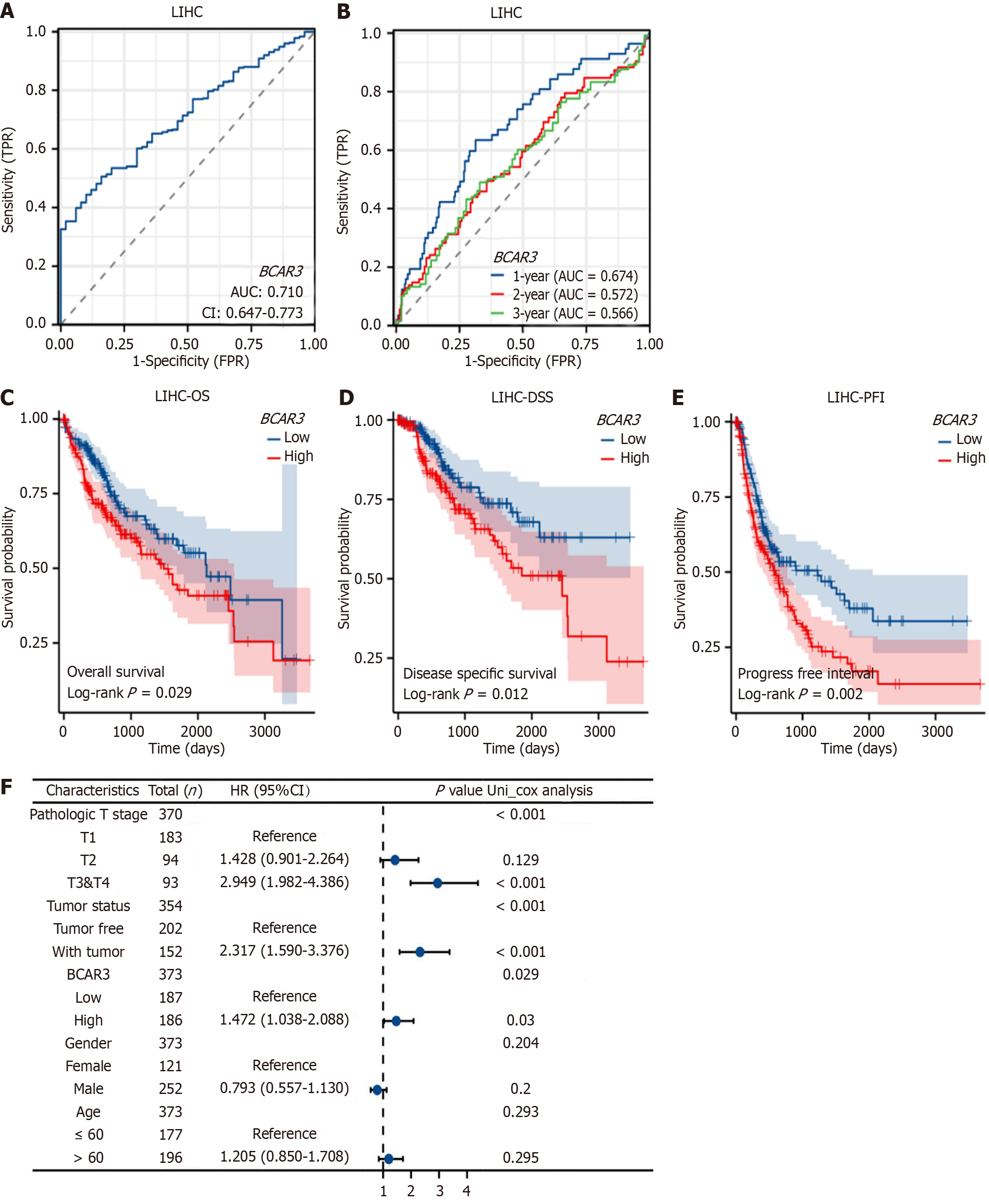
Subsequently, we analyzed the important value of the BCAR3 gene in HCC diagnosis and prognosis. The sensitivity analysis revealed that the diagnostic ROC curve of the target gene BCAR3 demonstrated an area under the curve (AUC) of 0.7, indicating that the high BCAR3 expression indicated the diagnostic value for HCC (Figure 3A). More importantly, the prognostic ROC curve of BCAR3 revealed that the AUCs were 0.674, 0.572, and 0.566 at 1, 2, and 3 years, respectively (Figure 3B). Additionally, we revealed that the OS, disease-specific survival, and progression-free interval of patients with HCC with high expression of BCAR3 were worse than those with low BCAR3 expression based on Kaplan-Meier curves and log-rank test analysis (Figure 3C-E, Table 1). Univariate Cox regression analysis revealed that high BCAR3 expression, tumor status, and tumor pathological T stage (T3 and T4) were correlated with poor OS in patients with HCC (Figure 3F), whereas multivariate Cox regression analysis indicated that BCAR3 expression level was not an independent prognostic risk factor for HCC (Table 2). In conclusion, BCAR3 was a prognostic risk factor for HCC.
| Characteristics | Low expression of BCAR3 | High expression of BCAR3 | P value |
| n | 187 | 187 | |
| Gender | 0.912 | ||
| Male | 127 (34) | 126 (33.7) | |
| Female | 60 (16) | 61 (16.3) | |
| Age | 0.275 | ||
| ≤ 60 | 94 (25.2) | 83 (22.3) | |
| > 60 | 93 (24.9) | 103 (27.6) | |
| Tumor status | 0.014 | ||
| Tumor free | 111 (31.3) | 91 (25.6) | |
| With tumor | 64 (18) | 89 (25.1) | |
| Pathologic T stage | 0.085 | ||
| T1 and T2 | 146 (39.4) | 132 (35.6) | |
| T3 | 37 (10) | 43 (11.6) | |
| T4 | 3 (0.8) | 10 (2.7) | |
| Pathologic M stage | 0.667 | ||
| M0 | 138 (50.7) | 130 (47.8) | |
| M1 | 3 (1.1) | 1 (0.4) | |
| AFP (ng/mL) | 0.056 | ||
| ≤ 400 | 110 (39.3) | 105 (37.5) | |
| > 400 | 42 (15) | 23 (8.2) | |
| Histological type | 0.996 | ||
| Fibrolamellar carcinoma | 1 (0.3) | 2 (0.5) | |
| Hepatocellular carcinoma | 183 (49.9) | 181 (49.3) | |
| Overall survival event | 0.017 | ||
| Alive | 133 (35.6) | 111 (29.7) | |
| Dead | 54 (14.4) | 76 (20.3) | |
| Disease specific survival event | 0.009 | ||
| No | 153 (41.8) | 134 (36.6) | |
| Yes | 29 (7.9) | 50 (13.7) | |
| Progression free interval event | 0.005 | ||
| No | 109 (29.1) | 82 (21.9) | |
| Yes | 78 (20.9) | 105 (28.1) |
| Characteristics | Total (N) | Univariate analysis | Multivariate analysis | ||
| Hazard ratio (95%CI) | P value | Hazard ratio (95%CI) | P value | ||
| Pathological T stage | 370 | < 0.001 | |||
| T1 | 183 | Reference | Reference | ||
| T2 | 94 | 1.428 (0.901-2.264) | 0.129 | 1.368 (0.846-2.212) | 0.201 |
| T3 and T4 | 93 | 2.949 (1.982-4.386) | < 0.001 | 2.458 (1.610-3.751) | < 0.001 |
| Tumor status | 354 | < 0.001 | |||
| Tumor free | 202 | Reference | Reference | ||
| With tumor | 152 | 2.317 (1.590-3.376) | < 0.001 | 1.864 (1.261-2.755) | 0.002 |
| BCAR3 | 373 | 0.029 | |||
| Low | 187 | Reference | Reference | ||
| High | 186 | 1.472 (1.038-2.088) | 0.030 | 1.245 (0.859-1.805) | 0.247 |
| Gender | 373 | 0.204 | |||
| Female | 121 | Reference | |||
| Male | 252 | 0.793 (0.557-1.130) | 0.200 | ||
| Age | 373 | 0.293 | |||
| ≤ 60 | 177 | Reference | |||
| > 60 | 196 | 1.205 (0.850-1.708) | 0.295 | ||
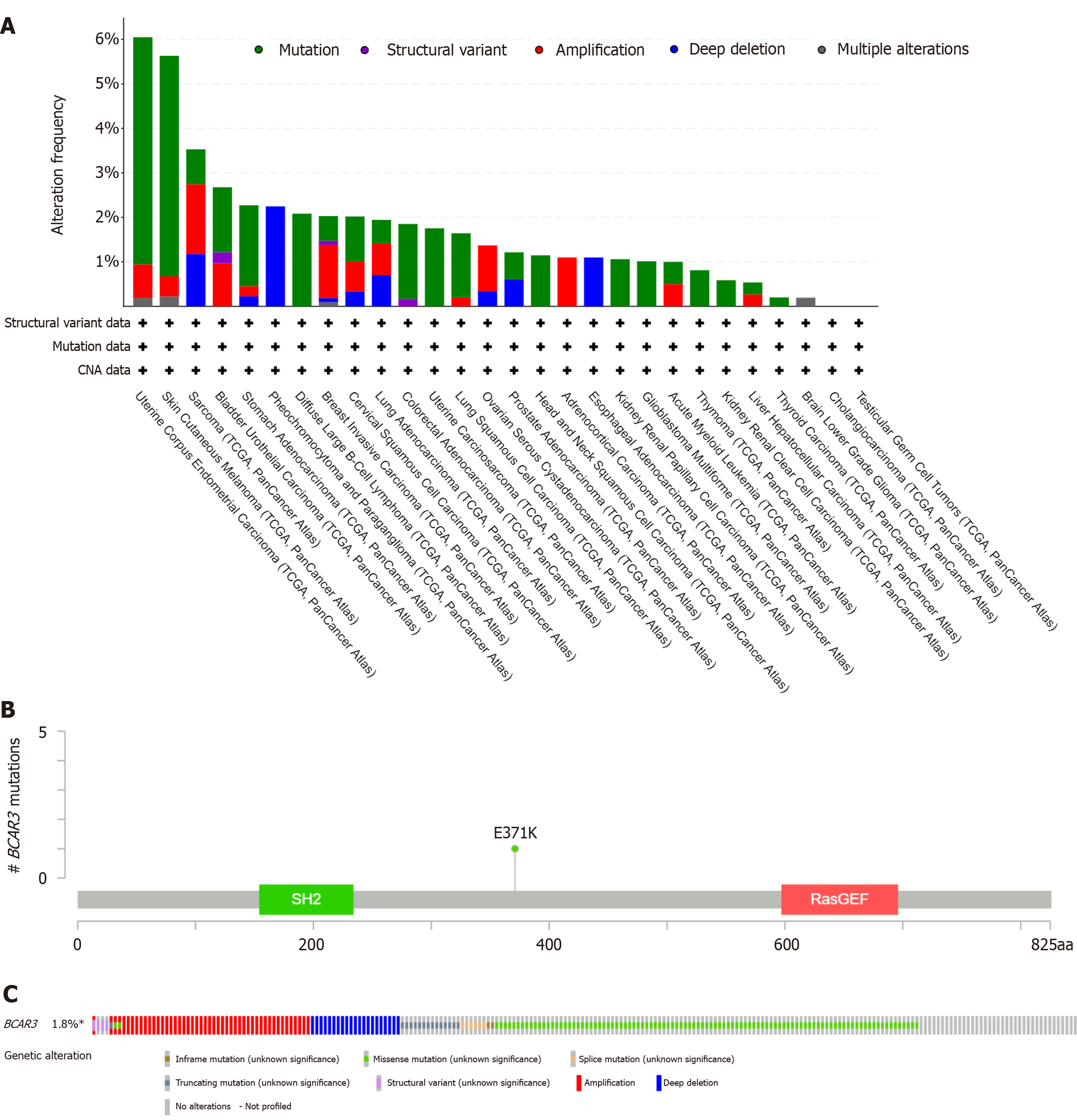
The genetically altered status of the BCAR3 gene in different types of cancers in the TCGA cohort was then investigated at the cBioPortal website. The highest alteration frequency of BCAR3 (approximately 6%) was observed in patients with UCEC with “mutation,” “amplification,” and “multiple alterations” as the primary changes. Additionally, genetic alterations of the BCAR3 gene were observed in HCC cases, mainly “mutation” and “amplification” (Figure 4A). Moreover, genetic changes of the BCAR3 gene were found in HCC cases, mainly “mutation” and “amplification,” with two mutation sites of “SH2” and “RasGEF,” the mutation rate was approximately 1.8% (Figure 4B and C).
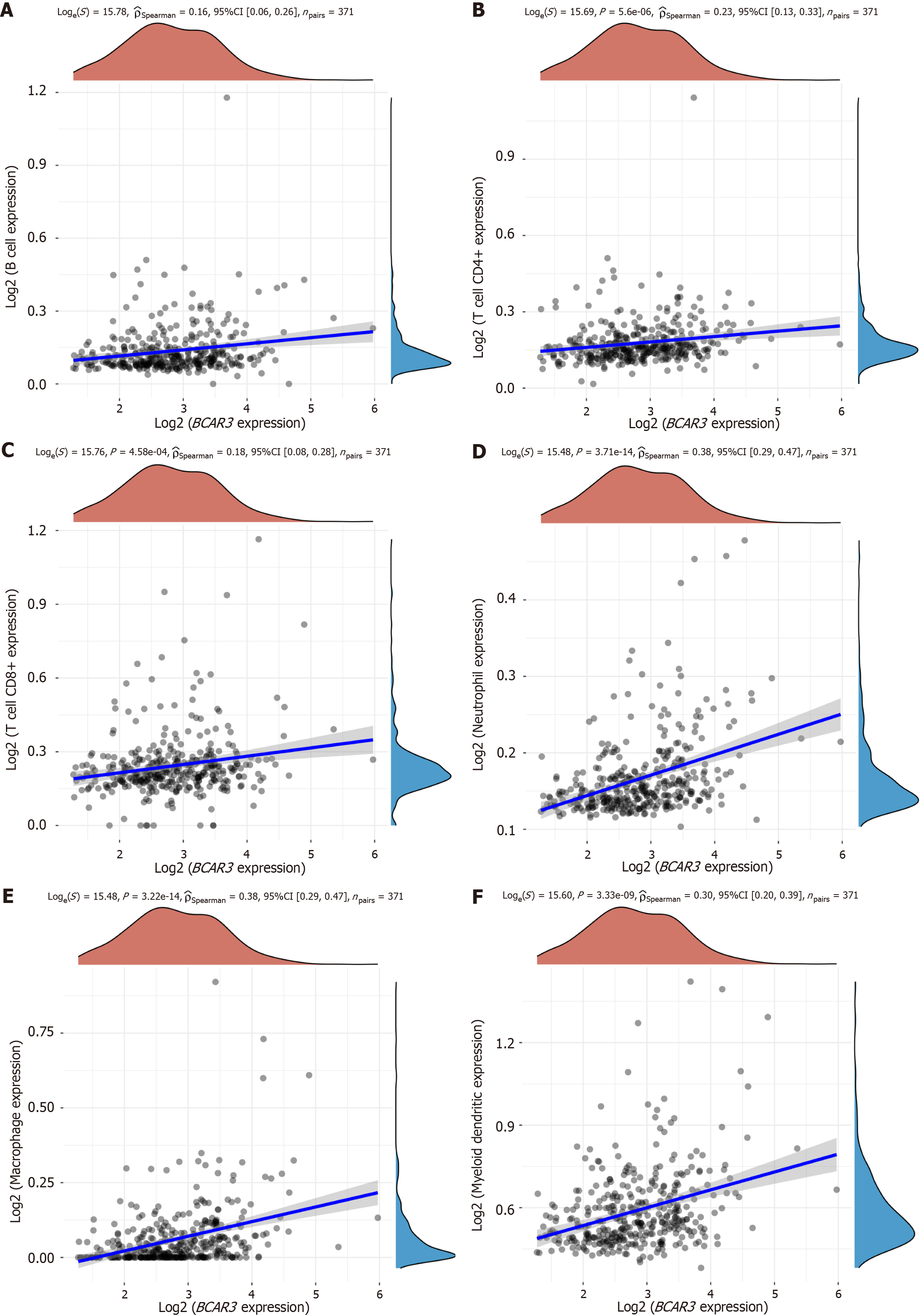
We analyzed the correlations between immune cells and the levels of immune infiltration for BCAR3 and revealed that B cells, T cells CD4+, T cells CD8+, neutrophil, macrophage, and myeloid dendritic cells were positively correlated with BCAR3 (Figure 5, R > 0.2, P < 0.01).
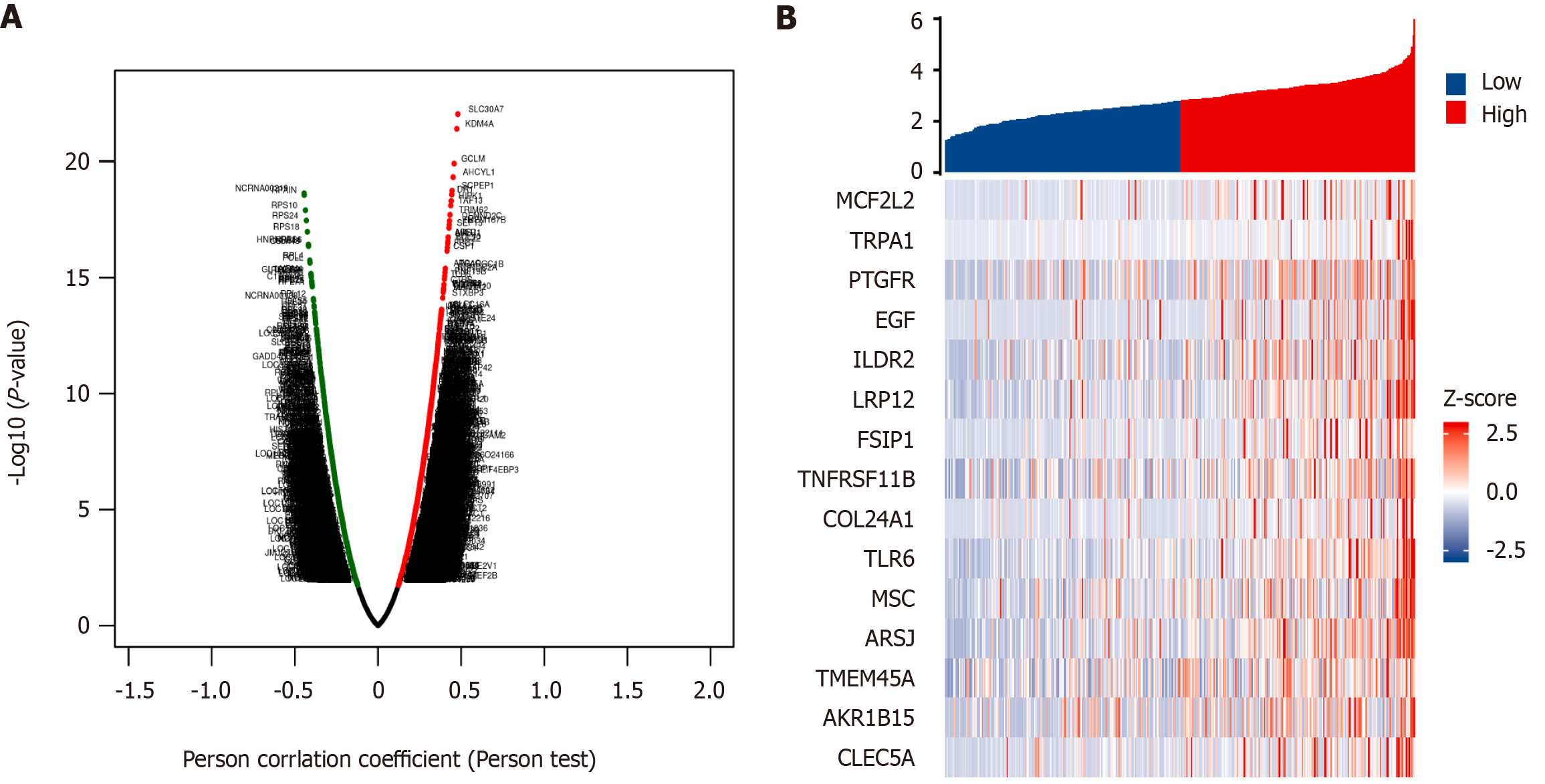
In HCC tissues, 451 co-expressed genes from the TCGA database that were significantly associated with BCAR3 were determined through the LinkedOmics database (false discovery rate < 0.05, P < 0.05, |cor| ≥ 0.3). Among them, 286 genes were positively correlated with BCAR3 expression, whereas 164 genes were negatively correlated with BCAR3 expression (Figure 6A). Subsequently, single-gene differential analysis of target gene BCAR3 was conducted through an online tool website (https://www.xiantao.love/), and 881 DEGs in HCC were obtained. After the intersection of DEGs and the above co-expressed genes, 16 overlapping genes and their volcano maps were collected for further analysis (Figure 6B).
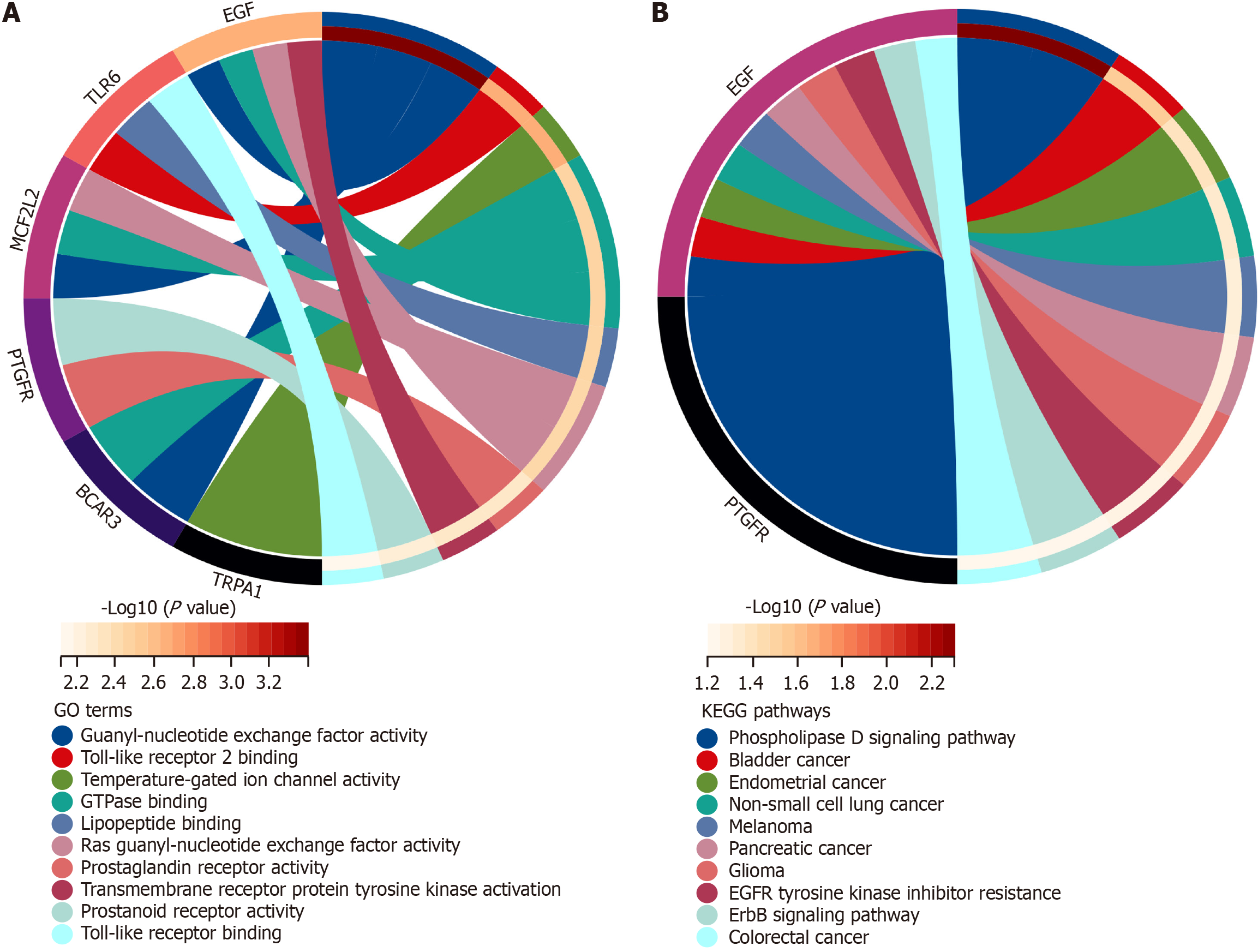
Metascape database was used for GO/KEGG analysis to investigate the biological functions of 16 overlapping genes. The results revealed that BCAR3 and its related genes were functional mediators of immune regulation, including positive epidermal growth factor receptor (EGFR) signaling pathway regulation, ERBB signaling pathway positive regulation, DNA replication positive regulation, guanyl-nucleotide exchange factor activity, GTPase regulator activity, and nucleoside-triphosphatase regulator activity (Figure 7A). These genes were involved in inflammatory processes, including responses to bacteria, tuberculosis, phagocytosis, macrophage activation, and endocytosis. Additionally, BCAR3 expression was associated with MAPK cascade regulation and cell death (Figure 7B).
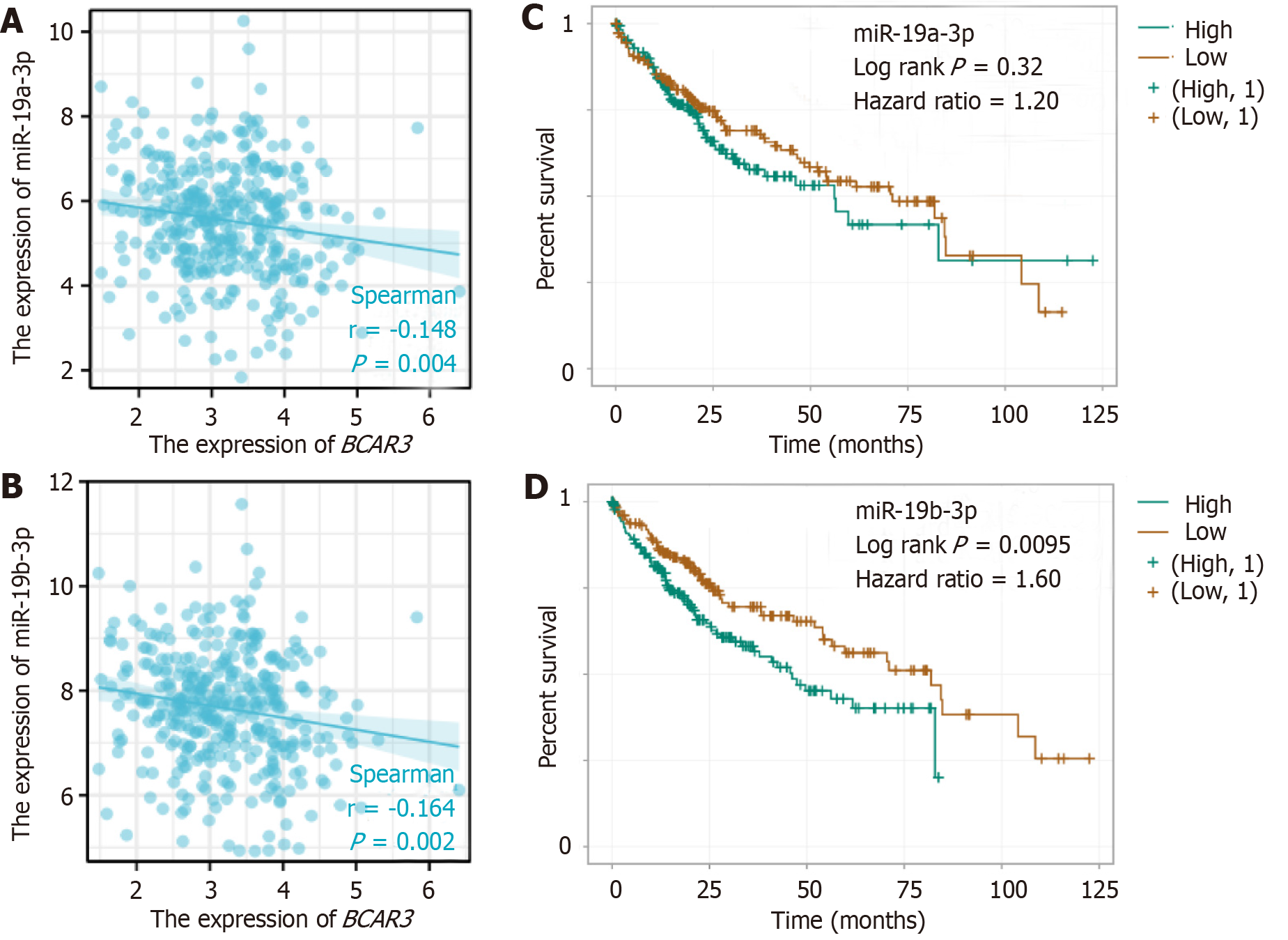
We screened two groups of miRNAs that were co-expressed and negatively correlated with BCAR3 (r < -0.1, P < 0.01) in preparation for the subsequent screening of potential targets and pathways after downloading the BCAR3-miRNA interaction data from the starBase database and using the R language package for the analysis (Figure 8A and B). Prognosis and survival curve analysis revealed that high expression level of miR-19b-3p-miRNA was associated with poor prognosis of patients with HCC. However, the expression level of miR-19a-3p-miRNA was not correlated with the prognosis of patients with HCC (Figure 8C and D).
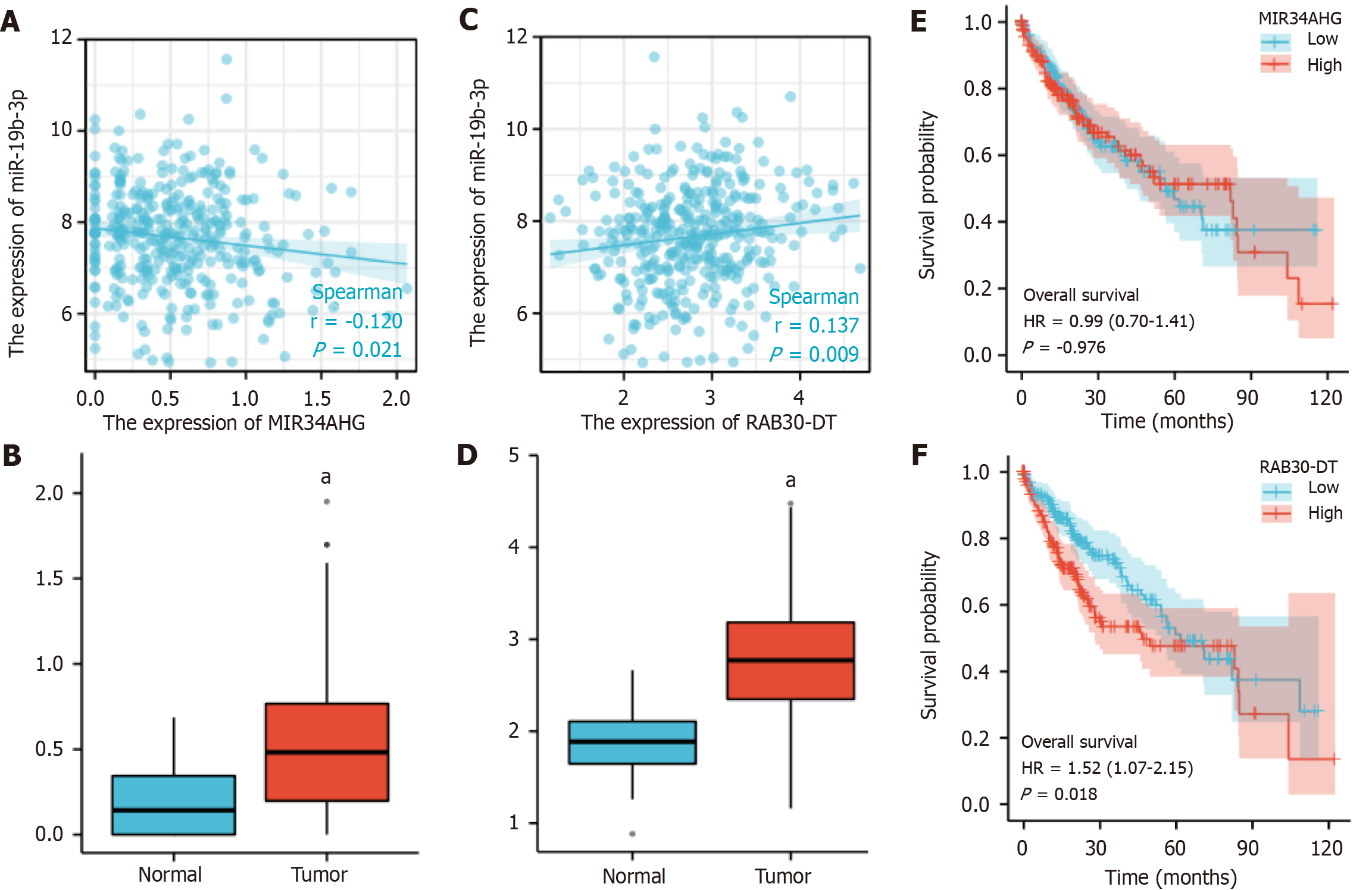
Thereafter, we downloaded the lncRNA data that interacted with miR-19b-3p from the starBase database and screened and analyzed the correlation coefficients. Finally, the miR-19b-3p-related lncRNAs, namely MIR34AHG (Figure 9A and B, r < -0.1, P < 0.05) and RAB30-DT (Figure 9C and D, r > 0.1, P < 0.01) were screened. The lncRNAs were differently expressed in between the normal and tumor groups, and high RAB30-DT expression was associated with poor prognosis (Figure 9E and F). Therefore, a BCAR3-related miRNA-lncRNA pathway was constructed to investigate HCC occurrence and development.
BCAR3 has been associated with several prevalent cancers, but its function in HCC remains unclear. First, we mainly discussed the differential expression of BCAR3 in HCC and its effect on the survival and prognosis of patients with HCC and revealed that the BCAR3 gene was differentially expressed in a variety of malignant tumors. Our study revealed that BCAR3 expression in HCC was observably higher in cancer tissues than in normal tissues, and the expression level was higher in HCC tissues with TP53 mutation compared to those without. TP53 is one of the most prevalent mutated genes in human cancers[14]. The immune response of HCC with TP53 mutation was significantly weakened compared with HCC without TP53 mutation, which was detrimental to the survival of patients with HCC[15]. Additionally, BCAR3 was positively correlated with various immune cells in HCC tissues and may play an important role in the immune micro-environment of tumors. However, the specific mechanism between BCAR3 expression and TP53 mutation in HCC remains unclear and may be associated with the immune landscape. Finally, we determined miRNAs and lncRNAs associated with BCAR3 mRNA through the starBase database and revealed that the ceRNA regulatory network, composed of BCAR3 mRNA-miR-19b-3p miRNA-RAB30-DT lncRNA, was significantly correlated with the prognosis of patients with HCC.
BCAR3, as a signal transduction regulator, was widely expressed in human tissues and was first discovered during the study of anti-estrogen resistance genes in breast cancer[16]. Further study revealed that BCAR3 was closely associated with a variety of cell biological processes in the human body, such as cell migration, proliferation, and survival. BCAR3 over-expression in breast cancer cells promoted cell migration and invasion[17]. BCAR3 effectively binds to p130Cas (BCAR1)[18], thereby activating Rac1[19], regulating Src/p130Cas association and Src family tyrosine kinase activity and rapidly disrupting adhesion and causing tumor invasion[20,21]. BCAR3 regulated the process of adhesion force between cells and extracellular matrix by affecting the organization of cytoskeleton and cell adhesion proteins, which was particularly crucial for cell migration and tissue remodeling. The over-expressed and hyper-activated protein of Rac1 in HCC played an important role in cancer cell proliferation, metastasis, and treatment resistance[22]. Therefore, BCAR3 probably plays a similar role by activating Rac1 signaling pathways, thereby affecting the prognosis of patients with HCC, but further studies are warranted.
Our study revealed that BCAR3 over-expression affected the immune infiltration of HCC cells and was involved in the positive regulation of the EGFR signaling pathway of tumors with related genes. Persistent and chronic liver inflammation promotes HCC development[23]. The previous study identified that the epidermal growth factor (EGF) promoted DNA synthesis and HCC cell regeneration, multiple inflammatory factor production and accumulation[24], and bound to EGFR to form EGF–EGFR pathway, which contributes to the formation of inflammation, angiogenesis, and distant HCC metastasis[25,26]. Thus, BCAR3 promoted HCC progression by regulating the EGFR signaling pathway, causing poor prognosis in patients with HCC.
In recent years, the role of ceRNA in HCC occurrence and development received increasing attention. Multiple ceRNAs (including lncRNAs, miRNAs, and circRNAs) and corresponding ceRNA networks have been identified, which affect HCC development by regulating specific miRNAs and their target genes[27]. MiR-122 is a hepato-specific miRNA. Studies revealed that lncRNA HOTAIR binds to miR-122, inhibits its activity through the “miRNA sponge effect,” and promotes HCC proliferation, migration, and invasion[28,29]. Additionally, ceRNA plays a role by regulating important cell signaling pathways, such as regulating Wnt/beta-catenin signaling pathway activation to drive the aggressiveness and metastasis of liver cancer cells[30]. UCA1 lncRNA promotes the survival of liver cancer cells by regulating downstream expression of Bcl-2 and Hexokinase 2 through interaction with miR-216b or miR-143[31]. Additionally, ceRNA regulates HCC development by affecting epigenetic mechanisms, thereby interfering with cell cycle and apoptosis[32]. In particular, MEG3 affects liver cancer cell invasion by interacting with miR-145-5p to up-regulate disabled-2 expression[33]. Challenges remain in terms of clinical application despite the clear regulatory effects of these ceRNAs and networks in cell and animal models. This study obtained BCAR3-related mRNAs and lncRNAs by calculating the Pearson correlation coefficient, revealing a BCAR3-related miRNA-lncRNA pathway.
The miRNA-associated BCAR3 mRNA was known as miR-19b-3p, which was a miRNA and a class of small molecular RNA that regulates gene expression. By regulating gene expression, miR-19b-3p could affect biological processes, such as cell growth, differentiation, and death, and be involved in cancer occurrence and development, including lung adenocarcinoma, esophageal squamous carcinoma, and intrahepatic cholangiocarcinoma[34-36]. Recent experimental studies revealed that the up-regulated miR-19b-3p expression level in HCC inhibited ferroptosis and promoted HCC cell proliferation by inhibiting RBMS1 expression[37], and was closely associated with HCC malignancy and prognosis. Additionally, miR-19b-3p overexpression activated the Wnt/β-catenin signaling pathway and played a role in promoting HCC development[38]. Therefore, miR-19b-3p may promote HCC occurrence and progression by regulating BCAR3 expression, but the specific mechanism remains unclear.
RAB30-DT is a lncRNA that belongs to a family of great interest in cancer, but information about this lncRNA is limited[39]. RAB30-DT demonstrated an abnormal expression level in HCC and a significantly higher expression level in HCC than that in normal liver tissue, which is significantly associated with the poor prognosis of patients with HCC. Some studies revealed that miR-19b-3p promoted tumor growth and intratumoral cell proliferation by targeting PTEN, causing PI3K/AKT signaling pathway activation[40]. Therefore, the possible mechanism by which RAB30-DT affected HCC occurrence and development was to reduce the inhibitory effect on the target gene PTEN by binding to miR-19b-3p, thereby improving the migration and invasion ability of HCC cells. More studies are warranted to investigate this process. In conclusion, BCAR3 probably promoted the RAB30-DT expression by regulating miR-19b-3p expression, thereby playing a role in HCC pathogenesis.
Our study used TCGA and starBase databases to investigate and analyze the role of BCAR3 and its related ceRNAs in HCC occurrence and development. Our results present very valuable direction and reference for the research of BCAR3, which may be utilized as a new potential biomarker to provide new ideas for the early diagnosis and treatment of HCC. However, our study has some limitations. Noteworthily, the study was based on bioinformatics analysis, so the validity of the results warrants further verification by basic experimental studies.
In conclusion, BCAR3 plays an important role in HCC occurrence and development and is closely associated with HCC diagnosis and prognosis. BCAR3 is expected to become a new prognostic indicator and therapeutic target and bring more accurate treatment for patients with HCC. However, the current study remains preliminary, and further research is required to investigate the mechanism of action of BCAR3 in HCC as well as the application of this knowledge to clinical treatment.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68881] [Article Influence: 13776.2] [Reference Citation Analysis (202)] |
| 2. | Ganesan P, Kulik LM. Hepatocellular Carcinoma: New Developments. Clin Liver Dis. 2023;27:85-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 301] [Article Influence: 100.3] [Reference Citation Analysis (1)] |
| 3. | Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400:1345-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1284] [Cited by in RCA: 1595] [Article Influence: 398.8] [Reference Citation Analysis (42)] |
| 4. | Alqahtani A, Khan Z, Alloghbi A, Said Ahmed TS, Ashraf M, Hammouda DM. Hepatocellular Carcinoma: Molecular Mechanisms and Targeted Therapies. Medicina (Kaunas). 2019;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 177] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 5. | van Agthoven T, van Agthoven TL, Dekker A, van der Spek PJ, Vreede L, Dorssers LC. Identification of BCAR3 by a random search for genes involved in antiestrogen resistance of human breast cancer cells. EMBO J. 1998;17:2799-2808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Casanova AG, Roth GS, Hausmann S, Lu X, Bischoff LJM, Froeliger EM, Belmudes L, Bourova-Flin E, Flores NM, Benitez AM, Chasan T, Caporicci M, Vayr J, Blanchet S, Ielasi F, Rousseaux S, Hainaut P, Gozani O, Le Romancer M, Couté Y, Palencia A, Mazur PK, Reynoird N. Cytoskeleton remodeling induced by SMYD2 methyltransferase drives breast cancer metastasis. Cell Discov. 2024;10:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 7. | Arras J, Thomas KS, Myers PJ, Cross AM, Osei AD, Vazquez GE, Atkins KA, Conaway MR, Jones MK, Lazzara MJ, Bouton AH. Breast Cancer Antiestrogen Resistance 3 (BCAR3) promotes tumor growth and progression in triple-negative breast cancer. Am J Cancer Res. 2021;11:4768-4787. [PubMed] |
| 8. | Hou J, Mi X, Liu N, Li X, Li XN, Yang Y, Lu X, Fang Y, Jin NY. MiR-199a/b-3p inhibits colorectal cancer cell proliferation, migration and invasion through targeting PAK4 and BCAR3. Eur J Med Res. 2022;27:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 9. | Zhang W, Lin Y, Liu X, He X, Zhang Y, Fu W, Yang Z, Yang P, Wang J, Hu K, Zhang X, Liu W, Yuan X, Jing H. Prediction and prognostic significance of BCAR3 expression in patients with multiple myeloma. J Transl Med. 2018;16:363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Qi X, Zhang DH, Wu N, Xiao JH, Wang X, Ma W. ceRNA in cancer: possible functions and clinical implications. J Med Genet. 2015;52:710-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 998] [Cited by in RCA: 1011] [Article Influence: 91.9] [Reference Citation Analysis (0)] |
| 11. | Liu XH, Sun M, Nie FQ, Ge YB, Zhang EB, Yin DD, Kong R, Xia R, Lu KH, Li JH, De W, Wang KM, Wang ZX. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer. 2014;13:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 659] [Cited by in RCA: 799] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 12. | Wang SH, Ma F, Tang ZH, Wu XC, Cai Q, Zhang MD, Weng MZ, Zhou D, Wang JD, Quan ZW. Long non-coding RNA H19 regulates FOXM1 expression by competitively binding endogenous miR-342-3p in gallbladder cancer. J Exp Clin Cancer Res. 2016;35:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 13. | Wang YG, Wang T, Ding M, Xiang SH, Shi M, Zhai B. hsa_circ_0091570 acts as a ceRNA to suppress hepatocellular cancer progression by sponging hsa-miR-1307. Cancer Lett. 2019;460:128-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 14. | Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA, Leiserson MDM, Miller CA, Welch JS, Walter MJ, Wendl MC, Ley TJ, Wilson RK, Raphael BJ, Ding L. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2931] [Cited by in RCA: 3473] [Article Influence: 267.2] [Reference Citation Analysis (0)] |
| 15. | Long J, Wang A, Bai Y, Lin J, Yang X, Wang D, Yang X, Jiang Y, Zhao H. Development and validation of a TP53-associated immune prognostic model for hepatocellular carcinoma. EBioMedicine. 2019;42:363-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 270] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 16. | van Agthoven T, Sieuwerts AM, Meijer-van Gelder ME, Look MP, Smid M, Veldscholte J, Sleijfer S, Foekens JA, Dorssers LC. Relevance of breast cancer antiestrogen resistance genes in human breast cancer progression and tamoxifen resistance. J Clin Oncol. 2009;27:542-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Guo J, Canaff L, Rajadurai CV, Fils-Aimé N, Tian J, Dai M, Korah J, Villatoro M, Park M, Ali S, Lebrun JJ. Breast cancer anti-estrogen resistance 3 inhibits transforming growth factor β/Smad signaling and associates with favorable breast cancer disease outcomes. Breast Cancer Res. 2014;16:476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Wallez Y, Riedl SJ, Pasquale EB. Association of the breast cancer antiestrogen resistance protein 1 (BCAR1) and BCAR3 scaffolding proteins in cell signaling and antiestrogen resistance. J Biol Chem. 2014;289:10431-10444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Gotoh T, Cai D, Tian X, Feig LA, Lerner A. p130Cas regulates the activity of AND-34, a novel Ral, Rap1, and R-Ras guanine nucleotide exchange factor. J Biol Chem. 2000;275:30118-30123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Makkinje A, Vanden Borre P, Near RI, Patel PS, Lerner A. Breast cancer anti-estrogen resistance 3 (BCAR3) protein augments binding of the c-Src SH3 domain to Crk-associated substrate (p130cas). J Biol Chem. 2012;287:27703-27714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Cross AM, Wilson AL, Guerrero MS, Thomas KS, Bachir AI, Kubow KE, Horwitz AR, Bouton AH. Breast cancer antiestrogen resistance 3-p130(Cas) interactions promote adhesion disassembly and invasion in breast cancer cells. Oncogene. 2016;35:5850-5859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Sauzeau V, Beignet J, Vergoten G, Bailly C. Overexpressed or hyperactivated Rac1 as a target to treat hepatocellular carcinoma. Pharmacol Res. 2022;179:106220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Berasain C, Perugorria MJ, Latasa MU, Castillo J, Goñi S, Santamaría M, Prieto J, Avila MA. The epidermal growth factor receptor: a link between inflammation and liver cancer. Exp Biol Med (Maywood). 2009;234:713-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 525] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 25. | Ogunwobi OO, Wang T, Zhang L, Liu C. Cyclooxygenase-2 and Akt mediate multiple growth-factor-induced epithelial-mesenchymal transition in human hepatocellular carcinoma. J Gastroenterol Hepatol. 2012;27:566-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Huang P, Xu X, Wang L, Zhu B, Wang X, Xia J. The role of EGF-EGFR signalling pathway in hepatocellular carcinoma inflammatory microenvironment. J Cell Mol Med. 2014;18:218-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 27. | Chen S, Zhang Y, Ding X, Li W. Identification of lncRNA/circRNA-miRNA-mRNA ceRNA Network as Biomarkers for Hepatocellular Carcinoma. Front Genet. 2022;13:838869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 28. | Cheng D, Deng J, Zhang B, He X, Meng Z, Li G, Ye H, Zheng S, Wei L, Deng X, Chen R, Zhou J. LncRNA HOTAIR epigenetically suppresses miR-122 expression in hepatocellular carcinoma via DNA methylation. EBioMedicine. 2018;36:159-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 135] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 29. | Li D, Zhang J, Li J. Role of miRNA sponges in hepatocellular carcinoma. Clin Chim Acta. 2020;500:10-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 30. | Huang G, Liang M, Liu H, Huang J, Li P, Wang C, Zhang Y, Lin Y, Jiang X. CircRNA hsa_circRNA_104348 promotes hepatocellular carcinoma progression through modulating miR-187-3p/RTKN2 axis and activating Wnt/β-catenin pathway. Cell Death Dis. 2020;11:1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 262] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 31. | Wang F, Ying HQ, He BS, Pan YQ, Deng QW, Sun HL, Chen J, Liu X, Wang SK. Upregulated lncRNA-UCA1 contributes to progression of hepatocellular carcinoma through inhibition of miR-216b and activation of FGFR1/ERK signaling pathway. Oncotarget. 2015;6:7899-7917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 316] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 32. | Xu G, Xu WY, Xiao Y, Jin B, Du SD, Mao YL, Zhang ZT. The emerging roles of non-coding competing endogenous RNA in hepatocellular carcinoma. Cancer Cell Int. 2020;20:496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Wei Q, Liu G, Huang Z, Huang Y, Huang L, Huang Z, Wu X, Wei H, Pu J. LncRNA MEG3 Inhibits Tumor Progression by Modulating Macrophage Phenotypic Polarization via miR-145-5p/DAB2 Axis in Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2023;10:1019-1035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 34. | Chen J, Zhang K, Zhi Y, Wu Y, Chen B, Bai J, Wang X. Tumor-derived exosomal miR-19b-3p facilitates M2 macrophage polarization and exosomal LINC00273 secretion to promote lung adenocarcinoma metastasis via Hippo pathway. Clin Transl Med. 2021;11:e478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 152] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 35. | Zhang Y, Lu W, Chen Y, Lin Y, Yang X, Wang H, Liu Z. The miR-19b-3p-MAP2K3-STAT3 feedback loop regulates cell proliferation and invasion in esophageal squamous cell carcinoma. Mol Oncol. 2021;15:1566-1583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Tang Y, Yang J, Wang Y, Tang Z, Liu S, Tang Y. MiR-19b-3p facilitates the proliferation and epithelial-mesenchymal transition, and inhibits the apoptosis of intrahepatic cholangiocarcinoma by suppressing coiled-coil domain containing 6. Arch Biochem Biophys. 2020;686:108367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Zhai H, Zhong S, Wu R, Mo Z, Zheng S, Xue J, Meng H, Liu M, Chen X, Zhang G, Zheng X, Du F, Li R, Zhou B. Suppressing circIDE/miR-19b-3p/RBMS1 axis exhibits promoting-tumour activity through upregulating GPX4 to diminish ferroptosis in hepatocellular carcinoma. Epigenetics. 2023;18:2192438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 38. | Zeng H, Chen YX. MiR-19b-3p Inhibits Hypoxia-Ischemia Encephalopathy by Inhibiting SOX6 Expression via Activating Wnt/β-catenin Pathway. Neurochem Res. 2023;48:874-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Santangelo A, Rossato M, Lombardi G, Benfatto S, Lavezzari D, De Salvo GL, Indraccolo S, Dechecchi MC, Prandini P, Gambari R, Scapoli C, Di Gennaro G, Caccese M, Eoli M, Rudà R, Brandes AA, Ibrahim T, Rizzato S, Lolli I, Lippi G, Delledonne M, Zagonel V, Cabrini G. A molecular signature associated with prolonged survival in glioblastoma patients treated with regorafenib. Neuro Oncol. 2021;23:264-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 40. | Chen CL, Tseng YW, Wu JC, Chen GY, Lin KC, Hwang SM, Hu YC. Suppression of hepatocellular carcinoma by baculovirus-mediated expression of long non-coding RNA PTENP1 and MicroRNA regulation. Biomaterials. 2015;44:71-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 183] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/