Published online Mar 15, 2024. doi: 10.4251/wjgo.v16.i3.857
Peer-review started: October 30, 2023
First decision: December 21, 2023
Revised: December 26, 2023
Accepted: January 29, 2024
Article in press: January 29, 2024
Published online: March 15, 2024
Processing time: 134 Days and 7.4 Hours
Recently, vessels encapsulating tumor clusters (VETC) was considered as a distinct pattern of tumor vascularization which can primarily facilitate the entry of the whole tumor cluster into the bloodstream in an invasion independent manner, and was regarded as an independent risk factor for poor prognosis in hepatocellular carcinoma (HCC).
To develop and validate a preoperative nomogram using contrast-enhanced computed tomography (CECT) to predict the presence of VETC+ in HCC.
We retrospectively evaluated 190 patients with pathologically confirmed HCC who underwent CECT scanning and immunochemical staining for cluster of differentiation 34 at two medical centers. Radiomics analysis was conducted on intratumoral and peritumoral regions in the portal vein phase. Radiomics features, essential for identifying VETC+ HCC, were extracted and utilized to develop a radiomics model using machine learning algorithms in the training set. The model’s performance was validated on two separate test sets. Receiver operating characteristic (ROC) analysis was employed to compare the identified performance of three models in predicting the VETC status of HCC on both training and test sets. The most predictive model was then used to constructed a radiomics nomogram that integrated the independent clinical-radiological features. ROC and decision curve analysis were used to assess the performance characteristics of the clinical-radiological features, the radiomics features and the radiomics nomogram.
The study included 190 individuals from two independent centers, with the majority being male (81%) and a median age of 57 years (interquartile range: 51-66). The area under the curve (AUC) for the combined radiomics features selected from the intratumoral and peritumoral areas were 0.825, 0.788, and 0.680 in the training set and the two test sets. A total of 13 features were selected to construct the Rad-score. The nomogram, combining clinical-radiological and combined radiomics features could accurately predict VETC+ in all three sets, with AUC values of 0.859, 0.848 and 0.757. Decision curve analysis revealed that the radiomics nomogram was more clinically useful than both the clinical-radiological feature and the combined radiomics models.
This study demonstrates the potential utility of a CECT-based radiomics nomogram, incorporating clinical-radiological features and combined radiomics features, in the identification of VETC+ HCC.
Core Tip: Vessels encapsulating tumor clusters (VETC) is an independent risk factor for poor prognosis in hepatocellular carcinoma (HCC) and currently determined only on histologic examination after surgical resection. We evaluated 190 patients with pathologically confirmed HCC and constructed a machine learning-based contrast-enhanced computed tomography radiomics model, performed canonical screening of features and multiple validations, and confirmed robustness on various data resources. The radiomics model showed remarkable performance in predicting the VETC subtype, and the results were reproducible, demonstrating that the approach may be applied to other patient samples. Radiomics could provide valuable information for assisting clinicians in pretreatment decision-making.
- Citation: Zhang C, Zhong H, Zhao F, Ma ZY, Dai ZJ, Pang GD. Preoperatively predicting vessels encapsulating tumor clusters in hepatocellular carcinoma: Machine learning model based on contrast-enhanced computed tomography. World J Gastrointest Oncol 2024; 16(3): 857-874
- URL: https://www.wjgnet.com/1948-5204/full/v16/i3/857.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i3.857
Hepatocellular carcinoma (HCC) is the fifth most frequently diagnosed cancer and the third cause of cancer-related mortality worldwide[1,2]. HCC accounts for 75%-90% of primary liver cancers and constitutes a major global health problem[3]; moreover, HCC is difficult to treat. As therapeutic strategies, liver transplantation (LT) and surgical resection remain the effective modalities for HCC. However, the long-term outcomes of patients after curative resection show marked diversity, which remains a substantial challenge in clinical management. The 5-year recurrence rate was more than 50%, even up to 70%[4], vs 25%-35% with LT[5]. Early metastasis is responsible for frequent relapse and high mortality of HCC[6].
As a typical solid tumor, angiogenesis of HCC is closely related to recurrence and metastasis. The sinusoidal structure of the tumor vasculature in HCC increases the propensity for blood-borne metastases to neighboring or distant sites[7,8]. The epithelial-mesenchymal transition (EMT) has been considered a key pattern in migration and invasion of HCC[9]. Recently, Fang et al[6] for the first time further emphasized this distinct pattern of tumor vascularization that is independent of EMT, which was characterized by the presence of cluster of differentiation 34 (CD34)+ vessels encapsulating tumor clusters (VETC) in pathological imaging. The VETC pattern plays a crucial role in enabling the entire tumor cluster to enter the bloodstream independently of invasion in HCC[10]. Several reports have shown that VETC is an independent risk factor for poor prognosis in HCC, and patients with VETC+ HCC show shorter overall survival and disease free survival and are more prone to progression and metastasis relative to patients with VETC- HCC[6,11,12]. In addition, Fang et al[13] indicated that the VETC pattern acts as a predictor of sorafenib benefit in patients with HCC. However, VETC is currently only determined only on histologic examination after surgical resection[14]. Therefore, preoperative diagnosis of VETC status in HCC is of great significance to help predict patient outcomes and decide on therapeutic strategies in HCC.
Radiomics presents a noninvasive methodology and holds significant potential in terms of sensitivity, selectivity, and experimental viability for the diagnosis of diseases, staging tumors, and predicting prognosis[15,16]. Radiomics has found use in HCC, including preoperative prediction of pathological indicators[17], differential diagnosis[18], evaluating curative effect, and prognosis prediction[19]. Recently, Yu et al[20] applied gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging (MRI) radiomics approach to evaluate VETC in HCC, Dong et al[21] attempted to develop deep learning radiomics model of dynamic contrast-enhanced MRI to predict VETC in HCC. As a routine examination method, the emergence of computed tomography (CT) has made a qualitative leap in the imaging diagnosis of liver cancer and driven the progress of liver surgery. CT images are clear and stable, and are used for routine diagnosis and follow-up examination of liver cancer after rehabilitation. We hypothesized that radiomics features based on contrast-enhanced CT (CECT) scans might provide a preoperative reference for accurate prediction of VETC status in patients with HCC. To our knowledge, no studies have determined whether CECT-based radiomics features can be used to predict VETC status with HCC patients. The objective of this study was to develop and validate a nomogram based on clinical-radiological and radiomics features from intratumoral and peritumoral regions for preoperative prediction of VETC+ HCC using data from a multicenter study.
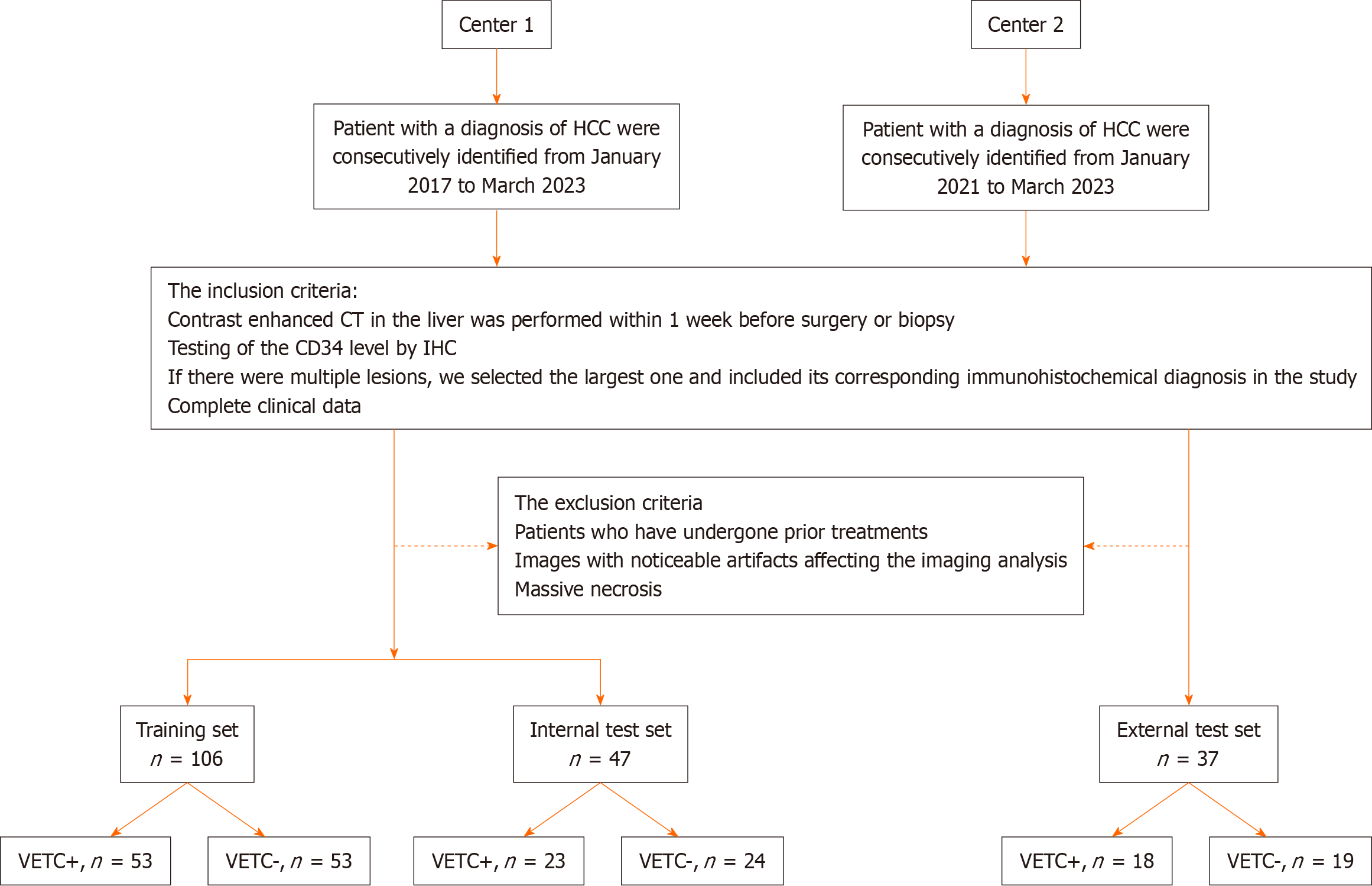
We retrospectively included consecutive patients who received a histological diagnosis of HCC between January 2017 and March 2023 for radiomics model construction, using two sample data sets from two separate hospitals: A training set and an internal test set from the Second Hospital of Shandong University (center 1), and an external test set from the Qilu Hospital of Shandong University (center 2). The institutional review board of the two centers approved this retrospective multicenter study and the requirement for informed consent was waived because of the retrospective data sets, IRB No. KYLL-2023LW044.
The inclusion criteria were as follows: (1) CECT in the liver was performed within 1 wk before surgery or biopsy; (2) Testing of the CD34 level by immunohistochemistry (IHC); (3) If there were multiple lesions, we selected the largest one and included its corresponding immunohistochemical diagnosis in the study; and (4) Complete clinical data. The exclusion criteria were as follows: (1) Patients who had undergone prior treatments, including anti-tumor therapies, radiofrequency ablation, transcatheter arterial chemoembolization, and other similar procedures; (2) Images with noticeable artifacts affecting the imaging analysis; and (3) Massive necrosis (a significant area of necrosis in HCC, with few solid components present.
Radcloud platform (version 7.2; Huiying Medical Technology Co., Ltd, Beijing, China) was used to manage the imaging data and conduct subsequent analysis of radiomics statistics. Finally, a total of 153 patients with HCC (121 men and 32 women; 76 VETC+ and 77 VETC-) from center 1 were enrolled into a training set and an internal test set. To ensure appropriate sample distribution, the dataset was randomly split into a training set and an internal test set using a ratio of 7:3 and a random seed of 39. Another cohort of 37 patients with HCC (32 men and 5 women; 18 VETC+ and 19 VETC-) from center 2 were enrolled into an external test set. For a visual representation of the patient recruitment process, please refer to Figure 1.
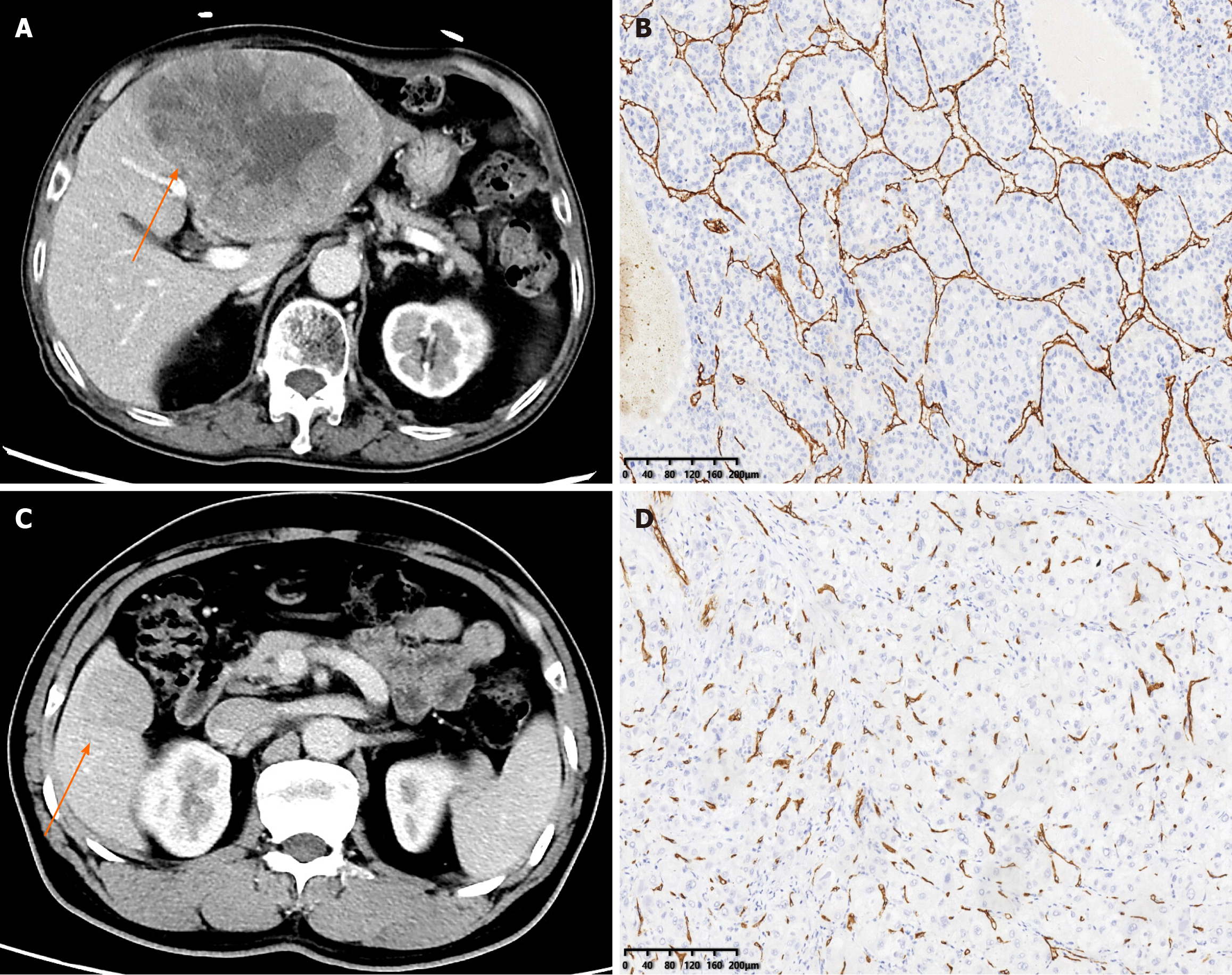
The VETC pattern of all 190 patients in this study was determined by IHC performed on surgical histopathology samples. A 7-point baseline sampling protocol was applied to sample specimens to measure HCC[22]. The definition of the VETC pattern is the presence of vessels that form cobweb-like networks and that encapsulate and separate individual tumor clusters an explicit and continuous lining of CD34-positive endothelium[12]. Under light microscopy (100 ×), the five most intensely vascularized fields were selected, and the total number of individual tumor clusters that were completely surrounded by endothelium was evaluated. The index of VETC was presented by the average number of encapsulated tumor clusters per field[6]. According to previous studies, cases with VETC index ≥ 5% in whole or part of the HCC section by CD34 immunostaining were identified as VETC+, and those with VETC index < 5% were identified as VETC-[23]. Two experienced pathologists, each with over 10 years of experience, conducted a qualitative and independent pathological assessment. Both the pathologists were blinded to the clinical, laboratory, and imaging results of the CECT. In cases where there was disagreement, a third pathologist was consulted, and the matter was discussed until a consensus was reached.
Contrast-enhanced liver CT was performed using a 256-section (GE Revolution; both GE Healthcare) or a 128-section (Siemens Somatom Definition; Siemens) multidetector CT scanner. The following CT acquisition parameters were used: Tube voltage 120 kVp, tube current 240 mAs, rotation time 0.5 s, matrix size 512 × 512, slice thickness 5 mm. Nonionic contrast agent (300 mg of iodine per milliliter, 3 mL/s, 1.5 mL/kg body weight, Omnipaque, GE Healthcare) was administered as a bolus rapidly via the antecubital vein using a syringe pump. The arterial phase (AP), portal vein phase, and delayed phase images were obtained during suspended respiration at 15 s, 30 s, and 180 s respectively.
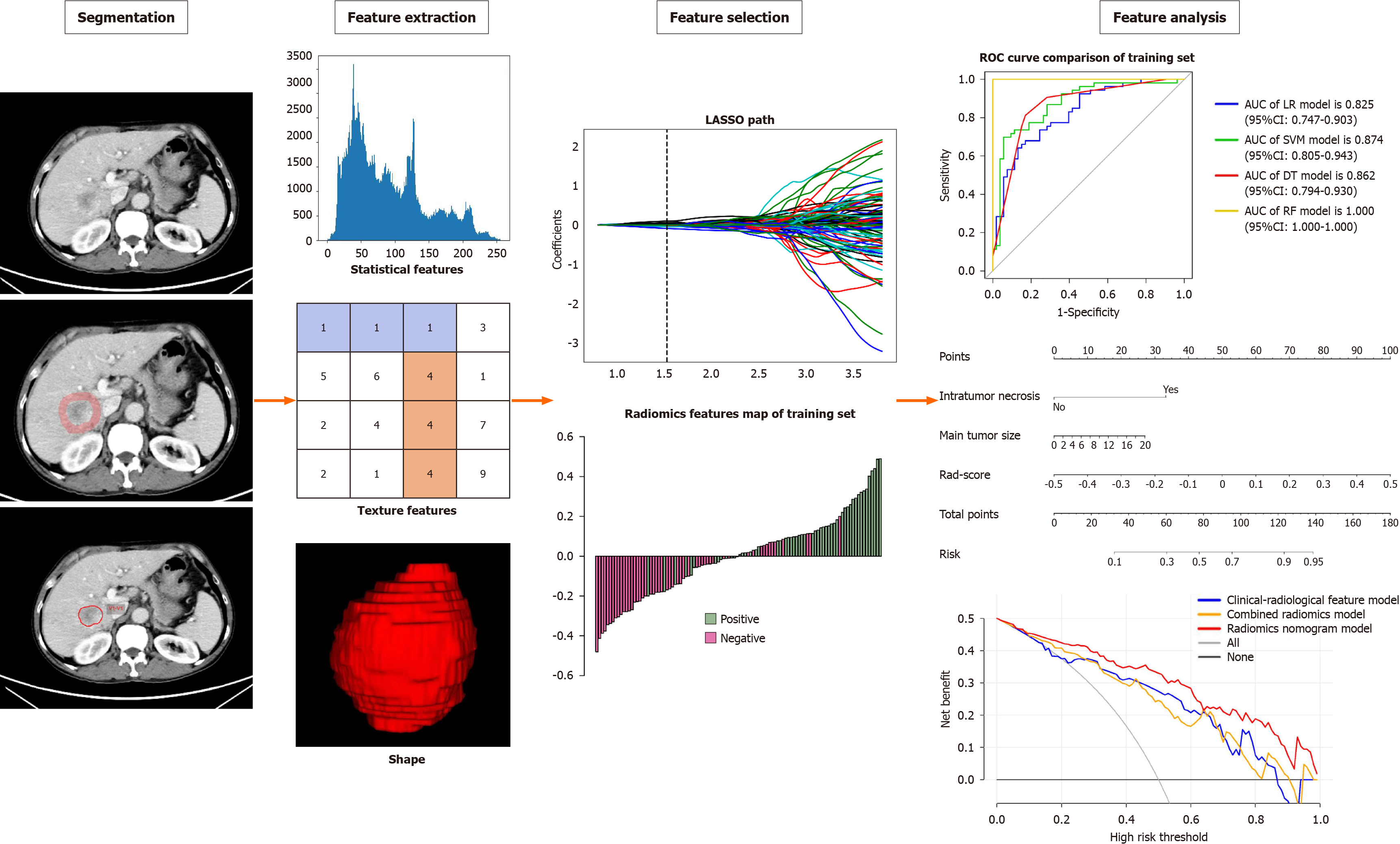
The Radcloud platform was used for image segmentation purposes. Two radiologists (reader 1, 8 years of liver imaging experience; reader 2, 10 years of liver imaging experience) who were blinded to the clinical and histopathologic data delineated in a slice-by-slice manner the volumes of interest (VOI) of HCC from the portal venous phase images to obtain a tumor segmentation[24]. When there was a disagreement between the two radiologists, a senior radiologist (reader 3, 15 years of liver imaging experience) was consulted. Figure 2 provides an illustrative example of the tumor segmentation achieved through this process. To account for the peritumoral region, a topology algorithm was employed to dilate the region by a radius of 10 mm, as illustrated in Figure 2. In instances where the VOI extended beyond the liver parenchyma after the dilation, manual removal of the excessive portion was performed.
Following the segmentation of VOI-1 from intratumoral regions and VOI-2 from peritumoral regions, radiomics features were extracted using the Radcloud platform. Subsequently, a total of 3376 quantitative imaging features were extracted, including first-order statistics, 3D shape features, gray-level co-occurrence matrix features, gray-level run length matrix features, gray-level size zone matrix features, neighboring gray tone difference matrix features, and gray-level dependence matrix features. Notably, although shape features were solely derived from the original images, the remaining features could also be extracted after applying various filters such as wavelet, square, square root, gradient, logarithm, exponential, local binary pattern in 2D (lbp-2D), and lbp-3D. To obtain textural features, the preprocessed CT images underwent wavelet filtering. This involved the use of a built-in stationary wavelet transform employing high or low-pass filters in the X-, Y-, and Z- directions. Moreover, the lbp-3D image type consisted of three subcategories. One of these subcategories was the kurtosis map (lbp-3D-k), whereas the other two were calculated using varying levels of spherical harmonics, namely lbp-3D-m1 and lbp-3D-m2. All these radiomics features adhered to the image biomarker standardization initiative[25]. In addition, the values of these radiomics features were normalized using the z-score method.
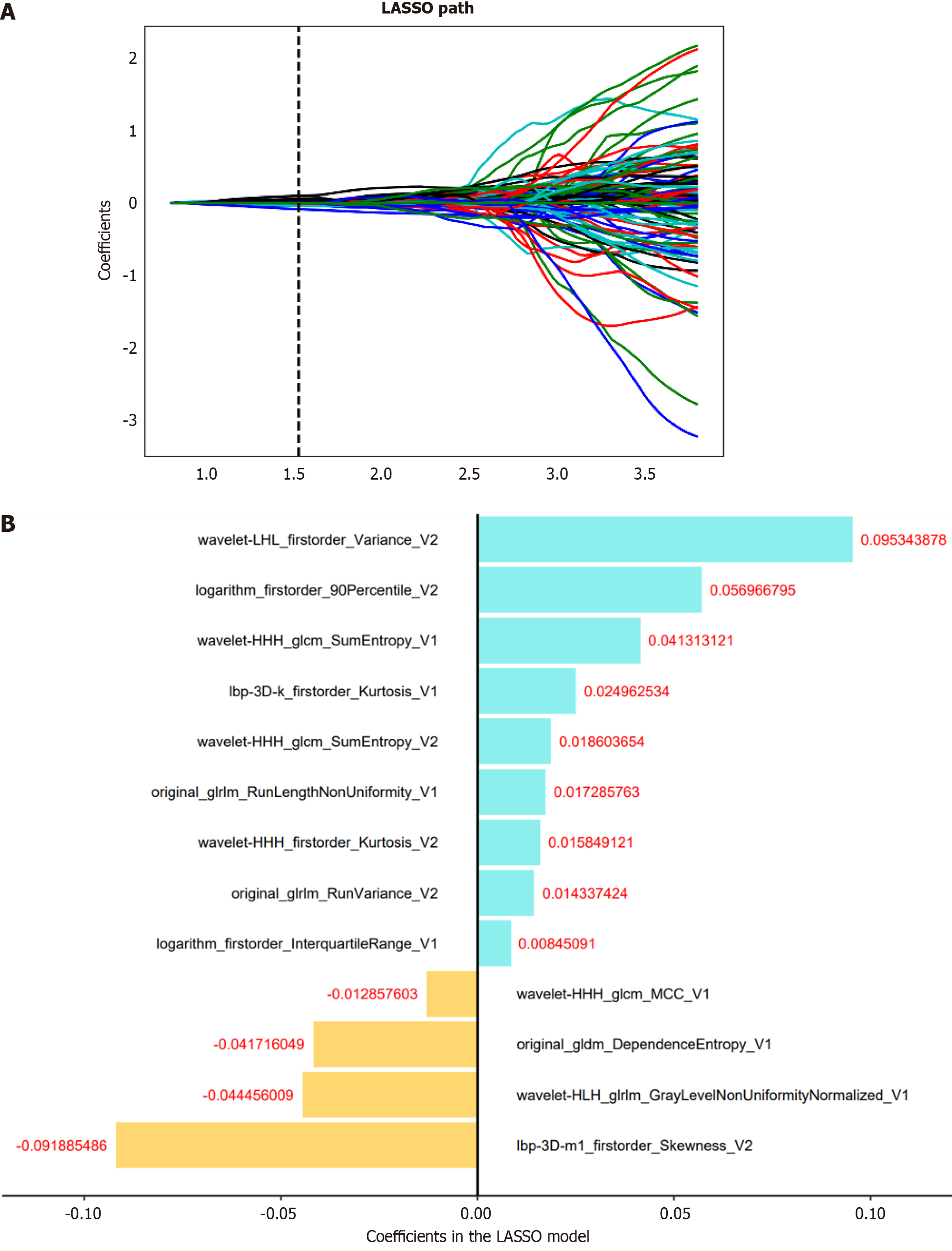
Dimension reduction techniques were used to select relevant features and mitigate potential issues such as overfitting and bias during construction of the radiomics signature using the training set data. The workflow for the radiomics analysis is visually depicted in Figure 2. To assess interobserver reproducibility, two radiologists (reader 1 and reader 2) independently repeated the segmentation process on 30 randomly selected lesions after a one-month interval. The radiomics features demonstrating good agreement [interclass correlation coefficient (ICC) > 0.8] between the two readers were included in subsequent analyses. Moreover, a variance threshold of 0.8 was applied to further refine the feature selection process. Subsequently, SelectKBest, a univariate analysis method, was used to select features with P values less than 0.05 for further analysis. Finally, the optimal feature subset was constructed using the least absolute shrinkage and selection operator (LASSO). Regularization parameter (alpha) tuning was performed through 10-fold cross-validation, and features with non-zero coefficients were selected for subsequent radiomics analysis.
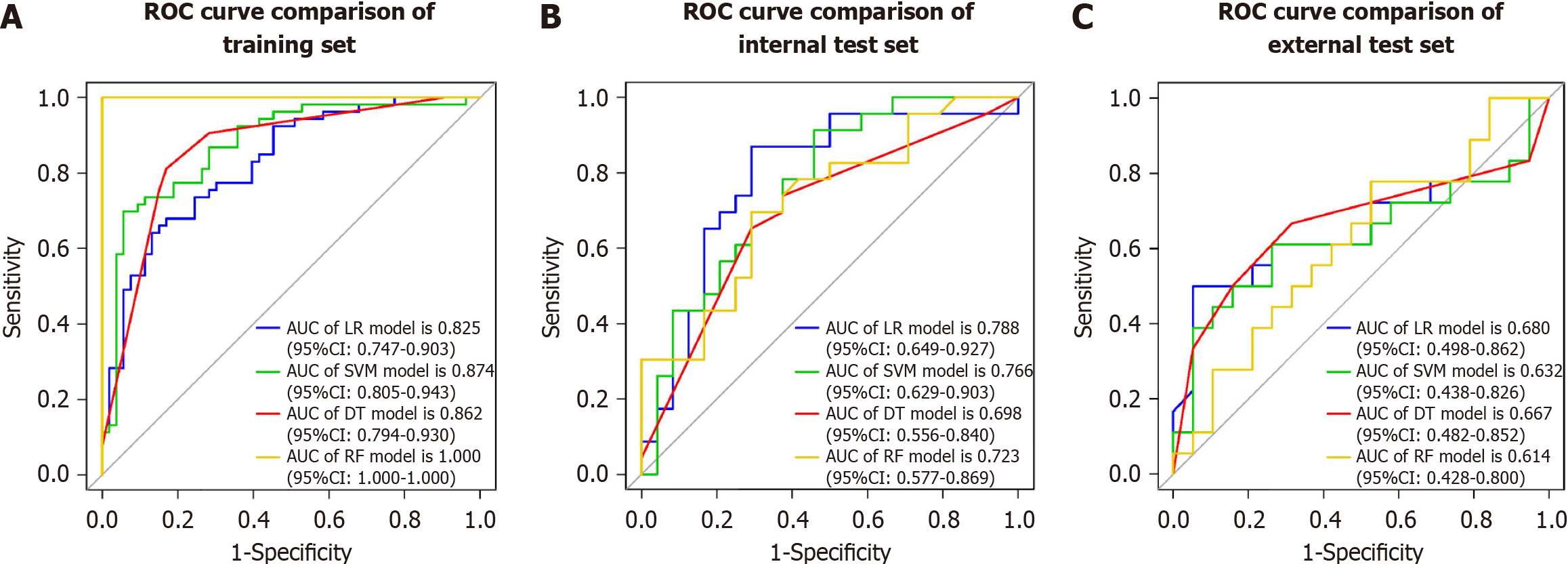
We selected relevant features extracted from intratumoral, peritumoral, and combined intratumoral and peritumoral regions. Subsequently, a radiomics score (Rad-score) was computed for each patient by using LASSO logistic regression (LR) on the features, where the coefficients were utilized for weighting (refer to Figure 3B). Multiple machine learning algorithms, including LR, support vector machine (SVM), decision tree (DT), and random forest (RF), were employed to establish radiomics models for intratumoral, peritumoral, and combined regions. The model demonstrating the highest predictive performance among these algorithms was chosen to construct a radiomics nomogram in conjunction with the independent clinical-radiological feature.
The study recorded clinical and laboratory data, which included age, sex, history of hepatic virus infection [negative, history of hepatitis B virus (HBV), HCV, or HBV and HCV], history of cirrhosis (absent, present), alanine aminotransferase, aspartate aminotransferase, gamma-glutamyl transferase, and alpha-fetoprotein. The radiologists (reader 1 and reader 2) also reviewed radiological feature descriptors of each lesion, such as main tumor size, single lobe involvement, non-smooth tumor margin, intratumor necrosis, intratumor hemorrhage, AP hyperenhancement, washout, and well-defined capsule; occasional cases with discrepancies were referred to reader 3, were resolved by consensus. After multiple LR analysis, significant risk factors were used to build a clinical-radiological feature model.
Statistical analyses were carried out with the R software (version 4.2.1; https://www.r-project.org/). The Mann-Whitney U test was employed to assess the differences in clinical and radiological data among the three groups. Inter-group comparisons were performed using either the χ2 test or one-way analysis of variance (ANOVA). Calibration curves were constructed based on 1000 iterations of bootstrap resampling, and the Hosmer-Lemeshow goodness-of-fit test was applied to evaluate the model calibration. To compare the estimated values of the area under the curve (AUC) for different prediction models, the non-parametric Delong test was utilized. All statistical tests were two-sided, and a significance level of P < 0.05 was considered statistically significant for the entire duration of the study.
The imaging of 190 preoperative patients with HCC was collected from two independent institutions in China. The training set included 106 patients from the center1 {male, 78%; median [interquartile range (IQR)] age 58.5 (51, 65.75)}, and the internal test set included 47 patients [male, 81%; median (IQR) age 56 (51.5, 67.5)]. The external test set came from the center2 [male, 86%; median (IQR) age 57 (50, 64)]. In the training set, 50% (53/106) of the patients were diagnosed with VETC+, 49% (23/47) were diagnosed with VETC+ in the internal set, and 49% (18/37) were diagnosed with VETC+ in the external set. There were no differences in clinical characteristics or radiological features between the training set and the two test sets (Table 1). The representative images of CECT and immunohistochemical staining for CD34 were shown in Figure 4.
| Variables | Total set (n = 190) | Training set (n = 106) | Internal test set (n = 47) | External test set (n = 37) | P value |
| VETC (%) | 0.986 | ||||
| Positive | 94 (49) | 53 (50) | 23 (49) | 18 (49) | |
| Negative | 96 (51) | 53 (50) | 24 (51) | 19 (51) | |
| Age (median, IQR) | 57 (51, 66) | 58.5 (51, 65.75) | 56 (51.5, 67.5) | 57 (50, 64) | 0.708 |
| Sex (%) | 0.555 | ||||
| Male | 153 (81) | 83 (78) | 38 (81) | 32 (86) | |
| Female | 37 (19) | 23 (22) | 9 (19) | 5 (14) | |
| Hepatitis (%) | 0.799 | ||||
| HBV or/and HCV | 171 (90) | 96 (91) | 41 (87) | 34 (92) | |
| Negative | 19 (10) | 10 (9) | 6 (13) | 3 (8) | |
| Cirrhosis (%) | 0.008 | ||||
| Present | 158 (83) | 85 (80) | 36 (77) | 37 (100) | |
| Absent | 32 (17) | 21 (20) | 11 (23) | 0 (0) | |
| ALT (median, IQR) | 32 (19, 51.75) | 28 (18, 49) | 40 (22.5, 63) | 33 (22, 45) | 0.094 |
| AST (median, IQR) | 37 (25, 61.5) | 36.5 (23.25, 1.75) | 49 (28.5, 78) | 30 (24, 46) | 0.041 |
| GGT (median, IQR) | 59.25 (31, 131.25) | 61.75 (30, 127) | 76 (38.5, 143.5) | 49 (27, 98) | 0.157 |
| AFP (median, IQR) | 49.06 (5.54, 9.25) | 62.74 (6.62, 9.25) | 56.78 (5.87, 16.5) | 34.74 (5.22, 798) | 0.516 |
| Main tumor size (median, IQR) | 5.7 (3.2, 9.28) | 6.05 (3.02, 9.3) | 6.9 (3.45, 11.15) | 4.11 (3.2, 6.5) | 0.098 |
| Multiplicity (%) | 0.037 | ||||
| ≥ 2 | 46 (24) | 29 (27) | 14 (30) | 3 (8) | |
| 1 | 144 (76) | 77 (73) | 33 (70) | 34 (92) | |
| Single lobe involvement (%) | 0.064 | ||||
| Present | 141 (74) | 74 (70) | 34 (72) | 33 (89) | |
| Absent | 49 (26) | 32 (30) | 13 (28) | 4 (11) | |
| Intratumor hemorrhage (%) | 0.179 | ||||
| Present | 12 (6) | 9 (8) | 3 (6) | 0 (0) | |
| Absent | 178 (94) | 97 (92) | 44 (94) | 37 (100) | |
| Intratumor necrosis (%) | 0.131 | ||||
| Present | 95 (50) | 57 (54) | 25 (53) | 13 (35) | |
| Absent | 95 (50) | 49 (46) | 22 (47) | 24 (65) | |
| Arterial phase hyper enhancement (%) | 0.701 | ||||
| Present | 179 (94) | 101 (95) | 44 (94) | 34 (92) | |
| Absent | 11 (6) | 5 (5) | 3 (6) | 3 (8) | |
| Well defined capsule (%) | 0.143 | ||||
| Present | 140 (74) | 75 (71) | 33 (70) | 32 (86) | |
| Absent | 50 (26) | 31 (29) | 14 (30) | 5 (14) | |
| Washout (%) | 0.249 | ||||
| Present | 187 (98) | 105 (99) | 45 (96) | 37 (100) | |
| Absent | 3 (2) | 1 (1) | 2 (4) | 0 (0) | |
| Non-smooth tumor margin (%) | 0.435 | ||||
| Present | 116 (61) | 69 (65) | 26 (55) | 21 (57) | |
| Absent | 74 (39) | 37 (35) | 21 (45) | 16 (43) |
Details of the clinical data and the radiological features in the training set are provided in Table 2. There was a statistically significant difference in the values of the 8 features selected by univariate analysis; these features were associated with VETC+ HCC and were considered as candidates for backward stepwise multivariate analysis. After multiple LR analysis, intratumor necrosis [P < 0.001, odds ratio (OR) = 7.947, 95% confidence interval (CI): 2.367-26.682] and main tumor size (P < 0.001, OR = 1.873, 95%CI: 0.629-5.581) were confirmed as independent predictors of VETC+ and were used to construct the clinical-radiological feature model (Table 2). Based on receiver operating characteristic (ROC) analysis, the AUCs for the clinical-radiological feature model was 0.833 (95%CI: 0.753-0.913), 0.781 (95%CI: 0.644-0.918), and 0.684 (95%CI: 0.498-0.862) in the training, internal test, and external test sets, respectively.
| Variables | VETC- (n = 53) | VETC+ (n = 53) | Univariate analysis | Multivariate analysis | ||
| OR | P value | OR | P value | |||
| Age, median (IQR) | 63 (50, 67) | 55 (51, 62) | 0.997 | 0.096 | ||
| Sex (%) | 1.344 | 0.637 | ||||
| Male | 40 (75) | 43 (81) | ||||
| Female | 13 (25) | 10 (19) | ||||
| Hepatitis (%) | 1.133 | 0.74 | ||||
| HBV or/and HCV | 47 (89) | 49 (92) | ||||
| Negative | 6 (11) | 4 (8) | ||||
| Cirrhosis (%) | 0.669 | 0.626 | ||||
| Present | 44 (83) | 41 (77) | ||||
| Absent | 9 (17) | 12 (23) | ||||
| ALT, median (IQR) | 23 (15, 38) | 36 (20, 52) | 1.010 | 0.011 | 1.001 | 0.738 |
| AST, median (IQR) | 29 (21, 50) | 40 (28, 68) | 0.989 | 0.022 | 0.991 | 0.450 |
| GGT, median (IQR) | 39 (27, 87) | 100 (40, 185) | 1.000 | 0.001 | 1.000 | 0.209 |
| AFP, median (IQR) | 62.23 (5.48, 446.4) | 78.51 (8.05, 8213) | 0.999 | 0.076 | ||
| Main tumor size, median (IQR) | 4.1 (2.4, 6.5) | 8.9 (5.6, 10.8) | 2.815 | < 0.001 | 1.873 | < 0.001 |
| Multiplicity (%) | 0.799 | 0.009 | 0.907 | 0.660 | ||
| ≥ 2 | 8 (15) | 21 (40) | ||||
| 1 | 45 (85) | 32 (60) | ||||
| Single lobe involvement (%) | 0.620 | < 0.001 | 0.952 | 0.617 | ||
| Present | 46 (87) | 28 (53) | ||||
| Absent | 7 (13) | 25 (47) | ||||
| Intratumor hemorrhage (%) | 0.609 | 1 | ||||
| Present | 4 (8) | 5 (9) | ||||
| Absent | 49 (92) | 48 (91) | ||||
| Intratumor necrosis (%) | 0.850 | < 0.001 | 7.947 | < 0.001 | ||
| Present | 13 (25) | 44 (83) | ||||
| Absent | 40 (75) | 9 (17) | ||||
| Arterial phase hyperenhancement (%) | 1.112 | 0.363 | ||||
| Present | 49 (92) | 52 (98) | ||||
| Absent | 4 (8) | 1 (2) | ||||
| Well defined capsule (%) | 1.018 | 1 | ||||
| Present | 38 (72) | 37 (70) | ||||
| Absent | 15 (28) | 16 (30) | ||||
| Washout (%) | 1.815 | 1 | ||||
| Present | 52 (98) | 53 (100) | ||||
| Absent | 1 (2) | 0 (0) | ||||
| Non-smooth tumor margin (%) | 1.717 | 0.014 | 1.109 | 0.881 | ||
| Present | 28 (53) | 41 (77) | ||||
| Absent | 25 (47) | 12 (23) | ||||
In total, 3376 radiomics features were extracted from two VOIs (1688 features for VOI-1, 1688 features for VOI-2). Among them, 1430 features from VOI-1 and 1328 features from VOI-2, both with an ICC > 0.8, were retained for subsequent feature selection. The selection process involved applying the variance threshold, the SelectKBest and LASSO regression (Figure 3A).
After eliminating highly collinear features, we constructed the intratumoral (11 intratumoral features used), peritumoral (10 peritumoral features used), and combined (7 intratumoral and 6 peritumoral features used) radiomics models on the training set with multivariate LR (Table 3). Based on selected radiomics features, we built the intratumoral, peritumoral, and combined radiomics models.
| Intratumoral radiomics model | Peritumoral radiomics model | Combined radiomics model |
| Original_GLDM_DependenceEntropy | Original_shape_Sphericity | Original_GLDM_DependenceEntropy1 |
| Lbp-3D-k_GLRLM_ShortRunHighGrayLevelEmphasis | Lbp-2D_firstorder_InterquartileRange | Original_GLRLM_RunLengthNonUniformity1 |
| Lbp-3D-k_GLDM_SmallDependenceEmphasis | Lbp-3D-k_firstorder_Minimum | Wavelet-HHH_GLCM_SumEntropy1 |
| Original_GLRLM_RunLengthNonUniformity | Wavelet-HHH_GLCM_SumEntropy | Wavelet-HHH_GLCM_SumEntropy2 |
| Wavelet-HHH_GLCM_SumEntropy | Original_GLRLM_RunVariance | Original_GLRLM_RunVariance2 |
| Lbp-3D-k_firstorder_Kurtosis | Wavelet-LHL_firstorder_Variance | Logarithm_firstorder_InterquartileRange1 |
| Wavelet-HLH_GLRLM_GrayLevelNonUniformityNormalized | Lbp-3D-m1_firstorder_Skewness | Wavelet-LHL_firstorder_Variance2 |
| Squareroot_firstorder_Minimum | Logarithm_firstorder_10Percentile | Wavelet-HHH_GLCM_MCC1 |
| Wavelet-LLH_GLCM_Imc2 | Squareroot_firstorder_10Percentile | Lbp-3D-k_firstorder_Kurtosis1 |
| Wavelet-LHL_GLCM_MaximumProbability | Wavelet-HLL_firstorder_Kurtosis | Wavelet-HHH_firstorder_Kurtosis2 |
| Wavelet-HLH_GLCM_MaximumProbability | Lbp-3D-m1_firstorder_Skewness2 | |
| Wavelet-HLH_GLRLM_GrayLevelNonUniformityNormalized_V11 | ||
| Logarithm_firstorder_90Percentile2 |
The performance of the combined radiomics model in predicting VETC was evaluated using LR, SVM, DT, and RF (Table 4 and Figure 3). Among these models, LR exhibited the best performance and was chosen as the classifier for all subsequent analyses in this article. The AUC for the intratumoral model was 0.772 (95%CI: 0.684-0.860) in the training set, 0.768 (95%CI: 0.628-0.908) in the internal test set, and 0.673 (95%CI: 0.495-0.851) in the external test set. For the peritumoral model, the AUC values were 0.823 (95%CI: 0.745-0.901) in the training set, 0.757 (95%CI: 0.615-0.899) in the internal test set, and 0.605 (95%CI: 0.418-0.792) in the external test set. The combined radiomics model demonstrated the highest predictive performance across the training set and both test sets, with AUC values of 0.825 (95%CI: 0.747-0.903) in the training set, 0.788 (95%CI: 0.649-0.927) in the internal test set, and 0.680 (95%CI: 0.498-0.862) in the external test set (Table 5 and Figure 5).
| Set | ML model | AUC (95%CI) | Accuracy | Sensitivity | Specificity | PPV | NPV |
| Training | |||||||
| LR | 0.825 (0.747-0.903) | 0.726 | 0.736 | 0.717 | 0.722 | 0.731 | |
| SVM | 0.874 (0.805-0.943) | 0.764 | 0.792 | 0.736 | 0.745 | 0.765 | |
| DT | 0.862 (0.794-0.930) | 0.820 | 0.811 | 0.830 | 0.827 | 0.815 | |
| RF | 1 (1.000-1.000) | 1 | 1 | 1 | 1 | 1 | |
| Internal test | |||||||
| LR | 0.788 (0.649-0.927) | 0.745 | 0.783 | 0.708 | 0.720 | 0.773 | |
| SVM | 0.766 (0.629-0.903) | 0.681 | 0.739 | 0.625 | 0.654 | 0.714 | |
| DT | 0.698 (0.556-0.840) | 0.659 | 0.696 | 0.625 | 0.640 | 0.682 | |
| RF | 0.723 (0.577-0.869) | 0.702 | 0.739 | 0.667 | 0.667 | 0.696 | |
| External test | |||||||
| LR | 0.680 (0.498-0.862) | 0.676 | 0.500 | 0.842 | 0.750 | 0.640 | |
| SVM | 0.632 (0.438-0.826) | 0.676 | 0.500 | 0.842 | 0.75 | 0.640 | |
| DT | 0.667 (0.482-0.852) | 0.676 | 0.500 | 0.842 | 0.750 | 0.640 | |
| RF | 0.614 (0.428-0.800) | 0.568 | 0.444 | 0.684 | 0.571 | 0.565 |
| Set | Model | AUC (95%CI) | Accuracy | Sensitivity | Specificity | PPV | NPV |
| Training | |||||||
| Intratumoral radiomics | 0.772 (0.684-0.860) | 0.689 | 0.736 | 0.642 | 0.673 | 0.708 | |
| Peritumoral radiomics | 0.823 (0.745-0.901) | 0.745 | 0.774 | 0.717 | 0.732 | 0.760 | |
| Combined radiomics | 0.825 (0.747-0.903) | 0.726 | 0.736 | 0.717 | 0.722 | 0.731 | |
| Internal test | |||||||
| Intratumoral radiomics | 0.768 (0.628-0.908) | 0.638 | 0.696 | 0.583 | 0.615 | 0.667 | |
| Peritumoral radiomics | 0.757 (0.615-0.899) | 0.702 | 0.783 | 0.625 | 0.750 | 0.667 | |
| Combined radiomics | 0.788 (0.649-0.927) | 0.745 | 0.783 | 0.708 | 0.720 | 0.773 | |
| External test | |||||||
| Intratumoral radiomics | 0.673 (0.495-0.851) | 0.568 | 0.556 | 0.579 | 0.556 | 0.579 | |
| Peritumoral radiomics | 0.605 (0.418-0.792) | 0.568 | 0.389 | 0.737 | 0.560 | 0.583 | |
| Combined radiomics | 0.680 (0.498-0.862) | 0.676 | 0.500 | 0.842 | 0.750 | 0.640 |
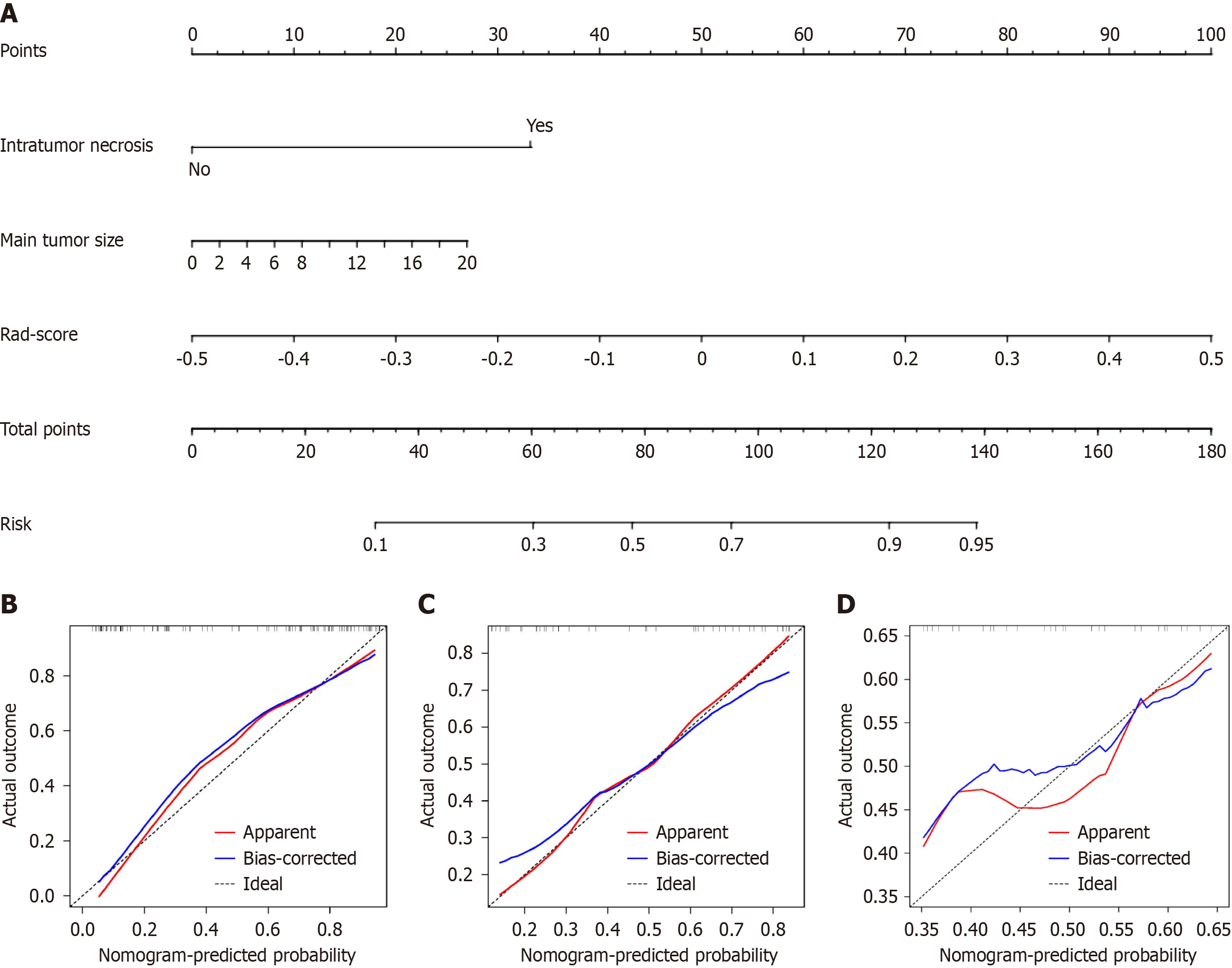
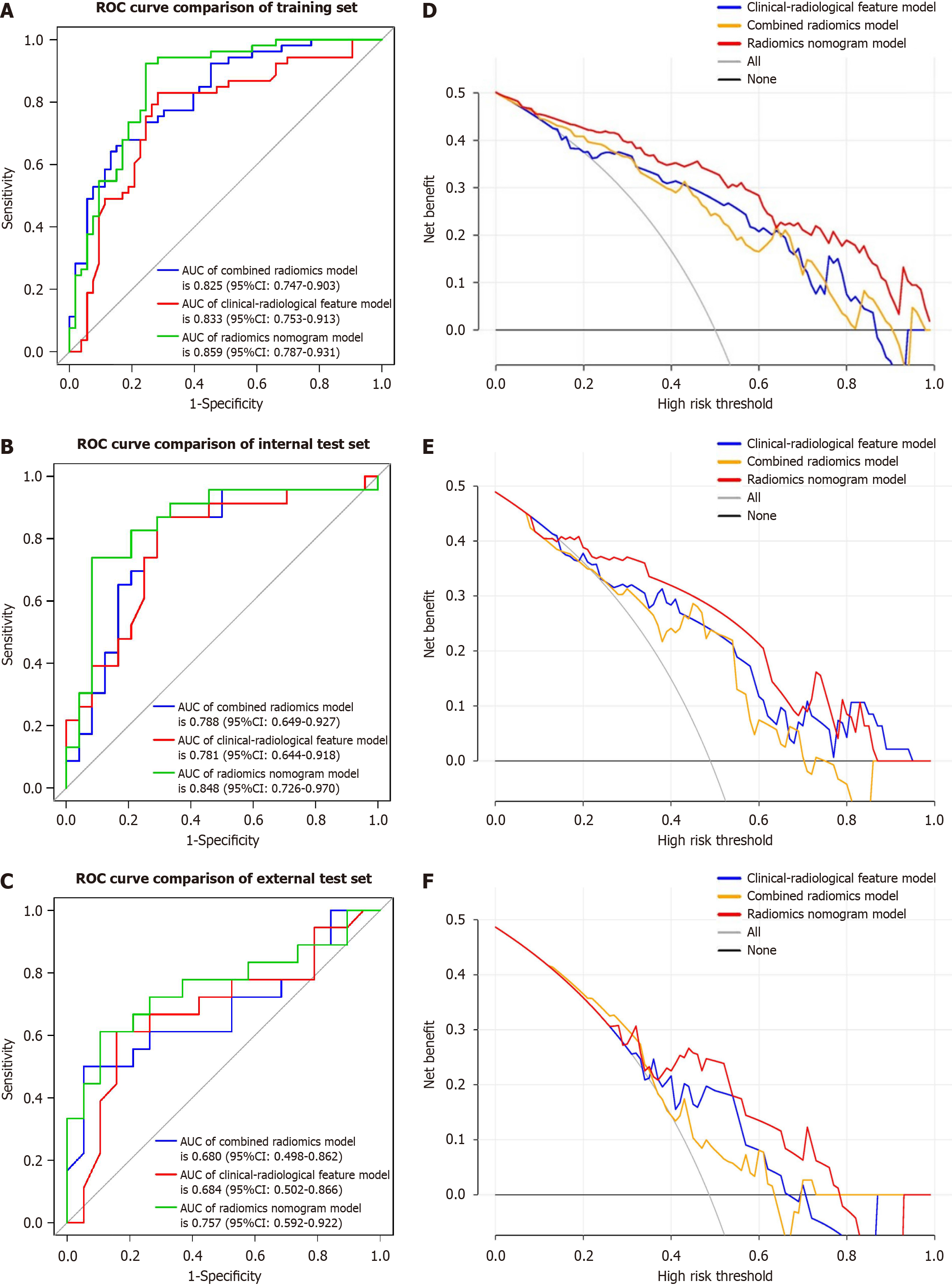
To develop a clinically applicable approach that could predict the probability of VETC+ HCC, the clinical-radiological and radiomics features were incorporated into the radiomics nomogram (Figure 6A). The Rad-score, calculated by applying LR to the combined radiomics features weighted by their coefficients, served as an indicator for each patient. The calibration curves of the radiomics nomogram demonstrated a satisfactory fit in the training, internal test and external test sets (Figure 6B-D), as evidenced by the Hosmer-Lemeshow test P-values of 0.8633, 0.7965, and 0.3205 respectively, which indicate the goodness-of-fit of the model. As shown in the nomogram (Figure 6A), by assigning each feature a value based on a point scale ranging from 0 to 100, one can obtain a total score by adding the scores for each feature. The risk of VETC+ HCC can be predicted by projecting the score to the bottom risk axis. The sensitivity, specificity, accuracy, and AUC of the clinical-radiological feature, combined radiomics, and radiomics nomogram models are shown in Table 6. The radiomics nomogram exhibited superior predictive performance, with an AUC of 0.859 (95%CI: 0.787-0.931) on the training set, 0.848 (95%CI: 0.726-0.970) on the internal test set, and 0.757 (95%CI: 0.592-0.922) on the external test set, and achieved better discriminatory performance than the clinical-radiological feature and the combined radiomics models (Figure 7A-C). The Delong test revealed statistically significant differences in AUCs among the clinical-radiological feature, the combined radiomics and the radiomics nomogram models on the internal test set (P = 0.004 and P < 0.001, respectively). The utility of the three predictive models was assessed using DCA, which calculated the net benefit at various probability thresholds (Figure 7D-F). The DCA results indicated that the radiomics nomogram model provided greater overall net benefit than either the radiological feature or the combined radiomics models, affirming the reliability of the nomogram as a clinical tool for predicting the risk of VETC+ HCC.
| Set | Model | AUC (95%CI) | Accuracy | Sensitivity | Specificity | PPV | NPV |
| Training | |||||||
| Clinical-radiological feature | 0.833 (0.753-0.913) | 0.792 | 0.830 | 0.754 | 0.737 | 0.776 | |
| Combined radiomics | 0.825 (0.747-0.903) | 0.726 | 0.736 | 0.717 | 0.722 | 0.731 | |
| Radiomics nomogram | 0.859 (0.787-0.931) | 0.792 | 0.830 | 0.754 | 0.772 | 0.816 | |
| Internal test | |||||||
| Clinical-radiological feature | 0.781 (0.644-0.918) | 0.744 | 0.782 | 0.708 | 0.720 | 0.773 | |
| Combined radiomics | 0.788 (0.649-0.927) | 0.745 | 0.783 | 0.709 | 0.720 | 0.773 | |
| Radiomics nomogram | 0.848 (0.726-0.970) | 0.787 | 0.826 | 0.750 | 0.760 | 0.818 | |
| External test | |||||||
| Clinical-radiological feature | 0.684 (0.498-0.862) | 0.676 | 0.500 | 0.842 | 0.750 | 0.64 | |
| Combined radiomics | 0.680 (0.502-0.866) | 0.676 | 0.500 | 0.842 | 0.750 | 0.640 | |
| Radiomics nomogram | 0.757 (0.592-0.922) | 0.729 | 0.611 | 0.842 | 0.750 | 0.783 |
The VETC pattern in HCC has been identified as a predictor of micro-metastasis, aggressive behavior, and unfavorable prognosis[13,26]. There is a lack of development and validation for CT radiomics model to preoperatively predict the VETC subtype of HCC, and the biologic underpinnings of the radiomics method deserve investigation. In our study, we established and validated a noninvasive CECT radiomics nomogram composed of radiomics features, and the clinical-radiological feature of intratumor necrosis, and main tumor size predict VETC+. Our results showed that the combined radiomics model showed no additional value over the clinical-radiological feature model, but that the nomogram showed good discrimination performance (AUC: 0.859) on the training set and the two test sets for prediction of VETC+ compared with the combined radiomics model or the clinical-radiological feature model.
Feng et al[27] had reported that the presence of VETC demonstrated a significant correlation with various clinical characteristics, including tumor size exceeding 5 cm and the occurrence of tumor necrosis. In our study, maximum tumor diameter and tumor necrosis were also independent predictors for VETC subtype. This agrees with the findings reported by them in the clinical-radiological feature model which achieved areas under the ROC curve of 0.833, 0.781, and 0.684 on the training set, the internal test set and the external test set, respectively. Angiogenesis activation is a mark of aggressive VETC HCC. Increased diffusion distances from the existing vascularity supply as the tumor expands and increased cellularity due to proliferative tumor cells result in hypoxia and necrosis. Hypoxia and neoangiogenesis result in obvious necrosis in fast-growing HCC[28,29]. Neovascularity mainly occurs on the periphery of the tumor, and rapidly reduces central perfusion, leading to central necrosis.
Radiomics has been known as an important digital biopsy method to predict several biological features of tumors[12]. In this study, we constructed a machine learning-based CECT radiomics model, performed canonical screening of features and multiple validations, and confirmed robustness on various data resources. The suboptimal performance on the external test set may be ascribed to differences in the CT scan protocol and to heterogeneity of the data set, which came from two different institutions. However, the radiomics model showed remarkable performance in predicting the VETC subtype, and the results were reproducible, demonstrating that the approach may be applied to other patient samples. VETC is a heterogeneous pattern of angiogenesis involved in HCC biological behavior[12]. This may account for why the radiomics model had a favorable predictive ability in predicting VETC. In this study, the intratumoral or peritumoral radiomics model achieved identified good performance in predicting VETC. As shown in our study, the peritumoral radiomics model was superior to the intratumoral model, which was consistent with previous reports[20,30]. This result might suggest that VETC is more likely to be found in the peritumoral region. Moreover, the combined intratumoral and peritumoral radiomics model exhibited better predictive performance than the intratumoral model in preoperative prediction of VETC in HCC. Furthermore, we combined the clinical-radiological feature and the radiomics models to create the radiomics nomogram model and validate its predictive power. Our study showed that the radiomics nomogram model had a higher predictive value than the single clinical-radiological feature model or the radiomics model with, AUC of 0.859, 0.848, and 0.757 on the training set, the internal test set and the external test set, respectively. Our result goes further by indicating a radiomics link between CT imaging and VETC subtype, which may facilitate the implementation of morphomolecular subtyping of HCC into clinical practice and application.
The radiomics model could reflect the heterogeneity of HCC[31]. First-order features mainly depend on the statistics of the intensity information. Texture analysis was recently found to provide a quantitative, objective assessment of tumor heterogeneity by analyzing the distribution and relationship of pixel or voxel grey levels and could reflect information on the lesion microenvironment[32]. In our study, of the eleven features in the intratumoral radiomics model, two were first-order features and nine were texture features. Of the ten features in the peritumoral radiomics model, seven were first-order features, one was a shape feature, and only two were texture features. The radiomics model had more texture features, especially the intratumoral radiomics model. The results demonstrated that VETC+ HCC have more diverse vascular patterns, including VETC, sinusoidal capillarization and other neovascularization patterns, which could lead to additional heterogeneity in texture compared with VETC- HCC.
Our study had several limitations. First, the radiomics model was constructed based on retrospective data with patients who underwent surgical or biopsy treatments at multiple institutions, which may have resulted in selection bias. Second, we defined “VETC ≥ 5%” as the VETC group[12,33] with reference to previous reports. However, the optimal cut-off value of VETC is not yet standardized. Future studies could be conducted to develop and verify the optimal cut-off value for HCC. Third, the sample size of our study was relatively small, especially the external validation group, and larger sample sizes are needed for radiomics analysis in future studies. Finally, the radiomics marker is limited by its complexity and lack of algorithmic standardization. In future studies, the development of a deep learning-based predictive model will be constructed and validated. Therefore, a further prospective study avoiding the above limitations is needed to validate those results.
In conclusion, the CECT radiomics model could noninvasively predict the VETC subtype in patients with HCC. The radiomics nomogram constructed from clinical-radiological features and combined radiomics features demonstrated good performance in preoperatively predicting VETC, and their combination showed superior predictive performance compared with the single model. Thus, this combination may be useful for the preoperative identification of VETC subtype in HCC, which could help select HCC patients with poor prognosis, early recurrence, and Sorafenib benefit. Therefore, it could provide valuable information for assisting clinicians in pretreatment decision-making.
Vessels encapsulating tumor clusters (VETC) is an independent risk factor for poor prognosis in hepatocellular carcinoma (HCC) and patients with VETC+ HCC show shorter overall survival and disease-free survival and are more prone to progression and metastasis relative to patients with VETC- HCC. So far, VETC is currently determined only on histologic examination after surgical resection.
Preoperative diagnosis of VETC status in HCC is of great significance for predicting the prognosis of HCC patients and determining treatment strategies.
This study aimed to develop and validate a preoperative nomogram based on contrast-enhanced computed tomography (CECT) scanning combined with radiomics and clinical-radiological features to provide a preoperative reference for accurate prediction of VETC status in patients with HCC.
This was a retrospective, diagnostic study conducted from January 2017 to March 2023, at two centers. The study included 190 (training set: 106; internal test set: 47; external test set: 37) HCC patients who underwent CECT. Variance threshold, SelectKBest, the least absolute shrinkage and selection operator algorithm and multivariable logistic regression analysis were used to select the useful features and transform them into models. Receiver operating characteristic analysis was employed to compare the identified performance of models in predicting the VETC status of HCC on both training and test sets.
Among 190 individuals used for radiomics modeling, with the majority being male (81%) and a median age of 57 years (interquartile range: 51-66), 94 (49%) were confirmed to have the VETC subtype. The nomogram model included clinical-radiological features and 13 radiomics features and showed good performance for predicting the VETC subtype, with area under the curves of 0.859, 0.848, and 0.757 in the training set, internal test set, and external test set, respectively. The radiomics nomogram outperformed any clinical-radiological feature and the combined radiomics models in terms of clinical predictive abilities, according to a decision curve analysis.
The findings of this research indicate that a nomogram, developed using clinical-radiological features and combined radiomics features, holds the capability to accurately forecast the VETC status of HCC.
Our findings may be useful for preoperative identification of VETC subtype in HCC, which could help select HCC patients with poor prognosis, early recurrence, and sorafenib benefit.
| 1. | Huang X, Long L, Wei J, Li Y, Xia Y, Zuo P, Chai X. Radiomics for diagnosis of dual-phenotype hepatocellular carcinoma using Gd-EOB-DTPA-enhanced MRI and patient prognosis. J Cancer Res Clin Oncol. 2019;145:2995-3003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | Shao CC, Zhao F, Yu YF, Zhu LL, Pang GD. Value of perfusion parameters and histogram analysis of triphasic computed tomography in pre-operative prediction of histological grade of hepatocellular carcinoma. Chin Med J (Engl). 2021;134:1181-1190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56683] [Article Influence: 7085.4] [Reference Citation Analysis (135)] |
| 4. | Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261:947-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 725] [Article Influence: 65.9] [Reference Citation Analysis (1)] |
| 5. | Ma X, Wei J, Gu D, Zhu Y, Feng B, Liang M, Wang S, Zhao X, Tian J. Preoperative radiomics nomogram for microvascular invasion prediction in hepatocellular carcinoma using contrast-enhanced CT. Eur Radiol. 2019;29:3595-3605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 177] [Article Influence: 25.3] [Reference Citation Analysis (1)] |
| 6. | Fang JH, Zhou HC, Zhang C, Shang LR, Zhang L, Xu J, Zheng L, Yuan Y, Guo RP, Jia WH, Yun JP, Chen MS, Zhang Y, Zhuang SM. A novel vascular pattern promotes metastasis of hepatocellular carcinoma in an epithelial-mesenchymal transition-independent manner. Hepatology. 2015;62:452-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 207] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 7. | Morse MA, Sun W, Kim R, He AR, Abada PB, Mynderse M, Finn RS. The Role of Angiogenesis in Hepatocellular Carcinoma. Clin Cancer Res. 2019;25:912-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 412] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 8. | Zhu AX, Duda DG, Sahani DV, Jain RK. HCC and angiogenesis: possible targets and future directions. Nat Rev Clin Oncol. 2011;8:292-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 429] [Cited by in RCA: 458] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 9. | Giannelli G, Koudelkova P, Dituri F, Mikulits W. Role of epithelial to mesenchymal transition in hepatocellular carcinoma. J Hepatol. 2016;65:798-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 487] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 10. | Zeng HX, Li Z, Chen JG, Fang JH, Zhuang SM. [Vessels Encapsulating Tumor Clusters (VETC): a New Mechanism of Tumor Metastasis and A New Target for Precision Medicine]. Chinese J Cell Biol. 2022;. |
| 11. | Chen ZY, Guo ZX, Lu LH, Mei J, Lin WP, Li SH, Wei W, Guo RP. The predictive value of vessels encapsulating tumor clusters in treatment optimization for recurrent early-stage hepatocellular carcinoma. Cancer Med. 2021;10:5466-5474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Renne SL, Woo HY, Allegra S, Rudini N, Yano H, Donadon M, Viganò L, Akiba J, Lee HS, Rhee H, Park YN, Roncalli M, Di Tommaso L. Vessels Encapsulating Tumor Clusters (VETC) Is a Powerful Predictor of Aggressive Hepatocellular Carcinoma. Hepatology. 2020;71:183-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 179] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 13. | Fang JH, Xu L, Shang LR, Pan CZ, Ding J, Tang YQ, Liu H, Liu CX, Zheng JL, Zhang YJ, Zhou ZG, Xu J, Zheng L, Chen MS, Zhuang SM. Vessels That Encapsulate Tumor Clusters (VETC) Pattern Is a Predictor of Sorafenib Benefit in Patients with Hepatocellular Carcinoma. Hepatology. 2019;70:824-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 14. | Zhu YY, Chen F. [A novel tumor vascular metastasis pathway and its imaging research progress in hepatocellular carcinoma: vessels encapsulating tumor clusters]. Chinese J Radiol. 2023;57:101-105. [DOI] [Full Text] |
| 15. | Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology. 2016;278:563-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4541] [Cited by in RCA: 6044] [Article Influence: 604.4] [Reference Citation Analysis (7)] |
| 16. | Yang L, Yang J, Zhou X, Huang L, Zhao W, Wang T, Zhuang J, Tian J. Development of a radiomics nomogram based on the 2D and 3D CT features to predict the survival of non-small cell lung cancer patients. Eur Radiol. 2019;29:2196-2206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 17. | Wu M, Tan H, Gao F, Hai J, Ning P, Chen J, Zhu S, Wang M, Dou S, Shi D. Predicting the grade of hepatocellular carcinoma based on non-contrast-enhanced MRI radiomics signature. Eur Radiol. 2019;29:2802-2811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (1)] |
| 18. | Wu J, Liu A, Cui J, Chen A, Song Q, Xie L. Radiomics-based classification of hepatocellular carcinoma and hepatic haemangioma on precontrast magnetic resonance images. BMC Med Imaging. 2019;19:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 19. | Shan QY, Hu HT, Feng ST, Peng ZP, Chen SL, Zhou Q, Li X, Xie XY, Lu MD, Wang W, Kuang M. CT-based peritumoral radiomics signatures to predict early recurrence in hepatocellular carcinoma after curative tumor resection or ablation. Cancer Imaging. 2019;19:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 142] [Article Influence: 20.3] [Reference Citation Analysis (1)] |
| 20. | Yu Y, Fan Y, Wang X, Zhu M, Hu M, Shi C, Hu C. Gd-EOB-DTPA-enhanced MRI radiomics to predict vessels encapsulating tumor clusters (VETC) and patient prognosis in hepatocellular carcinoma. Eur Radiol. 2022;32:959-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 21. | Dong X, Yang J, Zhang B, Li Y, Wang G, Chen J, Wei Y, Zhang H, Chen Q, Jin S, Wang L, He H, Gan M, Ji W. Deep Learning Radiomics Model of Dynamic Contrast‐Enhanced MRI for Evaluating Vessels Encapsulating Tumor Clusters and Prognosis in Hepatocellular Carcinoma. JMRI. 2023;59:108-119. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 22. | Cong WM, Bu H, Chen J, Dong H, Zhu YY, Feng LH; Guideline Committee. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol. 2016;22:9279-9287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 185] [Cited by in RCA: 312] [Article Influence: 31.2] [Reference Citation Analysis (1)] |
| 23. | Fan Y, Yu Y, Wang X, Hu M, Du M, Guo L, Sun S, Hu C. Texture Analysis Based on Gd-EOB-DTPA-Enhanced MRI for Identifying Vessels Encapsulating Tumor Clusters (VETC)-Positive Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2021;8:349-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Xia TY, Zhou ZH, Meng XP, Zha JH, Yu Q, Wang WL, Song Y, Wang YC, Tang TY, Xu J, Zhang T, Long XY, Liang Y, Xiao WB, Ju SH. Predicting Microvascular Invasion in Hepatocellular Carcinoma Using CT-based Radiomics Model. Radiology. 2023;307:e222729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 158] [Reference Citation Analysis (1)] |
| 25. | Zwanenburg A, Vallières M, Abdalah MA, Aerts HJWL, Andrearczyk V, Apte A, Ashrafinia S, Bakas S, Beukinga RJ, Boellaard R, Bogowicz M, Boldrini L, Buvat I, Cook GJR, Davatzikos C, Depeursinge A, Desseroit MC, Dinapoli N, Dinh CV, Echegaray S, El Naqa I, Fedorov AY, Gatta R, Gillies RJ, Goh V, Götz M, Guckenberger M, Ha SM, Hatt M, Isensee F, Lambin P, Leger S, Leijenaar RTH, Lenkowicz J, Lippert F, Losnegård A, Maier-Hein KH, Morin O, Müller H, Napel S, Nioche C, Orlhac F, Pati S, Pfaehler EAG, Rahmim A, Rao AUK, Scherer J, Siddique MM, Sijtsema NM, Socarras Fernandez J, Spezi E, Steenbakkers RJHM, Tanadini-Lang S, Thorwarth D, Troost EGC, Upadhaya T, Valentini V, van Dijk LV, van Griethuysen J, van Velden FHP, Whybra P, Richter C, Löck S. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology. 2020;295:328-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2090] [Cited by in RCA: 2689] [Article Influence: 448.2] [Reference Citation Analysis (0)] |
| 26. | Ding T, Xu J, Zhang Y, Guo RP, Wu WC, Zhang SD, Qian CN, Zheng L. Endothelium-coated tumor clusters are associated with poor prognosis and micrometastasis of hepatocellular carcinoma after resection. Cancer. 2011;117:4878-4889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Feng Z, Li H, Zhao H, Jiang Y, Liu Q, Chen Q, Wang W, Rong P. Preoperative CT for Characterization of Aggressive Macrotrabecular-Massive Subtype and Vessels That Encapsulate Tumor Clusters Pattern in Hepatocellular Carcinoma. Radiology. 2021;300:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 28. | Kim KR, Moon HE, Kim KW. Hypoxia-induced angiogenesis in human hepatocellular carcinoma. J Mol Med (Berl). 2002;80:703-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Villa E, Critelli R, Lei B, Marzocchi G, Cammà C, Giannelli G, Pontisso P, Cabibbo G, Enea M, Colopi S, Caporali C, Pollicino T, Milosa F, Karampatou A, Todesca P, Bertolini E, Maccio L, Martinez-Chantar ML, Turola E, Del Buono M, De Maria N, Ballestri S, Schepis F, Loria P, Enrico Gerunda G, Losi L, Cillo U. Neoangiogenesis-related genes are hallmarks of fast-growing hepatocellular carcinomas and worst survival. Results from a prospective study. Gut. 2016;65:861-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 212] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 30. | Dou TH, Coroller TP, van Griethuysen JJM, Mak RH, Aerts HJWL. Peritumoral radiomics features predict distant metastasis in locally advanced NSCLC. PLoS One. 2018;13:e0206108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 31. | Feng ST, Jia Y, Liao B, Huang B, Zhou Q, Li X, Wei K, Chen L, Li B, Wang W, Chen S, He X, Wang H, Peng S, Chen ZB, Tang M, Chen Z, Hou Y, Peng Z, Kuang M. Preoperative prediction of microvascular invasion in hepatocellular cancer: a radiomics model using Gd-EOB-DTPA-enhanced MRI. Eur Radiol. 2019;29:4648-4659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 160] [Article Influence: 22.9] [Reference Citation Analysis (1)] |
| 32. | Önner H, Abdülrezzak Ü, Tutuş A. Could the skewness and kurtosis texture parameters of lesions obtained from pretreatment Ga-68 DOTA-TATE PET/CT images predict receptor radionuclide therapy response in patients with gastroenteropancreatic neuroendocrine tumors? Nucl Med Commun. 2020;41:1034-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Choi SY, Kim SH, Park CK, Min JH, Lee JE, Choi YH, Lee BR. Imaging Features of Gadoxetic Acid-enhanced and Diffusion-weighted MR Imaging for Identifying Cytokeratin 19-positive Hepatocellular Carcinoma: A Retrospective Observational Study. Radiology. 2018;286:897-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ozdemir HI, Turkey S-Editor: Wang JJ L-Editor: A P-Editor: Zheng XM