Published online Mar 15, 2024. doi: 10.4251/wjgo.v16.i3.833
Peer-review started: October 11, 2023
First decision: December 5, 2023
Revised: December 19, 2023
Accepted: January 24, 2024
Article in press: January 24, 2024
Published online: March 15, 2024
Processing time: 153 Days and 2.1 Hours
Traditional lymph node stage (N stage) has limitations in advanced gastric remnant cancer (GRC) patients; therefore, establishing a new predictive stage is necessary.
To explore the predictive value of positive lymph node ratio (LNR) according to clinicopathological characteristics and prognosis of locally advanced GRC.
Seventy-four patients who underwent radical gastrectomy and lymphadenectomy for locally advanced GRC were retrospectively reviewed. The relationship between LNR and clinicopathological characteristics was analyzed. The survival analysis was performed using Kaplan-Meier survival curves and Cox regression model.
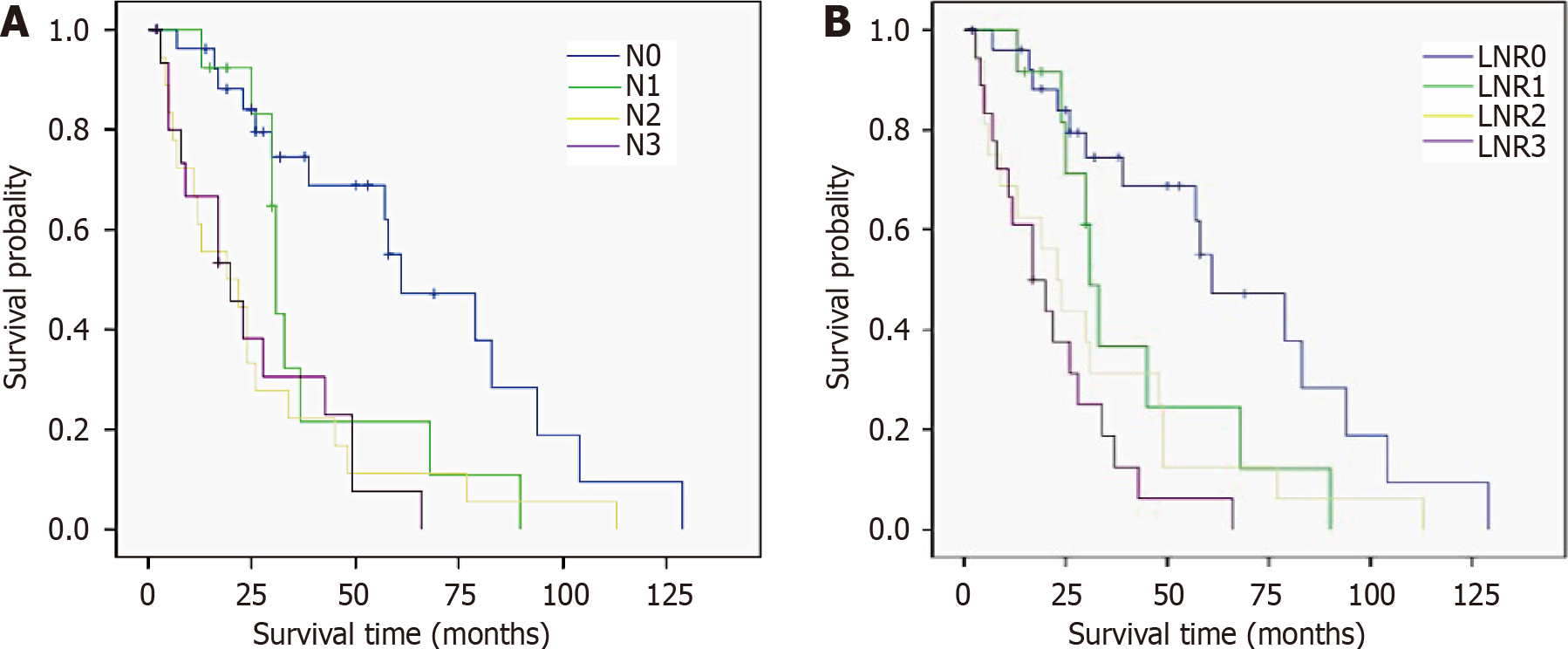
Number of metastatic LNs, tumor diameter, depth of tumor invasion, Borrmann type, serum tumor biomarkers, and tumor-node-metastasis (TNM) stage were correlated with LNR stage and N stage. Univariate analysis revealed that the factors affecting survival included tumor diameter, anemia, serum tumor biomarkers, vascular or neural invasion, combined resection, LNR stage, N stage, and TNM stage (all P < 0.05). The median survival time for those with LNR0, LNR1, LNR2 and LNR3 stage were 61, 31, 23 and 17 mo, respectively, and the differences were significant (P = 0.000). Anemia, tumor biomarkers and LNR stage were independent prognostic factors for survival in multivariable analysis (all P < 0.05).
The new LNR stage is uniquely based on number of metastatic LNs, with significant prognostic value for locally advanced GRC, and could better differentiate overall survival, compared with N stage.
Core Tip: Lymph node (LN) counts of gastric remnant cancer (GRC) patients are often insufficient, and the prognostic ability of traditional LN stage (N stage) is therefore limited. This study investigated the predictive value of LN ratio (LNR) according to clinicopathological characteristics and prognosis of patients with locally advanced GRC. Compared with N stage, the new LNR stage had significant prognostic value for patients with locally advanced GRC, and it could better differentiate overall survival in patients, compared with N stage.
- Citation: Zhuo M, Tian L, Han T, Liu TF, Lin XL, Xiao XY. Predictive value of positive lymph node ratio in patients with locally advanced gastric remnant cancer. World J Gastrointest Oncol 2024; 16(3): 833-843
- URL: https://www.wjgnet.com/1948-5204/full/v16/i3/833.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i3.833
Gastric remnant cancer (GRC) is currently defined as carcinoma in the remnant stomach following partial gastrectomy, regardless of the disease being benign or malignant[1]. According to the Chinese surgeons’ consensus opinion for the definition of gastric stump cancer (2018 edition), GRC is defined as carcinoma arising in the remnant stomach ≥ 5 years after gastrectomy for benign disease, or ≥ 10 years after gastrectomy for gastric cancer. GRC has been reported to represents 1%-3% of all gastric cancers[2-4]. According to the American Joint Committee on Cancer (AJCC) traditional lymph node (LN) stage (N stage) of gastric cancer has been well explored[5-7]. However, the evaluation of N stage in GRC remains uncertain, which is mainly because the number of LNs required to ensure accuracy needs to be at least 15[7,8]. Indeed, surgery for GRC usually fails to retrieve the 15 LNs necessary for the initial operation[5,9].
Since traditional N stage has its limitation in GRC patients, establishing a new predictive stage is necessary. Positive LN ratio (LNR) is defined as the ratio of the number of metastatic LNs to the total number of LNs retrieved. In patients with gastric cancer, LNR might be more appropriate than N stage in predicting clinicopathological characteristics and prognosis[10-12]. However, the value of LNR stage in patients with GRC remains unclear. The purpose of this study was to evaluate the impact of LNR on clinicopathological characteristics and prognosis in patients with GRC.
Patients with insufficient clinical data or no retrieved LNs were excluded from the study. From September 2003 to January 2016, 74 patients that underwent radical gastrectomy and lymphadenectomy for locally advanced GRC at Renji Hospital, were enrolled. Clinicopathological characteristics and overall survival were recorded. This retrospective study was approved by the Ethics Committee of Renji Hospital.
All histopathological information and tumor-node-metastasis (TNM) stages were assessed and confirmed by implementing the AJCC cancer staging manual 8th edition[13]. Patients were divided into two groups based on initial surgery for peptic ulcer (benign disease) or gastric cancer (malignant disease). Histological types were dichotomized into two categories: differentiated (papillary, moderately or well-differentiated carcinoma) and undifferentiated (poorly differentiated adenocarcinoma, signet ring cell carcinoma, and mucinous adenocarcinoma). The lesions were classified into anastomotic, nonanastomotic and total remnant stomach. Serum albumin < 35 g/L was defined as hypoproteinemia. Hemoglobin < 90 g/L was considered to indicate anemia. Serum tumor biomarkers including carcinoembryonic antigen (CEA), carbohydrate antigen (CA)19-9, CA72-4 and CA12-5 were all detected before the surgery.
The best cut-off point of LNR is still controversial[7,14]. LNR stages were categorized according to previous published cut-offs or quartiles. In this study, LNR was classified into four groups according to quartiles: LNR0 0.0, LNR1 0.01-0.20, LNR2 0.21-0.69 and LNR3 0.70-1.0.
Statistical analyses were performed using SPSS version 21.0 (IBM, Chicago, IL, USA). Continuous values were analyzed using independent t tests or one-way analysis of variance. χ2 and Fisher’s exact probability tests were applied for analysis of categorical variables. For survival analysis, univariate analysis was determined by log-rank test and curves were plotted using the Kaplan-Meier method. Multivariate survival analysis was conducted using Cox proportional hazards regression. P < 0.05 was considered statistically significant.
The mean age of the 74 patients was 66.24 ± 9.057 years, and 66 (89.2%) were male. A total of 64 patients (86.5%) underwent initial surgery for benign disease and 10 (13.5%) underwent initial surgery for gastric cancer. Billroth II anastomosis was performed in most patients (73.0%). The mean interval survival time was 29.32 ± 11.970 years, which was significantly longer in patients affected by a previous benign disease than those who suffered from a previous malignant disease (30.66 ± 11.044 years vs 20.80 ± 14.665 years, P = 0.014). GRC was most commonly located at the site of anastomosis (47/74, 63.5%). The baseline characteristics of all patients are shown in Table 1.
| Variables | Values |
| Gender | |
| Male | 66 (89.2) |
| Female | 8 (10.8) |
| Age (yr) (mean ± SD, range) | 66.24 ± 9.057 (41-86) |
| Initial surgery | |
| Benign | 64 (86.5) |
| Malignant | 10 (13.5) |
| Interval (yr) (mean ± SD, range) | 29.32 ± 11.97 (5-55) |
| Reconstruction | |
| Billroth I | 20 (27.0) |
| Billroth II | 54 (73.0) |
| Lesion location | |
| Anastomosis site | 47 (63.5) |
| Non-anastomotic | 22 (29.7) |
| Total remnant stomach | 5 (6.8) |
| Histological types | |
| Differentiated | 22 (29.7) |
| Undifferentiated | 52 (70.3) |
| Borrmann | |
| Borrmann I | 7 (9.4) |
| Borrmann II | 13 (17.6) |
| Borrmann III | 46 (62.2) |
| Borrmann IV | 8 (10.8) |
| Vascular or nerve invasion | |
| Yes | 33 (44.6) |
| No | 41 (55.4) |
| Tumor diameter (cm) (mean ± SD, range) | 5.59 ± 2.61 (0.50-12.00) |
| Combine resection | |
| Yes | 26 (35.1) |
| No | 48 (64.9) |
| Hypoproteinemia | |
| Yes | 15 (20.3) |
| No | 59 (79.7) |
| Anemia | |
| Yes | 24 (32.4) |
| No | 50 (67.6) |
| Serum tumor biomarkers level | |
| Normal | 47 (63.5) |
| Abnormal | 27 (36.5) |
| Depth of tumor invasion | |
| T2/T3 | 13 (17.6) |
| T4 | 61 (82.4) |
| N stage | |
| N0 | 27 (36.5) |
| N1 | 13 (17.6) |
| N2 | 18 (24.3) |
| N3 | 16 (21.6) |
| LNR stage | |
| LNR0 | 27 (36.5) |
| LNR1 | 12 (16.2) |
| LNR2 | 16 (21.6) |
| LNR3 | 19 (25.7) |
| TNM stage | |
| IB-II | 26 (35.1) |
| III | 48 (64.9) |
A total of 836 LNs were dissected in 74 patients, and the mean number was 11 (range 1-33). There were 274 metastatic LNs, and the mean was four (range 0-20). The mean number of retrieved LNs was 11 in the initial benign group and 10 in the initial malignant group (P = 0.607). Patients with < 15 LNs were predominantly located in the initial malignant group (80.0% vs 73.4%, P = 0.659).
The number of patients classified as N0, N1, N2, and N3 was 27, 13, 18 and 16, respectively. There were 27 patients classified as LNR0, 12 as LNR1, 16 as LNR2 and 19 as LNR3. The number of metastatic LNs, number of LNs dissected, tumor diameter, Borrmann type, depth of tumor invasion, serum tumor biomarkers, combined resection and TNM stage were correlated with N stage (Table 2). LNR stage was significantly associated with vascular or neural invasion, number of metastatic LNs, tumor diameter, depth of tumor invasion, serum tumor biomarkers, Borrmann type, and TNM stage (Table 3).
| Variables | N0 | N1 | N2 | N3 | F/χ2 | P value |
| Dissected lymph nodes | 9.85 ± 7.32 | 9.15 ± 5.89 | 9.83 ± 7.56 | 17.13 ± 8.22 | 4.294 | 0.008 |
| Metastatic lymph nodes | 0.00 ± 0.00 | 1.23 ± 0.44 | 4.06 ± 1.16 | 11.56 ± 4.77 | 91.523 | 0.000 |
| Tumor diameter | 3.96 ± 1.67 | 5.92 ± 2.56 | 6.83 ± 2.70 | 6.66 ± 2.64 | 7.363 | 0.000 |
| Borrmann | 17.450 | 0.042 | ||||
| Borrmann I | 3 | 0 | 2 | 2 | ||
| Borrmann II | 9 | 0 | 3 | 1 | ||
| Borrmann III | 15 | 12 | 9 | 10 | ||
| Borrmann IV | 0 | 1 | 4 | 3 | ||
| Depth of tumor invasion | 11.152 | 0.011 | ||||
| T2/T3 | 10 | 1 | 1 | 1 | ||
| T4 | 17 | 12 | 17 | 5 | ||
| Serum tumor biomarkers level | 9.019 | 0.029 | ||||
| Normal | 22 | 9 | 7 | 9 | ||
| Abnormal | 5 | 4 | 11 | 7 | ||
| TNM stage | 54.105 | 0.000 | ||||
| IB-II | 24 | 1 | 1 | 0 | ||
| III | 3 | 12 | 17 | 16 | ||
| Combine resection | 8.917 | 0.030 | ||||
| Yes | 4 | 6 | 10 | 6 | ||
| No | 23 | 7 | 8 | 10 |
| Variables | LNR0 | LNR1 | LNR2 | LNR3 | F/χ2 | P value |
| Metastatic lymph nodes | 0.00 ± 0.00 | 1.75 ± 1.29 | 6.63 ± 5.51 | 7.74 ± 5.12 | 21.263 | 0.000 |
| Tumor diameter | 3.96 ± 1.67 | 5.38 ± 2.05 | 6.66 ± 2.32 | 7.13 ± 3.01 | 8.755 | 0.000 |
| Borrmann | 21.878 | 0.009 | ||||
| Borrmann I | 3 | 0 | 0 | 4 | ||
| Borrmann II | 9 | 1 | 3 | 0 | ||
| Borrmann III | 15 | 10 | 11 | 10 | ||
| Borrmann IV | 0 | 1 | 2 | 5 | ||
| Depth of tumor invasion | 12.470 | 0.006 | ||||
| T2/T3 | 10 | 2 | 0 | 1 | ||
| T4 | 17 | 10 | 16 | 18 | ||
| Serum tumor biomarkers level | 8.654 | 0.034 | ||||
| Normal | 22 | 6 | 11 | 8 | ||
| Abnormal | 5 | 6 | 5 | 11 | ||
| TNM stage | 54.986 | 0.000 | ||||
| IB-II | 24 | 2 | 0 | 0 | ||
| III | 3 | 10 | 16 | 19 | ||
| Vascular or nerve invasion | 8.616 | 0.035 | ||||
| Yes | 8 | 8 | 5 | 2 | ||
| No | 19 | 4 | 11 | 7 |
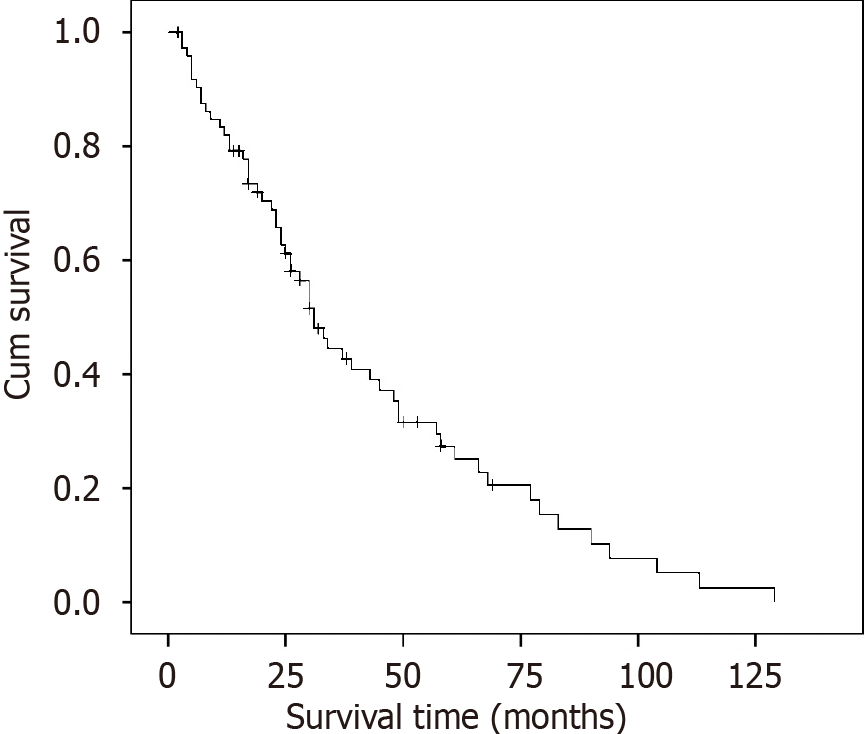
The median follow-up of the entire cohort was 26 mo (range 2-129 mo). The median survival time was 31.0 mo. Overall,
| Variable | n | Median survival time (months) | P value |
| Tumor diameter (cm) | 0.013 | ||
| < 5.5 | 35 | 48.0 | |
| ≥ 5.5 | 39 | 25.0 | |
| Anemia (HGB) < 90 g/L | 0.034 | ||
| Yes | 24 | 23.0 | |
| No | 50 | 37.0 | |
| Serum tumor biomarkers level | 0.014 | ||
| Normal | 47 | 37.0 | |
| Abnormal | 27 | 26.0 | |
| Vascular or nerve invasion | 0.020 | ||
| Yes | 33 | 25.0 | |
| No | 41 | 43.0 | |
| Combined resection | 0.021 | ||
| Yes | 26 | 25.0 | |
| No | 48 | 39.0 | |
| N stage | 0.000 | ||
| N0 | 27 | 61.0 | |
| N1 | 13 | 31.0 | |
| N2 | 18 | 19.0 | |
| N3 | 16 | 20.0 | |
| LNR stage | 0.000 | ||
| LNR0 | 27 | 61.0 | |
| LNR1 | 12 | 31.0 | |
| LNR2 | 16 | 23.0 | |
| LNR3 | 19 | 17.0 | |
| TMN stage | 0.000 | ||
| IB-II | 26 | 79.0 | |
| III | 48 | 25.0 |
| Variable | B | SE | Wald | df | P value | HR | 95%CI |
| LNR stage | 0.499 | 0.122 | 16.755 | 1 | 0.000 | 1.647 | 1.297-2.092 |
| Anemia | 0.656 | 0.287 | 5.216 | 1 | 0.022 | 1.926 | 1.097-3.381 |
| Serum tumor biomarkers level | 0.612 | 0.293 | 4.365 | 1 | 0.037 | 1.844 | 1.039-3.275 |
GRC was first described in 1922 by Balfour[15]. The prevalence of GRC continues to increase because of the long latency period after prior gastric surgery, including that for peptic ulcer or gastric cancer[16]. However, the clinicopathological characteristics and prognosis of GRC, especially the values of LN metastasis or N stage, are still controversial[3,5,7,8]. Our results showed that, compared with N stage, LNR stage was not related to the number of retrieved LNs and, according to the multivariable analysis, it played an independent role in prognosis.
Some studies have shown that the number of dissected LNs was significantly lower in patients with GRC, especially in patients with initial malignant cancer. This aspect was considered to be related to LN dissection during primary gastrectomy[6,17]. In the present study, the mean number of retrieved LNs and the proportion of patients with < 15 was similar to those reported in previous studies[5-7,9]. Although theses values did not differ significantly between the two groups, our series displayed a trend: The patients that underwent initial surgery for benign disease had more retrieved LNs and a lower proportion.
LN metastasis plays an important role in both gastric cancer and GRC. Since N stage seems inaccurate for the evaluation, other studies are suggesting an alternative to N stage, which is dependent on the absolute number of metastatic LNs required for GRC[5,9]. In our study, we found that the new LNR stage is uniquely based on the number of metastatic LNs. Other studies have demonstrated that this new staging system might be more accurate in predicting survival in different cancers, including primary gastric cancer, regardless of the number of retrieved LNs[10,18,19]. Thus, the value of LNR stage in GRC, due to its unique characteristics, is worthy of exploration.
The prognosis of GRC remains controversial. Some studies have reported that GRC shows similar prognosis to primary gastric cancer[20-22], whereas others have argued that the prognosis for GRC is worse[3,9,23]. In our study, we only enrolled patients with locally advanced GRC and 73.9% (67/74) of the patients were stage II or stage III. The survival rate was similar to that in other reports[5,9].
Other studies have confirmed that tumor size, combined resection, N stage, LNR stage and TNM stage were linked to prognosis, while the number of retrieved LNs had no effect[5-7,9]. GRC has higher rates of combine resection and the prognostic value is highly debated. Some studies have demonstrated that this factor has no influence[5,24], but others have reported a worse outcome[7,20]. In contrast, we were unable to demonstrate the predictive value of T stage, which may be due mainly to two factors: (1) we excluded patients with T1 stage; and (2) patients with T2 and T3 stage were combined as a whole cohort. With respect to the influence of primary disease and histological types on prognosis, we concluded that they do not affect survival. Kung et al[16] reported that prognosis was better in patients with initial malignant disease because of the regular follow-up. Son et al[5] reported that previous malignant disease meant poor 5-year survival rate. In addition, histological types were considered not to affect survival as their influence was reported as inconsistent in different studies[6,16].
Despite reports of worse outcomes in patients with vascular or neural invasion, this evidence remains unclear, due to the limited number of cases[16,25,26]. Our study demonstrated that anemia was an independent predictor of GRC. The estimated rate of preoperative anemia was 27%-44% in gastric cancer and predicted poor prognosis[27]. Due to lifelong vitamin B12 deficiency and iron absorption disorders due to gastrectomy[28], anemia may be more common in GRC. The rate of anemia was 55.4% in our cohort, being defined as hemoglobin < 90 g/L. This implies the need to improve nutritional status.
The abnormal rate of tumor biomarker level was 36.5% in our study. A correlation has been commonly observed between serum tumor biomarkers and prognosis and diagnosis of gastric cancer[29-31], but no consensus has been reached. Deng et al[30] reported that high serum tumor biomarker level was possibly a poor prognostic factor. A recent Chinese study with 92 GRC cases indicated that patients with high CEA level had an equivalent prognosis. Few studies have evaluated the association between serum tumor biomarker levels and GRC; therefore, more data are needed to clarify this aspect.
In the present cohort, we tried to demonstrate the superiority of LNR stage for GRC. Some studies have demonstrated that the prognostic ability of a new staging system (using the ratio of the number of metastatic LNs to the number of retrieved LNs) has not improved[5,7]. We confirmed some advantage of LNR stage in predicting median survival time in different groups. As shown in Figure 2, patients with N3 stage had a longer median survival time compared to those with N2 stage, while median survival time decreased with the increase of LNR stage. Moreover, LNR stage was still an independent predictive factor considering the multivariable analysis, but N stage and TNM stage (which is largely related to N stage) were not. Notwithstanding the limited number of cases and the diverse entry criteria, our results suggest that LNR stage has a better prognostic performance in all patients and those with different stages of GRC. This suggests that LNR stage is an ideal and effective staging system for patients with GRC, but whether the same staging system is suitable for all patients is still an open question.
There were several limitations to this study. First, GRC is a rare disease, and 74 cases are not sufficient to identify an optimal staging system. Second, it was a retrospective study conducted in single center. Third, overall survival is most significant in evaluating the prognosis of cancer patients[32,33]. Only the 5-year survival rate and median survival time were assessed, and we did not include disease-free survival. Therefore, it is crucial to perform future studies with large sample sizes in multiple institutions.
This study showed the limitation of traditional N staging. LNR stage was not correlated with the number of LNs dissected and had a better prognostic value. It might be more reliable than N stage in patients with GRC.
Some studies have shown that the number of dissected lymph nodes (LNs) was significantly lower in patients with gastric remnant cancer (GRC). Since traditional LN stage (N stage) seems inaccurate for the evaluation, other studies have suggested an alternative to N stage, which is dependent on the absolute number of metastatic LNs required for GRC.
To explore a superior predictor in surgically treated locally advanced GRC.
To evaluate the impact of LN ratio (LNR) on clinicopathological characteristics and prognosis in patients with GRC.
The relationship between LNR and clinicopathological characteristics was analyzed. The survival analysis was performed using Kaplan-Meier survival curves and Cox regression model.
The 1-, 3- and 5-year overall survival rates were 81.9%, 44.5% and 27.4%, and the median survival time was 31.0 mo. The median survival time for those with LNR0, LNR1, LNR2 and LNR3 stage was 61, 31, 23 and 17 mo, respectively, and the difference was significant. Univariate analysis revealed that the factors affecting survival included tumor diameter, anemia, serum tumor biomarkers, vascular or neural invasion, combined resection, N stage, LNR stage and TNM stage. Anemia, level of serum tumor biomarkers and LNR stage were independent prognostic factors for survival in multivariable analysis.
Compared with N stage, the new LNR stage is uniquely based on the number of metastatic LNs. LNR stage has significant prognostic value for patients with locally advanced GRC, and it could better differentiate overall survival in patients than N stage.
In the future, we will work with other hospitals to increase the number of samples and evaluate whether LNR is better at predicting the need for adjuvant treatment than N stage.
| 1. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1911] [Article Influence: 127.4] [Reference Citation Analysis (0)] |
| 2. | Mezhir JJ, Gonen M, Ammori JB, Strong VE, Brennan MF, Coit DG. Treatment and outcome of patients with gastric remnant cancer after resection for peptic ulcer disease. Ann Surg Oncol. 2011;18:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Mak TK, Guan B, Peng J, Chong TH, Wang C, Huang S, Yang J. Prevalence and characteristics of gastric remnant cancer: A systematic review and meta-analysis. Asian J Surg. 2021;44:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Katai H, Ishikawa T, Akazawa K, Isobe Y, Miyashiro I, Oda I, Tsujitani S, Ono H, Tanabe S, Fukagawa T, Nunobe S, Kakeji Y, Nashimoto A; Registration Committee of the Japanese Gastric Cancer Association. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001-2007). Gastric Cancer. 2018;21:144-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 379] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 5. | Son SY, Kong SH, Ahn HS, Park YS, Ahn SH, Suh YS, Park DJ, Lee HJ, Kim HH, Yang HK. The value of N staging with the positive lymph node ratio, and splenectomy, for remnant gastric cancer: A multicenter retrospective study. J Surg Oncol. 2017;116:884-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Lu J, Zheng ZF, Zhou JF, Xu BB, Zheng CH, Li P, Xie JW, Wang JB, Lin JX, Chen QY, Truty MJ, He QL, Huang CM. A novel prognosis prediction model after completion gastrectomy for remnant gastric cancer: Development and validation using international multicenter databases. Surgery. 2019;166:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Nakagawa M, Choi YY, An JY, Hong JH, Kim JW, Kim HI, Cheong JH, Hyung WJ, Choi SH, Noh SH. Staging for Remnant Gastric Cancer: The Metastatic Lymph Node Ratio vs. the UICC 7th Edition System. Ann Surg Oncol. 2016;23:4322-4331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Deng J, Liang H. Discussion of the applicability of positive lymph node ratio as a proper N-staging for predication the prognosis of gastric cancer after curative surgery plus extended lymphadenectomy. Ann Surg. 2012;256:e35-6; author reply e37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Irino T, Hiki N, Ohashi M, Nunobe S, Tokunaga M, Sano T, Yamaguchi T. Characteristics of gastric stump cancer: A single hospital retrospective analysis of 262 patients. Surgery. 2016;159:1539-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Zeng WJ, Hu WQ, Wang LW, Yan SG, Li JD, Zhao HL, Peng CW, Yang GF, Li Y. Lymph node ratio is a better prognosticator than lymph node status for gastric cancer: A retrospective study of 138 cases. Oncol Lett. 2013;6:1693-1700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Lu J, Zheng ZF, Xie JW, Wang JB, Lin JX, Chen QY, Cao LL, Lin M, Tu RH, Huang CM, Zheng CH, Li P. Is the 8th Edition of the AJCC TNM Staging System Sufficiently Reasonable for All Patients with Noncardia Gastric Cancer? A 12,549-Patient International Database Study. Ann Surg Oncol. 2018;25:2002-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Ilhan E ZB, Simsek H, Canpolat S, Yildirim M. Can the Ratio of Metastatic to Examined Lymph Nodes (N Ratio) be used as an Independent Prognostic Factor in Patients with Gastric Cancer? Is Hypothetical TRM (tumor-ratio-metastasis) Staging System an Alternative to TNM (tumor-node-metastasis) Staging System? PRZ Gastroenterol. 2013;8:247-256. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | In H, Solsky I, Palis B, Langdon-Embry M, Ajani J, Sano T. Validation of the 8th Edition of the AJCC TNM Staging System for Gastric Cancer using the National Cancer Database. Ann Surg Oncol. 2017;24:3683-3691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 271] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 14. | Attaallah W, Uprak K, Gunal O, Yegen C. Prognostic Impact of the Metastatic Lymph Node Ratio on Survival in Gastric Cancer. Indian J Surg Oncol. 2016;7:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Balfour DC. Factors influencing the life expectancy of patients operated on for gastric ulcer. Ann Surg. 1922;76:405-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Kung CY, Fang WL, Wang RF, Liu CA, Li AFY, Wu CW, Shyr YM, Chou SC, Huang KH. Prognosis and clinicopathologic features in patients with gastric stump cancer after curative surgery. Curr Oncol. 2020;27:e259-e264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Iguchi K, Kunisaki C, Sato S, Tanaka Y, Miyamoto H, Kosaka T, Akiyama H, Endo I, Rino Y, Masuda M. Evaluation of Optimal Lymph Node Dissection in Remnant Gastric Cancer Based on Initial Distal Gastrectomy. Anticancer Res. 2018;38:1677-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Tokgoz S, Bugdayci Basal F. The Prognostic Effect of Metastatic Lymph Node Ratio in Operated Gastric Cancer Patients. J Coll Physicians Surg Pak. 2020;30:1035-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Shin S, Kang D, Cho JH, Choi YS, Kim J, Zo JI, Shim YM, Kim HK. Prognostic impact of lymph node ratio in patients with pT1-2N1M0 non-small cell lung cancer. J Thorac Dis. 2020;12:5552-5560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Tran TB, Hatzaras I, Worhunsky DJ, Vitiello GA, Squires MH 3rd, Jin LX, Spolverato G, Votanopoulos KI, Schmidt C, Weber S, Bloomston M, Cho CS, Levine EA, Fields RC, Pawlik TM, Maithel SK, Norton JA, Poultsides GA. Gastric remnant cancer: A distinct entity or simply another proximal gastric cancer? J Surg Oncol. 2015;112:877-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Galata C, Ronellenfitsch U, Weiß C, Blank S, Reißfelder C, Hardt J. Surgery for Gastric Remnant Cancer Results in Similar Overall Survival Rates Compared with Primary Gastric Cancer: A Propensity Score-Matched Analysis. Ann Surg Oncol. 2020;27:4196-4203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Ramos MFKP, Pereira MCM, Oliveira YS, Pereira MA, Barchi LC, Dias AR, Zilberstein B, Ribeiro Junior U, Cecconello I. Surgical results of remnant gastric cancer treatment. Rev Col Bras Cir. 2020;47:e20202703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Tokunaga M, Sano T, Ohyama S, Hiki N, Fukunaga T, Yamada K, Yamaguchi T. Clinicopathological characteristics and survival difference between gastric stump carcinoma and primary upper third gastric cancer. J Gastrointest Surg. 2013;17:313-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Park YE, Kim SW. Clinicopathologic features of remnant gastric cancer after curative distal gastrectomy according to previous reconstruction method: a retrospective cohort study. World J Surg Oncol. 2019;17:203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Chowdappa R, Tiwari AR, Ranganath N, Kumar RV. Is there difference between anastomotic site and remnant stump carcinoma in gastric stump cancers?-a single institute analysis of 90 patients. J Gastrointest Oncol. 2019;10:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Matsuo K, Lee SW, Tanaka R, Imai Y, Honda K, Taniguchi K, Tomiyama H, Uchiyama K. T stage and venous invasion are crucial prognostic factors for long-term survival of patients with remnant gastric cancer: a cohort study. World J Surg Oncol. 2021;19:291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Huang XZ, Yang YC, Chen Y, Wu CC, Lin RF, Wang ZN, Zhang X. Preoperative Anemia or Low Hemoglobin Predicts Poor Prognosis in Gastric Cancer Patients: A Meta-Analysis. Dis Markers. 2019;2019:7606128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 28. | Lim CH, Kim SW, Kim WC, Kim JS, Cho YK, Park JM, Lee IS, Choi MG, Song KY, Jeon HM, Park CH. Anemia after gastrectomy for early gastric cancer: long-term follow-up observational study. World J Gastroenterol. 2012;18:6114-6119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Guo J, Chen S, Li S, Sun X, Li W, Zhou Z, Chen Y, Xu D. A novel classifier based on three preoperative tumor markers predicting the cancer-specific survival of gastric cancer (CEA, CA19-9 and CA72-4). Oncotarget. 2018;9:4814-4822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Deng K, Yang L, Hu B, Wu H, Zhu H, Tang C. The prognostic significance of pretreatment serum CEA levels in gastric cancer: a meta-analysis including 14651 patients. PLoS One. 2015;10:e0124151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 31. | Chen C, Chen Q, Zhao Q, Liu M, Guo J. Value of Combined Detection of Serum CEA, CA72-4, CA19-9, CA15-3 and CA12-5 in the Diagnosis of Gastric Cancer. Ann Clin Lab Sci. 2017;47:260-263. [PubMed] |
| 32. | Zheng ZF, Lu J, Zheng CH, Li P, Xie JW, Wang JB, Lin JX, Chen QY, Lin M, Huang CM. A Novel Prognostic Scoring System Based on Preoperative Sarcopenia Predicts the Long-Term Outcome for Patients After R0 Resection for Gastric Cancer: Experiences of a High-Volume Center. Ann Surg Oncol. 2017;24:1795-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 33. | Zheng ZF, Lu J, Wang W, Desiderio J, Li P, Xie JW, Wang JB, Lin JX, Parisi A, Zhou ZW, Huang CM, Zheng CH. Development and External Validation of a Simplified Nomogram Predicting Individual Survival After R0 Resection for Gastric Cancer: An International, Multicenter Study. Ann Surg Oncol. 2018;25:2383-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ilhan E, Turkey S-Editor: Gong ZM L-Editor: A P-Editor: Zhao S