Published online Mar 15, 2024. doi: 10.4251/wjgo.v16.i3.699
Peer-review started: October 3, 2023
First decision: December 5, 2023
Revised: December 22, 2023
Accepted: January 16, 2024
Article in press: January 16, 2024
Published online: March 15, 2024
Processing time: 160 Days and 17.5 Hours
There is scant literature on hepatocellular carcinoma (HCC) in patients with Budd-Chiari syndrome (BCS).
To assess the magnitude, clinical characteristics, feasibility, and outcomes of treatment in BCS-HCC.
A total of 904 BCS patients from New Delhi, India and 1140 from Mumbai, India were included. The prevalence and incidence of HCC were determined, and among patients with BCS-HCC, the viability and outcomes of interventional therapy were evaluated.
In the New Delhi cohort of 35 BCS-HCC patients, 18 had HCC at index presentation (prevalence 1.99%), and 17 developed HCC over a follow-up of 4601 person-years, [incidence 0.36 (0.22-0.57) per 100 person-years]. BCS-HCC patients were older when compared to patients with BCS alone (P = 0.001) and had a higher proportion of inferior vena cava block, cirrhosis, and long-segment vascular obstruction. The median alpha-fetoprotein level was higher in patients with BCS-HCC at first presentation than those who developed HCC at follow-up (13029 ng/mL vs 500 ng/mL, P = 0.01). Of the 35 BCS-HCC, 26 (74.3%) underwent radiological interventions for BCS, and 22 (62.8%) patients underwent treatment for HCC [transarterial chemoembolization in 18 (81.8%), oral tyrosine kinase inhibitor in 3 (13.6%), and transarterial radioembolization in 1 (4.5%)]. The median survival among patients who underwent interventions for HCC compared with those who did not was 3.5 years vs 3.1 mo (P = 0.0001). In contrast to the New Delhi cohort, the Mumbai cohort of BCS-HCC patients were predominantly males, presented with a more advanced HCC [Barcelona Clinic Liver Cancer C and D], and 2 patients underwent liver trans
HCC is not uncommon in patients with BCS. Radiological interventions and liver transplantation are feasible in select primary BCS-HCC patients and may improve outcomes.
Core Tip: Hepatocellular carcinoma is not uncommon in patients with Budd-Chiari syndrome. It may be the presenting feature or may develop later during illness. Early diagnosis and intervention are the keys to improving outcomes. Strategies for surveillance include serial alpha-fetoprotein and ultrasound assessment every 6 mo with biopsy in cases with high clinical suspicion. Endovascular intervention, which improves liver functions, is usually done prior to therapy for hepatocellular carcinoma. Liver transplantation and surgical resection have curative potential, while locoregional therapy may be offered to a select group with more advanced disease, which improves outcomes in these patients.
- Citation: Agarwal A, Biswas S, Swaroop S, Aggarwal A, Agarwal A, Jain G, Elhence A, Vaidya A, Gupte A, Mohanka R, Kumar R, Mishra AK, Gamanagatti S, Paul SB, Acharya SK, Shukla A, Shalimar. Clinical profile and outcomes of hepatocellular carcinoma in primary Budd-Chiari syndrome. World J Gastrointest Oncol 2024; 16(3): 699-715
- URL: https://www.wjgnet.com/1948-5204/full/v16/i3/699.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i3.699
Budd-Chiari syndrome (BCS) is characterized by obstruction of the hepatic venous outflow tract at any level from hepatic veins (HV) to the junction of the inferior vena cava (IVC) with the right atrium[1]. Prior to the turn of the century, hepatocellular carcinoma (HCC) was considered a rare event in the natural history of BCS[2]. The incidence of HCC in BCS patients varies between 2.0% to 51.6%[3]. Chronic vascular injury (hepatic congestion) has been postulated to contribute to the development of fibrosis and cirrhosis in BCS, resulting in dysplastic nodules and HCC. However, there remains uncertainty regarding the risk factors for HCC in BCS. Some studies have identified factors that might increase the risk of HCC in these patients, including male sex, cirrhosis, prolonged ischemia time, long segment IVC block, combined HV and IVC block, and duration of the BCS itself[4-9].
Multiple therapeutic options may be utilized to manage HCC, and the choice depends upon the stage of disease and the expertise of the treating center[10]. In a study by Gwon et al[11], 20 patients with HCC, who had membranous obstruction of IVC, underwent transarterial chemoembolization (TACE). The 3-year and 5-year survival rates were 61% and 46%, respectively, comparable to other etiologies of HCC. In another study by Liu et al[6], 14 patients underwent TACE, and 9 patients also had angioplasty for BCS. However, the chronology of angioplasty and intervention for HCC was not consistent across all the cases. It was performed with the first session of TACE in 5 patients and with the second session of TACE in the remaining 4 patients.
There is a paucity of data regarding the safety and efficacy of radiological interventions for HCC and BCS in patients with BCS-HCC. Also, it is unknown whether revascularization procedures can reduce the occurrence of new HCC and improve the results of locoregional therapies for HCC. Multifocal disease and new HCC lesions over time further add to the complexity of management options. Therefore, in this study, we described the clinical presentations, radiological interventions employed for management, and the clinical outcomes of BCS-HCC patients.
All consecutive patients diagnosed with primary BCS-HCC between January 1987 and January 2023 at the All-India Institute of Medical Sciences, New Delhi, India were assessed for inclusion for this retrospective analysis from a prospectively maintained database. Patients with secondary BCS, insufficient baseline data, and inaccessible follow-up data were excluded. In addition, patients with other etiologies for HCC were also excluded. The institute ethics committee approved the study (IEC/NP-458/12.12.2014, RP-22/2015). Written informed consent was waived (de-identified data). We also included BCS-HCC patients diagnosed at King Edward Memorial Hospital, Mumbai, Maharashtra, India.
BCS: Diagnosis of BCS was made when the IVC and/or HV (two out of three major HV) showed thrombosis/stenosis on either ultrasound doppler/computed tomography (CT)/magnetic resonance venogram. The site of the venous block was based on the available imaging and categorized as either HV/IVC alone or combined. The obstruction of the vein was classified as either short segment (< 3 cm) or long segment (≥ 3 cm)[9].
Cirrhosis: Diagnosed based on a combination of clinical, biochemical, and imaging findings, endoscopy, or liver biopsy[12].
HCC: Standard diagnostic criteria accepted at the period were used. Prior to 2001, HCC was diagnosed based on either fine needle biopsy or by demonstrating a liver lesion enhancing on the arterial phase of a CT scan together with raised serum alpha-fetoprotein alpha-fetoprotein (AFP) ≥ 300 ng/mL. Subsequently, the European Association for the Study of the Liver guidelines were followed[13,14]. The European Association for the Study of the Liver guidelines for the diagnosis of HCC recommend considering HCC as every nodule > 1 cm with arterial phase hyperenhancement and washout on the portal venous phase on one of the two dynamic imaging techniques [CT scan or magnetic resonance imaging (MRI)] and to biopsy a nodule > 1 cm when the imaging is inconclusive.
HCC at presentation: When HCC was diagnosed at index presentation to our clinic in a previously unevaluated case or within 6 mo of diagnosis of BCS.
HCC at follow-up: It was defined as patient(s) who developed HCC after at least 6 mo after the diagnosis of BCS.
Angiographic interventions: We have previously published our protocol for BCS management[15,16]. Our institutional policy is to offer the patients radiologic interventions upfront for decompression of hepatic vasculature, followed by management of HCC. In patients with focal obstruction of the IVC and/or HV, revascularization of the occluded segment was performed by angioplasty using balloon dilatation attempted via the transjugular or transfemoral route. Stenting of these focal obstructions was performed if there was residual stenosis or persistence of collaterals after balloon angioplasty. Patients with combined IVC and HV occlusion underwent angioplasty/stenting of both segments. Transjugular intrahepatic portosystemic shunt (TIPSS) was performed in patients with long-segment/diffuse involvement of all three HVs.
Anticoagulation protocol: Patients subjected to radiological interventions for BCS were anticoagulated initially with heparin infusion overlapping with oral anticoagulants (vitamin K antagonists). The dose of oral anticoagulants was titrated to maintain the international normalized ratio between 2.0 and 2.5. The underlying hypercoagulable state was managed in consultation with hematologists.
The management plan was decided by a multidisciplinary team, including a hepatologist, interventional radiologist, and liver transplant surgeon. Staging and treatment allocation in each HCC patient was completed per the Barcelona Clinic Liver Cancer (BCLC) staging classification, wherever applicable[10,17]. Response to treatment of HCC was assessed by the modified response evaluation criteria in solid tumors (mRECIST) on multiphasic CT/MRI[18]. For patients diagnosed with HCC prior to the publication of the BCLC and mRECIST criteria, their data was retrospectively analyzed to identify which BCLC class they belonged to as well as the radiological response after therapy. Subsequent radiological interventions were decided according to the clinical and radiological response. Surveillance with AFP in patients with HCC has been recommended by the expert guidelines. Our cohort dates to 1985 (19 years prior to the randomized controlled trial recommending surveillance), and hence the routine implementation of AFP or surveillance was not available for all patients. This may also have a potential impact on the survival estimates provided and is a limitation of the retrospective nature of our data.
A Doppler ultrasound was conducted on the day after the procedure in each case and repeated 3 mo thereafter or when required (clinical/biochemical worsening) to assess for restenosis. In cases where the patency of the veins could not be confidently ascertained by Doppler or an intrahepatic mass was suspected, a multiphasic CT/MRI was done.
Stent block was defined when the stent in IVC/HV showed thrombosis/stenosis. Time to restenosis was calculated by calculating the number of days between the diagnosis of the stent block and the initial intervention for BCS.
Total follow-up days were determined by calculating the number of days between the date of the first outpatient visit and the date of death or the last follow-up. Patients who were lost to follow-up were contacted by telephone, and the follow-up was updated; otherwise, the date of the last outpatient visit was considered as the last follow-up.
All clinical records, including the details of BCS, HCC, and management were retrieved from the electronic database. Data about clinical presentation and the following parameters were collected: hematological/biochemical tests (complete blood count, liver function tests, and kidney function tests) and serum AFP. Details of viral serology, including hepatitis B surface antigen, anti-hepatitis C virus (HCV) antibodies, tests for Wilson’s disease, autoimmune markers, and serum ferritin were noted.
Continuous data were expressed as mean, standard deviation for non-skewed and median (interquartile range) for skewed data and were compared among groups using Student’s t-test/Mann-Whitney U test, as appropriate. Categorical data were expressed as proportions and were compared using the χ2 test/Fisher’s exact test, as appropriate. Kaplan-Meir survival curve analysis was used to calculate the median survival of HCC among BCS patients in this study by using the available follow-up of each patient and compared using the log-rank test. A P < 0.05 was statistically significant. The data was analyzed using the IBM SPSS Statistics software (version 25.0, Chicago, IL, United States) and MedCalc Software (version 15.11.4, MedCalc Software, Ostend, Belgium).
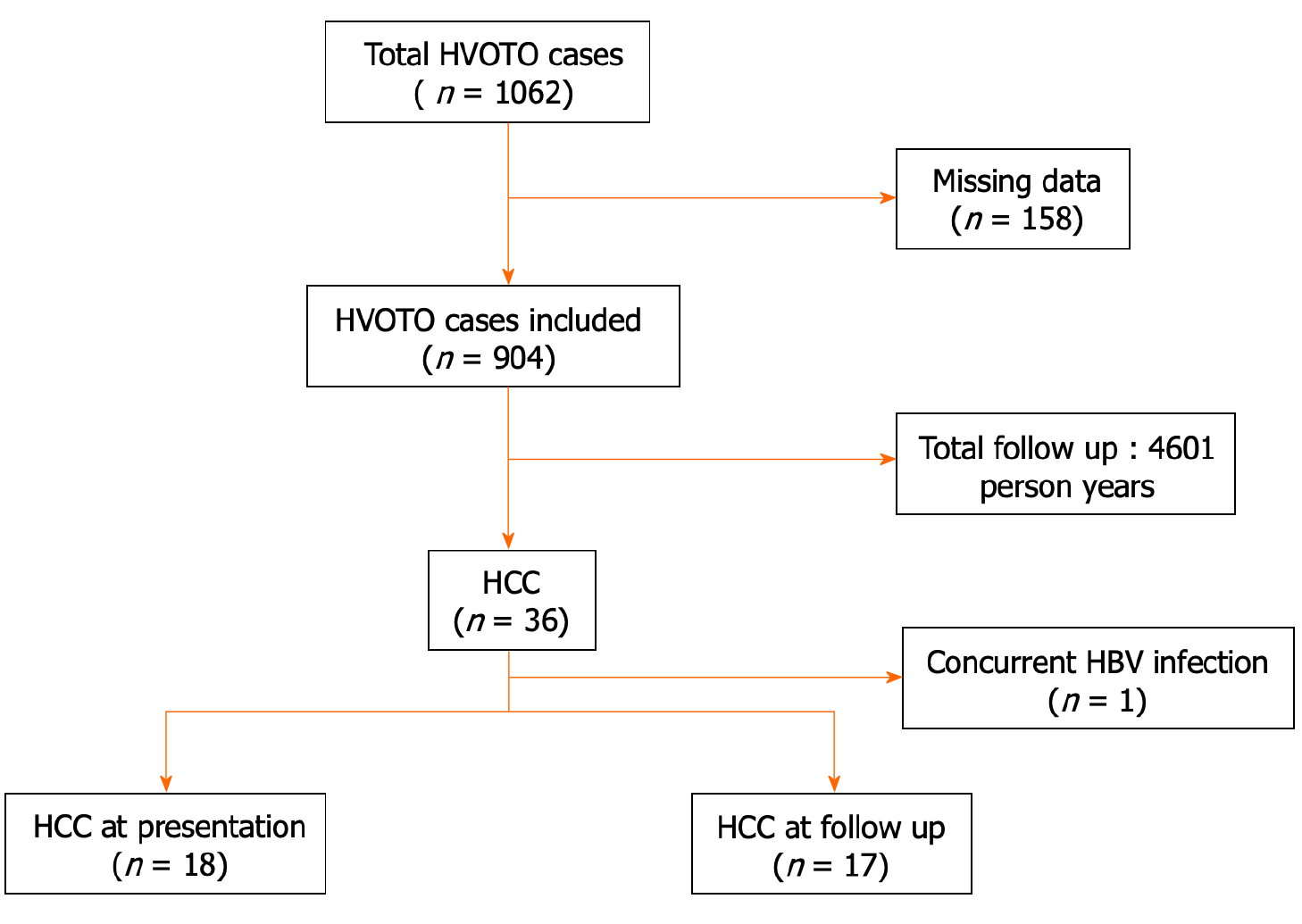
Of the total 1062 BCS patients initially evaluated, 904 were included in the final analysis after exclusion of 158 patients with missing data. Overall, 35 out of 904 (3.8%) had primary BCS-HCC (Figure 1). Of the 35 BCS-HCC patients, 19 (54.3%) were females. The proportions of patients in Child Turcotte Pugh (CTP) classes A, B, and C were 21 (60.0%), 11 (31.4%), and 3 (8.5%), respectively. Complete etiological evaluation for hypercoagulable states was available in 11 patients with BCS-HCC. However, none of the markers were found to be positive.
Of the 35 HCC patients, 18 had HCC at presentation [prevalence 18/904 (1.9%)] and 17 developed HCC (among 886) over a follow-up of 4601 person-years with an incidence of 0.36 (0.22-0.57) per 100 person-years. The cumulative incidence of HCC during the follow-up period was assessed after the exclusion of the 18 patients who had HCC at presentation.
BCS-HCC patients (n = 35), when compared to those with BCS alone (n = 869), were older (median age 32 years vs 26 years, P = 0.001) and had a higher prevalence of IVC block [7/35 (20.0%) vs 50/869 (5.7%), P = 0.02], cirrhosis [35/35 (100%) vs 716/869 (82.4%), P = 0.006], and long-segment vascular obstruction [27/35 (77.1%) vs 429/869 (49.4%), P = 0.001] (Table 1).
| Characteristic | Overall population, n = 904 | BCS w/o HCC, n = 869 | BCS-HCC, n = 35 | P value |
| Age in yr at first presentation | 26 (21-33) | 26 (20-33) | 32 (25-40) | 0.001 |
| Duration of symptoms in month | 12 (4-36) | 12 (4-36) | 3 (2-24) | < 0.001 |
| Male sex | 496 (54.87) | 480 (55.24) | 16 (45.71) | 0.270 |
| Site of block | ||||
| Inferior vena cava | 57 (6.31) | 50 (5.75) | 7 (20.00) | 0.020 |
| Hepatic vein | 398 (44.03) | 388 (44.65) | 10 (28.57) | |
| Combined inferior vena cava and hepatic vein | 449 (49.67) | 431 (49.60) | 18 (51.43) | |
| Type of block, BCS | ||||
| Short segment | 448 (49.56) | 440 (50.63) | 8 (22.86) | 0.001 |
| Long segment | 456 (50.44) | 429 (49.37) | 27 (77.14) | |
| Child class | ||||
| A | 420 (46.46) | 399 (45.91) | 21 (60.00) | 0.050 |
| B | 260 (28.76) | 249 (28.65) | 10 (28.57) | |
| C | 71 (7.85) | 68 (7.83) | 4 (11.40) | |
| Cirrhosis | 751 (83.08) | 716 (82.39) | 35 (100.00) | 0.006 |
| Pain in abdomen | 335 (37.06) | 321 (36.94) | 14 (40.00) | 0.710 |
| Ascites | 679 (75.11) | 658 (75.72) | 21 (60.00) | 0.035 |
| Gastrointestinal bleeding | 195 (21.57) | 189 (21.75) | 6 (17.14) | 0.510 |
| Jaundice | 200 (22.12) | 190 (21.86) | 10 (28.57) | 0.340 |
| Hepatic encephalopathy at presentation | 35 (3.87) | 34 (3.91) | 1 (2.86) | 0.750 |
| Hemoglobin in g/dL | 11.7 (9.9-13.4) | 11.7 (9.9-13.4) | 10.7 (9.2-12.8) | 0.150 |
| Total leucocyte count as /mm3 | 5560 (3850-7500) | 5600 (3880-7550) | 3800 (4600-6900) | 0.310 |
| Platelet count as × 103/mm3 | 151 (110-219) | 152 (110-225) | 130 (75-161) | 0.006 |
| Creatinine in mg/dL | 0.7 (0.6-0.9) | 0.7 (0.6-0.9) | 0.7 (0.6-0.9) | 0.330 |
| Total bilirubin in mg/dL | 1.5 (0.9-2.4) | 1.5 (0.9-2.4) | 1.7 (1.0-2.3) | 0.310 |
| Aspartate transaminase in IU/L | 42 (32-63) | 42 (31-62) | 59 (35-80) | 0.008 |
| Alanine transaminase in IU/L | 31 (22-47) | 31 (22-47) | 46 (27-65) | 0.010 |
| Albumin in g/dL | 3.9 (3.3-4.5) | 3.9 (3.3-4.5) | 4.2 (2.9-4.6) | 0.620 |
| Alkaline phosphatase in IU/L | 250.0 (159-372) | 250.5 (160.0-372.0) | 239.0 (148-314) | 0.820 |
| Alpha-fetoprotein | 3.3 (2.1-9.6) | 2.9 (1.9-4.3) | 1310.0 (237.0-14308.0) | < 0.001 |
| BCS intervention received | 654 (72.34) | 628 (72.26) | 26 (74.28) | 0.189 |
| Inferior vena cava | 317 (35.06) | 302 (34.70) | 15 (42.8) | |
| Hepatic vein | 106 (11.72) | 102 (11.73) | 4 (11.42) | |
| TIPSS | 198 (21.90) | 194 (22.32) | 4 (11.42) | |
| IVC + HV | 28 (3.09) | 26 (2.90) | 2 (5.70) | |
| PSRS | 5 (0.50) | 4 (0.46) | 1 (2.80) | |
| Stent block | 159/654 (24.31) | 151/637 (23.70) | 8/17 (47.05) | 0.063 |
| Time to restenosis in d | 496.0 (181.0-1253.0) | 496.0 (18.0-1198.0) | 692.5 (226.5-1540.0) | 0.550 |
| Follow-up in d | 1249.5 (503.5-2573.0) | 1248.0 (530.0-2513.0) | 1307.0 (158.0-3811.0) | 0.530 |
BCS-HCC patients, compared to those without HCC, had higher aspartate aminotransaminase and alanine aminotransferase levels. The platelet counts were lower among those with BCS-HCC. Ascites was more frequent in patients without HCC than those with HCC (75.7% vs 60.0%, P = 0.035). There were no significant differences in other clinical and biochemical parameters (Table 1).
Of the 906 patients with BCS, 676 (74.6%) patients underwent radiological interventions. Data for stent block was available for 654/676 (96.7%) patients until the last follow-up. A total of 159/654 (24.3%) patients developed stent blocks and required reintervention for BCS. The BCS-HCC group had a higher, though statistically insignificant, proportion of stent block (restenosis) than the BCS alone group (47.05% vs 23.70%, P = 0.063). The median time to restenosis was 692 d in the BCS-HCC group compared to 496 d in the BCS alone group (P = 0.55).
The BCS patients who developed HCC on follow-up were similar to those who presented with HCC (Table 2) in terms of age at onset of BCS, duration of symptoms, sex distribution, site of block, length of the block, presence of cirrhosis, and CTP class (P > 0.05 for all). The median serum AFP levels in the BCS-HCC group were 1310 ng/mL, with higher levels in patients with BCS-HCC at first presentation compared to those who developed HCC during follow-up (13029 ng/mL vs 500 ng/mL, P = 0.01). BCLC (A, B, C, and D) stages in patients developing HCC at follow-up compared to those at first presentation were 1 (5.8%), 10 (58.8%), 2 (11.7%), and 4 (23.5%) vs 4 (22.2%), 7 (38.8%), 7 (38.8%), and 0, respectively (P = 0.029).
| Characteristic | BCS-HCC on follow-up, n = 17 | BCS-HCC at first presentation, n = 18 | P value |
| Age in yr at onset of BCS | 30.0 (24.0-40.0) | 32.5 (26.0-38.0) | 0.640 |
| Duration of symptoms | 3 (2-18) | 3 (2-24) | 0.880 |
| Sex, males | 9 (52.90) | 7 (38.88) | 0.880 |
| Site of block | |||
| Inferior vena cava | 4 (23.50) | 3 (16.67) | 0.450 |
| Hepatic vein | 4 (23.50) | 6 (33.33) | |
| Combined inferior vena cava and hepatic vein | 9 (52.90) | 9 (50.00) | |
| Type of block, BCS | |||
| Short segment | 4 (23.50) | 4 (22.22) | 0.370 |
| Long segment | 13 (76.40) | 14 (77.78) | |
| Child class | |||
| A | 8 (47.1) | 13 (72.22) | 0.120 |
| B | 5 (29.40) | 5 (27.78) | |
| C | 4 (23.50) | 0 (0) | |
| Pain in abdomen | 7 (41.18) | 7 (38.89) | 0.890 |
| Ascites | 11 (64.71) | 10 (55.56) | 0.580 |
| Gastrointestinal bleeding | 2 (11.76) | 4 (22.22) | 0.660 |
| Hepatic encephalopathy at presentation | 1 (5.88) | 0 (0) | 0.490 |
| Hemoglobin in g/dL | 10.40 (8.50-11.50) | 12.10 (9.75-13.90) | 0.110 |
| Total leucocyte count in mm3 | 4300 (3500-6400) | 4735 (4050-6950) | 0.610 |
| Platelet count as × 103/mm3 | 101.0 (71.0-156.0) | 150.0 (113.0-166.5) | 0.210 |
| Creatinine in mg/dL | 0.7 (0.5-0.9) | 0.7 (0.6-0.9) | 0.550 |
| Total bilirubin in mg/dL | 1.8 (1.5-3.2) | 1.5 (0.8-2.1) | 0.160 |
| Aspartate transaminase in IU/L | 66.0 (46.0-120.0) | 51.5 (35.0-76.0) | 0.280 |
| Alanine transaminase in IU/L | 46.0 (27.0-59.0) | 42.5 (28.0-66.0) | 0.890 |
| Albumin in g/dL | 4.0 (2.9-4.7) | 4.3 (3.3-4.5) | 0.530 |
| Alkaline phosphatase in IU/L | 274.0 (178.0-394.0) | 210.5 (143.0-300.0) | 0.320 |
| Alpha-fetoprotein in ng/mL | 500.0 (66.6-1320.0) | 13029.0 (500.0-17943.0) | 0.010 |
| Intervention received | 14 (82.35) | 12 (66.67) | 0.170 |
| Inferior vena cava angioplasty/stenting | 9 (52.90) | 6 (33.33) | |
| Hepatic vein angioplasty/stenting | 2 (11.70) | 2 (11.11) | |
| IVC + HV angioplasty/stenting | 0 | 2 (11.11) | |
| TIPSS | 2 (11.70) | 2 (11.11) | |
| PSRS | 1 (5.80) | 0 | |
| Barcelona Clinic Liver Cancer staging | 0.029 | ||
| A | 1 (5.80) | 4 (22.20) | |
| B | 10 (58.80) | 7 (38.80) | |
| C | 2 (11.70) | 7 (38.80) | |
| D | 4 (23.50) | 0 |
Of the 35 BCS-HCC patients, 26 (74.3%) underwent radiological interventions for BCS, and 22 (62.8%) patients underwent treatment for HCC (Table 2). Twelve (34.2%) BCS-HCC patients were not treated for HCC for various reasons including advanced stage of disease (n = 7), and refusal for treatment (n = 5). One patient is currently under evaluation and awaiting multidisciplinary discussion.
The initial interventions for HCC included TACE in 18/35 (51.4%) patients, oral tyrosine kinase inhibitor (TKI) in 3 (8.5%) patients and transarterial radioembolization (TARE) in 1 (2.8%) patient. The maximum number of interventions a patient required for HCC was five in 1 patient, with a median of one (range 1-5) per patient. mRECIST was assessed for patients who underwent interventions (TACE and TARE). The response at 1 mo based on mRECIST criteria was available in 15/19 (78.9%) cases. Of these 15 patients, 5 (33.3%) patients showed complete response, 6 (40.0%) patients showed partial response, and 4 (26.7%) patients had progressive disease. One patient died within 1 mo of TACE.
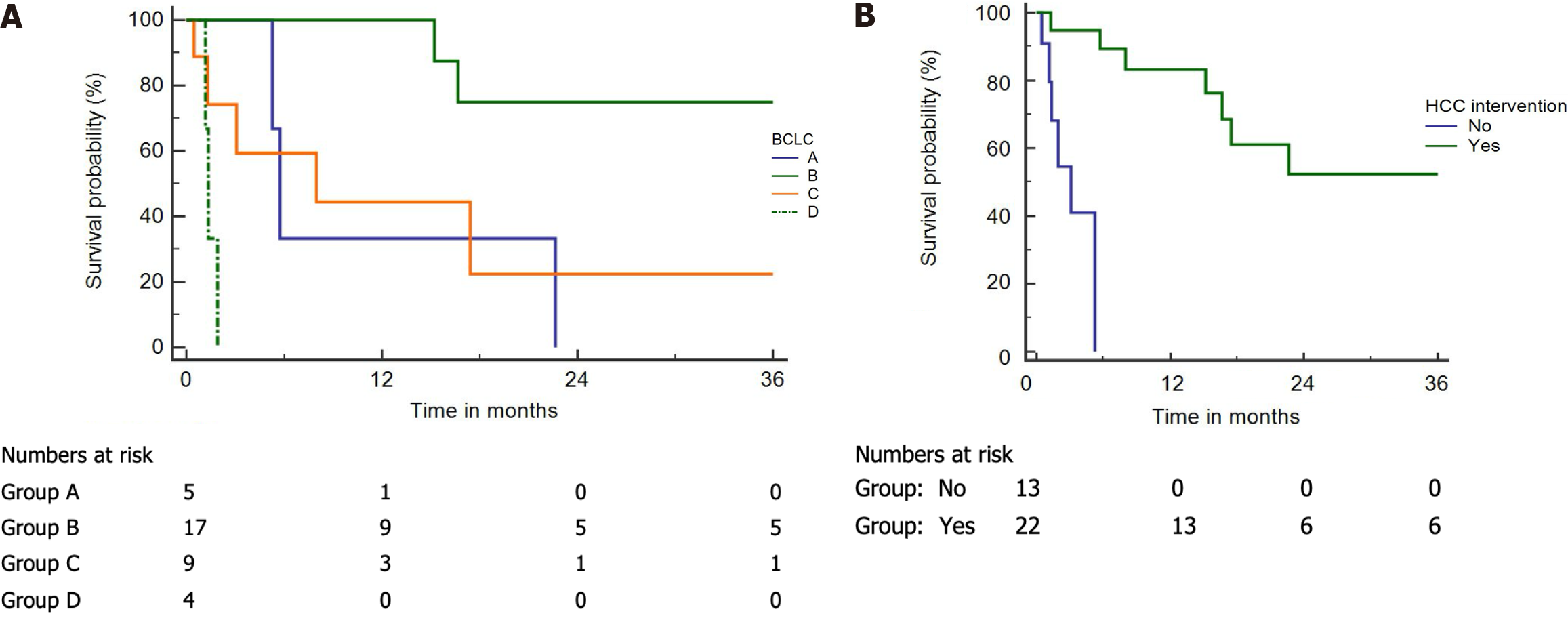
Median survival in those who underwent interventions for HCC was 3.5 years (Figure 2A). The median survival in patients as per BCLC stages A, B, C, and D was 172 d, 1352 d, 240 d, and 40 d, respectively (Figure 2B).
Two-thirds (12/18) of patients underwent endovascular intervention for BCS. Among them, 6 (33.3%) patients underwent IVC angioplasty, 2 (11.1%) patients each underwent HV angioplasty, combined IVC and HV angioplasty, and TIPSS (Table 3). The remaining 6 patients refused treatment. None of the patients had restenosis/stent block after the initial intervention for BCS.
| Age | Sex | Site of obstruction | Length of block | BCS management | AFP | BCLC | CTP | HCC initial management | HCC response | Survival after HCC diagnosis in d | No. of lesions/size in cm | Reinterventions for HCC |
| 35 | M | IVC + HV | Long | IVC + HV angioplasty | 115 | A | A | TACE | PR | 680 | 1 (10 cm × 10 cm) | 1, TACE |
| 38 | F | HV | Long | HV angioplasty | 239 | B | A | TACE | PR | 1656 | 2.0 (5.5 cm × 5.5 cm) | 4, TACE, followed by TKI |
| 37 | M | IVC | Short | IVC angioplasty | 17943 | C (lung metastasis) | A | TACE | NA | 39 | 1 (> 10 cm) | - |
| 30 | F | IVC | Short | IVC angioplasty | 11430 | B | A | TACE | PR | 1280 | 2 (5.2 cm × 5.4 cm) | - |
| 17 | F | IVC + HV | Long | TIPSS | 34930 | B | A | TACE | PR | 500 | 4 (4 cm × 5 cm) | 2, TACE |
| 38 | F | HV | Long | Medical management | 5905 | C (PVT) | B | SC | NA | 93 | NA | - |
| 52 | M | HV | Long | Medical management | 237 | A | A | SC | NA | 158 | 1.0 (1.6 cm × 1.8 cm) | - |
| 22 | F | IVC + HV | Long | Medical management | 56804 | C (PVT) | A | SC | NA | 14 | 1 (11 cm × 11 cm) | - |
| 48 | M | HV | Long | Medical management | NA | A | A | SC | NA | 51 | 1 (2.5 cm × 3.0 cm) | - |
| 26 | M | IVC + HV | Long | IVC angioplasty | 15314 | A | A | TACE | PD | 172 | 1 (2.5 cm × 3.5 cm) | TKI |
| 30 | F | IVC + HV | Long | Medical management | 31068 | C (PVT) | B | TKI | NA | 211 | Infiltrative | - |
| 63 | M | IVC | Short | IVC angioplasty | 20312 | C (Rt. PVT) | A | TARE | NA | 4881 | 3 (> 5 cm) | - |
| 32 | F | IVC + HV | Long | IVC angioplasty | 3153 | B | A | TACE | CR | 2291 | Multiple (2.9 cm × 2.8 cm) | 1, TACE |
| 32 | M | IVC + HV | Long | IVC angioplasty | 13211 | C (Lt. PVT) | A | TACE | PD | 240 | 3.0 (12.0 cm × 5.6 cm) | - |
| 25 | F | HV | Short | HV angioplasty | 16.6 | B | B | TACE | NA | 4021 | 2.0 (5.0 cm × 3.3 cm) | - |
| 29 | F | IVC + HV | Long | IVC + HV angioplasty | 13029 | C (PVT) | B | TKI | NA | 523 | 1.0 (9.5 cm × 7.1 cm) | - |
| 21 | F | HV | Long | TIPSS | 500 | B | A | SC | NA | 91 | 2 (6 cm × 8 cm) | - |
| 33 | F | IVC + HV | Long | Medical management | 14308 | B | B | SC | NA | 971 | 2.0 (6.9 cm × 5.4 cm) | - |
All 12 who underwent BCS intervention also received therapy for HCC. Of these, 9 (75.0%) patients underwent TACE, 2 (16.6%) received oral TKIs, and 1 patient underwent TARE. Repeat sessions of locoregional therapy for HCC were done in 4 patients The response at 1 mo based on mRECIST criteria was available for 7/10 (70%) patients. A partial response was seen in 4 (40%) patients, complete response in 1 patient, and progressive disease in 2 patients (1 patient received TKIs after progression). A total of 7 patients were alive at the last follow-up. The median duration of follow-up in those who underwent interventions was 201 d (39-500). The details of the patients are shown in Table 3.
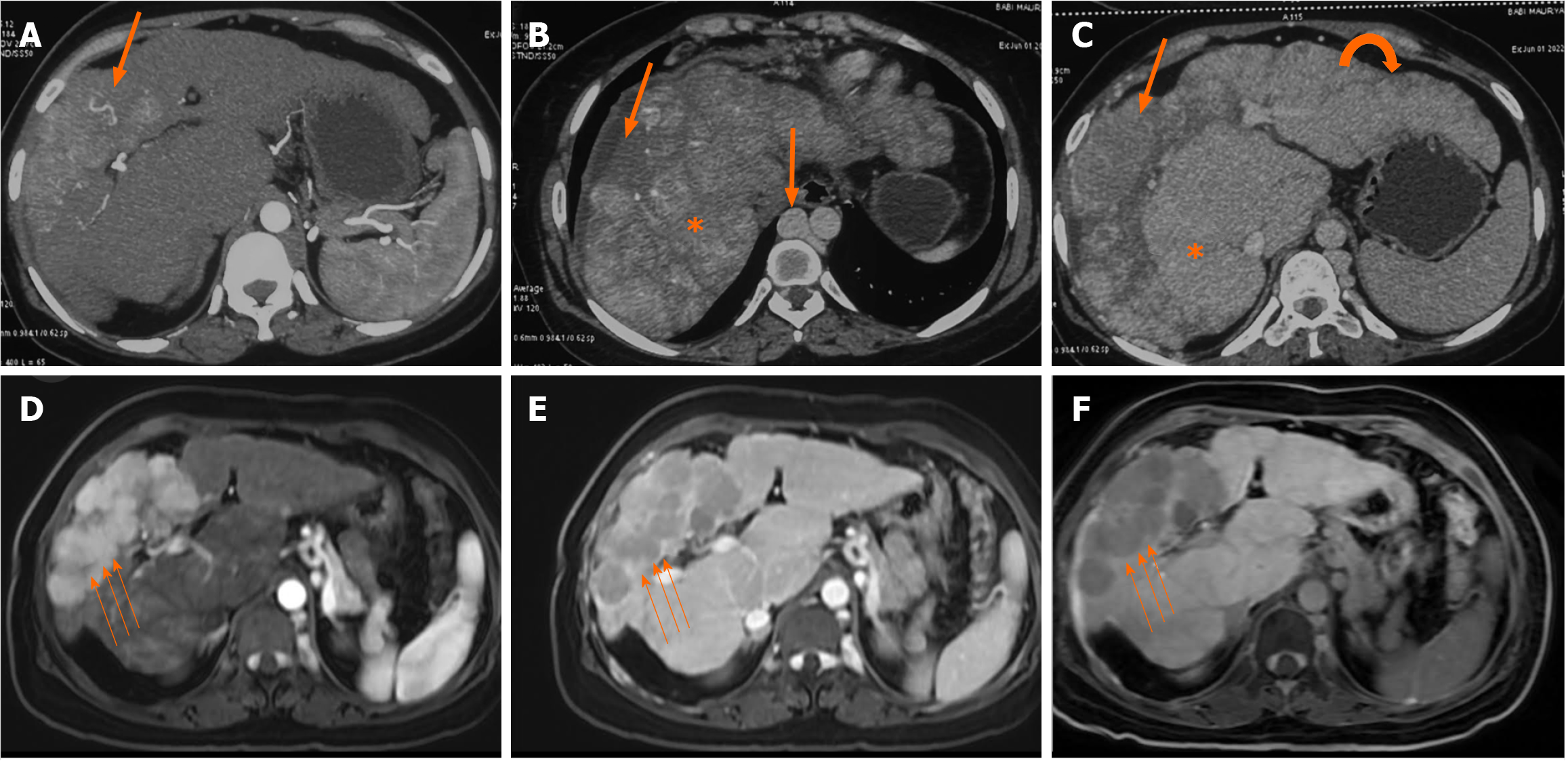
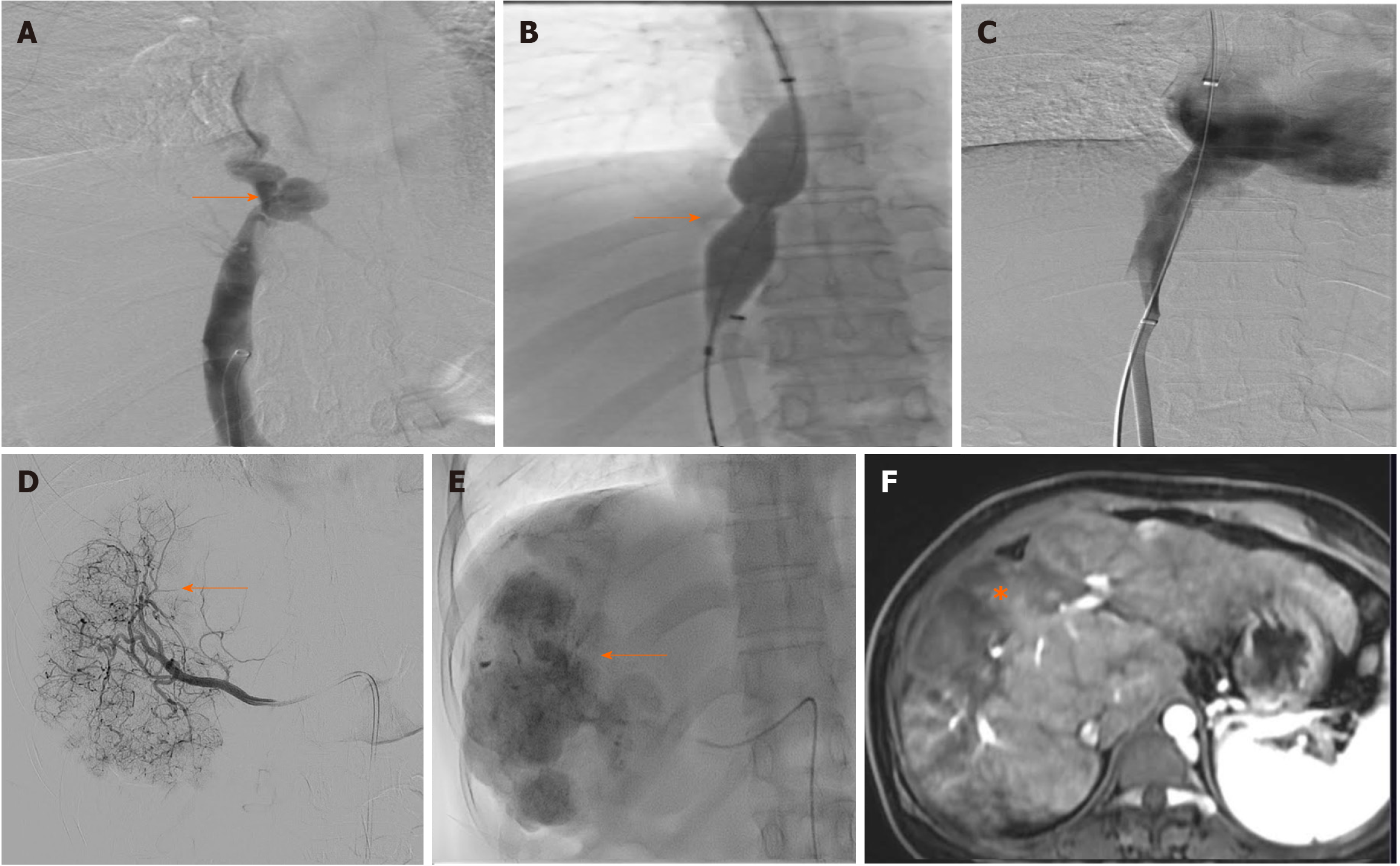
A representative image of a patient with BCS-HCC pretreatment (Figure 3) and post-treatment (Figure 4) highlights the imaging findings and management of both BCS and HCC.
A total of 14/17 (82.3%) patients had undergone intervention for BCS. Nine (52.9%) patients had IVC angioplasty, 2 (11.7%) patients each underwent HV angioplasty and TIPSS, and 1 patient underwent a surgical proximal splenorenal shunt. Of the remaining 3 patients, 2 (11.7%) patients chose anticoagulation alone, and 1 patient denied treatment. Eight (47.05%) patients had restenosis/stent blocks after the initial intervention for the BCS and required reintervention.
Ten out of seventeen (58.8%) patients received treatment for HCC. Nine out of ten (90.0%) patients underwent TACE, and 1 (10%) patient received oral TKIs. Of the 7 patients who did not receive HCC treatment, 4 had BCLC-D disease, and 3 refused treatment. Re-interventions for HCC were done in 3 patients. The response at 1 mo based on mRECIST criteria was available for 8/9 patients who underwent radiological interventions. There was a complete response in 4 patients and partial response and progressive disease in 2 patients each. A total of 12 patients were alive at the last follow-up. The details of the patients are shown in Table 4.
| Age | Sex | Site of obstruction | Length of block | BCS intervention | BCS: Restenosis | BCS: Time between intervention and stent block in d | No. of reinterventions for BCS | Time between BCS and HCC diagnosis in d | AFP | BCLC | CTP | Initial HCC management | HCC response | Survival after diagnosis of HCC until last follow-up in d | No. of lesion/size | Reintervention for HCC |
| 20 | M | IVC + HV | Long | Medical management | No | 2374 | 1320.0 | B | B | TACE | PD | 456 | 2.0 (3.0 cm × 3.6 cm) | 2 (PAI, TACE) | ||
| 35 | F | IVC | Short | IVC angioplasty | Yes | 1411 | 1 (IVC angioplasty) | 2396 | 8.8 | B | A | TACE | CR | 3061 | 3.0 (2.9 cm × 2.5 cm) | - |
| 40 | M | IVC + HV | Long | Medical management | No | 186 | 8489.0 | B | A | SC | NA | 5731 | 3 (6 cm × 7 cm) | - | ||
| 52 | M | HV | Long | HV angioplasty | Yes | 116 | 1 (RHV stenting) | 2148 | 4.0 | B | B | TACE | CR | 5241 | 2 (9 cm × 5 cm) | - |
| 35 | F | IVC | Long | IVC angioplasty | No | 4273 | 3888.0 | B | A | TACE | PD | 1471 | 7.0 (4.0 cm × 3.9 cm) | - | ||
| 47 | F | IVC + HV | Long | IVC angioplasty | Yes | 169 | 2 (IVC angioplasty, IVC angioplasty) | 788 | 1240.0 | B | A | TACE | PR | 39281 | 3 (7 cm × 7 cm) | 5 (TACE, TACE, PAI, TACE, TACE) |
| 24 | M | IVC + HV | Short | Medical management | No | 3771 | 177910.0 | D | C | SC | NA | 58 | Infiltrative | - | ||
| 21 | M | HV | Long | TIPSS | Yes | 166 | 1 (TIPSS revision) | 424 | 4.4 | B | A | TACE | CR | 23741 | Multiple (3.1 cm × 2.3 cm) | - |
| 22 | F | IVC | Long | IVC angioplasty | Yes | 973 | 2 (TIPSS, TIPSS revision) | 2062 | 1300.0 | C | B | TACE | NA | 241 | 1 (11.2 cm × 8.8 cm) | - |
| 45 | F | IVC + HV | Long | IVC angioplasty | Yes | 412 | 2 (Balloon dilatation, balloon) | 4603 | 500.0 | B | A | SC | NA | 61 | Multiple/NA | - |
| 26 | F | IVC + HV | Long | IVC angioplasty | No | 4018 | 315.0 | C | B | TKI | NA | 2164 | Infiltrative | - | ||
| 52 | F | IVC + HV | Long | IVC angioplasty | Yes | 284 | 1 (IVC angioplasty) | 1768 | 14951.0 | D | C | SC | NA | 401 | Multiple | - |
| 30 | M | HV | Short | HV angioplasty | No | 306 | 66.6 | B | A | TACE | CR | 1352 | 4.0 (4.1 cm × 3.6 cm) | 2 (TACE, TACE) | ||
| 40 | M | IVC + HV | Long | IVC angioplasty | No | 262 | 131.0 | D | C | SC | NA | 35 | Infiltrative with PVT | - | ||
| 32 | F | IVC | Short | IVC angioplasty | No | 3762 | 2.2 | A | A | SC | NA | 491 | 2 (2 cm × 1 cm) | - | ||
| 30 | M | IVC + HV | Long | PSRS | Yes | 5484 | 1 (TIPSS) | 5599 | 353.0 | B | B | TACE | PR | 171 | 2.0 (4.0 cm × 4.2 cm) | TKI |
| 28 | M | HV | Long | TIPSS | No | 4023 | 500.0 | D | C | SC | NA | 221 | Infiltrative with PVT | - |
A total of 9 out of 1140 BCS patients were diagnosed with HCC (85% males). CTP class B and C proportions were 5 (55.5%) and 4 (44.4%), respectively. All had a long segment block and a combined IVC and HV block, except 1 patient who had a short segment HV block. All except 1 patient were diagnosed with HCC during follow-up. The median AFP was 900 ng/mL (25-1450). In comparison with the New Delhi cohort, there were no differences in the median age of onset of BCS (32 years vs 30 years), duration of symptoms, length, site of block (long segment and combined blocks), and number of nodules (multinodular disease). In contrast to the New Delhi Centre, HCC was found predominantly in males (85% vs 45%, P = 0.09), and presented with a more advanced HCC (BCLC C and D, 6 (66.7%) vs 13 (37.1%), P = 0.14), and patients were not amenable to any locoregional therapy at the time of presentation.
Three out of nine (33%) patients underwent TIPSS for BCS; the rest were managed with anticoagulation alone. Only 1 patient had restenosis/stent block after the initial intervention for BCS, for which reintervention was done. Two patients underwent liver transplantation for HCC (alive at last follow-up of 3 years and 8 years, respectively). A total of 7 patients were alive at the last follow-up (Table 5).
| Age | Sex | Site of obstruction | Length of block | BCS intervention | BCS: Restenosis | BCS: Time between intervention and stent block | No. of Reinterventions for HVOTO | Time between BCS and HCC diagnosis in d | AFP | BCLC | CTP | Initial HCC management | HCC response | Survival after diagnosis of HCC until last follow-up in d | No. of lesion | Reintervention for HCC |
| 30 | M | IVC + HV | Long | Medical management | No | 3500 | 58000 | C | C | Conservative | 1201 | 4 | None | |||
| 46 | M | IVC+ HV | Long | Medical management | No | 150 | 25.60 | D | C | Conservative | 3651 | 4 | None | |||
| 51 | M | HV | Short | Medical management | No | 350 | 1.16 | D | C | Conservative | 901 | 4 | None | |||
| 22 | M | IVC + HV | Long | Medical management | No | 460 | 600 | C | B | Conservative | 1501 | 3 | None | |||
| 35 | M | IVC + HV | Long | TIPSS | No | 3400 | 1200 | D | C | Conservative | 30 | 4 | None | |||
| 15 | F | IVC + HV | Long | Medical management | No | 240 | NA | B | B | LT | 10001 | 1 | None | |||
| 16 | M | IVC + HV | Long | TIPSS | Yes | 150 | IVC angioplasty | 3300 | NA | B | B | LT | 29001 | 2 | None | |
| NA | NA | IVC + HV | Long | TIPSS | No | 1200 | NA | B | B | Conservative | 601 | 1 | None | |||
| NA | NA | IVC +HV | Long | Medical management | 400 | 1450 | D | B | Conservative | 1801 | 4 | None |
The present study highlighted that HCC can manifest at the time of diagnosis of BCS or develop later. Radiological interventions are feasible in a select group of patients and may improve outcomes. In this study, the prevalence of primary HCC at index presentation was 1.99%, and the incidence was 0.36 (0.22-0.57) per 100 person-years.
In a meta-analysis of 1487 articles on BCS, only 16 studies provided the frequency of HCC[3]. Heterogeneity among the included studies was statistically significant and arose due to variable periods of inclusion, discrepancy in diagnostic criteria and methods, different follow-up periods, and inclusion of studies with concomitant viral hepatitis. The variability in the prevalence of HCC in BCS is significant (2.0%-46.2% in 12 Asian studies, 40.0%-51.6% in 2 African studies, 11.3% in 1 European study, and 11.1% in 1 American study). The analysis showed that among BCS patients with viral hepatitis, the pooled prevalence of HCC was 17.6% (95% confidence interval: 10.1%-26.7%), whereas among those with BCS alone, the pooled prevalence of HCC was 15.4% (95% confidence interval: 6.8%-26.7%). Table 6 details studies reporting the prevalence of BCS-HCC among patients with BCS. The higher prevalence in Africa and Japan compared to Western countries and India may be due to differences in the patient populations, the follow-up periods, the underlying etiology of BCS, the severity of cirrhosis at the time of diagnosis, and the exclusion or inclusion of patients with chronic viral hepatitis[19,20]. Many previous studies that reported the association of HCC with BCS included cases with HBV or HCV co-infection[7,21-23]. Therefore, the present study on primary BCS cases without other known risk factors of HCC assumes importance.
| Ref. | Country | Patients, n | Incidence of HCC, n (%) | Risk factors | Treatment offered |
| Nakamura et al[31], 1968 | Japan | 64 | 28 (43.7) | Not provided | Details not provided |
| Nakamura and Takezawa et al[32], 1982 | Japan | 13 | 6 (46.0) | Age at BCS diagnosis < 44 yr; complete obstruction of IVC | Details not provided |
| Simson et al[33], 1982 | South Africa | 101 | 48 (47.5) | Not provided | Details not provided |
| Rector et al[34], 1985 | United States | 10 | 2 (20.0) | Not provided | Details not provided |
| Kew et al[35], 1989 | South Africa | 15 | 6 (40.0) | Not provided | Details not provided |
| Okuda et al[1], 1998 | Japan | 148 | 10 (6.7) | Not provided | Details not provided |
| Shrestha et al[36], 1996 | Nepal | 150 | 7 (4.6) | Not provided | Details not provided |
| Bayraktar et al[24], 1998 | Turkey | 56 (prospective study duration 10 years) | 3 (5.3) | Hepatic vein thrombosis | Details not provided |
| Dilawari et al[37], 1994 | India | 115 | 9 (7.8) | Not provided | Details not provided |
| Matsui et al[38], 2000 | Japan | 12 | 3 (25.0) | Chronic congestion | Resection (1); TACE (1); chemotherapy (1) |
| Shin et al[23], 2004 | South Korea | 73 | 15 (20.5), 6 de novo/9 follow up | Female sex not provided | TACE (11); resection (2); conservative (2) |
| Moucari et al[4], 2008 | France | 97 | 11 (11.3) | Male, factor V Leiden mutation, IVC obstruction | TACE (7); LT (3); conservative (1) |
| Amarapurkar et al[25], 2008 | India | 35 (prospective study); follow-up 42 mo | 1 (2.8) | Not provided | Details not provided |
| Shrestha et al[39], 2009 | Nepal | 56 | 6 (10.7) | HV block | PAI (1); supportive (5) |
| Gwon et al[11], 2010 | Korea | 98 | 23 (23.4) | Female sex | TACE (20); TACE + LT (3) |
| Park et al[5], 2012 | Korea | 95 | 17, annual incidence 2.8 | Elevated HVPG | Intraarterial chemotherapy (3); conservative (3); LT (2) |
| Liu et al[6], 2013 | China | 246 | 14 (5.6) | IVC block + stricture of hepatic venous outflow | TACE (14) |
| Paul et al[9], 2015 | India | 421 | 16 (3.8) | Cirrhosis; combined IVC and HV block; long segment IVC block | TACE (11); TKI (1); conservative (4) |
| Sakr et al[8], 2017 | Egypt | 348 | 15 (4.3) | Male, older age; cigarette smoking, AFP > 24.5 | TACE (4); TACE + RFA (2); TACE + TKI (1); conservative (8) |
| Li et al[7], 2022 | China | 113 | 12 (10.6) | IVC block + stricture of hepatic venous outflow | Resection (2); RFA (1); Conservative (4); TACE (5) |
Our study found a relatively low prevalence of 1.9% of primary BCS-HCC (18/904 cases of HCC at first presentation). The sample sizes in the previously reported studies were small and may have been associated with selection bias. Two previous studies reported the incidence of HCC in BCS prospectively as 2.85% and 5.30%, respectively, and both were limited by a low number of patients and short follow-up durations (7 years and 10 years, respectively)[24,25]. Our results suggest that the incidence of BCS-HCC is low compared to viral hepatitis-related HCC[21,26,27].
Four previous studies have reported the presence of long-segment IVC strictures and combined IVC and HV blocks as risk factors for the development of HCC in patients with BCS[4,6,7,9]. In agreement with the reported studies, we found that a long segment IVC block and combined IVC and HV block were associated with HCC. Long segment block may be associated with more hepatic congestion and extensive fibrosis due to persistent, progressive parenchymal injury.
In our study cases, a liver lesion might have gone undetected on Doppler ultrasound, as the scans were focused on assessing stent patency. This could have affected our incidence estimates. It is difficult to biopsy suspicious lesions in BCS, as there is a high risk of bleeding consequent to anticoagulation and the risk of recurrent thrombosis upon its discontinuation. Therefore, the decision to biopsy is generally taken on a case-by-case basis. The above facts highlight the difficulties in screening patients with BCS having liver cirrhosis for development of HCC and may explain why most of these patients were detected in advanced stages of liver cancer, as in our study.
The serum AFP levels in BCS-HCC were significantly higher than BCS patients without HCC (1310.0 ng/mL vs 2.9 ng/mL). The median serum AFP in patients with BCS-HCC at presentation was higher than those who developed HCC at follow-up (13029 ng/mL vs 500 ng/mL). Not all HCCs produce AFP; at the same time, false positive results due to raised AFP levels are not infrequent, such as in active viral hepatitis[28-30].
Of all BCS patients, approximately 70% of patients in both HCC and non-HCC groups received an intervention for vascular decompression, with no significant differences in the rates of stent block or reintervention on follow-up.
In our cohort, most BCS-HCC patients presented as BCLC-B, had multinodular disease, underlying cirrhosis, and portal hypertension and were not eligible for curative resection or ablation. Our data suggests that the choice of modalities of therapy varies between centers. This may be related to the choice of therapy as per the treating physician, expertise of interventional radiologist, cost of the procedure, and the availability of liver transplantation. The most common treatment modality was TACE in 18 (81.8%) patients. Shin et al[23] suggested that HCC patients associated with BCS were responsive to interventions such as TACE. Gwon et al[11] showed that, for HCC patients with membranous obstruction of the IVC who underwent TACE, the 3-year and 5-year survival rates were 61% and 46%, respectively. In our study, the median survival among cases with BCLC stages A, B, C, and D who underwent intervention was 6 mo, 45 mo, 8 mo, and 40 d, respectively. The apparent low survival of patients in the BCLC-A group may be attributed to fewer patients in this subgroup, potentially skewing the results. Also, only 2 patients among these received treatment for HCC. Therefore, it seems that the survival of patients with BCS-HCC is comparable to patients with other etiologies of HCC.
Treatment of BCS is associated with improvement in liver function tests, whereas interventions for HCC carry a risk of decompensation. Therefore, as a protocol, we treat BCS-HCC patients with interventions first for BCS, followed by HCC. There is no consensus in the literature regarding the methods and timing of treatment of HCC in patients with BCS. It remains unclear whether angioplasty and stenting can reduce the occurrence of new HCC and improve the results of TACE and other interventional procedures for HCC.
Our study has a few limitations. Our analysis included patients with BCS spanning more than three decades. The diagnostic modalities and our understanding of BCS and HCC have evolved over this period. In the initial part of the study, radiological investigations and interventions were unavailable for diagnosing and managing BCS and HCC. Hence, this lack of uniformity may have impacted the results of this study. It is possible that we might have missed BCS-HCC lesions in the initial part of the study. We excluded 158 (14.8%) patients because of missing data. The relationship between prothrombotic disorders, venous outflow obstruction level, and HCC development could not be assessed, as the data for all patients was unavailable at the time of analysis. Future studies need to assess the differences in the risk of development of HCC among patients with and without hypercoagulable states. Patients were assessed for hepatitis B surface antigen and anti-HCV antibody but not for the presence of hepatitis B virus-DNA and HCV-RNA in serum, which is a shortcoming. We diagnosed HCC based on imaging, elevated alpha-fetoprotein levels, and an increase in the size of the liver lesion over time. A lack of biopsy in the majority of patients for the diagnosis of HCC was another limitation of this study.
HCC is not uncommon in patients with BCS. Radiological interventions and liver transplantation are feasible in primary BCS patients with HCC and may improve outcomes.
Hepatocellular carcinoma (HCC) is a cancer with poor survival outcomes. Budd-Chiari syndrome (BCS) is a disease of the liver that leads to cirrhosis and may lead to HCC. Current guidelines are not clear regarding management of patients with both BCS and HCC. In clinical practice there can be barriers to providing treatments that can improve outcomes for those with HCC. Liver transplant or curative surgery are not an option for those diagnosed with advanced disease. Treatment protocols include managing BCS first followed by treating HCC. Locoregional therapies (e.g., transarterial chemoembolization) is feasible in a selected group of patients and improves outcomes.
There is very little data to decide management of HCC in BCS. Therefore, research into this area is needed due to the complexity of treating patients with both HCC and BCS. We hypothesize that treating BCS first followed by treatment of HCC should be one of the strategies to improve outcomes in these patients.
To investigate the magnitude, clinical characteristics, and treatment outcomes in patients with HCC and BCS.
We conducted a retrospective cohort study including patients diagnosed with BCS over a span of more than 30 years We used Kaplan-Meir survival curve analysis to calculate the median survival of HCC among BCS patients using the available follow-up of each patient.
In a study of 904 BCS patients, 35 developed BCS-associated HCC (BCS-HCC). Prevalence stood at 3.8%, with an HCC incidence of 0.36 per 100 person-years. BCS-HCC patients were older, had increased complications, and higher liver enzyme levels compared to BCS alone. Most underwent BCS interventions (74.3%), with 62.8% receiving HCC treatment. Those undergoing interventions exhibited prolonged median survival (3.5 years) as compared to those who did not (3.1 mo).
We found that HCC is not uncommon in patients with BCS. A significant proportion of them present as advanced disease precluding them from liver transplant or curative surgeries. Improvement in survival was statistically significant in patients receiving treatment for HCC as compared to ones who did not. Locoregional therapies were suitable in these patients and improves outcomes.
This study, a retrospective analysis of clinical records, observed that locoregional therapies are feasible in patients with HCC due to BCS, consequently leading to improved treatment outcomes. This further validates the role of locoregional therapies in patients with BCS-HCC.
| 1. | Okuda K, Kage M, Shrestha SM. Proposal of a new nomenclature for Budd-Chiari syndrome: hepatic vein thrombosis versus thrombosis of the inferior vena cava at its hepatic portion. Hepatology. 1998;28:1191-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 116] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Afredj N, Debzi N. Hepatocellular Carcinoma in Budd-Chiari Syndrome. In: Qi X, editor. Budd-Chiari Syndrome [Internet]. Singapore: Springer, 2020: 113-129. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Ren W, Qi X, Yang Z, Han G, Fan D. Prevalence and risk factors of hepatocellular carcinoma in Budd-Chiari syndrome: a systematic review. Eur J Gastroenterol Hepatol. 2013;25:830-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Moucari R, Rautou PE, Cazals-Hatem D, Geara A, Bureau C, Consigny Y, Francoz C, Denninger MH, Vilgrain V, Belghiti J, Durand F, Valla D, Plessier A. Hepatocellular carcinoma in Budd-Chiari syndrome: characteristics and risk factors. Gut. 2008;57:828-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Park H, Yoon JY, Park KH, Kim DY, Ahn SH, Han KH, Chon CY, Park JY. Hepatocellular carcinoma in Budd-Chiari syndrome: a single center experience with long-term follow-up in South Korea. World J Gastroenterol. 2012;18:1946-1952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Liu FY, Wang MQ, Duan F, Fan QS, Song P, Wang Y. Hepatocellular carcinoma associated with Budd-Chiari syndrome: imaging features and transcatheter arterial chemoembolization. BMC Gastroenterol. 2013;13:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Li KS, Guo S, Chen YX, Zhang ZL. Budd-Chiari syndrome and its associated hepatocellular carcinoma: Clinical risk factors and potential immunotherapeutic benefit analysis. Front Oncol. 2022;12:1075685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Sakr M, Abdelhakam SM, Dabbous H, Hamed A, Hefny Z, Abdelmoaty W, Shaker M, El-Gharib M, Eldorry A. Characteristics of hepatocellular carcinoma in Egyptian patients with primary Budd-Chiari syndrome. Liver Int. 2017;37:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Paul SB, Shalimar, Sreenivas V, Gamanagatti SR, Sharma H, Dhamija E, Acharya SK. Incidence and risk factors of hepatocellular carcinoma in patients with hepatic venous outflow tract obstruction. Aliment Pharmacol Ther. 2015;41:961-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 3133] [Article Influence: 783.3] [Reference Citation Analysis (61)] |
| 11. | Gwon D, Ko GY, Yoon HK, Sung KB, Kim JH, Lee SS, Lee JM, Ohm JY, Shin JH, Song HY. Hepatocellular carcinoma associated with membranous obstruction of the inferior vena cava: incidence, characteristics, and risk factors and clinical efficacy of TACE. Radiology. 2010;254:617-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Brown JJ, Naylor MJ, Yagan N. Imaging of hepatic cirrhosis. Radiology. 1997;202:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 92] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J; EASL Panel of Experts on HCC. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3252] [Cited by in RCA: 3258] [Article Influence: 130.3] [Reference Citation Analysis (1)] |
| 14. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6422] [Article Influence: 802.8] [Reference Citation Analysis (9)] |
| 15. | Hemachandran N, Shalimar, Acharya S, Kedia S, Gunjan D, Saraya A, Sharma R, Gamanagatti S. Long-Term Outcomes of Endovascular Interventions in More than 500 patients with Budd-Chiari Syndrome. J Vasc Interv Radiol. 2021;32:61-69.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Shalimar, Kumar A, Kedia S, Sharma H, Gamanagatti SR, Gulati GS, Nayak B, Thakur B, Acharya SK. Hepatic venous outflow tract obstruction: treatment outcomes and development of a new prognostic score. Aliment Pharmacol Ther. 2016;43:1154-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Llovet JM, Fuster J, Bruix J; Barcelona-Clínic Liver Cancer Group. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004;10:S115-S120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 516] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 18. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3439] [Article Influence: 214.9] [Reference Citation Analysis (43)] |
| 19. | Valla DC. Hepatic venous outflow tract obstruction etiopathogenesis: Asia vs the West. J Gastroenterol Hepatol. 2004;19:S204-11. [RCA] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Chaubal A, Shukla A. Budd-Chiari Syndrome: East vs West. Budd-Chiari Syndrome. 2019;231-243. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Shrestha SM, Shrestha S, Shrestha A, Tsuda F, Endo K, Takahashi M, Okamoto H. High prevalence of hepatitis B virus infection and inferior vena cava obstruction among patients with liver cirrhosis or hepatocellular carcinoma in Nepal. J Gastroenterol Hepatol. 2007;22:1921-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Valla DC. Budd-Chiari syndrome/hepatic venous outflow tract obstruction. Hepatol Int. 2018;12:168-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 23. | Shin SH, Chung YH, Suh DD, Shin JW, Jang MK, Ryu SH, Park NH, Lee HC, Lee YS, Suh DJ. Characteristic clinical features of hepatocellular carcinoma associated with Budd-Chiari syndrome: evidence of different carcinogenic process from hepatitis B virus-associated hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2004;16:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Bayraktar Y, Egesel T, Sağlam F, Balkanci F, Van Thiel DH. Does hepatic vein outflow obstruction contribute to the pathogenesis of hepatocellular carcinoma? J Clin Gastroenterol. 1998;27:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Amarapurkar DN, Punamiya SJ, Patel ND. Changing spectrum of Budd-Chiari syndrome in India with special reference to non-surgical treatment. World J Gastroenterol. 2008;14:278-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Lin L, Yan L, Liu Y, Qu C, Ni J, Li H. The Burden and Trends of Primary Liver Cancer Caused by Specific Etiologies from 1990 to 2017 at the Global, Regional, National, Age, and Sex Level Results from the Global Burden of Disease Study 2017. Liver Cancer. 2020;9:563-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 27. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 4463] [Article Influence: 892.6] [Reference Citation Analysis (4)] |
| 28. | Trevisani F, D'Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P, Domenicali M, De Notariis S, Roda E, Bernardi M. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol. 2001;34:570-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 524] [Article Influence: 21.0] [Reference Citation Analysis (4)] |
| 29. | Farinati F, Marino D, De Giorgio M, Baldan A, Cantarini M, Cursaro C, Rapaccini G, Del Poggio P, Di Nolfo MA, Benvegnù L, Zoli M, Borzio F, Bernardi M, Trevisani F. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol. 2006;101:524-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 319] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 30. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, Rodés J, Bruix J; Barcelona Liver Cancer Group. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2654] [Article Influence: 110.6] [Reference Citation Analysis (1)] |
| 31. | Nakamura T, Nakamura S, Aikawa T, Suzuki O, Onodera A, Karoji N. Obstruction of the inferior vena cava in the hepatic portion and the hepatic veins. Report of eight cases and review of the Japanese literature. Angiology. 1968;19:479-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 58] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Nakamura S, Takezawa Y. Obstruction of the inferior vena cava in the hepatic portion and hepatocellular carcinoma. Tohoku J Exp Med. 1982;138:119-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 33. | Simson IW. Membranous obstruction of the inferior vena cava and hepatocellular carcinoma in South Africa. Gastroenterology. 1982;82:171-178. [PubMed] [DOI] [Full Text] |
| 34. | Rector WG Jr, Xu YH, Goldstein L, Peters RL, Reynolds TB. Membranous obstruction of the inferior vena cava in the United States. Medicine (Baltimore). 1985;64:134-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 51] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Kew MC, McKnight A, Hodkinson J, Bukofzer S, Esser JD. The role of membranous obstruction of the inferior vena cava in the etiology of hepatocellular carcinoma in Southern African blacks. Hepatology. 1989;9:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 48] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Shrestha SM, Okuda K, Uchida T, Maharjan KG, Shrestha S, Joshi BL, Larsson S, Vaidya Y. Endemicity and clinical picture of liver disease due to obstruction of the hepatic portion of the inferior vena cava in Nepal. J Gastroenterol Hepatol. 1996;11:170-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 78] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Dilawari JB, Bambery P, Chawla Y, Kaur U, Bhusnurmath SR, Malhotra HS, Sood GK, Mitra SK, Khanna SK, Walia BS. Hepatic outflow obstruction (Budd-Chiari syndrome). Experience with 177 patients and a review of the literature. Medicine (Baltimore). 1994;73:21-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 220] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 38. | Matsui S, Ichida T, Watanabe M, Sugitani S, Suda T, Takahashi T, Asakura H. Clinical features and etiology of hepatocellular carcinoma arising in patients with membranous obstruction of the inferior vena cava: in reference to hepatitis viral infection. J Gastroenterol Hepatol. 2000;15:1205-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Shrestha SM. Liver cirrhosis and hepatocellular carcinoma in hepatic vena cava disease, a liver disease caused by obstruction of inferior vena cava. Hepatol Int. 2009;3:392-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Niu XK, China; Rong GH, China S-Editor: Chen YL L-Editor: Filipodia P-Editor: Zhang XD