Published online Mar 15, 2024. doi: 10.4251/wjgo.v16.i3.1084
Peer-review started: December 24, 2023
First decision: January 10, 2024
Revised: January 18, 2024
Accepted: January 27, 2024
Article in press: January 27, 2024
Published online: March 15, 2024
Processing time: 78 Days and 18.6 Hours
The advanced first-line regimen for advanced gastric cancer is based on a combination of fluoropyrimidine and platinum and/or paclitaxel (PTX), forming a two- or three-drug regimen. Compared to conventional PTX, nanoparticle albumin-bound PTX (Nab-PTX) has better therapeutic effects and fewer adverse effects reported in studies. Nab-PTX is a great option for patients presenting with advanced gastric cancer. Herein, we highlight an adverse event (hemorrhagic cystitis) of Nab-PTX in advanced gastric cancer.
A 55-year-old male was diagnosed with lymph node metastasis after a laparoscopic-assisted radical gastrectomy for gastric cancer that was treated by Nab-PTX and S-1 (AS). On the 15th day after treatment with AS, he was diagnosed with hemorrhagic cystitis.
Physicians should be aware that hemorrhagic cystitis is a potential adverse event associated with Nab-PTX treatment.
Core Tip: Chemotherapy is an important treatment method for advanced gastric cancer. Up to now, nanoparticle albumin-bound paclitaxel (Nab-PTX) has shown promising responses in advanced gastric cancer. We aim to draw attention to a rarely treatment-related adverse event after Nab-PTX.
- Citation: Zhang XJ, Lou J. Hemorrhagic cystitis in gastric cancer after nanoparticle albumin-bound paclitaxel: A case report. World J Gastrointest Oncol 2024; 16(3): 1084-1090
- URL: https://www.wjgnet.com/1948-5204/full/v16/i3/1084.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i3.1084
There were approximately 1089103 new cases of gastric cancer and 768693 gastric cancer-related deaths in 2020, making gastric cancer the 5th most common cancer worldwide and the 4th leading cause of death[1]. Due to the difficulty in diagnosing early-stage gastric cancer, patients with advanced gastric cancer often have metastases, resulting in poor treatment outcomes and a 5-year survival rate < 10%[2]. Current treatments for advanced gastric cancer include radiotherapy, chemotherapy, immunotherapy, and targeted therapy. Moreover, fluoropyrimidine, platinum, and paclitaxel (PTX) are the main therapeutic drugs for advanced gastric cancer. Usually, the advanced first-line regimen is based on a combination of fluoropyrimidine and platinum and/or PTX, forming a two- or three-drug regimen[3]. PTX is a compound extracted from Taxus chinensis that promotes the assembly of microtubule proteins into microtubules, prevents microtubule dissociation, blocks cell cycle progression, prevents mitosis, and inhibits cancer cell growth[4]. Nanoparticle albumin-bound PTX (Nab-PTX) has a 33% greater uptake in tumors than PTX[5]. Nab-PTX is rapidly dissolved and released after entering the bloodstream and exists in the form of free PTX and albumin-bound PTX complexes. The vast majority of Nab-PTX complexes enter the intercellular matrix through active transportation and binds to the Sparc protein expressed in tumor tissues. Nab-PTX is enriched in tumor tissues to exert anti-tumor effects with relative targeting[6]. Moreover, Nab-PTX does not contain solvents, such as ethanol and polyoxyethylene castor oil, so steroids or antihistamines can prevent allergic reactions and can be used in patients with an alcohol allergy.
The combination of Nab-PTX and S-1 (AS), an oral 5-FU derivative, has been reported to have a synergistic anti-tumor effect in preclinical mouse models[7]. In a phase III non-inferiority clinical study of advanced gastric cancer, the median overall survival of patients treated with Nab-PTX was not inferior to PTX[8]. In a multicenter, randomized, phase III clinical study on advanced gastric cancer, the efficacy of Nab-PTX combined with S-1 was compared to oxaliplatin combined with S-1 (SOX). The results showed a median progression-free survival of 9.03 months in the AS group and 5.07 months in the SOX group. Of note, the study was discontinued due to slow enrollment and a change in treatment strategies; however, the mid-term results indicated that progression-free survival after AS treatment was superior to SOX treatment[9]. The most common adverse events reported in Nab-PTX clinical trials are like conventional PTX and include bone marrow suppression, sensory neuropathy, hair loss, and fatigue[8,9].
A 55-year-old male presented to the hospital with hemorrhagic cystitis after chemotherapy.
Over the past 2 wk, the patient experienced worsening frequency, urgency, and dysuria with gross hematuria.
The patient had a history of cystitis in 2021.
The patient denied any family history of malignant tumors.
The physical examination revealed normal vital signs.
The urine routine showed a 1+ white blood cell count, 3+ occult blood count, and 4+ red blood cell count. No abnormalities were detected on routine blood analyses. The urine bacterial culture showed no bacterial growth and acid-fast staining was negative for bacteria. A tuberculosis-specific enzyme-linked immunospot assay (T-SPOT.TB) examination showed negative results. No malignant cells were detected in the urine cytologic evaluation.
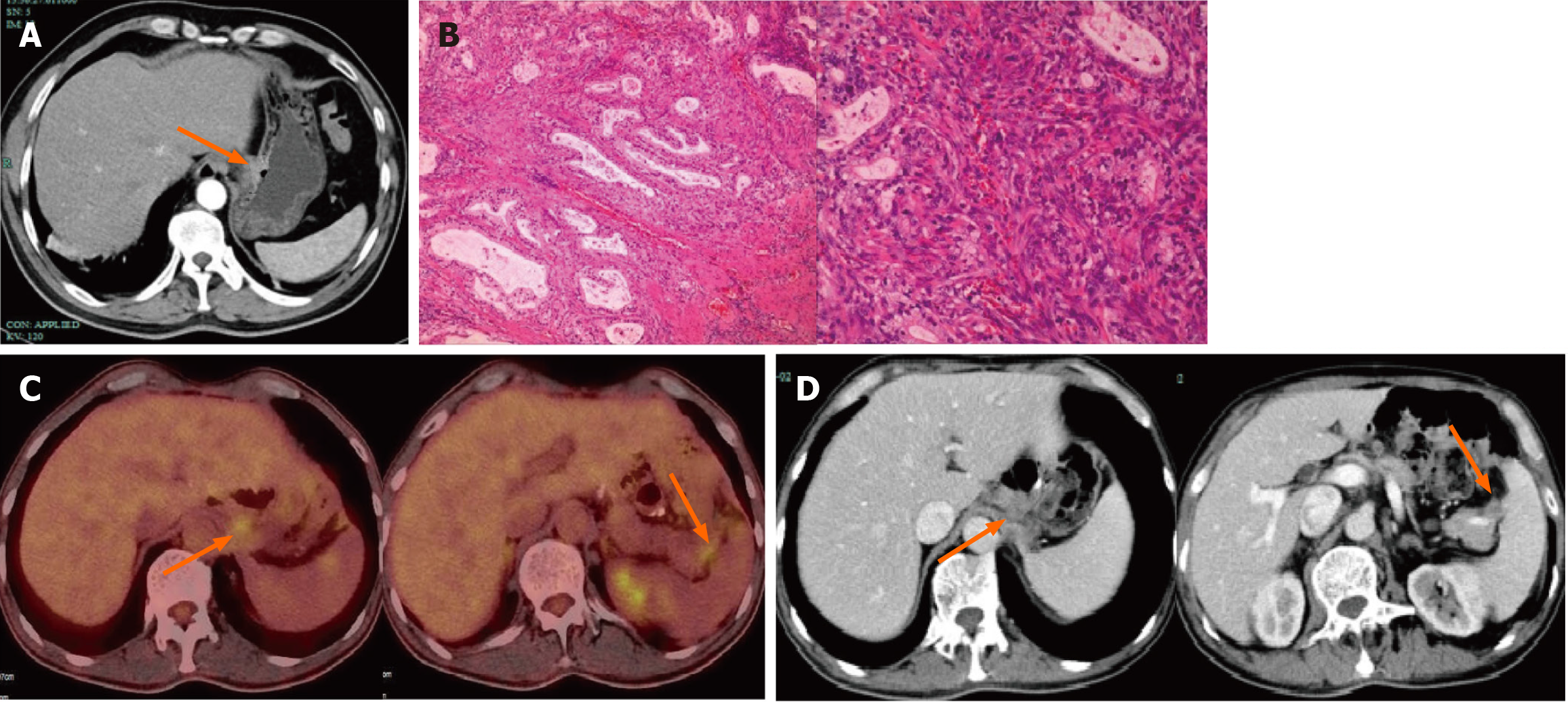
An abdominal enhanced computer tomography (CT) scan revealed thickening of the gastric wall near the lesser curvature of the stomach and ulcerations, suggesting a malignant tumor on December 25, 2020 (Figure 1A).
The postoperative pathologic evaluation after a laparoscopic-assisted radical gastrectomy for gastric cancer performed on January 10, 2021 revealed that the histologic type was a moderately differentiated adenocarcinoma. Immunohistochemistry of omental adipose tissue revealed the following: P53+ (wild-type); Ki-67+ (approximately 40%); CerbB-2 (2+); MSH2+; MSH6+; PMS2+; MLH1+; S-100−; SALL4−; AFP±; SATB2−; CDX2−; and CK20± (Figure 1B). Staging of gastric cancer according to the 8th edition of the AJCC was as follows: T3N2M0, stage IIIA. The patient received six cycles of the SOX regimen [S-1 (60 mg po bid d1-d14) and oxaliplatin (150 mg d1)] after surgery. During this period, numbness and discomfort occurred in the hands and feet, suggesting peripheral neurotoxicity caused by oxaliplatin.
On April 27, 2023, a follow-up abdominal enhanced CT scan revealed multiple enlarged lymph nodes in the abdominal cavity. On May 3, 2023, a positron emission tomography-CT scan revealed enlarged lymph nodes in the left upper peritoneum and splenopancreatic space after gastric cancer surgery, with increased 18 F-fluorodeoxyglucose metabolism, suggesting metastasis (Figure 1C).
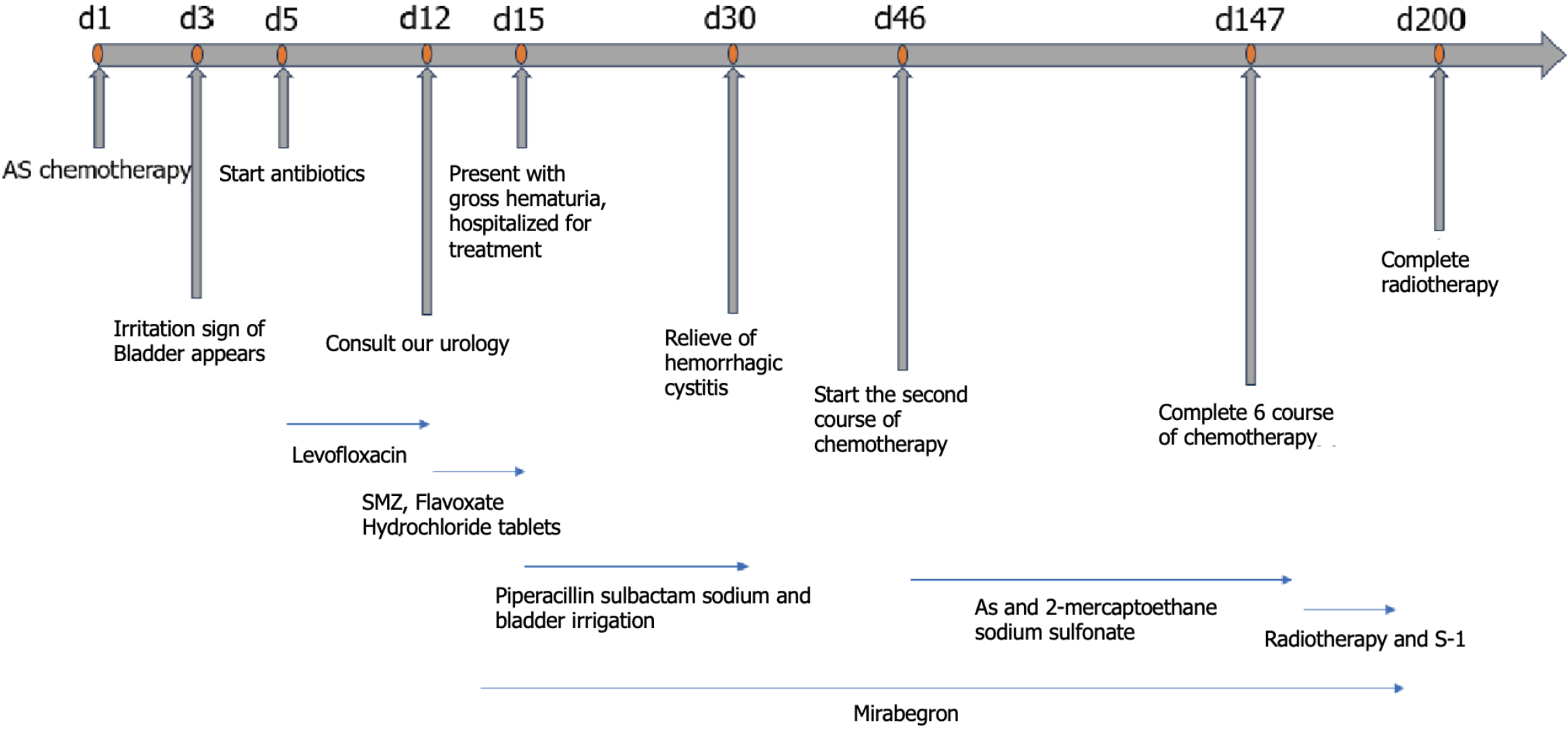
After surgery, fluorescence in situ hybridization for human epidermal growth factor receptor 2 was negative with no gene amplification. The tumor cell proportion score < 1% and programmed cell death ligand-1 programmed death ligand-1 Yielded the combined positive score < 1. On May 5, 2023, the first course of Nab-PTX (130 mg/m2 on days 1 and 8) combined with S-1 chemotherapy (60 mg twice daily for 14 d) was initiated.
The patient developed bladder symptoms, including frequent urination, urgency, and pain, on the third day after treatment began but did not seek medical attention at that time. On the 5th day, he purchased levofloxacin tablets (500 mg qd orally for 1 wk) at a pharmacy because the symptoms persisted, but there was no improvement. Due to symptoms of cystitis, he did not return to the hospital to complete Nab-PTX chemotherapy on the 8th day. On the 12th day after treatment began, the patient had a consultation in the Urology Department. On the 15th day the frequency, urgency, and dysuria worsened with gross hematuria.
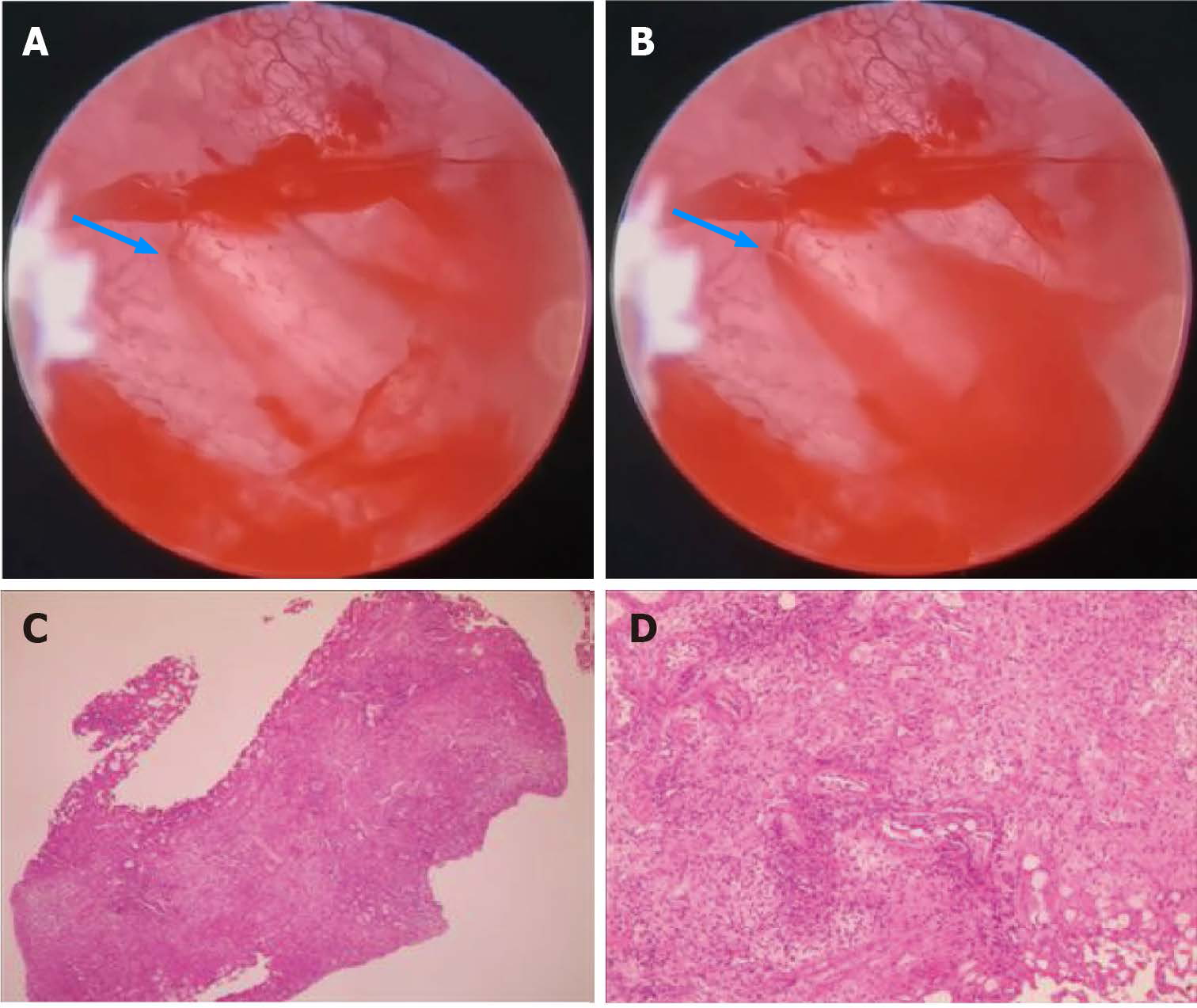
Cystoscopy revealed edema, inflammation, and bleeding of the bladder mucosa, suggesting hemorrhagic cystitis. The pathologic results of a biopsy under cystoscopy revealed chronic inflammation of the bladder mucosa with granulation tissue formation (Figure 2).
Hemorrhagic cystitis associated with Nab-PTX was the final diagnosis.
Sustained-release mirabelon tablets and piperacillin sulbactam sodium were administered and the bladder was irrigated. Hemorrhagic cystitis was relieved 2 wk later.
Considering the limited availability of drugs for advanced gastric cancer, the patient continued to receive 5 cycles Nab-PTX combined with S-1 chemotherapy. The bladder was protected with 2-mercaptoethane sodium sulfonate on the day of intravenous chemotherapy.
During the treatment period regular abdominal enhanced CT scans were performed. On August 31, 2023, an abdominal enhanced CT scan showed a reduction in the size of abdominal lymph nodes (Figure 1D). During the treatment period the patient had repeated episodes of frequent urination, urgency, and pain, but no hematuria was observed. After completing six cycles of Nab-PTX combined with S-1 chemotherapy, the patient underwent 25 sessions of radiation therapy on enlarged lymph nodes in the abdominal cavity and received synchronous oral chemotherapy with S-1. There was no further visual hematuria after radiotherapy, and the frequent urination, urgency, and pain improved significantly. A routine urinalysis revealed no white blood cells, red blood cells, or occult blood. The patient’s treatment process is shown in Figure 3.
Nab-PTX is a cytotoxic drug used clinically for anti-tumor activity. Nab-PTX is widely used in solid tumors, such as breast, gastric, ovarian, pancreatic, and non-small cell lung cancers. Since 1992, PTX has been approved by the Food and Drug Administration (FDA) for the treatment of ovarian cancer and various forms, such as docetaxel, albumin-bound PTX, and liposome PTX, have been approved for cancer treatment. Clinical studies have shown that compared to conventional PTX, Nab-PTX exhibits good efficacy and safety[10].
The combination of oxaliplatin and fluoropyrimidine has a Class 1A level of evidence in the first-line treatment of advanced gastric cancer, making the oxaliplatin and fluoropyrimidine combination a first-line treatment for metastatic gastric cancer[3]. However, oxaliplatin has dose-limiting toxicity, and when the cumulative dose reaches 800 mg/m2, oxaliplatin may cause permanent sensory abnormalities and functional impairment. In our case, the patient underwent six cycles of the SOX regimen followed by adjuvant chemotherapy after surgery and developed numbness in the hands and feet during the adjuvant treatment period, which persisted until recurrence and metastasis. Considering the better safety of Nab-PTX, we treated our patient with the AS chemotherapy regimen in the advanced frontline. The patient had previously received oral S-1 chemotherapy during adjuvant therapy, completing a total of 6 cycles without any symptoms of hemorrhagic cystitis. Moreover, after completion of a Nab-PTX intravenous infusion, the cystitis symptoms gradually improved during maintenance chemotherapy with S-1. Therefore, we are of the opinion that the hemorrhagic cystitis was mainly related to Nab-PTX. We use Naranjo’s algorithm (adverse drug reaction probability scale) to evaluate the causality of adverse events of Nab-PTX. The total score was 8 points, which is means that hemorrhagic cystitis probably caused by Nab-PTX.
Hemorrhagic cystitis is a severe adverse event that can occur in cancer patients receiving cytotoxic drug treatment. The common symptoms include bleeding, frequent urination, urgency, and dysuria, which severely affect a patient’s quality of life. Treatment mainly includes continuous bladder flushing, hydration, alkalization of urine with sodium bicarbonate, protection of bladder with sodium mesilate, and anti-infectives. It has also been reported that hyperbaric oxygen treatment temporarily alleviates hemorrhagic cystitis caused by cyclophosphamide in breast cancer patients[11].
In non-grassroots invasive bladder urothelial carcinoma, hemorrhagic cystitis is common in response to BCG and mitomycin C bladder infusion chemotherapy[12], which is caused by direct contact between bladder infusion chemotherapy and the bladder mucosa, leading to bladder mucosa damage. Hemorrhagic cystitis has been reported after chemotherapy with cyclophosphamide and ifosfamide, mainly related to the metabolite, acrolein[13], but there are few reports involving other chemotherapy drugs[14]. A 73-year-old male patient with prostate cancer developed hemorrhagic cystitis after receiving chemotherapy with docetaxel. After continuous bladder irrigation and fluid replacement, the condition improved after 2 wk[15]. A phase II clinical study using PTX combined with carboplatin in the treatment of advanced lung cancer reported that approximately 1% of patients developed hemorrhagic cystitis during treatment[16]. Wang and Liu[17] analyzed the adverse events of Nab-PTX using the United States FDA adverse event reporting system and identified 1659 adverse events, including 18 cases of urinary tract infections and no reported adverse events consistent with hemorrhagic cystitis. Only one case involving a 69-year-old breast cancer patient who received Nab-PTX chemotherapy developed hemorrhagic cystitis has been reported[14]. He improved after drug withdrawal, bladder irrigation, and oral glucocorticoid for 2 wk. Interestingly, the breast cancer patient had a history of cystitis, as occurred in our patient.
The mechanism underlying hemorrhagic cystitis caused by Nab-PTX is not clear. After entering the body, 94% of nab-PTX is bound to plasma proteins and 6% is unbound to PTX. Nab-PTX is mainly metabolized by CYP2C8 in liver microsomes, with < 1% metabolized by the kidneys. According to the pharmacokinetics and pharmacologic toxicity of Nab-PTX[18,19], we speculate that this may be related to oxidative stress, inflammatory response and disruption of the urothelial barrier, especially in patients with a history of cystitis. In conclusion, further research is needed to determine the mechanisms underlying the occurrence and development of hemorrhagic cystitis in patients treated with Nab-PTX.
Physicians should be aware that hemorrhagic cystitis is a potential adverse event associated with Nab-PTX treatment, especially in patients with a history of cystitis.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68650] [Article Influence: 13730.0] [Reference Citation Analysis (201)] |
| 2. | Yang L, Ying X, Liu S, Lyu G, Xu Z, Zhang X, Li H, Li Q, Wang N, Ji J. Gastric cancer: Epidemiology, risk factors and prevention strategies. Chin J Cancer Res. 2020;32:695-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 159] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 3. | Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, Zhang J, Sun LY, Lin RB, Qiu H, Wang C, Qiu MZ, Cai MY, Wu Q, Liu H, Guan WL, Zhou AP, Zhang YJ, Liu TS, Bi F, Yuan XL, Rao SX, Xin Y, Sheng WQ, Xu HM, Li GX, Ji JF, Zhou ZW, Liang H, Zhang YQ, Jin J, Shen L, Li J, Xu RH. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). 2021;41:747-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 515] [Article Influence: 103.0] [Reference Citation Analysis (2)] |
| 4. | Zhu L, Chen L. Progress in research on paclitaxel and tumor immunotherapy. Cell Mol Biol Lett. 2019;24:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 382] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 5. | Chan DL, Sjoquist KM, Goldstein D, Price TJ, Martin AJ, Bang YJ, Kang YK, Pavlakis N. The effect of anti-angiogenic agents on overall survival in metastatic oesophago-gastric cancer: A systematic review and meta-analysis. PLoS One. 2017;12:e0172307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Yardley DA. nab-Paclitaxel mechanisms of action and delivery. J Control Release. 2013;170:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 373] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 7. | Li JA, Xu XF, Han X, Fang Y, Shi CY, Jin DY, Lou WH. Nab-Paclitaxel Plus S-1 Shows Increased Antitumor Activity in Patient-Derived Pancreatic Cancer Xenograft Mouse Models. Pancreas. 2016;45:425-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Shitara K, Takashima A, Fujitani K, Koeda K, Hara H, Nakayama N, Hironaka S, Nishikawa K, Makari Y, Amagai K, Ueda S, Yoshida K, Shimodaira H, Nishina T, Tsuda M, Kurokawa Y, Tamura T, Sasaki Y, Morita S, Koizumi W. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): an open-label, randomised, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2:277-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 156] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 9. | Dai YH, Yu XJ, Xu HT, Zhuang L, Zhang MS, Zou YM, Fu Q, Qiu H, Yuan XL. Nab-paclitaxel plus S-1 versus oxaliplatin plus S-1 as first-line treatment in advanced gastric cancer: results of a multicenter, randomized, phase III trial (GAPSO study). Ther Adv Med Oncol. 2022;14:17588359221118020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 10. | Yoneshima Y, Morita S, Ando M, Miura S, Yoshioka H, Abe T, Kato T, Kondo M, Hosomi Y, Hotta K, Yamamoto N, Kishimoto J, Nakanishi Y, Okamoto I. Treatment Rationale and Design for J-AXEL: A Randomized Phase 3 Study Comparing Nab-Paclitaxel With Docetaxel in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. Clin Lung Cancer. 2017;18:100-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Tanaka T, Nakashima Y, Sasaki H, Masaki M, Mogi A, Tamura K, Takamatsu Y. Severe Hemorrhagic Cystitis Caused by Cyclophosphamide and Capecitabine Therapy in Breast Cancer Patients: Two Case Reports and Literature Review. Case Rep Oncol. 2019;12:69-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Shelley MD, Court JB, Kynaston H, Wilt TJ, Coles B, Mason M. Intravesical bacillus Calmette-Guerin versus mitomycin C for Ta and T1 bladder cancer. Cochrane Database Syst Rev. 2003;CD003231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Korkmaz A, Topal T, Oter S. Pathophysiological aspects of cyclophosphamide and ifosfamide induced hemorrhagic cystitis; implication of reactive oxygen and nitrogen species as well as PARP activation. Cell Biol Toxicol. 2007;23:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 197] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 14. | Ichioka E, Iguchi-Manaka A, Oikawa T, Sawa A, Okazaki M, Saito T, Kiyomatsu H, Ikeda T, Bando H, Hara H. A case of hemorrhagic cystitis caused by nab-paclitaxel. Int Cancer Conf J. 2016;5:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Ntekim AI, Ajekigbe A. Hemorrhagic cystitis in a patient receiving docetaxel for prostate cancer. Clin Med Insights Oncol. 2010;4:11-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Langer CJ, Leighton J, McAleer C, Comis R, O'Dwyer P, Ozols R. Paclitaxel and carboplatin in the treatment of advanced non-small cell lung cancer. Semin Oncol. 1995;22:64-69. [PubMed] |
| 17. | Wang Y, Liu X. Safety signals of albumin-bound paclitaxel: Data mining of the Food and Drug Administration adverse event reporting system. Indian J Pharmacol. 2023;55:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 18. | Liourdi D, Kallidonis P, Kyriazis I, Tsamandas A, Karnabatidis D, Kitrou P, Spyroulias GA, Kostopoulou ON, Marousis K, Kalpaxis DL, Goumenos DS, Liatsikos E. Evaluation of the distribution of Paclitaxel by immunohistochemistry and nuclear magnetic resonance spectroscopy after the application of a drug-eluting balloon in the porcine ureter. J Endourol. 2015;29:580-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Çomaklı S, Özdemir S, Güloğlu M. Chrysin attenuates paclitaxel-induced hepatorenal toxicity in rats by suppressing oxidative damage, inflammation, and apoptosis. Life Sci. 2023;332:122096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ghannam WM, Egypt; Tangsuwanaruk T, Thailand S-Editor: Fan JR L-Editor: A P-Editor: Zhang XD