Published online Mar 15, 2024. doi: 10.4251/wjgo.v16.i3.1006
Peer-review started: October 26, 2023
First decision: December 31, 2023
Revised: January 4, 2024
Accepted: January 31, 2024
Article in press: January 31, 2024
Published online: March 15, 2024
Processing time: 138 Days and 1 Hours
Colorectal cancer (CRC) is one very usual tumor together with higher death rate. Ubiquitin-specific protease 21 (USP21) has been confirmed to take part into the regulation of CRC progression through serving as a facilitator. Interestingly, the promotive function of USP21 has also discovered in the progression of CRC. ZEB1 has illustrated to be modulated by USP7, USP22 and USP51 in cancers. However, the regulatory functions of USP21 on ZEB1 in CRC progression need more investigations.
To investigate the relationship between USP21 and ZEB1 in CRC progression.
The mRNA and protein expressions were assessed through RT-qPCR, western blot and IHC assay. The interaction between USP21 and ZEB1 was evaluated through Co-IP and GST pull down assays. The cell proliferation was detected through colony formation assay. The cell migration and invasion abilities were determined through Transwell assay. The stemness was tested through sphere formation assay. The tumor growth was evaluated through in vivo mice assay.
In this work, USP21 and ZEB1 exhibited higher expression in CRC, and resulted into poor prognosis. Moreover, the interaction between USP21 and ZEB1 was further investigated. It was demonstrated that USP21 contributed to the stability of ZEB1 through modulating ubiquitination level. In addition, USP21 streng
For the first time, these above findings manifested that USP21 promoted tumorigenicity and stemness of CRC by deubiquitinating and stabilizing ZEB1. This discovery suggested that USP21/ZEB1 axis may provide novel sights for the treatment of CRC.
Core Tip: Ubiquitin-specific protease 21 (USP21) and ZEB1 had been discovered to exhibit higher expressions in colorectal cancer (CRC) tissues and cells, and result into poor prognosis. USP21 contributed to the stability of ZEB1 through modulating ubiquitination level. Our findings proved that USP21 promoted tumorigenicity and stemness of CRC by deubiquitinating and stabilizing ZEB1. Moreover, it was uncovered that USP21/ZEB1 axis aggravated tumor growth in vivo.
- Citation: Lin JJ, Lu YC. Ubiquitin-specific protease 21 promotes tumorigenicity and stemness of colorectal cancer by deubiquitinating and stabilizing ZEB1. World J Gastrointest Oncol 2024; 16(3): 1006-1018
- URL: https://www.wjgnet.com/1948-5204/full/v16/i3/1006.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i3.1006
Colorectal cancer (CRC) is a kind of prevalent reason for causing cancer-related mortality globally[1]. Some great improvements in CRC treatment (such as radiotherapy, immunotherapy and chemotherapy) have done, but high rate of tumor recurrence and metastasis of postoperative CRC patients still result into poor outcome[2]. Hence, it is urgently needed to look for the effective bio-targets and associated molecular pathways in improving CRC.
Ubiquitination is a sort of pivotal signal transduction mechanism, which modulates immune response and numerous biological processes[3]. It can be regulated by ubiquitinases and de-ubiquitinases to form a kind of pivotal post-translational modification, and this is a reversible process[4]. Ubiquitination affects the stability and activity of protein, and takes part into homeostatic cellular functions[5]. Many ubiquitin-specific proteases have been clarified to join into the progression of CRC. For example, Ubiquitin-specific protease (USP) 38 affects HDAC3 in CRC to modulate the stemness and chemoresistance[6]. Moreover, USP5 stabilizes Tu translation elongation factor to affect tumor growth in CRC[7]. USP22 reduces the mTOR activity to retard the progression of CRC[8]. Besides, USP25 aggravates tumorigenesis in CRC[9].
Ubiquitin-specific protease 21 (USP21) is one de-ubiquitinase that participates into the malignant progression of diversified cancers. For instance, USP21 deubiquitinase strengthens macropinocytosis in pancreatic cancer to trigger oncogenic KRAS bypass[10]. Furthermore, USP21 modulates the STAT3/FOXO1 pathway to accelerate cell proliferation and glycolysis in esophageal cancer[11]. Additionally, USP21 contributes to cell proliferation and migration in cholangiocarcinoma[12]. USP21 has also verified in affecting CRC progression. For instance, USP21 regulates the ubiquitination of Fra-1 to accelerate metastasis in CRC[13]. Besides, LINC00174 enhances USP21 expression to facilitate cell proliferation and invasion in CRC[14]. However, the regulatory functions of USP21 in CRC progression need more investigations.
In this work, it was demonstrated that USP21 accelerated tumorigenicity and stemness of colorectal cancer by deubiquitinating and stabilizing ZEB1. Our findings manifested that this discovery may be helpful for CRC treatment.
Twenty paired CRC tissues and adjacent normal tissues from Chaohu Hospital of Anhui Medical University were utilized for this work. These CRC patients have not received treatment, and have signed the informed consents. This study was approved by the Ethics Committee of Chaohu Hospital of Anhui Medical University (No. KYXM-2022-10-011). These gained tissues were kept in liquid nitrogen for next work.
The normal colonic epithelial cell line (NCM460) and CRC cell lines (HCT-116, SW480, SW620, LoVo) were brought from American Tissue Culture Collection (ATCC, United States). The culturing of these cells was made with RPMI-1640 medium (Gibco, United States) including 10% fetal bovine serum (FBS, Gibico, United States) in an atmosphere with 5% CO2 at 37 °C.
The shRNAs targeting USP21 (sh-USP21) with negative control (sh-NC) and pcDNA3.1 targeting ZEB1 (pcDNA3.1/ZEB1) with negative control (pcDNA3.1), were purchased from GenePharma (Shanghai, China). The transfection of these plasmids into HCT-116 and SW480 cells was made through Lipofectamine 2000 (Invitrogen, United States).
The extraction of RNAs from CRC tissues and cells was made through the TRIzol reagent (Invitrogen, USA). Then, the SuperScript™ II Reverse Transcriptase Kit (Invitrogen, USA) was utilized for doing the transcription from RNA to cDNA. The SYBR Premix Ex Taq™ (Takara, Dalian, China) was adopted to conduct qRT-PCR. The mRNA expression was assessed through the 2−∆∆Ct method. The primer sequences: USP21: forward, 5′-GCAGGATGCCCAAGAGTT-3′, and reverse, 5′-GCAGGGACAGGTCACAAAA-3′; ZEB1: forward, 5′-AGAAGCCAGTGGTCATGATG-3′, and reverse, 5′-CCTCAACAACCTCGTGGAAGCATAC-3′; GAPDH (the internal reference): forward, 5′-GAAGGTGAAGGTCGGAGTC-3′, and reverse, 5′-GAAGATGGTGATGGGATTTC-3′.
The extracted proteins from CRC cells were performed through RIPA buffer. Next, the separation of proteins was done under 10% SDS-PAGE, then the transferring of proteins to PVDF membranes (Beyotime, Shanghai, China) was conducted. Post sealing by non-fat milk, the primary antibodies against USP21 (1 µg/mL; ab112014; Abcam, Shanghai, China), ZEB1 (1:1,000; ab32503) and GAPDH (the internal reference, 1:2,000; ab9485) were mixed into the membranes for 12 h at 4 °C. Next, the appropriate secondary antibodies (1:1,000; ab7090) were also mixed into the membranes for 2 h. Lastly, the chemiluminescence detection kit (Thermo Fisher Scientific, Inc., United States) was adopted for assessing the blots.
HCT-116 and SW480 cells (1,000 cells/well) were put into the 6-well plate, and cultivated for 2 wk. Next, the fixing (4% paraformaldehyde) and staining (0.1% crystal violet) for colonies were made. The images were gained under a microscope.
The cell invasion or migration abilities were evaluated with using Transwell chambers (Corning Life Sciences, Corning, NY, United States) covered with (or not) the Matrigel (Becton Dickinson, United States). The upper chambers were added with HCT-116 and SW480 cells (1 × 105) and RPMI-1640 medium (200 μL), and the lower chambers were added with the DMEM medium (600 μL) with 20% FBS. Post 24 h, the invaded and migrated cells were made for the fixing (90% ethanol) and dyeing (0.1% crystal violet). Eventually, the invaded and migrated cells were counted through a microscope (Olympus Corporation, Tokyo, Japan).
The DMEM/F12 (Gibco, United States) including 1% FBS, 20 ng/mL epithelial growth factor, and 20 ng/mL fibroblast growth factor added into the ultra-low-attachment culture dishes (Corning, United States) was utilized for culturing the HCT-116 and SW480 cells. After 15 days, the spheroids (diameter > 50 µm) were figured up under one microscope (Olympus, Japan).
The lysis of cells was performed under the lysis buffer (P0013, Beyotime) with protease inhibitor cocktail (HY-K0010, MedChemExpress). The cell lysates were added with the indicated antibodies, immunoprecipitation at 4 °C overnight, and then mixed with protein A/G (P2055, Beyotime) at 4 °C for 3 h. After washing, the immunoprecipitates were determined by western blot.
The glutathione-S-transferase (GST, 100 μg) (ab89494, Abcam, Shanghai, China) and the GST-USP21 fusion protein were mixed in 50 μL glutathione agarose for 1 h. His-ZEB1 fusion protein was mixed into immobilized GST-USP21 and GST. Then, the fusion protein (100 μg) was added. With gentle shaking at 4°C for 12 h, the bound proteins were eluted through elution buffer (10 mmol/L glutathione in PBS, pH 8.0), and examined by immunoblotting.
The cell lysates were immunoprecipitated with anti-ZEB1 or anti-ubiquitin antibodies for 24 h at 4 °C. Then, the Protein A-Sepharose beads were appended for 2 h incubation at 4 °C. After being washed with lysis buffer, targeted proteins were collected, and analyzed by western blot.
The Animal Care and Use Committee of Beijing Viewsolid Biotechnology Co. LTD (VS2126A00153) approved this work. Male BALB/c nude mice (5-week-old, n = 15) were bought from the Vital River company (Beijing, China). Mice were randomly separated into three groups (n = 5 for each group). The mice were subcutaneously injected at the right flanks with the transfected CRC cells. Post 28 d, mice were sacrificed, the size, volume and weight of tumors were recorded.
The paraffin-embedded sections (4 μm) of tumor tissues were performed for dewaxing and re-hydration. After blocking, the sections were added with primary antibody Ki67 (ab16667, 1/200, Abcam, Shanghai, China), USP21 (ab246948, 1/500), ZEB1 (ab203829, 1/100) at 4°C for 12 h, and then added with secondary antibody (1:1000, ab7090). Furthermore, the sections were subjected to the staining by diaminobenzidine (DAB) and re-staining by hematoxylin. At last, images were obtained under a microscope (Nikon, Tokyo, Japan).
SPSS 22.0 statistical software (IBM Corp., Armonk, NY, United States) was employed to make the statistical analysis. The data were presented as the mean ± SD. Each experiment was repeated for three times. Pearson correlation analysis was adopted to analyze the correlation between the expressions of USP21 and ZEB1. The survival rate was analyzed through the Kaplan-Meier method. The Student’s t-test or one-way analysis of variance (ANOVA) was utilized for comparisons in two or more groups. P < 0.05 was deemed as statistically significant.
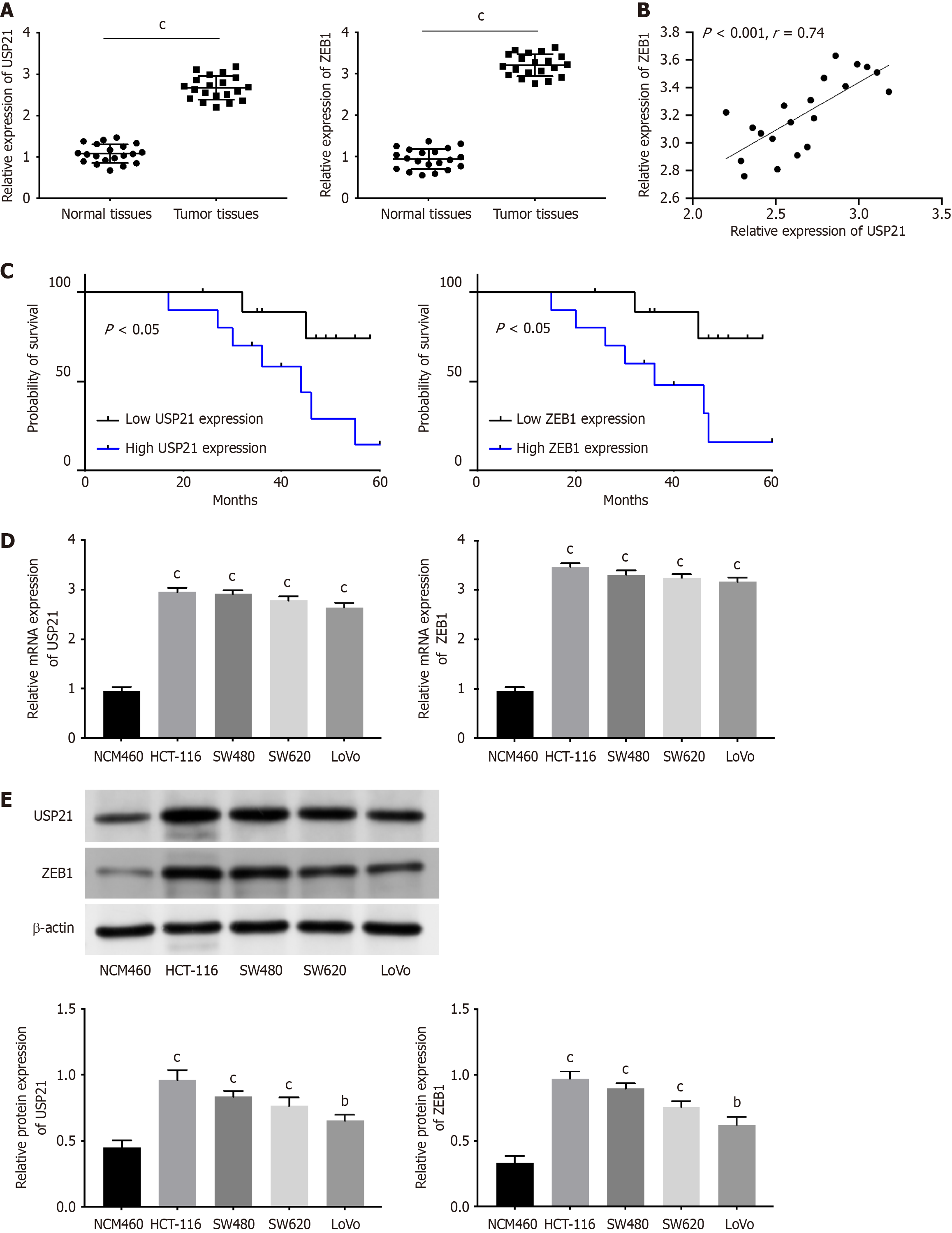
At first, the mRNA expressions of USP21 and ZEB1 were found to be both up-regulated in CRC tissues compared with adjacent normal tissues (Figure 1A). Additionally, there was a positive correlation between USP21 expression and ZEB1 expression in CRC tissues (Figure 1B). The CRC patients with higher USP21 (or ZEB1) expression had poor prognosis (Figure 1C). The mRNA and protein expressions of USP21 and ZEB1 were both higher in CRC cells (HCT-116, SW480, SW620, LoVo) than that in normal colonic epithelial cells (NCM460) (Figure 1D and E). These data uncovered that USP21 and ZEB1 exhibited higher expression, and resulted into poor prognosis.
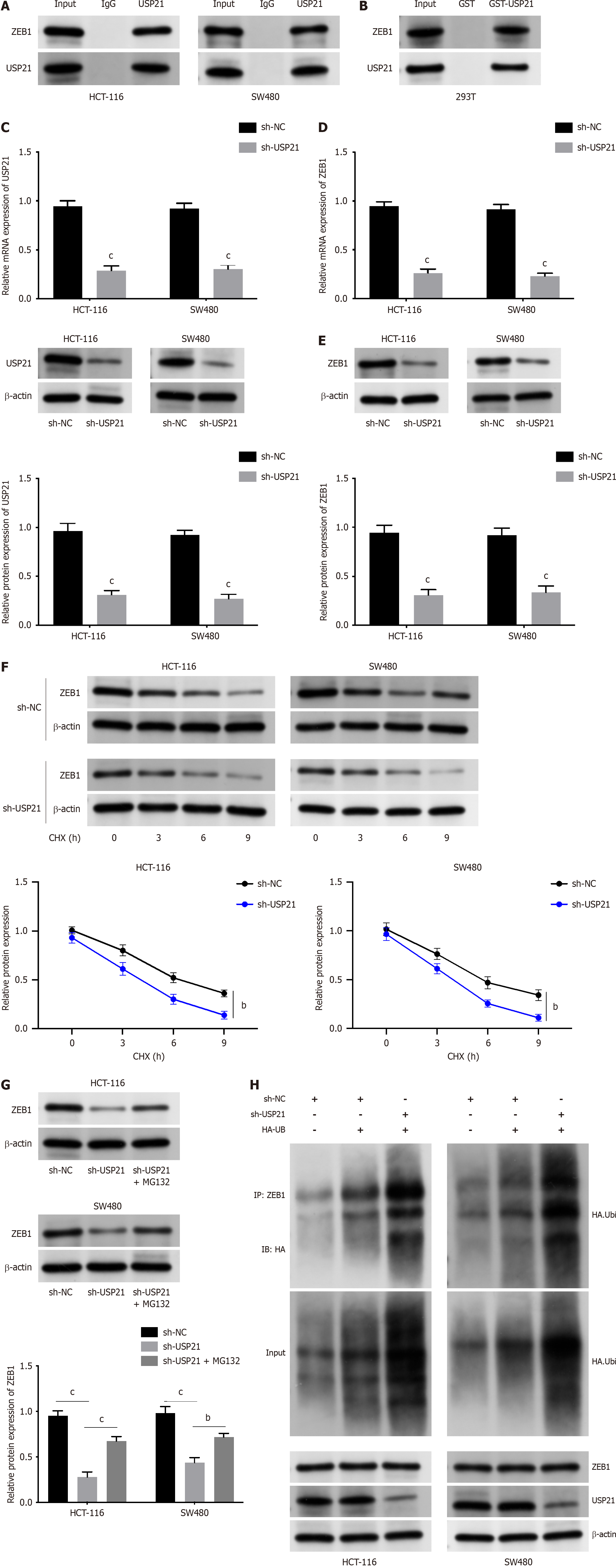
Through Co-IP and GST pull down assays, it was proved that USP21 interacted with ZEB1 (Figure 2A and B). The knockdown efficiency of USP21 was notarized in Figure 2C. Next, it was discovered that ZEB1 mRNA and protein expressions were both decreased after silencing USP21 (Figure 2D and E). After CHX treatment, the stability of ZEB1 was attenuated after USP21 knockdown (Figure 2F). Moreover, ZEB1 protein expression was reduced after USP21 knockdown, but this change was reversed after MG132 treatment (Figure 2G). The ubiquitination level of ZEB1 was strengthened after USP21 inhibition (Figure 2H). Taken together, USP21 contributed to the stability of ZEB1.
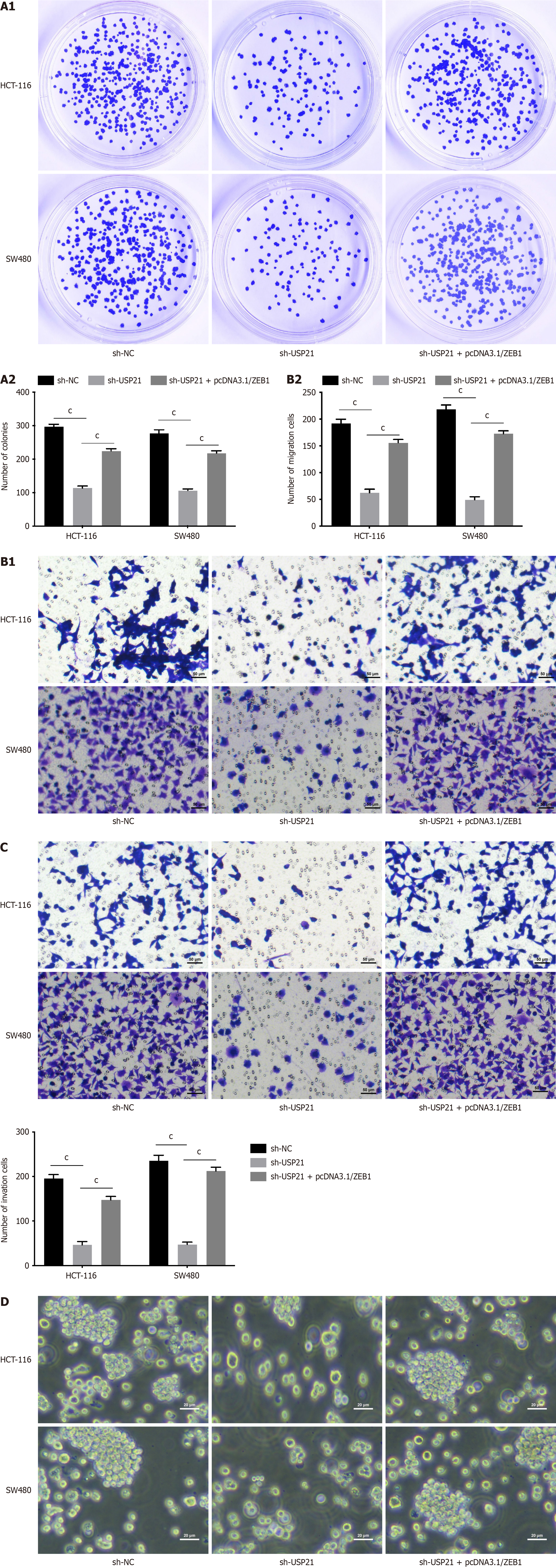
Functional experiments were conducted to verify the regulatory effects of USP21/ZEB1 axis in CRC progression. The cell proliferation was decreased after USP21 knockdown, but this effect was offset after ZEB1 overexpression (Figure 3A). In addition, the cell migration and invasion abilities were attenuated after suppressing USP21, but these changes were reversed after overexpressing ZEB1 (Figure 3B and C). The stemness was reduced after silencing USP21, but this effect was rescued after augmenting ZEB1 (Figure 3D). In a word, USP21 strengthened cell proliferation, migration and stemness through regulating ZEB1.
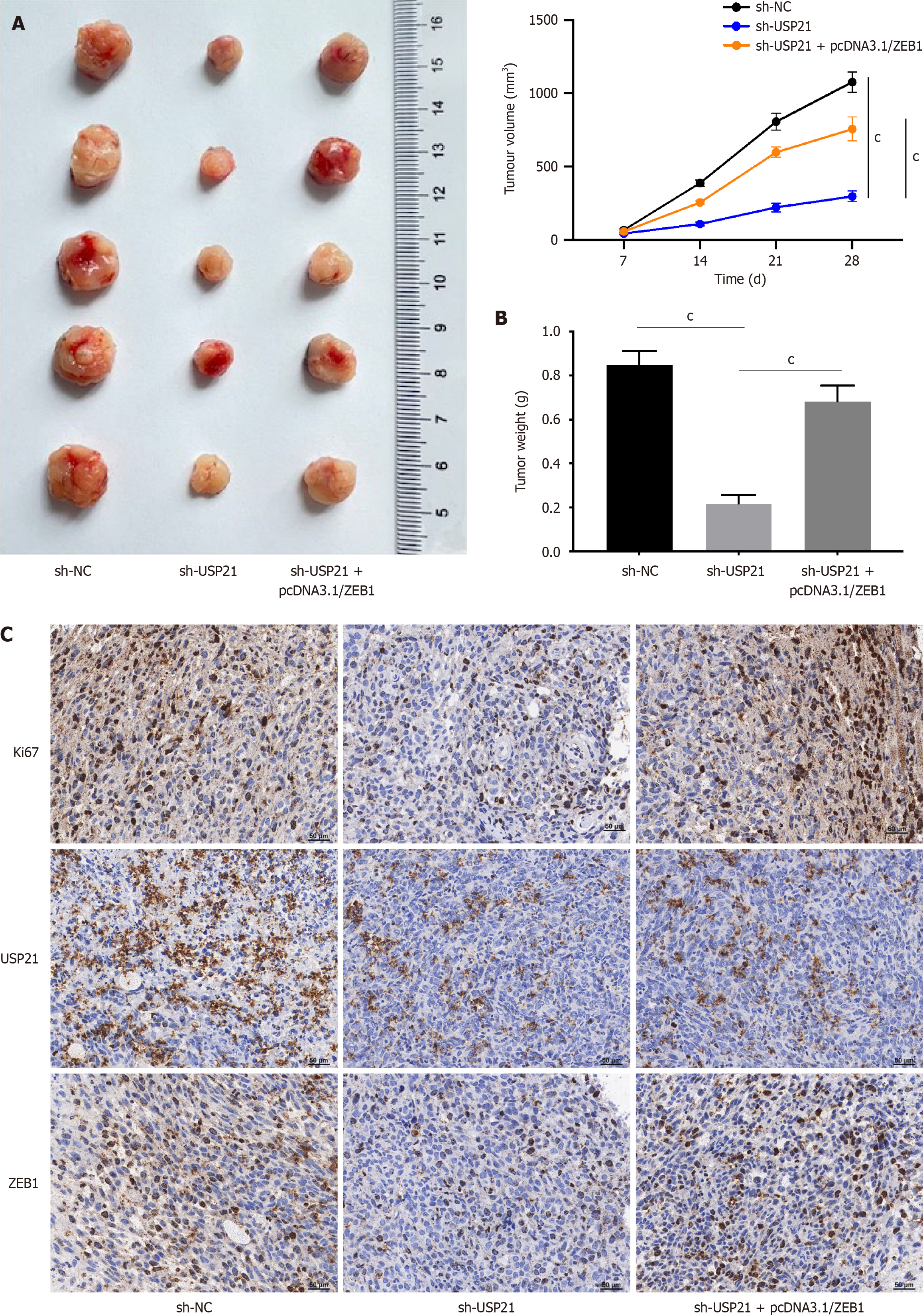
Last, in vivo assays were made. The tumor size, volume and weight were all decreased after USP21 suppression, but these changes were reversed after ZEB1 amplification (Figure 4A and B). Furthermore, the protein expressions of Ki67, USP21 and ZEB1 were down-regulated after silencing USP21, and the down-regulated Ki67 and ZEB1 expressions were rescued after overexpressing ZEB1 (Figure 4C). These findings certified that USP21/ZEB1 axis aggravated tumor growth in vivo.
Abundant reports have confirmed that USP21 takes part into the regulation of cancers, also in CRC[13,14]. USP21 has been proved to regulate the ubiquitination and stability of proteins in multiple cancers. For example, USP21 regulates the deubiquitinating and stabilizing of AURKA, thereby facilitating the progression of laryngeal cancer[15]. USP21 deubiquitinates and stabilizes FOXD1 to accelerate self-renewal and tumorigenicity in glioblastoma[16]. In addition, USP21 deubiquitinates FOXM1 to strengthen radioresistance in cervical cancer through the Hippo signaling pathway[17]. Moreover, USP21 decreases the EZH2 ubiquitination to accelerate cell proliferation and metastasis in bladder carcinoma[18]. However, the regulatory functions of USP21 in CRC progression need more investigations.
Zinc finger E-box-binding homeobox 1 (ZEB1, a transcription factor) has been uncovered to participate in various malignant tumors. For example, the EMT-activator Zeb1 affects cell plasticity and contributes to metastasis in pancreatic cancer[19]. Additionally, CHFR destabilizes ZEB1 to modulate chemoresistance in triple-negative breast cancer[20]. ZEB1 transcriptionally activates PFKM and heightens Warburg effect, thereby aggravating tumorigenesis and metastasis in hepatocellular carcinoma[21]. Furthermore, ZEB1 reduces SLC3A2 to strengthen chemoresistance to cisplatin in ovarian cancer[22]. Interestingly, USP22 exhibits deubiquitinase activity to affect the maintenance of ZEB1 stability[23]. Therefore, we suspected that USP21 can also regulate ZEB1 ubiquitination and stability. However, the regulatory effects of USP21 on ZEB1 in CRC progression keep unclear. In this study, USP21 and ZEB1 exhibited higher expression in CRC, and resulted into poor prognosis. Moreover, the interaction between USP21 and ZEB1 was further verified, and USP21 contributed to the stability of ZEB1 through modulating ubiquitination level.
Stemness is a key process, and USP21 has been testified to affect stemness in cancers. For example, USP21 combines with GATA3 to affect MAPK1 expression in gastric cancer, thereby modulating tumor growth and stemness[24]. USP21 affects stem cell pluripotency through deubiquitylating Nanog[25]. Besides, USP21 affects the activation of the Wnt pathway to facilitate stemness in pancreas cancer[26]. However, the regulatory effects of USP21/ZEB1 axis on stemness in CRC keep indistinct, and need more investigations. In this work, it was verified that USP21 strengthened cell proliferation, migration and stemness through regulating ZEB1. At last, through in vivo assays, it was illustrated that USP21/ZEB1 axis aggravated tumor growth.
For the first time, our findings proved that USP21 promoted tumorigenicity and stemness of CRC by deubiquitinating and stabilizing ZEB1. Nevertheless, there are still some limitations in this study. In the future, more experiments were conducted to notarize the other regulatory effects of USP21/ZEB1 axis on CRC progression.
Previous studies have illustrated that ubiquitin-specific protease 21 (USP21) and ZEB1 has been confirmed to take part into the regulation of cancers’ progression through serving as a facilitator. However, the regulatory functions of USP21, and the relationship between USP21 and ZEB1 in colorectal cancer (CRC) progression need more investigations.
To search useful bio-targets for CRC prognosis and treatment.
In order to probe the regulatory functions and the relationship between USP21 and ZEB1 in CRC progression.
The expressions of USP21 and ZEB1 in CRC were evaluated through real time-quantitative polymerase chain reaction (RT-qPCR) and western blot. The prognosis of GC patients with high or low USP21 (or ZEB1) expression was evaluated. The relationship between USP21 and ZEB1 in CRC progression was validated. The regulatory of USP21/ZEB1 axis in CRC progression was assessed via in vitro and in vivo experiments.
USP21 and ZEB1 exhibited higher expression in CRC, and resulted into poor prognosis. USP21 contributed to the stability of ZEB1 through modulating ubiquitination level. Furthermore, results revealed that USP21 strengthened cell proliferation, migration and stemness through regulating ZEB1.
USP21 promoted tumorigenicity and stemness of CRC by deubiquitinating and stabilizing ZEB1.
Other regulatory functions and related molecular mechanisms of USP21/ZEB1 axis in CRC progression may be investigated in the future, and its application in CRC treatment will be extended.
| 1. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1570] [Cited by in RCA: 3428] [Article Influence: 489.7] [Reference Citation Analysis (4)] |
| 2. | Baidoun F, Elshiwy K, Elkeraie Y, Merjaneh Z, Khoudari G, Sarmini MT, Gad M, Al-Husseini M, Saad A. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr Drug Targets. 2021;22:998-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 195] [Article Influence: 39.0] [Reference Citation Analysis (3)] |
| 3. | Popovic D, Vucic D, Dikic I. Ubiquitination in disease pathogenesis and treatment. Nat Med. 2014;20:1242-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 1095] [Article Influence: 91.3] [Reference Citation Analysis (0)] |
| 4. | Mevissen TET, Komander D. Mechanisms of Deubiquitinase Specificity and Regulation. Annu Rev Biochem. 2017;86:159-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 498] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 5. | Cockram PE, Kist M, Prakash S, Chen SH, Wertz IE, Vucic D. Ubiquitination in the regulation of inflammatory cell death and cancer. Cell Death Differ. 2021;28:591-605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 386] [Article Influence: 77.2] [Reference Citation Analysis (0)] |
| 6. | Zhan W, Liao X, Liu J, Tian T, Yu L, Li R. USP38 regulates the stemness and chemoresistance of human colorectal cancer via regulation of HDAC3. Oncogenesis. 2020;9:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 7. | Xu X, Huang A, Cui X, Han K, Hou X, Wang Q, Cui L, Yang Y. Ubiquitin specific peptidase 5 regulates colorectal cancer cell growth by stabilizing Tu translation elongation factor. Theranostics. 2019;9:4208-4220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 8. | Kosinsky RL, Zerche M, Saul D, Wang X, Wohn L, Wegwitz F, Begus-Nahrmann Y, Johnsen SA. USP22 exerts tumor-suppressive functions in colorectal cancer by decreasing mTOR activity. Cell Death Differ. 2020;27:1328-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Wang XM, Yang C, Zhao Y, Xu ZG, Yang W, Wang P, Lin D, Xiong B, Fang JY, Dong C, Zhong B. The deubiquitinase USP25 supports colonic inflammation and bacterial infection and promotes colorectal cancer. Nat Cancer. 2020;1:811-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 10. | Hou P, Ma X, Yang Z, Zhang Q, Wu CJ, Li J, Tan L, Yao W, Yan L, Zhou X, Kimmelman AC, Lorenzi PL, Zhang J, Jiang S, Spring D, Wang YA, DePinho RA. USP21 deubiquitinase elevates macropinocytosis to enable oncogenic KRAS bypass in pancreatic cancer. Genes Dev. 2021;35:1327-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Wu Y, Guo Y, Wang Q. USP21 accelerates the proliferation and glycolysis of esophageal cancer cells by regulating the STAT3/FOXO1 pathway. Tissue Cell. 2022;79:101916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 12. | Zhou P, Song T, Sun C, He N, Cheng Q, Xiao X, Ran J, Liu M, Xie S. USP21 upregulation in cholangiocarcinoma promotes cell proliferation and migration in a deubiquitinase-dependent manner. Asia Pac J Clin Oncol. 2021;17:471-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Yun SI, Hong HK, Yeo SY, Kim SH, Cho YB, Kim KK. Ubiquitin-Specific Protease 21 Promotes Colorectal Cancer Metastasis by Acting as a Fra-1 Deubiquitinase. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Liang W, Liu D, Wu J. c-JUN-induced upregulation of LINC00174 contributes to colorectal cancer proliferation and invasion through accelerating USP21 expression. Cell Biol Int. 2023;47:1782-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Wang QD, Shi T, Xu Y, Liu Y, Zhang MJ. USP21 contributes to the aggressiveness of laryngeal cancer cells by deubiquitinating and stabilizing AURKA. Kaohsiung J Med Sci. 2023;39:354-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 16. | Zhang Q, Chen Z, Tang Q, Wang Z, Lu J, You Y, Wang H. USP21 promotes self-renewal and tumorigenicity of mesenchymal glioblastoma stem cells by deubiquitinating and stabilizing FOXD1. Cell Death Dis. 2022;13:712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Li Z, Liu X, Yu H, Wang S, Zhao S, Jiang G. USP21 regulates Hippo signaling to promote radioresistance by deubiquitinating FOXM1 in cervical cancer. Hum Cell. 2022;35:333-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Chen Y, Zhou B, Chen D. USP21 promotes cell proliferation and metastasis through suppressing EZH2 ubiquitination in bladder carcinoma. Onco Targets Ther. 2017;10:681-689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Krebs AM, Mitschke J, Lasierra Losada M, Schmalhofer O, Boerries M, Busch H, Boettcher M, Mougiakakos D, Reichardt W, Bronsert P, Brunton VG, Pilarsky C, Winkler TH, Brabletz S, Stemmler MP, Brabletz T. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat Cell Biol. 2017;19:518-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 771] [Article Influence: 85.7] [Reference Citation Analysis (0)] |
| 20. | Luo H, Zhou Z, Huang S, Ma M, Zhao M, Tang L, Quan Y, Zeng Y, Su L, Kim J, Zhang P. CHFR regulates chemoresistance in triple-negative breast cancer through destabilizing ZEB1. Cell Death Dis. 2021;12:820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Zhou Y, Lin F, Wan T, Chen A, Wang H, Jiang B, Zhao W, Liao S, Wang S, Li G, Xu Z, Wang J, Zhang J, Ma H, Lin D, Li Q. ZEB1 enhances Warburg effect to facilitate tumorigenesis and metastasis of HCC by transcriptionally activating PFKM. Theranostics. 2021;11:5926-5938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 22. | Cui Y, Qin L, Tian D, Wang T, Fan L, Zhang P, Wang Z. ZEB1 Promotes Chemoresistance to Cisplatin in Ovarian Cancer Cells by Suppressing SLC3A2. Chemotherapy. 2018;63:262-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Zeng K, Xie W, Wang C, Wang S, Liu W, Su Y, Lin L, Zou R, Sun G, Zhou B, Wang M, Luan R, Bai Y, Huo Y, Kato S, Zhong X, Zhao Y. USP22 upregulates ZEB1-mediated VEGFA transcription in hepatocellular carcinoma. Cell Death Dis. 2023;14:194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 24. | Guo Q, Shi D, Lin L, Li H, Wei Y, Li B, Wu D. De-Ubiquitinating Enzymes USP21 Regulate MAPK1 Expression by Binding to Transcription Factor GATA3 to Regulate Tumor Growth and Cell Stemness of Gastric Cancer. Front Cell Dev Biol. 2021;9:641981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Liu X, Yao Y, Ding H, Han C, Chen Y, Zhang Y, Wang C, Zhang X, Zhai Y, Wang P, Wei W, Zhang J, Zhang L. USP21 deubiquitylates Nanog to regulate protein stability and stem cell pluripotency. Signal Transduct Target Ther. 2016;1:16024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Hou P, Ma X, Zhang Q, Wu CJ, Liao W, Li J, Wang H, Zhao J, Zhou X, Guan C, Ackroyd J, Jiang S, Zhang J, Spring DJ, Wang YA, DePinho RA. USP21 deubiquitinase promotes pancreas cancer cell stemness via Wnt pathway activation. Genes Dev. 2019;33:1361-1366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sahin TT, Turkey; Sato H, Japan S-Editor: Gong ZM L-Editor: A P-Editor: Zheng XM