Published online Feb 15, 2024. doi: 10.4251/wjgo.v16.i2.386
Peer-review started: October 26, 2023
First decision: December 2, 2023
Revised: December 14, 2023
Accepted: January 10, 2024
Article in press: January 10, 2024
Published online: February 15, 2024
Processing time: 98 Days and 12.5 Hours
The prognostic value of the Systemic Inflammation Response Index (SIRI) in advanced pancreatic cancer is recognized, but its correlation with patients´ nutritional status and outcomes remains unexplored.
To study the prognostic significance of SIRI and weight loss in metastatic pancreatic cancer.
The PANTHEIA-Spanish Society of Medical Oncology (SEOM) study is a multicentric (16 Spanish hospitals), observational, longitudinal, non-interventional initiative, promoted by the SEOM Real World-Evidence work group. This pilot study sought to analyze the association between weight loss and inflammatory status as defined by SIRI. The cohort stems from a proof-of-concept pilot study conducted at one of the coordinating centers. Patients with pathologically confirmed metastatic pancreatic adenocarcinoma, treated from January 2020 to January 2023, were included. The index was calculated using the product of neutrophil and monocyte counts, divided by lymphocyte counts, obtained within 15 days before initiation chemotherapy. This study evaluated associations between overall survival (OS), SIRI and weight loss.
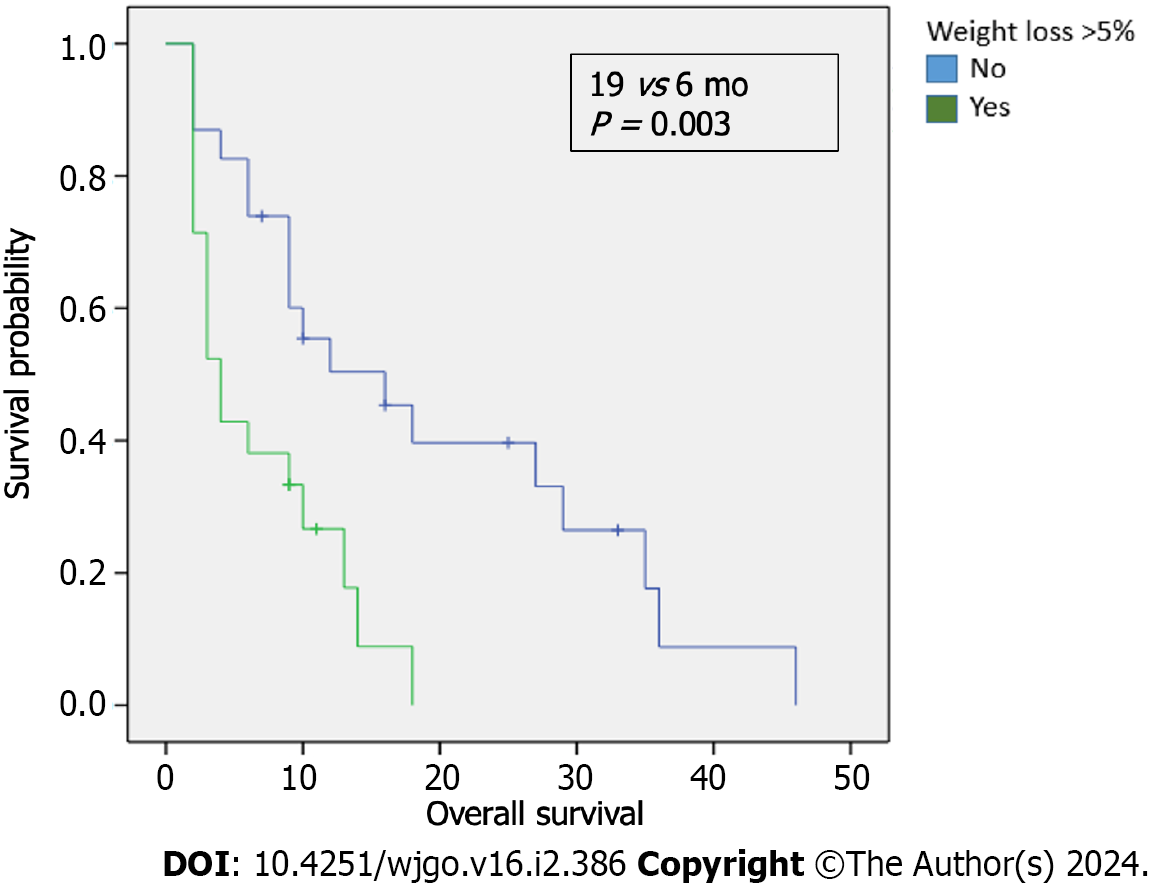
A total of 50 patients were included. 66% of these patients were male and the median age was 66 years. Metastasis sites: 36% liver, 12% peritoneal carcinomatosis, 10% lung, and 42% multiple locations. Regarding the first line palliative chemotherapy treatments: 50% received gemcitabine plus nab-paclitaxel; 28%, modified fluorouracil, leucovorin, irinotecan and oxaliplatin, and 16% were administered gemcitabine. 42% had a weight loss > 5% in the three months (mo) preceding diagnosis. 21 patients with a SIRI ≥ 2.3 × 103/L exhibited a trend towards a lower median OS compared to those with a SIRI < 2.3 × 103/L (4 vs 18 mo; P < 0.000). Among 21 patients with > 5% weight loss before diagnosis, the median OS was 6 mo, in contrast to 19 mo for those who did not experience such weight loss (P = 0.003). Patients with a weight loss > 5% showed higher SIRI levels. This difference was statistically significant (P < 0.000). For patients with a SIRI < 2.3 × 103/L, those who did not lose > 5% of their weight had an OS of 20 mo, compared to 11 mo for those who did (P < 0.001). No association was found between carbohydrate antigen 19-9 levels ≥ 1000 U/mL and weight loss.
A higher SIRI was correlated with decreased survival rates in patients with metastatic pancreatic cancer and associated with weight loss. An elevated SIRI is suggested as a predictor of survival, emphasizing the need for prospective validation in the upcoming PANTHEIA-SEOM study.
Core Tip: Advancements in the therapeutic landscape for pancreatic cancer have been modest in the recent years and the prognostic potential of the Systemic Inflammation Response Index (SIRI) in advanced pancreatic cancer has garnered interest. Originating from the PANTHEIA-Spanish Society of Medical Oncology (SEOM) initiative, a multicentric, observational, study promoted by the SEOM Real World-Evidence work group, our pilot research seeks to bridge this knowledge gap. Specifically, we focused on the interplay between weight loss and the inflammatory state as demarcated by SIRI. A significant correlation between elevated SIRI levels and decreased survival in patients with metastatic pancreatic cancer, with a notable association with weight loss, was observed.
- Citation: Pacheco-Barcia V, Custodio-Cabello S, Carrasco-Valero F, Palka-Kotlowska M, Mariño-Mendez A, Carmona-Bayonas A, Gallego J, Martín AJM, Jimenez-Fonseca P, Cabezon-Gutierrez L. Systemic Inflammation Response Index and weight loss as prognostic factors in metastatic pancreatic cancer: A concept study from the PANTHEIA-SEOM trial. World J Gastrointest Oncol 2024; 16(2): 386-397
- URL: https://www.wjgnet.com/1948-5204/full/v16/i2/386.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i2.386
In recent years, advances in the therapeutic landscape for pancreatic cancer have been modest[1]. Surgical resection remains the sole potential curative treatment; however, only 20% of patients are resectable upon diagnosis[2]. Furthermore, even among those who undergo surgery, the prognosis is unfavorable[3]. Most patients are diagnosed at an advanced stage due to the absence of screening protocols for early diagnosis and the silent nature of the disease. The disease continues to entail a poor overall 5-year survival rate, with less than 5% survival rate in the metastatic setting[4].
Chemotherapy regimens, such as gemcitabine combined with nab-paclitaxel[5] and fluorouracil, leucovorin, irinotecan and oxaliplatin (FOLFIRINOX)[6] have increased response rates and overall survival (OS) and both regimens are the standard of care for first-line treatment. However, considering the suboptimal outcomes and the toxicity associated with these treatments, there is a pressing need to explore prognostic factors to administer these therapies to patients most likely to benefit. Such insights would help categorize patients into distinct subgroups and open paths to more individualized therapeutic strategies based on prognosis. Carbohydrate antigen 19-9 (CA 19-9), a tumor-associated antigen, holds clinical significance as a biomarker to monitor pancreatic cancer survival. In addition, its role as a prognostic biomarker in the advanced disease setting has already been established[7-9]. Nevertheless, it is important to note that CA 19-9 is not tumor-specific. It has a low positive predictive value, may be undetectable in Lewis antigen-negative individuals[10], and could result in false positives during infection or inflammation[11,12]. Consequently, continued research into additional biomarkers that can aid in patient stratification is imperative. Specifically, pancreatic cancer tumorigenesis is known to be associated with a pronounced pro-inflammatory state, and with readily accessible mediators that may serve as prognostic indicators.
Recent studies have highlighted the role of both local immune response and systemic inflammation in cancer progression and OS[13-18]. Cancer-related inflammation manifests both at the site of the tumor and systemically, releasing acute phase proteins and circulating immune cells into the bloodstream. This inflammatory milieu is further enriched by cytokines secreted within the tumor microenvironment, which not only directly affect tumor cells, but also accelerate tumor progression through their interaction with the surrounding chemokine system and by stimulating the epithelial to mesenchymal transition[19]. Against this inflammatory backdrop, the Systemic Inflammatory Response Index (SIRI) based on neutrophil, monocyte, and lymphocyte counts, has emerged as a prognostic factor in pancreatic cancer[14,15,17,20-22]. Its utility extends to predicting OS in those undergoing chemotherapy, reflecting the status of both the immune response and systemic inflammation. This is critical, as systemic inflammation, denoted by elevated markers such as the neutrophil-to-lymphocyte ratio (NLR)[23] or C-reactive protein[24], can modulate chemotherapy responses and thereby impact prognosis.
One of the most conspicuous and clinically evident outcomes of the pro-inflammatory state is the anorexia-cachexia syndrome[25,26]. While malnutrition and cachexia are acknowledged as adverse prognostic factors, the correlation between serum pro-inflammatory mediators and nutritional parameters, as well as the combined or individual contribution of each to prognosis, has yet to be clearly defined.
For patients with advanced pancreatic cancer, the systemic inflammatory response triggered by the disease often precipitates malnutrition[25,26]. This is evident in cancer cachexia, a multifactorial hypermetabolic state characterized by involuntary weight loss, skeletal muscle depletion, and potentially, reduction in body fat. Unfortunately, traditional nutritional interventions might not effectively counteract this condition[25,27].
Therefore, the aim of this pilot study, conducted as part of the PANTHEIA-Spanish Society of Medical Oncology (SEOM) initiative, is to analyze the utility of SIRI as a prognostic factor for response in patients with metastatic pancreatic cancer and to investigate its association with weight loss.
The PANTHEIA-SEOM study, promoted by the SEOM Real World-Evidence working group, is a longitudinal, observational, non-interventional study. Data collection is facilitated electronically through the PANDORA-SEOM tool. The data presented are derived from a pilot study, serving as a proof-of-concept from one of the coordinating centers.
In this pilot study focused on exploratory analysis, we carried out a series of univariate crosses. We also conducted a multivariate analysis to ascertain whether any prognostic effects are additive. Specifically, we aimed to determine whether SIRI and weight loss influence each other or contribute independently to the prognosis.
The study adhered to the ethical standards of the committee responsible for human experimentation (both institutional and national) and aligned with the 1975 Helsinki Declaration, as updated in 2008. The local ethics committee and Institutional Review Board approved the study under version 3.5, code PANT-SP-2023-01. The study was undertaken according to the Strengthening the Reporting of Observational studies in Epidemiology guidelines.
This pilot study encompasses data from 50 consecutive patients diagnosed with pathologically confirmed metastatic pancreatic adenocarcinoma, all of whom underwent chemotherapy at the Hospital Universitario de Torrejon in Madrid between January 2020 to January 2023.
Metastatic disease is consistent with stage IV pancreatic cancer by the International-Union Against Cancer TNM Classification of Malignant Tumors (eighth edition)[28]. For all the patients, the diagnosis of metastases was confirmed through contrast-enhanced abdominal computed tomography (CT) scans performed according to the National Comprehensive Cancer Network guidelines[29].
Data sourced from institutional electronic medical records included routine practice information such as sex, age, comorbidities, Eastern Cooperative Group Performance Status (ECOG-PS), circulating tumor markers, hematology details including neutrophil, lymphocyte, and monocyte counts. Treatment choices were based on clinical judgment and adhered to established guidelines[29]. Laboratory data were obtained from analyses within 15 days before the start of chemotherapy. The upper limit of normal for CA 19-9 is set at 37 U/mL.
Patients received standard chemotherapy combinations or monotherapy based on the treating oncologist’s discretion and according to the department’s usual practice. To evaluate tumor response, CT scans were performed both at the time of diagnosis and approximately 12 weeks later, according to the Response Evaluation Criteria in Solid Tumors criteria[30].
The SIRI was calculated using the formula of pretreatment neutrophil × monocyte/lymphocyte, as previously described[14,15,17]. Based on our earlier findings, the optimal SIRI cutoff value was established at 2.3 × 103/L[14,15]. Weight loss was determined as a percentage by comparing the weight at the time of cancer diagnosis with the patient’s weight in the three months prior.
OS was measured as the time from the start of chemotherapy to either death or the date of last follow-up if the patient was still alive. Progression free survival (PFS) was defined as the period from the beginning of treatment to the date when disease progression was detected or to the last follow-up date if no progression was noted. To analyze these clinical endpoints, the Kaplan-Meier method was used and comparison was performed with the log-rank test. Any variables with a P value < 0.3 in univariate analysis, along with those of clinical significance, were included in the multivariate Cox proportional-hazard model to determine if SIRI operates as an independent prognostic factor for OS and PFS. In the multivariate Cox-regression framework, the “enter” method was chosen to input independent variables. Associations between variables were discerned with Pearson χ2 tests. All statistical analyses were performed using the Statistical Package for Social Sciences, version 22.0 (SPSS Inc., Chicago IL, United States). A two-sided P value < 0.05 was considered statistically significant.
Table 1 summarizes the demographics of the 50 patients included in this study. The median age was 66 years (range, 32-85). Most (66%) were men and half had an ECOG-PS of 1 upon diagnosis. A considerable 78% of patients were devoid of comorbidities as determined by the Charlson Index. Weight loss exceeding 5% in the three months prior to diagnosis was present in 42% (n = 21). All had stage IV disease and received first-line chemotherapy. In 36% of the cases, metastases were located in the liver, 12% in peritoneal carcinomatosis, 10% in the lungs, and 42% had metastases in multiple locations. Regarding treatment specifics, 25 patients received gemcitabine (1000 mg/m2) plus nab-paclitaxel (125 mg/m2) on days 1, 8, and 15 every 4 weeks; 14 patients were treated bi-weekly with modified FOLFIRINOX (mFOLFIRINOX) including fluorouracil (2400 mg/m2), leucovorin (400 mg/m2), irinotecan (180 mg/m2), and oxaliplatin (85 mg/m2); eight patients underwent monotherapy with gemcitabine (1000 mg/m2) on days 1, 8, and 15 every 4 weeks, and the remaining three patients received capecitabine (1000 mg/m2) twice daily for 14 days in a 21 days cycle.
| N = 50 | ||
| Sex | Male | 32 (64%) |
| Female | 18 (36%) | |
| Age | mean ± SD | 63.74 ± 11.2 |
| Median (p25-p75) | 66.7 (58-71) | |
| Range | 32-85 | |
| ECOG-PS | 0 | 16 (32%) |
| 1 | 25 (50%) | |
| 2 | 9 (18%) | |
| Comorbidities: Charlson Index | No | 39 (78%) |
| Yes | 11 (22%) | |
| Weight loss > 5% of body weight1 | No | 23 (46%) |
| Yes | 21 (42%) | |
| N/A | 6 (12%) | |
| 1st line palliative chemotherapy | mFOLFIRINOX | 14 (28%) |
| Gemcitabine + Nab/Paclitaxel | 25 (50%) | |
| Gemcitabine | 8 (16%) | |
| Capecitabine | 3 (6%) | |
| Stage | 4 | 50 (100%) |
| Site of distant metastases | Only liver | 18 (36%) |
| Only peritoneal carcinomatosis | 6 (12%) | |
| Only lung | 5 (10%) | |
| > 1 location | 21 (42%) |
The median OS for the entire cohort was 7 months (mo) with 42 events and 95% confidence interval (CI) of 3.976-10.024. Median PFS was 4 mo with 47 events and a 95%CI of 1.741-6.259. Notably, SIRI demonstrated a significant association with OS: Patients with a SIRI value < 2.3 × 103/L exhibited a median OS of 18 mo, compared to a median of 4 mo for those with SIRI values ≥ 2.3 × 103/L [hazard ratio (HR) = 5.021, 95%CI: 2.375-10.614, P < 0.000] (Figure 1A). Furthermore, SIRI also displayed prognostic relevance for PFS: Patients with a SIRI value < 2.3 × 103/L had a median PFS of 8 mo while those with values of ≥ 2.3 × 103/L showed a median PFS of 4 mo (HR = 4.048, 95%CI: 1.989-8.238, P < 0.000) (Figure 1B).
Median OS varied based on the primary chemotherapy regimens, such that patients treated with gemcitabine plus nab-paclitaxel had a median OS of 6 mo, 9 mo for those on mFOLFIRINOX, and 2 mo for those given gemcitabine alone (P = 0.184). In the exploratory analysis examining the association between the number of metastatic sites and SIRI, a trend towards poorer OS was noted in patients with elevated SIRI and increased tumor burden, indicated by metastases in > 1 site as compared to those with only liver metastasis or peritoneal carcinomatosis (OS of 2 mo vs 4 mo vs 6 mo, respectively; P = 0.42). However, no significant survival differences were found among metastatic sites in patients with low SIRI.
Univariate analysis predicting OS (Table 2) highlighted a statistically significant association between weight loss of > 5% in the three months prior to diagnosis and a SIRI value of ≥ 2.3 × 103/L with OS. However, factors such as sex, age, and CA 19-9 values did not demonstrate prognostic significance for OS. The multivariate analysis demonstrated SIRI to be an independent prognostic factor for OS (Table 3).
| Variable | OS | PFS | ||||||
| Sig | HR | Lower | Upper | Sig | HR | Lower | Upper | |
| Female | 0.373 | 1.34 | 0.701 | 2.573 | 0.236 | 1.457 | 0.782 | 2.713 |
| Age | 0.392 | 0.97 | 0.934 | 1.027 | 0.857 | 0.99 | 0.951 | 1.042 |
| Comorbidities | 0.795 | 1.10 | 0.536 | 2.259 | 0.106 | 1.80 | 0.882 | 3.687 |
| ECOG PS | 0.194 | 1.32 | 0.865 | 2.036 | 0.079 | 1.51 | 0.953 | 2.418 |
| Weight loss > 5%1 | 0.007 | 2.77 | 1.316 | 5.848 | 0.120 | 1.68 | 0.874 | 3.234 |
| Albumin levels | 0.366 | 1.40 | 0.674 | 2.915 | 0.08 | 1.84 | 0.918 | 3.705 |
| Site of metastases | 0.542 | 1.07 | 0.851 | 1.359 | 0.84 | 0.97 | 0.779 | 1.228 |
| CA 19-9 ≥ 37 U/mL | 0.776 | 1.13 | 0.465 | 2.788 | 0.531 | 1.30 | 0.570 | 2.974 |
| CA 19-9 ≥ 1000 U/mL | 0.258 | 1.49 | 0.744 | 3.016 | 0.215 | 1.51 | 0.786 | 2.910 |
| Chemotherapy | 0.264 | 1.16 | 0.892 | 1.519 | 0.017 | 1.432 | 1.065 | 1.924 |
| SIRI ≥ 2.3 × 103 | 0.000 | 5.02 | 2.375 | 10.614 | 0.000 | 4.04 | 1.989 | 8.238 |
| Variable | OS | PFS | ||||||
| Sig | HR | Lower | Upper | Sig | HR | Lower | Upper | |
| Weight loss > 5%1 | 0.158 | 1.94 | 0.772 | 4.901 | 0.838 | 1.08 | 0.481 | 2.465 |
| ECOG PS | 0.512 | 0.754 | 0.325 | 1.753 | 0.043 | 0.381 | 0.150 | 0.968 |
| SIRI ≥ 2.3 × 109 | 0.001 | 4.97 | 1.882 | 13.138 | 0.000 | 8.75 | 2.771 | 27.668 |
| Chemotherapy | 0.118 | 1.55 | 0.894 | 2.714 | 0.001 | 2.867 | 1.524 | 5.396 |
Median PFS for patients treated with gemcitabine plus nab-paclitaxel was 5 mo; with mFOLFIRINOX it was 6 mo, and with gemcitabine alone, it was 2 mo (P = 0.077). The univariate analysis for PFS (Table 2) revealed a statistically significant association with a SIRI value ≥ 2.3 × 103/L, ECOG PS, and chemotherapy. Further, in the multivariate analysis (Table 3), SIRI was the only significant prognostic factor for OS. In the multivariate analysis for PFS (Table 3), a statistically significant association was observed for SIRI, ECOG-PS, and chemotherapy.
We examined the survival outcomes of patients based on weight loss prior to diagnosis. The median OS for patients who experienced a weight loss > 5% in the three months before diagnosis was notably shorter than for those who did not, 6 months compared to 19 mo, respectively, P = 0.003 (Figure 2). In addition, we evaluated the association between SIRI levels and weight loss, finding that patients with a weight loss of > 5% exhibited higher SIRI levels; this difference was statistically significant, P < 0.000 (Table 4). No significant correlation was found between low albumin levels and SIRI (χ² = 1.534; P = 0.21, Table 5). However, within the low SIRI subgroup (< 2.3 × 10³/L), patients with low albumin levels (< 3.5 g/dL) tended to have worse OS (9 mo) compared to those with higher albumin levels (16 mo, P = 0.869). In patients with high SIRI values, albumin levels did not significantly affect survival outcomes. In the exploratory analysis of survival outcomes based on weight loss and SIRI values (Figure 3), the most striking difference was among patients with low SIRI levels (< 2.3 × 103/L): those without weight loss of > 5% had a median OS of 20 mo compared to 11 mo for those who did have a weight loss of > 5%.
| SIRI ≥ 2.3 | ||||
| No | Yes | Total | ||
| Weight loss > 5%1 | No | 20 | 6 | 26 |
| Yes | 3 | 21 | 18 | |
| Total | 23 | 21 | 44 | |
In this pilot study, an elevated SIRI was suggested to be predictive of lower OS, PFS, and increased weight loss. A previous study from our group retrospectively examined SIRI as a prognostic factor in a cohort of patients with advanced pancreatic cancer treated with combination chemotherapy[14,15]. We found that SIRI was predictive of the response to mFOLFIRINOX, a finding later observed by Kamposioras et al[20]. In their study, SIRI demonstrated prognostic value for patients with advanced pancreatic adenocarcinoma treated with mFOLFIRINOX. Notably, their cohort included patients with locally advanced disease and not exclusively those with metastatic disease.
On the other hand, nutritional status and the association between anorexia-cachexia syndrome and systemic inflammatory response measured by SIRI has not yet been studied. Shimizu et al[31] identified a prognostic significance of the lymphocyte monocyte ratio (LMR) and malnutrition measured by the prognostic nutritional index (PNI) in unresectable pancreatic cancer. Specifically, a low LMR and low PNI were linked to poor prognosis. However, key limitations of the study were that patients treated with gemcitabine plus nab/paclitaxel, which is a standard of care, were not included and the LMR is a non-specific marker of inflammation which does not account for the neutrophil count as SIRI does. In contrast to previously reported inflammatory markers, such as LMR and NLR, SIRI has shown a greater ability to predict OS compared to tumor staging or CA 19-9 levels in patients undergoing chemotherapy and can be assessed by routine peripheral blood tests[32-34]. One of the main aspects of SIRI is its consideration of both circulating neutrophils and monocytes. Neutrophils have been demonstrated to be useful in the prognosis of many types of cancers, including pancreatic cancer, while, monocytes have been associated with an increased risk of tumor progression and poor survival[13].
Retrospective series have demonstrated that SIRI is a prognostic factor for pancreatic cancer[14,15,17,20,22]. Qi et al[17] found that higher SIRI levels were associated with worse prognosis in advanced pancreatic cancer. However, the extrapolation of these results to the Western context is difficult because the study had three main limitations: It was conducted in an exclusively Asian population, that might respond differently to chemotherapy compared to Caucasian patients; patients were treated exclusively with gemcitabine monotherapy, which does not represent the standard first-line chemotherapy regimens of mFOLFIRINOX or gemcitabine plus nab/paclitaxel[5,6,29]; and the study included patients with locally advanced disease, who have shown different prognoses compared to metastatic patients[28]. In our study, a trend toward decreased survival was observed in patients exhibiting high SIRI values accompanied by more than one metastatic site, in contrast to those with high SIRI values but with metastases confined to the liver or peritoneal carcinomatosis. This suggests a significant impact of the metastatic site and tumor burden in relation to SIRI values, underscoring the potential importance of these factors in patient prognosis. A correlation was observed between SIRI and cancer-related inflammatory cytokines and chemokines [interleukin-10, C-C motif ligand 17 (CCL17), CCL18, and CCL22]. High SIRI values tended to present higher serum inflammatory cytokine/chemokine levels and shorter PFS.
On the other hand, previous studies have shown an association between systemic inflammatory response and malnutrition in cancer patients. In a cohort of 117 untreated advanced pancreatic cancer patients, Fearon et al[35] ob
Pancreatic cancer patients frequently present malnutrition and body weight loss, mainly during tumor progression[38,39]. Solely assessing weight loss could be insufficient to evaluate malnutrition before initiation chemotherapy. However, it may be beneficial to mitigate the confounding effects of ascites and edema throughout the course of the disease[40,41]. The onset of tumor cachexia depends on factors such as tumor type, stage, location, degree of inflammatory response provoked by the tumor, dietary intake, and the therapeutic approach chosen[25,42]. Tumor cachexia increases morbidity and mortality and impairs quality of life for cancer patients[42,43]. Two studies used the Khorana score, primarily based on analytical parameters such as hemoglobin, platelet and leucocytes, to predict early mortality in resectable pancreatic cancer[44,45]. This score has proved effective in predicting mortality and PFS in cancer patients, specifically those with pancreatic cancer. Therefore, it could be hypothesized that the inclusion of hemoglobin and platelets could enhance the predictive ability of SIRI or build a new mortality prediction model by combining these parameters. Previous studies have identified a prognostic significance linked to body weight changes, suggesting that a body weight loss of > 5% could adversely impact OS[35,46]. A key limitation of studies examining the association between cachexia, inflammation, and survival is their inclusion of patients with both locally advanced and metastatic disease[31,35].
In this analysis, patients with low SIRI levels exhibited significant survival differences when categorized based on weight loss. Specifically, those without a weight loss of > 5% had a survival of 20 mo compared to just 11 mo for patients who lost > 5% of their body weight. While these findings don’t establish a cause-and-effect association, they do hint at the potential roles of both SIRI and pre-diagnosis weight loss as prognostic indicators in the management of the cachexia syndrome. Furthermore, our exploratory analysis revealed that patients with low albumin levels, when coupled with low SIRI values, exhibited worse survival outcomes. This could further validate the role of SIRI as a prognostic marker. Early identification of patients at heightened risk for malnutrition could enable clinicians to optimize treatment strategies in metastatic conditions. Our study offers proof-of-concept evidence that SIRI, a marker of systemic inflammation, may correlate with the nutritional trajectories of patients with metastatic pancreatic cancer. This connection warrants prospective validation in the upcoming PANTHEIA-SEOM study.
This study possesses several limitations worth noting. Primarily, its retrospective and non-randomized nature makes it susceptible to selection bias. The potential use of SIRI to predict nutritional outcome and its association with malnutrition may be influenced by the diverse clinical and biochemical profiles across patients. To mitigate these potential disparities, we performed a multivariate analysis, which highlighted SIRI as an independent prognostic factor for OS. Additionally, the relatively small sample size not only restricts the generalizability of our findings, but also limits our capacity to evaluate more prognostic factors. The data being derived from a single center further narrows the broad applicability of the results. Weight loss was used as a marker of malnutrition, even though malnutrition typically requires a comprehensive assessment that includes clinical, dietary, biochemical, and anthropometric evaluations. Nevertheless, to the best of our knowledge, this study is the first to illustrate the significant predictive ability of SIRI and its association with weight loss in real-world settings for patients with metastatic pancreatic cancer undergoing chemotherapy. The multicentric PANTHEIA-SEOM study will prospectively evaluate the prognostic potential of SIRI and its link to malnutrition.
In conclusion, in this single-center pilot study of patients with metastatic pancreatic cancer, a higher SIRI was correlated with poorer survival outcomes and greater weight loss prior to diagnosis. These findings underscore the importance of prospective validation in the upcoming PANTHEIA-SEOM study.
Cancer-related inflammation manifests both at the site of the tumor and systemically, releasing acute phase proteins and circulating immune cells into the bloodstream. Against this inflammatory backdrop, the systemic inflammatory response index (SIRI) based on neutrophil, monocyte, and lymphocyte counts, has emerged as a prognostic factor in pancreatic cancer. Its utility extends to predicting overall survival (OS) in those undergoing chemotherapy, reflecting the status of both the immune response and systemic inflammation. One of the most conspicuous and clinically evident outcomes of the pro-inflammatory state is the anorexia-cachexia syndrome. While malnutrition and cachexia are acknowledged as adverse prognostic factors, the correlation between serum pro-inflammatory mediators and nutritional parameters, as well as the combined or individual contribution of each to prognosis, has yet to be clearly defined.
For patients with advanced pancreatic cancer, the systemic inflammatory response triggered by the disease often precipitates malnutrition. This is evident in cancer cachexia, a multifactorial hypermetabolic state characterized by involuntary weight loss, skeletal muscle depletion, and potentially, reduction in body fat. Unfortunately, traditional nutritional interventions might not effectively counteract this condition.
The primary objective of this pilot study, conducted as part of the PANTHEIA-Spanish Society of Medical Oncology (SEOM) initiative, is to analyze the utility of SIRI as a prognostic factor for response in patients with metastatic pancreatic cancer and to investigate its association with weight loss.
This study included patients with pathologically confirmed metastatic pancreatic adenocarcinoma, treated from January 2020 to January 2023. The index was calculated using the product of neutrophil and monocyte counts, divided by lymphocyte counts, obtained 15 d before initiation chemotherapy. Originating from the PANTHEIA-SEOM initiative, a multicentric, observational, study promoted by the SEOM Real World-Evidence work group, our pilot research seeks to bridge this knowledge gap. Specifically, we focused on the interplay between weight loss and the inflammatory state as demarcated by SIRI.
We examined the survival outcomes of patients based on weight loss prior to diagnosis. The median OS for patients who experienced a weight loss > 5% in the three months before diagnosis was notably shorter than for those who did not, 6 mo compared to 19 mo, respectively, P = 0.003. However, within the low SIRI subgroup (< 2.3 × 10³/L), patients with low albumin levels (< 3.5 g/dL) tended to have worse OS (9 mo) compared to those with higher albumin levels (16 mo, P = 0.869). In patients with high SIRI values, albumin levels did not significantly affect survival outcomes. In the exploratory analysis of survival outcomes based on weight loss and SIRI values, the most striking difference was among patients with low SIRI levels (< 2.3 × 103/L): Those without weight loss of > 5% had a median OS of 20 mo compared to 11 mo for those who did have a weight loss of > 5%. In this pilot study, an elevated SIRI was suggested to be predictive of lower OS, progression free survival, and increased weight loss.
Our findings indicate a significant correlation between elevated SIRI levels and decreased survival in patients with metastatic pancreatic cancer, with a notable association with weight loss. Furthermore, SIRI emerges as an independent prognostic factor of survival. By viewing SIRI as an emblem of systemic inflammation, we shed light on its potential link with the nutritional trajectories of these patients.
This revelation is paramount, as discerning the intricate web of systemic inflammation, weight loss and the cachexia-anorexia syndrome could pave the way for early interventions, potentially enhancing patient prognosis.
We are grateful to the PANDORA-SEOM tool for data collection and the technology team at IRICOM for its development. Our gratitude extends to all investigators involved in the PANTHEIA-SEOM study.
| 1. | Chiaravalli M, Reni M, O'Reilly EM. Pancreatic ductal adenocarcinoma: State-of-the-art 2017 and new therapeutic strategies. Cancer Treat Rev. 2017;60:32-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 2. | Hartwig W, Werner J, Jäger D, Debus J, Büchler MW. Improvement of surgical results for pancreatic cancer. Lancet Oncol. 2013;14:e476-e485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 287] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 3. | Khorana AA, Mangu PB, Berlin J, Engebretson A, Hong TS, Maitra A, Mohile SG, Mumber M, Schulick R, Shapiro M, Urba S, Zeh HJ, Katz MH. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34:2541-2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 291] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 4. | Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11065] [Cited by in RCA: 12229] [Article Influence: 1358.8] [Reference Citation Analysis (3)] |
| 5. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 5153] [Article Influence: 396.4] [Reference Citation Analysis (12)] |
| 6. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5913] [Article Influence: 394.2] [Reference Citation Analysis (24)] |
| 7. | Bauer TM, El-Rayes BF, Li X, Hammad N, Philip PA, Shields AF, Zalupski MM, Bekaii-Saab T. Carbohydrate antigen 19-9 is a prognostic and predictive biomarker in patients with advanced pancreatic cancer who receive gemcitabine-containing chemotherapy: a pooled analysis of 6 prospective trials. Cancer. 2013;119:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Hess V, Glimelius B, Grawe P, Dietrich D, Bodoky G, Ruhstaller T, Bajetta E, Saletti P, Figer A, Scheithauer W, Herrmann R. CA 19-9 tumour-marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomised controlled trial. Lancet Oncol. 2008;9:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 179] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 9. | Pelzer U, Hilbig A, Sinn M, Stieler J, Bahra M, Dörken B, Riess H. Value of carbohydrate antigen 19-9 in predicting response and therapy control in patients with metastatic pancreatic cancer undergoing first-line therapy. Front Oncol. 2013;3:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Tempero MA, Uchida E, Takasaki H, Burnett DA, Steplewski Z, Pour PM. Relationship of carbohydrate antigen 19-9 and Lewis antigens in pancreatic cancer. Cancer Res. 1987;47:5501-5503. [PubMed] |
| 11. | Mann DV, Edwards R, Ho S, Lau WY, Glazer G. Elevated tumour marker CA19-9: clinical interpretation and influence of obstructive jaundice. Eur J Surg Oncol. 2000;26:474-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 216] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Marrelli D, Caruso S, Pedrazzani C, Neri A, Fernandes E, Marini M, Pinto E, Roviello F. CA19-9 serum levels in obstructive jaundice: clinical value in benign and malignant conditions. Am J Surg. 2009;198:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 174] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 13. | Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493-e503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 951] [Cited by in RCA: 1723] [Article Influence: 156.6] [Reference Citation Analysis (0)] |
| 14. | Pacheco-Barcia V, Mondéjar Solís R, France T, Asselah J, Donnay O, Zogopoulos G, Bouganim N, Guo K, Rogado J, Martin E, Alcindor T, Colomer R. A systemic inflammation response index (SIRI) correlates with survival and predicts oncological outcome for mFOLFIRINOX therapy in metastatic pancreatic cancer. Pancreatology. 2020;20:254-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 15. | Pacheco-Barcia V, Mondéjar Solís R, France T, Asselah J, Donnay O, Zogopoulos G, Bouganim N, Guo K, Rogado J, Martin E, Alcindor T, Colomer R. A Systemic Inflammation Response Index Could be a Predictive Factor for mFOLFIRINOX in Metastatic Pancreatic Cancer. Pancreas. 2019;48:e45-e47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Wei L, Xie H, Yan P. Prognostic value of the systemic inflammation response index in human malignancy: A meta-analysis. Medicine (Baltimore). 2020;99:e23486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 17. | Qi Q, Zhuang L, Shen Y, Geng Y, Yu S, Chen H, Liu L, Meng Z, Wang P, Chen Z. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. 2016;122:2158-2167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 461] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 18. | Bittoni A, Pecci F, Mentrasti G, Crocetti S, Lupi A, Lanese A, Pellei C, Ciotti C, Cantini L, Giampieri R, Lenci E, Giglio E, Bini F, Copparoni C, Meletani T, Baleani MG, Berardi R. Systemic immune-inflammation index: a prognostic tiebreaker among all in advanced pancreatic cancer. Ann Transl Med. 2021;9:251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Germano G, Allavena P, Mantovani A. Cytokines as a key component of cancer-related inflammation. Cytokine. 2008;43:374-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 253] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 20. | Kamposioras K, Papaxoinis G, Dawood M, Appleyard J, Collinson F, Lamarca A, Ahmad U, Hubner RA, Wright F, Pihlak R, Damyanova I, Razzaq B, Valle JW, McNamara MG, Anthoney A. Markers of tumor inflammation as prognostic factors for overall survival in patients with advanced pancreatic cancer receiving first-line FOLFIRINOX chemotherapy. Acta Oncol. 2022;61:583-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Kim JS, Choi M, Kim SH, Hwang HK, Lee WJ, Kang CM. Systemic Inflammation Response Index correlates with survival and predicts oncological outcome of resected pancreatic cancer following neoadjuvant chemotherapy. Pancreatology. 2022;22:987-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 22. | Li S, Xu H, Wang W, Gao H, Li H, Zhang S, Xu J, Zhang W, Xu S, Li T, Ni Q, Yu X, Wu C, Liu L. The systemic inflammation response index predicts survival and recurrence in patients with resectable pancreatic ductal adenocarcinoma. Cancer Manag Res. 2019;11:3327-3337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Garcea G, Ladwa N, Neal CP, Metcalfe MS, Dennison AR, Berry DP. Preoperative neutrophil-to-lymphocyte ratio (NLR) is associated with reduced disease-free survival following curative resection of pancreatic adenocarcinoma. World J Surg. 2011;35:868-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 24. | Wang DS, Luo HY, Qiu MZ, Wang ZQ, Zhang DS, Wang FH, Li YH, Xu RH. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol. 2012;29:3092-3100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 25. | Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, Deutz NEP, Erickson N, Laviano A, Lisanti MP, Lobo DN, McMillan DC, Muscaritoli M, Ockenga J, Pirlich M, Strasser F, de van der Schueren M, Van Gossum A, Vaupel P, Weimann A. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. 2017;36:1187-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 850] [Article Influence: 94.4] [Reference Citation Analysis (0)] |
| 26. | Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, Compher C, Correia I, Higashiguchi T, Holst M, Jensen GL, Malone A, Muscaritoli M, Nyulasi I, Pirlich M, Rothenberg E, Schindler K, Schneider SM, de van der Schueren MA, Sieber C, Valentini L, Yu JC, Van Gossum A, Singer P. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36:49-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 908] [Cited by in RCA: 1627] [Article Influence: 162.7] [Reference Citation Analysis (0)] |
| 27. | Thompson KL, Elliott L, Fuchs-Tarlovsky V, Levin RM, Voss AC, Piemonte T. Oncology Evidence-Based Nutrition Practice Guideline for Adults. J Acad Nutr Diet. 2017;117:297-310.e47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 28. | Liao X, Zhang D. The 8th Edition American Joint Committee on Cancer Staging for Hepato-pancreato-biliary Cancer: A Review and Update. Arch Pathol Lab Med. 2021;145:543-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 29. | National Comprehensive Cancer Network. National Comprehensive Cancer Network (NCCN) guidelines - Pancreatic Adenocarcinoma. [cited 7 October 2023]. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1455. |
| 30. | Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, Hayes W, Hodi FS, Hoekstra OS, Huang EP, Lin N, Liu Y, Therasse P, Wolchok JD, Seymour L. RECIST 1.1-Update and clarification: From the RECIST committee. Eur J Cancer. 2016;62:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 1467] [Article Influence: 146.7] [Reference Citation Analysis (0)] |
| 31. | Shimizu T, Taniguchi K, Asakuma M, Tomioka A, Inoue Y, Komeda K, Hirokawa F, Uchiyama K. Lymphocyte-to-Monocyte Ratio and Prognostic Nutritional Index Predict Poor Prognosis in Patients on Chemotherapy for Unresectable Pancreatic Cancer. Anticancer Res. 2019;39:2169-2176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Imtiaz F, Shafique K, Mirza SS, Ayoob Z, Vart P, Rao S. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int Arch Med. 2012;5:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 408] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 33. | Ahn KS, Hwang JY, Han HS, Kim ST, Hwang I, Chun YO. The impact of acute inflammation on progression and metastasis in pancreatic cancer animal model. Surg Oncol. 2018;27:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Hlubocky FJ, Taylor LP, Marron JM, Spence RA, McGinnis MM, Brown RF, McFarland DC, Tetzlaff ED, Gallagher CM, Rosenberg AR, Popp B, Dragnev K, Bosserman LD, Dudzinski DM, Smith S, Chatwal M, Patel MI, Markham MJ, Levit K, Bruera E, Epstein RM, Brown M, Back AL, Shanafelt TD, Kamal AH. A Call to Action: Ethics Committee Roundtable Recommendations for Addressing Burnout and Moral Distress in Oncology. JCO Oncol Pract. 2020;16:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 35. | Fearon KC, Voss AC, Hustead DS; Cancer Cachexia Study Group. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr. 2006;83:1345-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 516] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 36. | Saroul N, Puechmaille M, Lambert C, Hassan AS, Biau J, Lapeyre M, Mom T, Bernadach M, Gilain L. Prognosis in Head and Neck Cancer: Importance of Nutritional and Biological Inflammatory Status. Otolaryngol Head Neck Surg. 2022;166:118-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Sachlova M, Majek O, Tucek S. Prognostic value of scores based on malnutrition or systemic inflammatory response in patients with metastatic or recurrent gastric cancer. Nutr Cancer. 2014;66:1362-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Schrader H, Menge BA, Belyaev O, Uhl W, Schmidt WE, Meier JJ. Amino acid malnutrition in patients with chronic pancreatitis and pancreatic carcinoma. Pancreas. 2009;38:416-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Goonetilleke KS, Siriwardena AK. Systematic review of peri-operative nutritional supplementation in patients undergoing pancreaticoduodenectomy. JOP. 2006;7:5-13. [PubMed] |
| 40. | Falconer JS, Fearon KC, Ross JA, Elton R, Wigmore SJ, Garden OJ, Carter DC. Acute-phase protein response and survival duration of patients with pancreatic cancer. Cancer. 1995;75:2077-2082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 41. | Wigmore SJ, Plester CE, Richardson RA, Fearon KC. Changes in nutritional status associated with unresectable pancreatic cancer. Br J Cancer. 1997;75:106-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 174] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 42. | Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4623] [Cited by in RCA: 4089] [Article Influence: 272.6] [Reference Citation Analysis (0)] |
| 43. | Pressoir M, Desné S, Berchery D, Rossignol G, Poiree B, Meslier M, Traversier S, Vittot M, Simon M, Gekiere JP, Meuric J, Serot F, Falewee MN, Rodrigues I, Senesse P, Vasson MP, Chelle F, Maget B, Antoun S, Bachmann P. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br J Cancer. 2010;102:966-971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 348] [Cited by in RCA: 474] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 44. | Khorana AA, Kuderer NM, McCrae K, Milentijevic D, Germain G, Laliberté F, MacKnight SD, Lefebvre P, Lyman GH, Streiff MB. Cancer associated thrombosis and mortality in patients with cancer stratified by khorana score risk levels. Cancer Med. 2020;9:8062-8073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 45. | Sohal DP, Shrotriya S, Glass KT, Pelley RJ, McNamara MJ, Estfan B, Shapiro M, Wey J, Chalikonda S, Morris-Stiff G, Walsh RM, Khorana AA. Predicting early mortality in resectable pancreatic adenocarcinoma: A cohort study. Cancer. 2015;121:1779-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Ryan AM, Prado CM, Sullivan ES, Power DG, Daly LE. Effects of weight loss and sarcopenia on response to chemotherapy, quality of life, and survival. Nutrition. 2019;67-68:110539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 152] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kitamura K, Japan; Pisani LF, Italy S-Editor: Wang JJ L-Editor: A P-Editor: Zhang XD