Published online Dec 15, 2024. doi: 10.4251/wjgo.v16.i12.4778
Revised: September 19, 2024
Accepted: October 12, 2024
Published online: December 15, 2024
Processing time: 83 Days and 0.9 Hours
Colorectal cancer (CRC) ranks third in the number of cancers mainly because of the inability to diagnose it at an early stage. The pathogenesis of CRC is com
Core Tip: The expression of cyclin-dependent kinase 9 (CDK9) was correlated with elevated autophagy levels in colon cancer, and high expression of CDK9 indicates a poor prognosis in colorectal cancer (CRC). The incidence of autophagy and the expressions of Beclin 1 and ATP binding cassette transporter G2 were different between left and right colon cancer. The roles of CDK9, ATP binding cassette transporter G2 and Beclin 1 in CRC were clarified, underlining the linkages among them and providing potential therapeutic targets of CRC.
- Citation: Shao ZB, He K, Su YB, Shi Z. Crosslink among cyclin-dependent kinase 9, ATP binding cassette transporter G2 and Beclin 1 in colorectal cancer. World J Gastrointest Oncol 2024; 16(12): 4778-4781
- URL: https://www.wjgnet.com/1948-5204/full/v16/i12/4778.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i12.4778
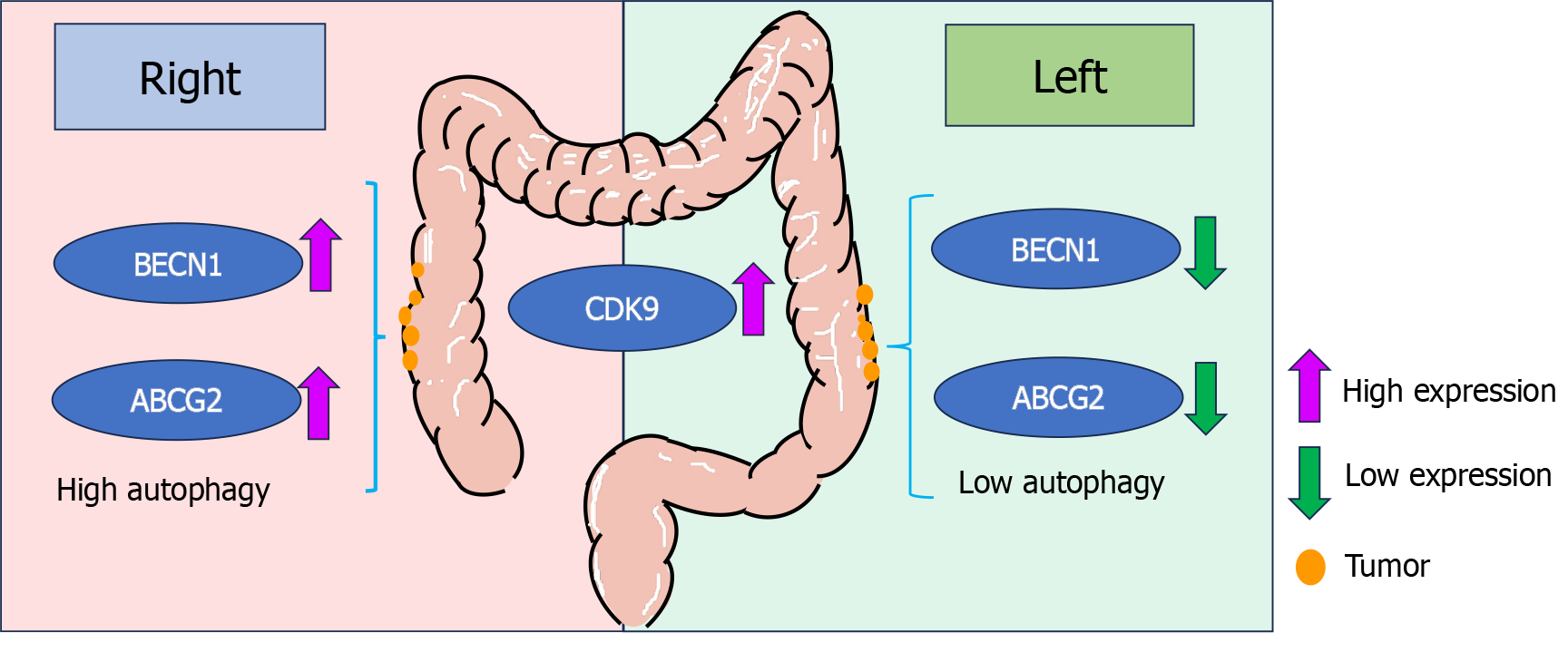
A clinical and translational study by Zheng et al[1], reported that the rate of autophagy and the expressions of Beclin 1 (BECN1) and ATP binding cassette transporter G2 (ABCG2) differed between left and right colon cancer tissues. Autophagy may be associated with chemotherapy resistance in colorectal cancer (CRC) patients. And cyclin dependent kinase 9 (CDK9) is highly expressed in CRC that can be used as a prognostic marker in CRC patients. This research could provide a theoretical basis for the exploration of CDK9 and autophagy inhibitors in combination therapy to enhance tumor cell sensitivity to chemotherapy.
CRC ranks third in the number of cancers mainly owing to the inability to diagnose it at an early stage[2]. The pathogenesis of CRC is complexed, which is the result of the complex interaction of a lot of genetic and environmental factors[3]. At present, one of the main treatments for CRC is chemotherapy. But the primary cause of CRC treatment failure is drug resistance[4]. Consequently, elucidating research into molecular mechanisms of drug resistance can be beneficial to develop new diagnostic or therapeutic strategies to overcome the challenges in the treatment of CRC.
ABCG2, as an important member of the ATP-binding cassette transmembrane transporter superfamily, plays a significant role in cancer multidrug resistance[5]. Several agents have been reported to be able to reverse ABCG2-mediated multidrug resistance in CRC cells by inhibiting the transporter activity of ABCG2[6,7]. Now, we are interested in the authors’ new finding that the expression level of ABCG2 in right colon cancer was higher than that in paracarcinoma tissue, but the expression level of ABCG2 was not significantly different between left colon cancer and paracarcinoma tissue. These findings might be useful for gaining insight into the pathogenesis of left and right colon cancer and improving treatment strategies for CRC therapy.
Autophagy refers to a catabolic process in which macromolecular substances such as misfolded proteins and damaged organelles are transported to lysosomes for degradation[8]. It can prevent genome damage and induce cancer cell death. And on the other hand, autophagy is a pro-oncogenic mechanism that provides drug resistance to cancer cells and promotes cancer cell growth[9]. The sensitivity of cancer cells to chemotherapeutic drugs can be restored by the use of autophagy inhibitors, such as chloroquine, or by the knockdown of autophagy-related proteins, including BECN1, autophagy-related gene 7, and autophagy-related gene 10[10]. Currently, autophagy inhibitors are promising for cancer treatment. Some small molecule autophagy inhibitors have been discovered according to the autophagy process[10]. Recent research has revealed FDW028 (a novel FUT8 inhibitor) exhibits potent anti CRC effects by facilitating lysosomal degradation of CD276 through the chaperone-mediated autophagy pathway[11]. Erianin (a natural product) can induce autophagy-dependent ferroptosis and inhibit tumor growth and metastasis in KRASG13D CRC[12]. Strigolactones are endogenous plant hormones that can act as a potential autophagy inhibitor by blocking autophagosome-lysosome fusion in HCT116 CRC cells[13]. BECN1, a key autophagy regulator, serves as a potential therapeutic target and is associated with chemotherapeutic resistance in cancers[14]. Previous studies have demonstrated that JAK2-depended BECN1 phosphorylation may confer chemotherapy resistance in CRC[15]. Based on Zheng et al’s research, the expression of BECN1 may be different between left and right colon cancer[1]. This research provided new ideas for further investigation on the drug resistance in CRC.
CDK family, a large class of serine/threonine protein kinases, plays a vital role in cell cycle progression and gene transcription regulation. There have been some reports on CDK inhibitors in the treatment of CRC. Zeng et al[16] proposed that CDK1 serves as a potential target for oxaliplatin-resistant CRC treatment. Lee et al[17] reported that the combination of palbociclib (CDK 4/6 inhibitor) and gedatolisib (phosphatidylinositol 3-kinase/mammalian target of rapamycin dual inhibitor) has synergistic anti-proliferative effects in both wild-type and mutated CRC cell lines. In a recent study, Wang et al[18] revealed that CDK3, CDK5 and CDK8 functioned as potential diagnostic markers for CRC. These findings give rationale for the application of CDK inhibitors in CRC treatment. CDK9 is an important member of the CDK family that regulates the transcription of genes such as chemoresistant genes in tumors, and some CDK9 inhibitors have entered clinical trials in combination with other drugs[19]. According to Zheng et al[1], the expression of CDK9 is positively correlated with autophagy in colon cancer. This finding may provide valuable information for further research on targeting CDK9 as a therapeutic strategy for CRC. The relationships between CDK9, BECN1, ABCG2 and autophagy are show in Figure 1.
The expression of CDK9 was correlated with elevated autophagy levels in colon cancer. Additionally, the expressions of ABCG2 and BECN1 were different between left and right colon cancer patients. Targeting CDK9, ABCG2 and BECN1 might be potential therapeutic strategies for CRC.
Thanks to all authors for their efforts in this work.
| 1. | Zheng L, Lu J, Kong DL. Expression of cyclin-dependent kinase 9 is positively correlated with the autophagy level in colon cancer. World J Gastrointest Oncol. 2024;16:314-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (2)] |
| 2. | Zhang Y, Wang Y, Zhang B, Li P, Zhao Y. Methods and biomarkers for early detection, prediction, and diagnosis of colorectal cancer. Biomed Pharmacother. 2023;163:114786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 89] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 3. | Ionescu VA, Gheorghe G, Bacalbasa N, Chiotoroiu AL, Diaconu C. Colorectal Cancer: From Risk Factors to Oncogenesis. Medicina (Kaunas). 2023;59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 106] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 4. | Zhang L, Lu X, Xu Y, La X, Tian J, Li A, Li H, Wu C, Xi Y, Song G, Zhou Z, Bai W, An L, Li Z. Tumor-associated macrophages confer colorectal cancer 5-fluorouracil resistance by promoting MRP1 membrane translocation via an intercellular CXCL17/CXCL22-CCR4-ATF6-GRP78 axis. Cell Death Dis. 2023;14:582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 5. | Li W, Zhang H, Assaraf YG, Zhao K, Xu X, Xie J, Yang DH, Chen ZS. Overcoming ABC transporter-mediated multidrug resistance: Molecular mechanisms and novel therapeutic drug strategies. Drug Resist Updat. 2016;27:14-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 514] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 6. | Yu ZZ, Xu BQ, Wang YY, Zhang PW, Shu YB, Shi Z. GSK2606414 Sensitizes ABCG2-Overexpressing Multidrug-Resistant Colorectal Cancer Cells to Chemotherapeutic Drugs. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 7. | Liu K, Chen Y, Shi XB, Xing ZH, He ZJ, Wang ST, Li YC, Liu WJ, Zhang PW, Yu ZZ, Mo XM, Shi XY, Chen ZS, Shi Z. Inhibiting the Activity of ABCG2 by KU55933 in Colorectal Cancer. Recent Pat Anticancer Drug Discov. 2022;17:387-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Reference Citation Analysis (0)] |
| 8. | Cao W, Li J, Yang K, Cao D. An overview of autophagy: Mechanism, regulation and research progress. Bull Cancer. 2021;108:304-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 292] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 9. | Kocaturk NM, Akkoc Y, Kig C, Bayraktar O, Gozuacik D, Kutlu O. Autophagy as a molecular target for cancer treatment. Eur J Pharm Sci. 2019;134:116-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 291] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 10. | Chen JL, Wu X, Yin D, Jia XH, Chen X, Gu ZY, Zhu XM. Autophagy inhibitors for cancer therapy: Small molecules and nanomedicines. Pharmacol Ther. 2023;249:108485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 11. | Wang M, Zhang Z, Chen M, Lv Y, Tian S, Meng F, Zhang Y, Guo X, Chen Y, Yang M, Li J, Qiu T, Xu F, Li Z, Zhang Q, Yang J, Sun J, Zhang H, Zhang H, Li H, Wang W. FDW028, a novel FUT8 inhibitor, impels lysosomal proteolysis of B7-H3 via chaperone-mediated autophagy pathway and exhibits potent efficacy against metastatic colorectal cancer. Cell Death Dis. 2023;14:495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 12. | Miao Q, Deng WQ, Lyu WY, Sun ZT, Fan SR, Qi M, Qiu SH, Zhu YR, Lin JP, Chen MF, Deng LJ. Erianin inhibits the growth and metastasis through autophagy-dependent ferroptosis in KRAS(G13D) colorectal cancer. Free Radic Biol Med. 2023;204:301-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 13. | Yang ST, Fan JB, Liu TT, Ning S, Xu JH, Zhou YJ, Deng X. Development of Strigolactones as Novel Autophagy/Mitophagy Inhibitors against Colorectal Cancer Cells by Blocking the Autophagosome-Lysosome Fusion. J Med Chem. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Prerna K, Dubey VK. Beclin1-mediated interplay between autophagy and apoptosis: New understanding. Int J Biol Macromol. 2022;204:258-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 133] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 15. | Hu F, Song D, Yan Y, Huang C, Shen C, Lan J, Chen Y, Liu A, Wu Q, Sun L, Xu F, Hu F, Chen L, Luo X, Feng Y, Huang S, Hu J, Wang G. IL-6 regulates autophagy and chemotherapy resistance by promoting BECN1 phosphorylation. Nat Commun. 2021;12:3651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 183] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 16. | Zeng K, Li W, Wang Y, Zhang Z, Zhang L, Zhang W, Xing Y, Zhou C. Inhibition of CDK1 Overcomes Oxaliplatin Resistance by Regulating ACSL4-mediated Ferroptosis in Colorectal Cancer. Adv Sci (Weinh). 2023;10:e2301088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 100] [Reference Citation Analysis (0)] |
| 17. | Lee CL, Cremona M, Farrelly A, Workman JA, Kennedy S, Aslam R, Carr A, Madden S, O'Neill B, Hennessy BT, Toomey S. Preclinical evaluation of the CDK4/6 inhibitor palbociclib in combination with a PI3K or MEK inhibitor in colorectal cancer. Cancer Biol Ther. 2023;24:2223388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Wang D, Zhou Y, Hua L, Li J, Zhu N, Liu Y. CDK3, CDK5 and CDK8 Proteins as Prognostic and Potential Biomarkers in Colorectal Cancer Patients. Int J Gen Med. 2022;15:2233-2245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Mandal R, Becker S, Strebhardt K. Targeting CDK9 for Anti-Cancer Therapeutics. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/