Published online Dec 15, 2024. doi: 10.4251/wjgo.v16.i12.4728
Revised: August 11, 2024
Accepted: August 20, 2024
Published online: December 15, 2024
Processing time: 191 Days and 5.1 Hours
Transhepatic arterial chemoembolization (TACE), as a local treatment, has been widely used in the treatment of unresectable liver cancer. The introduction of drug carrier microspheres has brought new hope for the therapeutic effect of TACE. Microspheres can realize the slow release and directional delivery of drugs, reduce systemic toxicity and improve local curative effect.
To compare the effectiveness of traditional transcatheter arterial chemoembolization against microsphere-assisted transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma that is incurable.
We searched the PubMed, Embase, Cochrane Library, and CNKI databases for clinical trials of drug-luting beads TACE (DEB-TACE) vs conventional TACE (cTACE) for the treatment of unresectable liver cancer. We screened references based on inclusion and exclusion criteria and then selected valid data for meta-analysis using RevMan 53 software. The complete response (CR) rate, partial response (PR) rate, postoperative stable disease (SD) rate, and 6-month and 12-month survival rates were compared.
A total of 12 articles were included, including 1177 patients, 519 of whom received DEB-TACE and 658 of whom received cTACE. The CR rate in the DEB-TACE group was much greater than that in the cTACE group [relative risk (RR) = 1.42, 95%CI: 1.18–1.72, P = 0.0002]. The 12-month survival rate significantly increased (RR = 1.09; 95%CI: 1.01- 1.17, P = 0.03); the PR rate (RR = 1.13; 95%CI: 0.97-1.30, P = 0.12); the SD rate (RR = 0.82; 95%CI: 0.64-1.05, P = 0.12); and the 6-month survival rate (RR = 1.05; 95%CI: 1.00-1.10, P = 0.07). There was no significant difference (P < 0.05).
Compared with those of iodized oil TACE, the drug-loaded microspheres tended to have therapeutic advantages.
Core Tip: This study systematically evaluated the efficacy and safety of transhepatic arterial chemoembolization with drug-loaded microspheres in the treatment of unresectable primary liver cancer. Through a meta-analysis of relevant literature, the effect of this treatment in prolonging survival, relieving symptoms and improving quality of life of patients was discussed, and the incidence of adverse reactions and complications was evaluated, so as to provide more scientific treatment recommendations for clinicians.
- Citation: Deng J, Mi YH, Xie L, Sun XX, Liu DH, Long HJ, He LY, Wu DH, Shang HC. Efficacy and safety of transhepatic arterial chemoembolization with drug-loaded microspheres in unresectable primary liver cancer. World J Gastrointest Oncol 2024; 16(12): 4728-4737
- URL: https://www.wjgnet.com/1948-5204/full/v16/i12/4728.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i12.4728
Each year, nearly 500000 hepatocelluar carcinoma (HCC) patients receive a diagnosis, accounting for more than 5% of all cancer cases. The recurrence rate of HCC is as high as 50% within 3 years and 70% at 5 years after hepatectomy[1-4]. Despite recent advances in surgical techniques and preoperative medical treatment, the incidence of postoperative complications in patients with cirrhosis is still as high as 42%[5]. Transhepatic arterial chemoembolization (TACE) is widely used as a treatment for patients with clinically unresectable HCC. It is composed of conventional TACE (cTACE) and the drug-luting beads TACE (DEB-TACE), which use iodide and DEB, respectively, as chemotherapy drug carriers[6]. DEB-TACE, a novel drug delivery and embolization system, delivers local, controlled, and sustained doses of chemotherapeutic agents to the tumor site through the blood vessels of highly vascularized malignancies. Numerous studies[7-9] have shown that DEB-TACE has a significant advantage in terms of overall survival or tumor response and can reduce the occurrence of adverse events. Despite the extensive studies conducted on cTACE and DEB-TACE, the role of DEB-TACE in comparison to that of cTACE remains relatively inconsistent.
Our search time was July 2023, and we searched the PubMed, Embase, Cochrane Library, and CNKI databases for controlled clinical studies on DEB-TACE and TACE for comparative treatment of unresectable liver cancer. Using a combination of subject words and free words, we ended the sentence with "DEB" OR "drug eluting" OR "drug eluting microsphere" OR "doxorubicin eluting". The search terms used were "TACE "OR "transcatheterarterial chemoembolization" AND "hepatocellular carcinoma" OR "adenocarcinoma" OR "carcinoma" OR "cancer" OR "neoplasm" OR " tumor".
Inclusion criteria: (1) Clinically controlled studies comparing DEB-TACE with cTACE for the treatment of unresectable liver cancer, regardless of age, sex, or nationality; (2) Had confirmed unresectable liver cancer for which a radiological or histopathological diagnosis was made in addition to alpha-fetoprotein levels; and (3) Had complete primary outcomes, including a complete response (CR) rate, a partial response (PR) rate, a stable disease (SD) rate, and 6- and 12-month survival.
Exclusion criteria: (1) Were conference papers, case reports, editorials, and nonhuman studies; and (2) Had repeated publications of the same data or incomplete data in the literature.
Two researchers independently screened the literature, extracted the data and cross-checked the data. Disagreements were resolved through discussion or by discussion with a third party. The following data were extracted: (1) Basic information of the trial, author name, nationality, publication time, sample size, patient sex, age, tumor diameter, and Child-Pugh grade; (2) Main results, namely, the CR rate, PR rate, SD rate, 6-month survival rate, and 12-month survival rate; and (3) The Newcastle-Ottawa Scale (NOS) score, which was used to assess the risk of bias.
RevMan 5.3 was used to determine the relative risk in each study [relative risk (RR), 95%CI]. A P > 0.05 indicated that there was no significant difference between the DEB-TACE and cTACE groups. Each study used the value of I2, which represents the percentage of total variation, to assess statistical heterogeneity. Generally, we used the fixed-effects model for analysis when I2 was less than 50%, assuming no significant difference in heterogeneity. A random effects model was used to analyze the heterogeneity when the I2 was greater than 50%. We used a forest plot to graphically represent and evaluate the treatment effect and the symmetry of the funnel plot to visually assess the presence of bias risk.
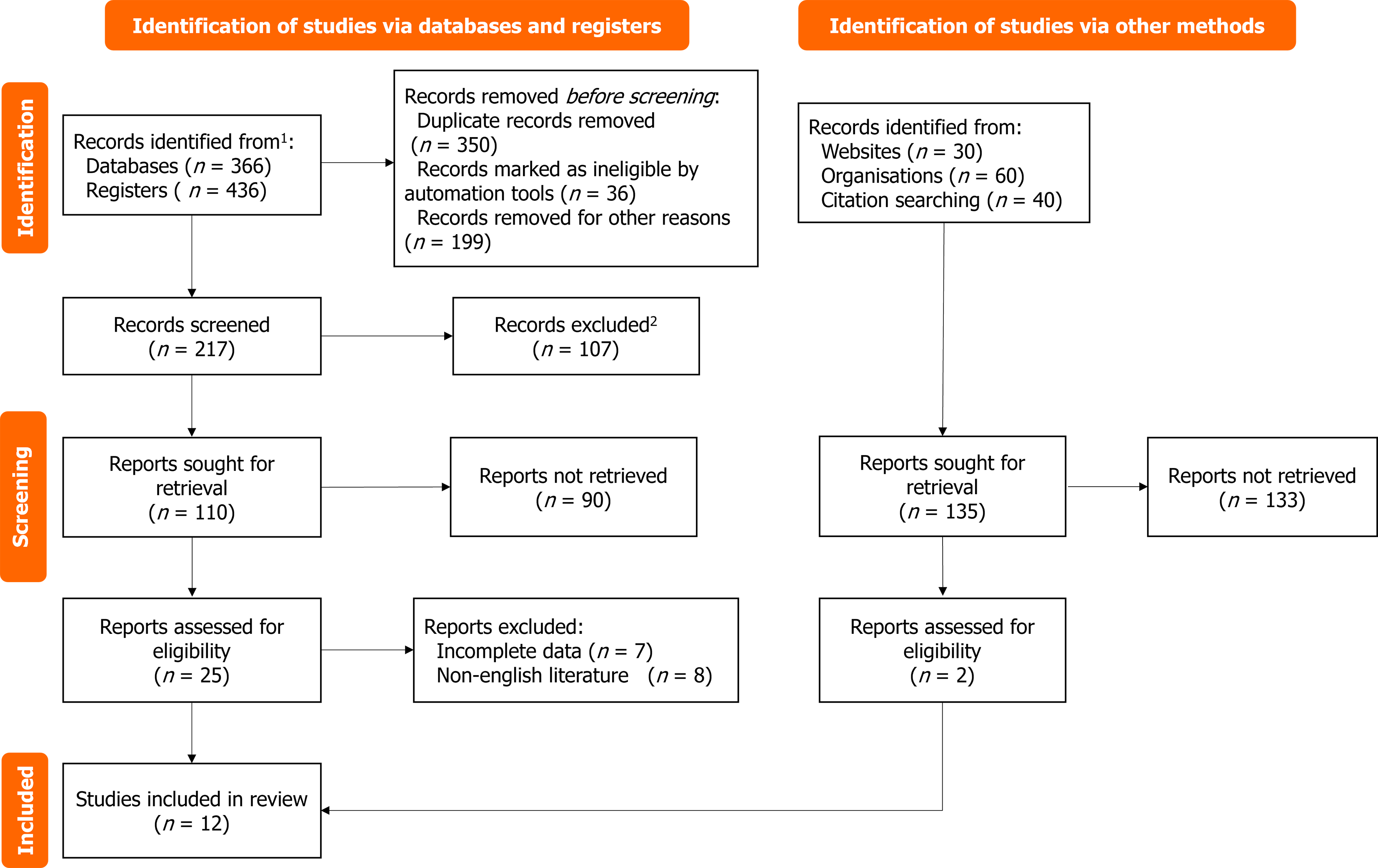
A total of 1220 pieces of literature were initially retrieved, 865 duplicated pieces were excluded, 105 reviews were excluded, only 32 abstracts were excluded, 12 animal experiments were excluded, 10 case reports were reviewed, 10 reviews were reviewed, and 3 brief investigations were conducted. Upon reviewing the titles and abstracts, we excluded 171 irrelevant studies, resulting in a total of 1208 excluded studies. The final meta-analysis included 12 studies[10-21]. Table 1 displays the characteristics of the included studies (Figure 1). There were 1177 patients (519 patients who received DEB-TACE and 658 patients who received cTACE), and all studies showed no statistically significant differences in age, sex ratio, Child-Pugh grade, tumor stage, or tumor characteristics between the two groups. Of the 12 studies, 7 were prospective, and the remaining 5 were retrospective. Three studies used epirubicin as a drug-carrying microsphere, and four studies used doxubicin.
| Ref. | Cases | Average age (years) | Gender (male/female) | Tumor size (mm) | Child-Pugh grading (A/B) | Newcastle-Ottawa Scale | |||||
| DEB-TACE | cTACE | DEB-TACE | cTACE | DEB-TACE | cTACE | DEB-TACE | cTACE | DEB-TACE | cTACE | ||
| Chen et al[10] | 22 | 20 | N/A | N/A | 19/3 | 17/3 | N/A | N/A | 15/7 | 17/3 | 6 |
| Chi et al[11] | 50 | 50 | 60.3 ± 12.3 | 35/15 | 40/10 | 42.5 ± 12.3 | 43.2 ± 11.8 | 35/15 | 40/10 | 8 | |
| Ferrer Puchol et al[12] | 47 | 25 | 59.84 ± 11.216 | 60.01 ± 12.79 | 34/13 | 18/7 | 61.5 ± 38.4 | 60.4 ± 39.6 | 30/17 | 17/8 | 7 |
| Huang et al[13] | 30 | 30 | 59.2 ± 6.3 | 58.3 ± 6.4 | 18/12 | 20/10 | 6 | ||||
| Lammer et al[14] | 93 | 108 | 67.3 ± 9.1 | 67.4 ± 8.8 | 79/14 | 95/13 | 88.9 ± 52.1 | 89.2 ± 59.3 | 77/16 | 89/19 | 9 |
| Lencioni et al[15] | 32 | 188 | 67.1 ± 10.56 | 18/14 | 100/88 | 67.2 ± 34.2 | 65.7 ± 33.6 | 17/15 | 100/88 | 8 | |
| Malagari et al[16] | 41 | 43 | 70.7 ± 6.9 | 70.0 ± 7.9 | 31/10 | 34/9 | 83.5 ± 27.5 | 81.0 ± 28.0 | 23/18 | 26/17 | 7 |
| Sacco et al[17] | 33 | 34 | 70.0 ± 7.7 | 20/13 | 21/13 | 63.1 ± 32.5 | 60.8 ± 31.1 | 20/13 | 22/12 | 9 | |
| Song et al[18] | 60 | 69 | 61.7 ± 9.8 | 59.4 ± 11.2 | 42/18 | 51/18 | 42.0 ± 28.0 | 50.0 ± 31.0 | 56/4 | 62/6 | 7 |
| Wiggermann et al[19] | 22 | 22 | 69.02+8.1 | 18/4 | 19/3 | 74.4 ± 33.7 | 69.8 ± 38.1 | 22/0 | 22/0 | 8 | |
| You et al[20] | 44 | 43 | 65 | 63 | 30/14 | 26/17 | N/A | N/A | 24/20 | 24/19 | 8 |
| Dhanasekaran et al[21] | 45 | 26 | 59.96 ± 11.45 | 58.96 ± 13.30 | 35/10 | 19/7 | 60.7 ± 45.2 | 54.9 ± 42.9 | 28/17 | 15/11 | 8 |
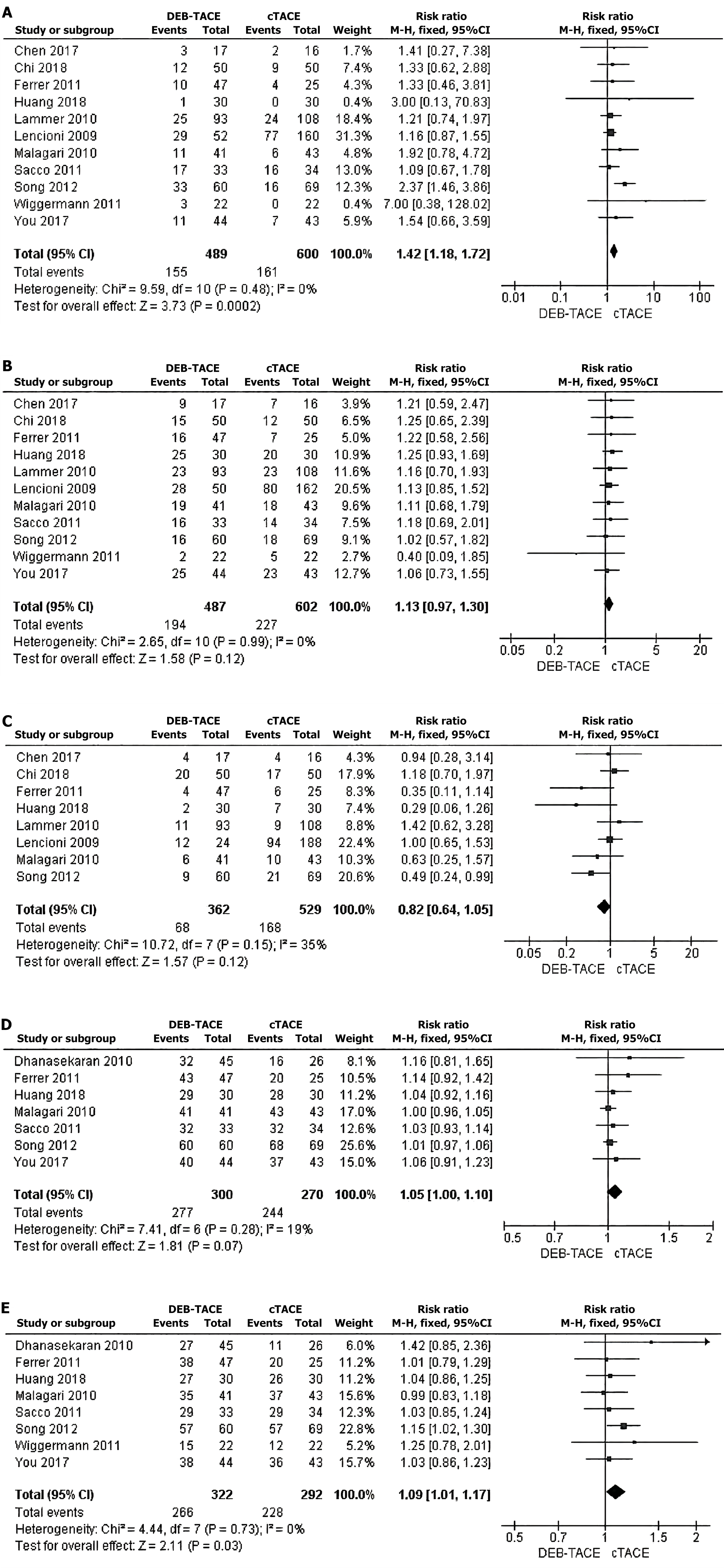
Postoperative CR rate: We conducted a comparative study on the CR rate based on mRECIST criteria one month after surgery. Eleven studies[10-20] reported the number of CR patients, and there was no significant heterogeneity among the studies. A fixed-effects model was used for analysis. The results of the meta-analysis showed that, compared with that in the cTACE group, the postoperative CR rate in the DEB-TACE group was significantly greater (RR = 1.42, 95%CI: 1.18-1.72, Z = 3.73, P = 0.0002) (Figure 2A).
PR rate after surgery: One month after surgery, the PR rate was compared according to the mRECIST standard. Eleven studies[10-20] reported the number of PR patients, and there was no substantial heterogeneity among the investigations. The analysis was conducted using a fixed-effects model. There was no significant difference in the PR rate between the cTACE group and the DEB-TACE group (RR = 1.13, 95%CI: 0.97-1.30, Z = 1.58, P = 0.12) (Figure 2B).
Comparison of the SD rate after surgery: Based on the mRECIST standard eight studies[10-16,18] reported the number of SD patients, and there was no significant heterogeneity among the studies. An analysis was conducted using a fixed-effects model. The meta-analysis results indicated that there was no statistically significant disparity in the SD rate between the cTACE group and the DEB-TACE group (RR = 0.02, 95%CI: 0.64-1). The values for Z and P are 1.57 and 0.12, respectively, as shown in Figure 2C.
Comparison of the 6-month survival rate after surgery: Seven studies[12,13,16-18,20,21] reported 6-month postoperative survival rates, with no significant heterogeneity among the studies. Fixed-effect response model analysis was used. The results of the meta-analysis showed that there was no significant difference in the 6-month survival rate between the cTACE group and the DEB-TACE group (RR = 1.05, 95%CI: 1.00-1.10, Z = 1.81, P = 0.07) (Figure 2D).
Comparison of the 12-month survival rate after surgery: Eight studies[12,13,16-21] reported the survival rate at 12 months after surgery, and there was no significant heterogeneity among the studies. A fixed-effects model was used for analysis. The meta-analysis revealed that the 12-month survival rate was significantly greater in the DEB-TACE group than in the cTACE group (RR = 1.99, 95%CI: 1.01-1.17; Z = 2.11; P = 0.03) (Figure 2E).
Subgroup analysis was performed based on Barcelona clinic liver cancer (BCLC) stage, Child-Pugh grade, and drug delivery status to further evaluate 12-month survival. First, subgroup meta-analyses were performed for the DEB-TACE group and the cTACE group according to BCLC stage, and the results are shown in the table. For HCC patients with BCLC stage A and BCLC stage B disease, the 12-month survival rate was significantly greater in the DEB-TACE group than in the cTACE group (RR = 1.29, 1.42, 95%CI = 1.03-1.61, 1.07-1. Overall, the 12-month survival rate of patients in the DEB-TACE group was significantly greater than that of patients in the cTACE group (RR = 1.18, 95%CI: 1.08-1.29, P = 0.02). Second, a subgroup meta-analysis was conducted between the DEB-TACE group and the cTACE group according to the Child-Pugh classification. For people with Child-Pugh grade A or B liver cancer, there was no significant difference in the 12-month survival rate between the DEB-TACE group and the cTACE group (RR = 1.42). 1.25, 95%CI = 0.79-2.54. The 12-month survival rate of the DEB-TACE group was, however, considerably higher than that of the cTACE group when the two groups were merged (RR = 1.16, 95%CI: 1.06-1.27). Patients in the DEB-TACE group had a significantly higher 12-month survival rate than those in the cTACE group when doxorubicin was administered (RR = 1.15, 95%CI: 1.03–1.28, P = 0.03). Patient in the cTACE group and those in the DEB-TACE group who received epirubicin had different 12-month survival rates. The storage rate did not differ significantly (RR = 1.07, 95%CI: 0.94-1.22, P = 0.06). Overall, the DEB-TACE group's 12-month survival rate was much higher than the cTACE group's (RR = 1.13, 95%CI: 1.01-1.25; P = 0.03). Based on the above subgroup analysis results, the 12-month survival rate of the DEB-TACE group was greater than that of the cTACE group.
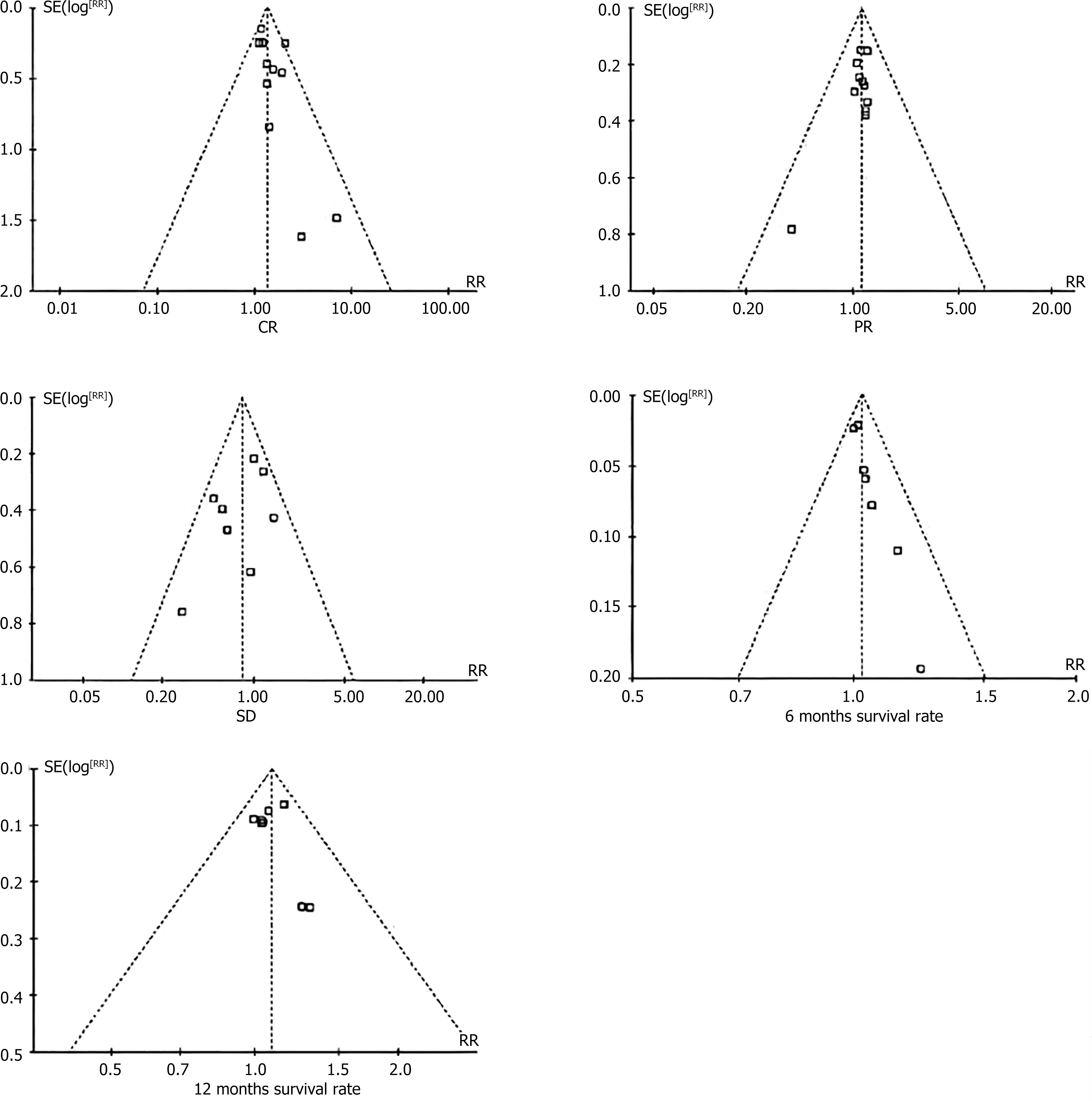
Bias risk assessment: A funnel plot was used to observe the bias risk of the five included studies, and the results showed that the risk of bias on the left and right sides of the five groups of images was basically symmetrical (Figure 3). The NOS scores of 12 studies ranged from 6 to 9, indicating that the results of the meta-analysis had good authenticity, so the conclusions were relatively reliable.
TACE has been used as a standard treatment for patients with unresectable liver cancer. The fundamental idea behind TACE is that the combination of intra-arterial chemotherapy with iodide and chemotherapy drugs, along with selective vascular embolization, produces a potent cytotoxic effect and ischemia, leading to a favorable therapeutic outcome and a high survival rate[22-24]. In recent years, developers have developed DEB-TACE to deliver higher doses of chemotherapeutic agents and extend the contact time with tumors, ensuring controlled and sustained release[25]. Compared with those of regular cTACE, the amount of drug needed to reach the systemic circulation of doxorubicin-loaded microspheres treated with DEB-TACE greatly decreased[26]. They also greatly decreased adverse events related to the drug[27-29]. There was no significant difference in the PR rate, SD rate, or 6-month survival rate between the two groups.
In this meta-analysis, the postoperative CR rate in the DEB-TACE group was significantly greater than that in the cTACE group, which was consistent with the results of one meta-analysis and four retrospective studies. Therefore, DEB-TACE may be an effective treatment for HCC[30]. The pharmacokinetic properties of DEB-TACE, which permits higher doses of chemotherapeutic agents and extended contact time with cancer cells, may account for this difference[31-33]. Malagari et al[16] reported that there was more tumor necrosis 7–14 days after DEB-TACE treatment, and during this period, the proportion of damaged and necrotic cells was close to 100%, and the plasma amycin concentration was the lowest. These findings suggest that DEB-TACE is a more effective surgical procedure than cTACE.
In addition, DEB-TACE was superior to cTACE in terms of treatment response and tumor progression[34]. Recently, a meta-analysis revealed three previous studies comparing the efficacy of DEB-TACE and cTACE for the treatment of HCC. In terms of the number of included studies[35-37], the meta-analysis of Gao et al[3] included 7 studies, including 693 patients. In contrast, this meta-analysis included a larger number of studies and patients (12 studies, 1177 patients). Previous meta-analyses, based on the quality of the included studies, used the NOS, Egger, and Beger tests for risk assessment, whereas this meta-analysis employed the NOS scale for the same purpose. Previous meta-analyses revealed that patients who underwent DEB-TACE had better 1-year and 2-year survival rates than those who underwent cTACE, while the 6-month and 3-year survival rates were similar. In contrast, this meta-analysis revealed that 12-month survival was significantly greater in the DEB-TACE group than in the cTACE group. A meta-subgroup analysis of the 12-month survival rate after a randomized controlled trial. The results showed that the 1- and 2-year survival rates of the DEB-TTACE group were significantly greater than those of the cTACE group in the randomized controlled trial, but there was no significant difference in the 3-year survival rate after surgery[38]. Several studies[39,40] have shown that the Child-Pugh grade, BCLC stage, Eastern Cooperative Oncology Group score, and serum bilirubin level are associated with survival. Song et al's study showed that in patients with mid-stage HCC, the 1-year survival rate in the DEB-TACE group was significantly greater than that in the cTACE group, while in patients with early-stage HCC, there was no significant difference in the survival rate between the two groups[18]. Similarly, Dhanasekaran et al[21] reported that among HCC patients with BCLC stage A and BCLC stage B disease, the 1-year survival rate was significantly greater in the DEB-TACE group than in the cTACE group. The results of these two studies were similar to those of this meta-subgroup analysis. Compared with that of patients with early-stage liver cancer, overall survival between the DEB-TACE group and the cTACE group was significantly different only for patients with early-stage liver cancer. Therefore, this paper conducted a subgroup analysis of the 12-month postoperative survival rate according to BCLC stage, Child-Pugh grade, and drugs in drug-loaded microcapsules, resulting in a more substantial and reliable meta-analysis. It is concluded that DEB-TACE is more advantageous for the treatment of HCC.
The limitations of this study include the following: (1) There were fewer studies than DEB-TACE and cTACE, and most of the clinical studies were retrospective studies, suggesting that unmeasured confounding factors and selection bias may have affected the results of these studies; (2) The number of participants is limited, and a large number of samples will increase the accuracy of the results; (3) Heterogeneity of baseline characteristics, such as age, Child-Pugh grade, and tumor diameter, may cause potential bias; (4) Inclusion criteria vary from study to study and may lead to different results; (5) In the selected studies, there were no comprehensive criteria for type, dose, and drug-carrying microsphere size, which may affect the accuracy of the results; and (6) Some studies used the European Society of Hepatology criteria or computed tomography to assess tumor response, which may not take into account the microstructure of necrosis.
This meta-analysis compared the efficacy of drug-loaded microspheres and traditional iodized oil in treating unresectable liver cancer via hepatic arterial chemoembolization. The results showed that the drug-loaded microsphere treatment had a significant advantage in terms of efficacy, and compared with those in the traditional iodized oil group, the tumor shrinkage rate was greater, survival was significantly longer, and side effects were fewer. Further analysis showed that drug-loaded microspheres had a better local tumor control effect, could release drugs more effectively, reduced damage to normal liver tissue, and improved the safety and tolerability of treatment. Therefore, our results support the idea of using drug-loaded microspheres for hepatic arterial chemoembolization in people with liver cancer that cannot be removed. This should be a better and safer way to treat cancer and provide doctors with important information for their work.
| 1. | Wang Y, Benzina A, Molin DG, Akker Nv, Gagliardi M, Koole LH. Preparation and structure of drug-carrying biodegradable microspheres designed for transarterial chemoembolization therapy. J Biomater Sci Polym Ed. 2015;26:77-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Wu L, Zheng Y, Liu J, Luo R, Wu D, Xu P, Wu D, Li X. Comprehensive evaluation of the efficacy and safety of LPV/r drugs in the treatment of SARS and MERS to provide potential treatment options for COVID-19. Aging (Albany NY). 2021;13:10833-10852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 3. | Gao F, Rafiq M, Cong H, Yu B, Shen Y. Current research status and development prospects of embolic microspheres containing biological macromolecules and others. Int J Biol Macromol. 2024;267:131494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 4. | Sun J, Li WG, Wang Q, He WP, Wang HB, Han P, Zhang T, Zhang AM, Fan YZ, Sun YZ, Duan XZ. Hepatic Resection Versus Stereotactic Body Radiation Therapy Plus Transhepatic Arterial Chemoembolization for Large Hepatocellular Carcinoma: A Propensity Score Analysis. J Clin Transl Hepatol. 2021;9:672-681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Cheng KL, Cheng YM, Chan CY, Wang CC. Predictors of Liver Dysfunction After Transhepatic Arterial Chemo-embolization in Hepatocellular Carcinoma Patients. Dig Dis Sci. 2023;68:3467-3472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | Wu L, Zhong Y, Wu D, Xu P, Ruan X, Yan J, Liu J, Li X. Immunomodulatory Factor TIM3 of Cytolytic Active Genes Affected the Survival and Prognosis of Lung Adenocarcinoma Patients by Multi-Omics Analysis. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 75] [Reference Citation Analysis (0)] |
| 7. | Chen NY, Chen KH, Wang YW, Tsai HH, Lee WC, Weng LC. The impact of symptom distress on health-related quality of life in liver cancer patients receiving arterial chemoembolization: the mediating role of hope. BMC Gastroenterol. 2022;22:456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 8. | Liu S, Yu G, Wang Q, Li L, Liu Y, Du K, Zhang F, Zhao B, Zhao G. CalliSpheres(®) microspheres drug-eluting bead transhepatic artery chemoembolization with or without sorafenib for the treatment of large liver cancer: a multi-center retrospective study. Am J Transl Res. 2021;13:13931-13940. [PubMed] |
| 9. | Wang W, Li F, Gan P, Li B, Li S. Callispheres drug-eluting bead transhepatic artery chemoembolization with oral delivery of sorafenib for the treatment of unresectable liver cancer. Front Surg. 2022;9:981116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 10. | Chen G, Zhang D, Ying YC, Wang ZF, Tao W, Zhu H, Zhang JF, Peng ZY. [Clinical study on the treatment of unresectable primary liver cancer by transarterial chemoembolization with Chinese drug-carrying microspheres]. hejiang Daxue Xuebao; Yixue Kexueban. 2017;46:44-51. |
| 11. | Chi Q, Zhang L, Liu B, Zhang GA. [Efficacy of Epirubicin combined with iodide and Hepas phere drug-loaded microspheres in the treatment of TACE in patients with liver cancer]. Xiandai Zhongliu Yixue. 2018;26:2090-2093. |
| 12. | Ferrer Puchol MD, la Parra C, Esteban E, Vaño M, Forment M, Vera A, Cosín O. [Comparison of doxorubicin-eluting bead transarterial chemoembolization (DEB-TACE) with conventional transarterial chemoembolization (TACE) for the treatment of hepatocellular carcinoma]. Radiologia. 2011;53:246-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Huang YJ, Ling YZ, Qin ZL. [Efficacy of HepaSphere drug-loaded microspheres in the treatment of primary liver cancer]. Linchuang Yixue Zazhi. 2018;38:35-36. |
| 14. | Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S, Benhamou Y, Avajon Y, Gruenberger T, Pomoni M, Langenberger H, Schuchmann M, Dumortier J, Mueller C, Chevallier P, Lencioni R; PRECISION V Investigators. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1063] [Cited by in RCA: 1218] [Article Influence: 71.6] [Reference Citation Analysis (1)] |
| 15. | Lencioni R, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Lammer J. A randomized phase II trial of a drug eluting bead in the treatment of hepatocellular carcinoma by transcatheter arterial chemoembolization. J Clin Oncol. 2009;27:4523. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Malagari K, Pomoni M, Kelekis A, Pomoni A, Dourakis S, Spyridopoulos T, Moschouris H, Emmanouil E, Rizos S, Kelekis D. Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2010;33:541-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 293] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 17. | Sacco R, Bargellini I, Bertini M, Bozzi E, Romano A, Petruzzi P, Tumino E, Ginanni B, Federici G, Cioni R, Metrangolo S, Bertoni M, Bresci G, Parisi G, Altomare E, Capria A, Bartolozzi C. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2011;22:1545-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 18. | Song MJ, Chun HJ, Song DS, Kim HY, Yoo SH, Park CH, Bae SH, Choi JY, Chang UI, Yang JM, Lee HG, Yoon SK. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol. 2012;57:1244-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 196] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 19. | Wiggermann P, Sieron D, Brosche C, Brauer T, Scheer F, Platzek I, Wawrzynek W, Stroszczynski C. Transarterial Chemoembolization of Child-A hepatocellular carcinoma: drug-eluting bead TACE (DEB TACE) vs. TACE with cisplatin/lipiodol (cTACE). Med Sci Monit. 2011;17:CR189-CR195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | You JX, Wang JB, Ai ST, Fan XD, Zheng LZ, Su LX, Wen MZ, Yang XT. [Effect of microspheres combined with liodoembolization in the treatment of hepatocellular carcinoma]. Jieru Fangshexue Zazhi. 2017;26:531-534. |
| 21. | Dhanasekaran R, Kooby DA, Staley CA, Kauh JS, Khanna V, Kim HS. Comparison of conventional transarterial chemoembolization (TACE) and chemoembolization with doxorubicin drug eluting beads (DEB) for unresectable hepatocelluar carcinoma (HCC). J Surg Oncol. 2010;101:476-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 167] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 22. | Wu L, Liu Q, Ruan X, Luan X, Zhong Y, Liu J, Yan J, Li X. Multiple Omics Analysis of the Role of RBM10 Gene Instability in Immune Regulation and Drug Sensitivity in Patients with Lung Adenocarcinoma (LUAD). Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 68] [Reference Citation Analysis (0)] |
| 23. | Yuan Y, He W, Yang Z, Qiu J, Huang Z, Shi Y, Lin Z, Zheng Y, Chen M, Lau WY, Li B, Yuan Y. TACE-HAIC combined with targeted therapy and immunotherapy versus TACE alone for hepatocellular carcinoma with portal vein tumour thrombus: a propensity score matching study. Int J Surg. 2023;109:1222-1230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 96] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 24. | Brown AM, Kassab I, Massani M, Townsend W, Singal AG, Soydal C, Moreno-Luna L, Roberts LR, Chen VL, Parikh ND. TACE versus TARE for patients with hepatocellular carcinoma: Overall and individual patient level meta analysis. Cancer Med. 2023;12:2590-2599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 25. | Liu YM, Ren YQ, Song SL, Zheng CS. Pyogenic liver abscess in non-liver cancer patients and liver cancer patients treated with TACE: Etiological characteristics, treatment, and outcome analysis. Kaohsiung J Med Sci. 2023;39:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 26. | Wu L, Zheng Y, Ruan X, Wu D, Xu P, Liu J, Wu D, Li X. Long-chain noncoding ribonucleic acids affect the survival and prognosis of patients with esophageal adenocarcinoma through the autophagy pathway: construction of a prognostic model. Anticancer Drugs. 2022;33:e590-e603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 27. | Aarts BM, Muñoz FMG, Wildiers H, Dezentjé VO, Baetens TR, Schats W, Lopez-Yurda M, Dresen RC, Wit-van der Veen BJ, Deroose CM, Maleux G, Beets-Tan RGH, Klompenhouwer EG. Intra-Arterial Therapies for Liver Metastatic Breast Cancer: A Systematic Review and Meta-Analysis. Cardiovasc Intervent Radiol. 2021;44:1868-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 28. | Wu L, Zhong Y, Yu X, Wu D, Xu P, Lv L, Ruan X, Liu Q, Feng Y, Liu J, Li X. Selective poly adenylation predicts the efficacy of immunotherapy in patients with lung adenocarcinoma by multiple omics research. Anticancer Drugs. 2022;33:943-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 29. | Wang J, Xu H, Wang Y, Feng L, Yi F. Efficacy and Safety of Drug-Eluting Bead TACE in the Treatment of Primary or Secondary Liver Cancer. Can J Gastroenterol Hepatol. 2023;2023:5492931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 30. | Wu L, Li H, Liu Y, Fan Z, Xu J, Li N, Qian X, Lin Z, Li X, Yan J. Research progress of 3D-bioprinted functional pancreas and in vitro tumor models. IJB. 2024;10:1256. [DOI] [Full Text] |
| 31. | Kim D, Lee JH, Moon H, Seo M, Han H, Yoo H, Seo H, Lee J, Hong S, Kim P, Lee HJ, Chung JW, Kim H. Development and evaluation of an ultrasound-triggered microbubble combined transarterial chemoembolization (TACE) formulation on rabbit VX2 liver cancer model. Theranostics. 2021;11:79-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | Wang Y, Lin W, Huang G, Nie S, Yu Q, Hou F, Zong S. The therapeutic principle of combined clearing heat and resolving toxin plus TACE on primary liver cancer: A systematic review and meta-analysis. J Ethnopharmacol. 2024;319:117072. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 33. | Bosi C, Rimini M, Casadei-Gardini A, Giorgio Ercolani. Understanding the causes of recurrent HCC after liver resection and radiofrequency ablation. Expert Rev Anticancer Ther. 2023;23:503-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 34. | Cao KK, Ding N, Li XW, Zhong JM, Zhai J, Qu ZQ, Zhang XL. Incidence And Risk Factors Of Contrast Nephropathy After Tace In Patients With Liver Cancer And Chronic Kidney Disease. Clin Invest Med. 2021;44:E19-E24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 35. | Wu L, Li X, Qian X, Wang S, Liu J, Yan J. Lipid Nanoparticle (LNP) Delivery Carrier-Assisted Targeted Controlled Release mRNA Vaccines in Tumor Immunity. Vaccines (Basel). 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 75] [Reference Citation Analysis (0)] |
| 36. | Wang J, Huang A, Fu P, Chen X, Zhang X, Yin Y, Zhou J. Effects of TACE combined with microwave ablation on T lymphocyte subsets and prognosis in patients with liver cancer and analysis of safety. J BUON. 2020;25:1883-1889. [PubMed] |
| 37. | Liu B, Zhang Y, Chen H, Li W, Tsochatzis E. The combination of transcatheter arterial chemoembolisation (TACE) and thermal ablation versus TACE alone for hepatocellular carcinoma. Cochrane Database Syst Rev. 2022;1:CD013345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Wu L, Chen X, Zeng Q, Lai Z, Fan Z, Ruan X, Li X, Yan J. NR5A2 gene affects the overall survival of LUAD patients by regulating the activity of CSCs through SNP pathway by OCLR algorithm and immune score. Heliyon. 2024;10:e28282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 39. | Chan KS, Tay WX, Cheo FY, Shelat VG. Preoperative transarterial chemoembolization (TACE) + liver resection versus upfront liver resection for large hepatocellular carcinoma (≥5 cm): a systematic review and meta-analysis. Acta Chir Belg. 2023;123:601-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Yin L, Liu KC, Lv WF, Lu D, Tan YL, Wang GX, Dai JY, Zhu XH, Jiang B. Comparing the effectiveness and safety of Sorafenib plus TACE with Apatinib plus TACE for treating patients with unresectable hepatocellular carcinoma: a multicentre propensity score matching study. Cancer Imaging. 2023;23:52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/