Published online Oct 15, 2024. doi: 10.4251/wjgo.v16.i10.4274
Revised: August 20, 2024
Accepted: August 28, 2024
Published online: October 15, 2024
Processing time: 152 Days and 21.5 Hours
Patient-derived organoids (PDOs) have been demonstrated to predict the res
A 59-year-old woman was admitted to the hospital due to upper abdominal pain for over 8 months. According to relevant examinations, she was diagnosed as pe
PDOs for drug sensitivity contribute to screening effective chemotherapy drugs for advanced pCCA, promoting conversion therapy and improving the prognosis.
Core Tip: The patient-derived organoids (PDOs) have been demonstrated to predict the response to drugs in multiple cancer types. Here we first descried a patient with advanced perihilar cholangiocarcinoma (pCCA) who successfully underwent surgical resection after use of the PDO-guided gemcitabine and cisplatin in combination with toripalimab and lenvatinib and achieved good prognosis. For advanced pCCA patients, the PDO-based drug sensitivity testing contributes to screening effective chemotherapy drugs to promote the personalized treatment, which not only creates opportunities for surgical resection by lessening the tumor, but also offers a novel platform for improving the patient’s prognosis.
- Citation: He YG, Zhang LY, Li J, Wang Z, Zhao CY, Zheng L, Huang XB. Conversion therapy in advanced perihilar cholangiocarcinoma based on patient-derived organoids: A case report. World J Gastrointest Oncol 2024; 16(10): 4274-4280
- URL: https://www.wjgnet.com/1948-5204/full/v16/i10/4274.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i10.4274
Cholangiocarcinoma (CCA) is a heterogeneous group of malignancies arising from the epithelium of the bile ducts, with pathological characteristics of biliary tract differentiation[1]. Anatomically, CCA can be classified as intrahepatic, peri
Although two dimensional cultures for CCA cell lines and xenograft animal models are the standard experimental models in CCA studies, they fail to recapitulate the key characteristics of an in-vivo growing tumor. Organoids, an in-vivo three dimensional (3D) culture technology, have been demonstrated to recapitulate both the pathology of the cells in patient-derived tissues and the native physiology of the cells in healthy tissues of origin[5], and can be generated from liver biopsies of patients with primary liver cancers including CCA, with the capability of highly retaining the histo
A 59-year-old woman was admitted to the hospital because of upper abdominal pain for over 8 months.
Upper abdominal pain lasted for more than 8 months.
The patient previously underwent tubal ligation, and had chronic hepatitis B.
The patient denied any family history of malignant tumors.
Laboratory examinations showed alanine aminotransferase of 374.3 IU/L, aspartate aminotransferase of 264.3 IU/L, total bilirubin of 65.2 μmol/L, direct bilirubin of 41.4 μmol/L, alpha fetal protein (AFP) of 2.23 ng/mL, carcinoembryonic antigen (CEA) of 16.68 ng/mL, carbohydrate antigen (CA) 15-3 of 112 U/mL, CA19-9 of 136.13 U/mL and CA125 of 1 000 U/mL (Table 1).
| Indicators | AFP (ng/mL) | CA19-9 (U/mL) | CEA (ng/mL) | CA125 (U/mL) | CA15-3 (U/mL) |
| On admission | 2.23 | 136.13 | 16.68 | 1000 | 112 |
| After treatment for 2 weeks | 5.86 | 127.24 | 20.25 | 1210 | 122 |
| After organoid-based treatment for 4 cycles | 2.12 | 43.84 | 4.21 | 16 | 12.2 |
| First year after surgery | 2.3 | 39.67 | 2.62 | 21.2 | 10.6 |
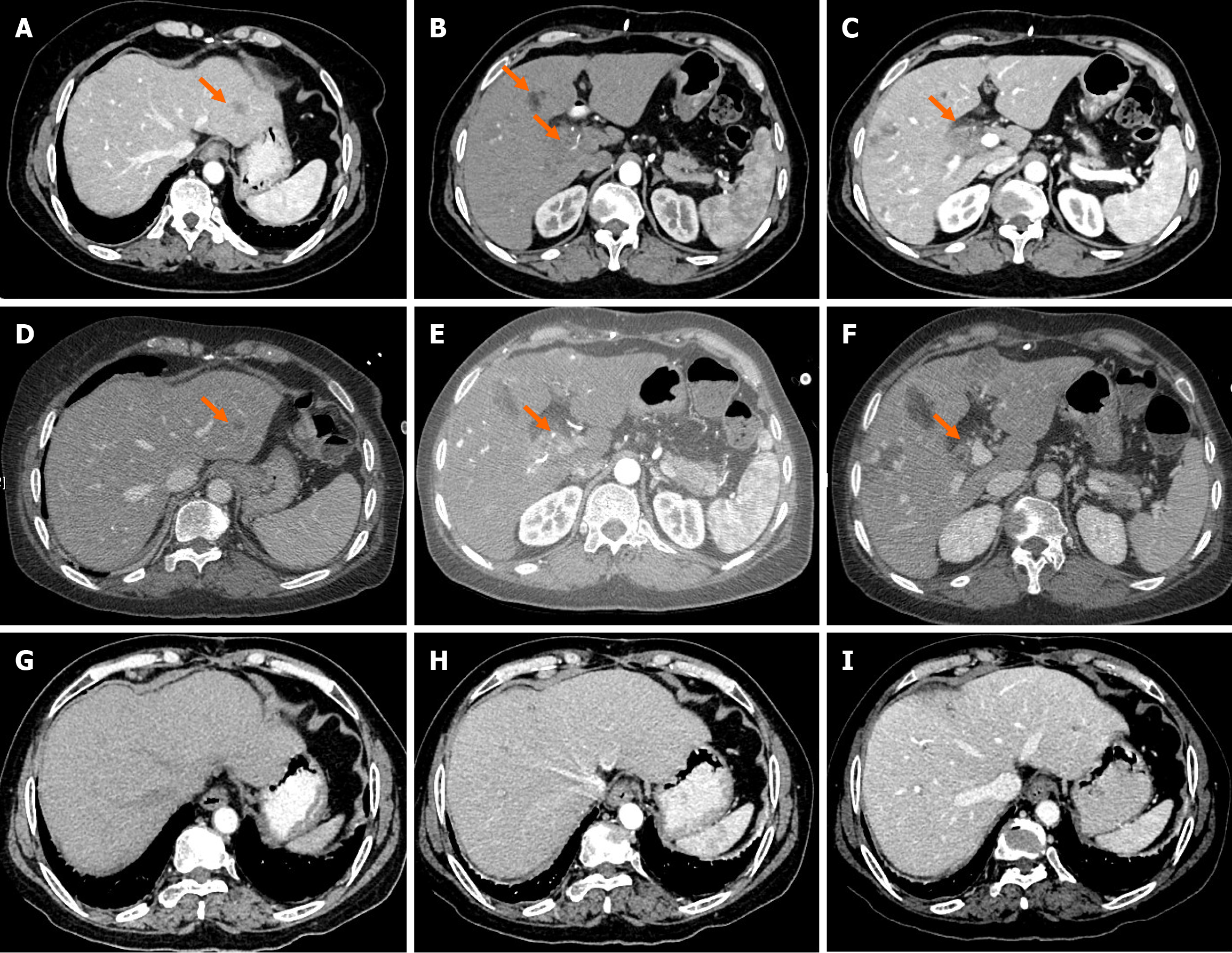
Computed tomography (CT) showed a space-occupying lesion in the hepatic hilar region and multiple hemangiomas in the liver, with the possibility of metastatic tumors in the liver S2 and S8 segments (Figure 1A-C). Magnetic resonance imaging (MRI) revealed space-occupying lesions in the liver S2 and S8 segments, various swollen lymph nodes in the hepatic hilar region, as well as multiple hemangiomas in the liver S4-6 segments. In combination with positron emission tomography-CT, pCCA with intrahepatic metastasis and perihilar lymphadenectasis was diagnosed.
In combination with relevant examinations, the patient was finally diagnosed with pCCA with intrahepatic metastasis and perihilar lymphatic metastasis.
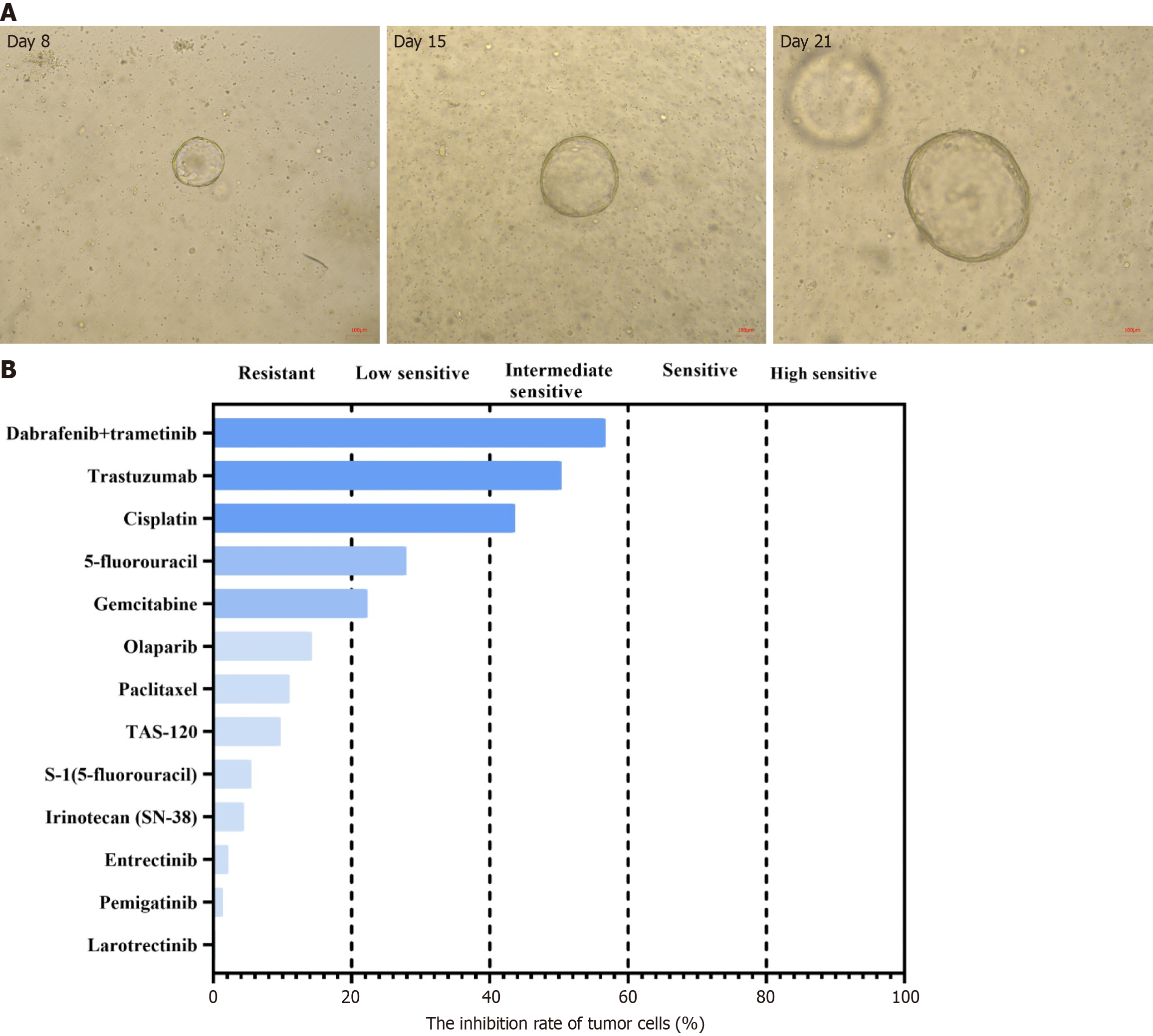
After multidisciplinary team discussion, percutaneous transhepatic cholangiodrainage was performed to relieve biliary obstruction, and needle biopsy was conducted to confirm the pathological diagnosis. Meanwhile, the organoids from biopsy samples were cultured after the informed consent form was obtained from the patient. Briefly, the tumor tissues obtained were washed with precooled phosphate buffer saline and then minced. After 30-minute digestion, cell pellets were collected via centrifugation. Pipettes were used to seed the cells and Matrigel suspension onto 6-well plates (2 mL per well) following addition of Matrigel, and the plates were placed in a 37 °C incubator for 15 min. When the droplets were fully solidified, the culture medium (Kingbio Medical Co., Ltd., Chongqing, China) was added. Subsequently, the plates were placed into an incubator (37 °C, 5% carbon dioxide) for culture. The culture medium was replaced every 2-3 days, and drug sensitivity testing was performed until organoids grew like solid spheroids with a diameter of about 70 µm (Figure 2A).
According to the patient’s condition, transarterial chemoembolization with nab-paclitaxel (125 mg/m2) was used in combination with toripalimab (240 mg, once every 3 weeks) and lenvatinib (40 mg per day). Two weeks later, the levels of all tumor markers above went up by varying degrees except for slightly decreased CA19-9 (Table 1). The organoid drug sensitivity testing showed sensitive to gemcitabine and cisplatin (Figure 2B). Based on this, the chemotherapy regimen was adjusted to gemcitabine (1000 mg/m2) combined with cisplatin (25 mg/m2), but the use of toripalimab and lenvatinib remained unchanged. After 4 cycles of treatment, the levels of AFP, CEA, CA15-3, CA19-9 and CA125 all returned to the normal range (Table 1). CT and MRI both indicated significantly lessened liver tumor diameter (Figure 1D-F). The tumor was assessed as resectable according to response evaluation criteria in solid tumors (version 1.1). Postoperative patho
At 12 months postoperatively, the patient was still alive, and the CT scan showed no recurrence or metastasis (Figure 1G-I).
As the most common type of CCA, pCCA originates from the extrahepatic biliary tree proximal to the origin of the cystic duct and is borne by a complex diagnostic iter[8]. Although great development has been achieved in surgical strategies over the past decades, the postoperative 5-year survival rate remains to be low, often close to 20%[9]. Surgery is the preferred treatment method, but most patients with pCCA are unresectable at the time of diagnosis. In recent years, use of aggressive approaches based on various imaging modalities and specific perioperative management has been confirmed to improve the prognosis of pCCA patients by converting the palliative therapies to the radical surgery[10,11]. In this study, under the guidance of the pCCA organoid for drug screening, the patient successfully received surgical resection after use of gemcitabine and cisplatin in combination with toripalimab and lenvatinib, and no recurrence and metastasis occurred 1 year after surgery.
Except for surgical resection, pre- and post-operative multidisciplinary treatment for CCA plays a crucial role in survival improvement. Neoadjuvant therapies involving chemotherapy and transarterial embolization have been demonstrated to downgrade the unresectable intrahepatic CCA, thus allowing for the implementation of radical surgical resection[12,13]. For patients with advanced CCA, the purpose of neoadjuvant therapy is to convert the unresectable tumors into the resectable ones to ameliorate the long-term prognosis. McMasters et al[14] found that preoperative chemoradiation for extrahepatic CCA contributed to producing significant antitumor responses, which might improve the capability of obtaining the tumor-free resection margin. When the neoadjuvant therapy with gemcitabine and S-1 chemotherapy was used for pCCA, the 5-year disease-specific survival was 50.3% in the resected and 30.0% in the borderline resected but only 16.5% in the locally advanced patients[15]. Moreover, in an open-label, single-arm, phase 2 study, gemcitabine/S-1 neoadjuvant therapy was identified to effective and tolerable in patients with borderline resectable pCCA, respectively with the median survival time of 50.1 months for the resected and 14.8 months for the unresected[16]. To date, however, the evidence of neoadjuvant therapy for CCA, especially pCCA, has not been built completely.
With the emergence of more novel and effective chemotherapy, targeted therapy, and immunotherapy, multi-drug combination therapy is proposed to treat advanced CCA. Although gemcitabine combined with cisplatin is considered as the first-line treatment for advanced CCA, the median overall survival was still less than 1 year[17], and no standard treatment is recommended beyond the first-line chemotherapy. In this study, in addition to gemcitabine and cisplatin, toripalimab and lenvatinib were also used as the neoadjuvant therapy, and the tumor was converted into the resectable, suggesting that gemcitabine and cisplatin in combination with toripalimab and lenvatinib may be a promising conversion therapy strategy for patients with advanced pCCA. Importantly, this patient did not experience any recurrence and metastasis 1 year following treatment.
The PDO, a novel 3D preclinical model, has the capability of highly preserving the histopathological, genetic, and molecular characteristics of the original tumor, which can simulate in vivo and ex vivo responses observed in patients and open the new way for guiding therapeutic decisions[18]. It can be established in a clinically meaningful time of 6-10 weeks, with the positive predictive value of 88% and negative predictive value of 100% in predicting the patient’s response[19]. Importantly, the neoadjuvant treatment response could also be predicted by the PDOs[20]. By comparing responses to antitumor agents in PDOs and PDO-based xenograft models with those of patients in clinical trials, Vlachogiannis et al[19] found that PDOs could recapitulate the patients’ clinical response and could be conducted in precision medicine protocols. Based on pharmaco-typing, PDOs was confirmed to be a predictive biomarker for clinical treatment responses to standard-of-care chemotherapeutics[21].
Recently, several studies on the clinical application of PDOs in CCA have all shown that CCA PDOs contribute to identification of effective cancer drugs to guide the individualized treatment, highlighting the importance of PDOs as in vitro models of CCA [6,7,22]. It was reported that the generation success rates of organoids from hepatocellular carcinoma and intrahepatic CCA needle biopsies were 33% and 60%, respectively[7], lower than 75%-83% in pancreatic cancer[23] and 90% in colorectal cancer[24], which might be associated with absence of epithelial stem cell features in hepatocytes. In this study, we successfully established the pCCA organoids from needle biopsy samples and found that chemotherapeutic drugs gemcitabine and cisplatin were potential candidates for the patient. Accordingly, the neoadjuvant chemo
For advanced pCCA patients, the PDO-based drug sensitivity testing contributes to screening effective chemotherapy drugs to promote the personalized treatment, which not only creates opportunities for surgical resection by lessening the tumor, but also offers a novel platform for improving the patient’s prognosis.
| 1. | Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, Pawlik TM, Gores GJ. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 862] [Cited by in RCA: 1122] [Article Influence: 93.5] [Reference Citation Analysis (0)] |
| 2. | Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1072] [Cited by in RCA: 1460] [Article Influence: 121.7] [Reference Citation Analysis (4)] |
| 3. | Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L, Geier A, Calvisi DF, Mertens JC, Trauner M, Benedetti A, Maroni L, Vaquero J, Macias RI, Raggi C, Perugorria MJ, Gaudio E, Boberg KM, Marin JJ, Alvaro D. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13:261-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 1037] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 4. | Elvevi A, Laffusa A, Scaravaglio M, Rossi RE, Longarini R, Stagno AM, Cristoferi L, Ciaccio A, Cortinovis DL, Invernizzi P, Massironi S. Clinical treatment of cholangiocarcinoma: an updated comprehensive review. Ann Hepatol. 2022;27:100737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 118] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 5. | Prior N, Inacio P, Huch M. Liver organoids: from basic research to therapeutic applications. Gut. 2019;68:2228-2237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 267] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 6. | Broutier L, Mastrogiovanni G, Verstegen MM, Francies HE, Gavarró LM, Bradshaw CR, Allen GE, Arnes-Benito R, Sidorova O, Gaspersz MP, Georgakopoulos N, Koo BK, Dietmann S, Davies SE, Praseedom RK, Lieshout R, IJzermans JNM, Wigmore SJ, Saeb-Parsy K, Garnett MJ, van der Laan LJ, Huch M. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med. 2017;23:1424-1435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 905] [Cited by in RCA: 1060] [Article Influence: 117.8] [Reference Citation Analysis (0)] |
| 7. | Nuciforo S, Fofana I, Matter MS, Blumer T, Calabrese D, Boldanova T, Piscuoglio S, Wieland S, Ringnalda F, Schwank G, Terracciano LM, Ng CKY, Heim MH. Organoid Models of Human Liver Cancers Derived from Tumor Needle Biopsies. Cell Rep. 2018;24:1363-1376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 351] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 8. | Dondossola D, Ghidini M, Grossi F, Rossi G, Foschi D. Practical review for diagnosis and clinical management of perihilar cholangiocarcinoma. World J Gastroenterol. 2020;26:3542-3561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (2)] |
| 9. | Kambakamba P, DeOliveira ML. Perihilar cholangiocarcinoma: paradigms of surgical management. Am J Surg. 2014;208:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Fruscione M, Pickens RC, Baker EH, Martinie JB, Iannitti DA, Hwang JJ, Vrochides D. Conversion therapy for intrahepatic cholangiocarcinoma and tumor downsizing to increase resection rates: A systematic review. Curr Probl Cancer. 2021;45:100614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Witzigmann H, Berr F, Ringel U, Caca K, Uhlmann D, Schoppmeyer K, Tannapfel A, Wittekind C, Mossner J, Hauss J, Wiedmann M. Surgical and palliative management and outcome in 184 patients with hilar cholangiocarcinoma: palliative photodynamic therapy plus stenting is comparable to r1/r2 resection. Ann Surg. 2006;244:230-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 200] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 12. | Buettner S, Koerkamp BG, Ejaz A, Buisman FE, Kim Y, Margonis GA, Alexandrescu S, Marques HP, Lamelas J, Aldrighetti L, Gamblin TC, Maithel SK, Pulitano C, Bauer TW, Shen F, Poultsides GA, Marsh JW, IJzermans JN, Pawlik TM. The effect of preoperative chemotherapy treatment in surgically treated intrahepatic cholangiocarcinoma patients-A multi-institutional analysis. J Surg Oncol. 2017;115:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Rayar M, Sulpice L, Edeline J, Garin E, Levi Sandri GB, Meunier B, Boucher E, Boudjema K. Intra-arterial yttrium-90 radioembolization combined with systemic chemotherapy is a promising method for downstaging unresectable huge intrahepatic cholangiocarcinoma to surgical treatment. Ann Surg Oncol. 2015;22:3102-3108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 14. | McMasters KM, Tuttle TM, Leach SD, Rich T, Cleary KR, Evans DB, Curley SA. Neoadjuvant chemoradiation for extrahepatic cholangiocarcinoma. Am J Surg. 1997;174:605-8; discussion 608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 147] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Kuriyama N, Usui M, Gyoten K, Hayasaki A, Fujii T, Iizawa Y, Kato H, Murata Y, Tanemura A, Kishiwada M, Sakurai H, Mizuno S, Isaji S. Neoadjuvant chemotherapy followed by curative-intent surgery for perihilar cholangiocarcinoma based on its anatomical resectability classification and lymph node status. BMC Cancer. 2020;20:405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Matsuyama R, Mori R, Ota Y, Homma Y, Yabusita Y, Hiratani S, Murakami T, Sawada Y, Miyake K, Shimizu Y, Kumamoto T, Endo I. Impact of Gemcitabine Plus S1 Neoadjuvant Chemotherapy on Borderline Resectable Perihilar Cholangiocarcinoma. Ann Surg Oncol. 2022;29:2393-2405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 17. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J; ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3345] [Article Influence: 209.1] [Reference Citation Analysis (15)] |
| 18. | Amato F, Rae C, Prete MG, Braconi C. Cholangiocarcinoma Disease Modelling Through Patients Derived Organoids. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Vlachogiannis G, Hedayat S, Vatsiou A, Jamin Y, Fernández-Mateos J, Khan K, Lampis A, Eason K, Huntingford I, Burke R, Rata M, Koh DM, Tunariu N, Collins D, Hulkki-Wilson S, Ragulan C, Spiteri I, Moorcraft SY, Chau I, Rao S, Watkins D, Fotiadis N, Bali M, Darvish-Damavandi M, Lote H, Eltahir Z, Smyth EC, Begum R, Clarke PA, Hahne JC, Dowsett M, de Bono J, Workman P, Sadanandam A, Fassan M, Sansom OJ, Eccles S, Starling N, Braconi C, Sottoriva A, Robinson SP, Cunningham D, Valeri N. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 2018;359:920-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 1457] [Article Influence: 182.1] [Reference Citation Analysis (0)] |
| 20. | Hsu KS, Adileh M, Martin ML, Makarov V, Chen J, Wu C, Bodo S, Klingler S, Sauvé CG, Szeglin BC, Smith JJ, Fuks Z, Riaz N, Chan TA, Nishimura M, Paty PB, Kolesnick R. Colorectal Cancer Develops Inherent Radiosensitivity That Can Be Predicted Using Patient-Derived Organoids. Cancer Res. 2022;82:2298-2312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 21. | Seppälä TT, Zimmerman JW, Suri R, Zlomke H, Ivey GD, Szabolcs A, Shubert CR, Cameron JL, Burns WR, Lafaro KJ, He J, Wolfgang CL, Zou YS, Zheng L, Tuveson DA, Eshlemann JR, Ryan DP, Kimmelman AC, Hong TS, Ting DT, Jaffee EM, Burkhart RA. Precision Medicine in Pancreatic Cancer: Patient-Derived Organoid Pharmacotyping Is a Predictive Biomarker of Clinical Treatment Response. Clin Cancer Res. 2022;28:3296-3307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 22. | Li L, Knutsdottir H, Hui K, Weiss MJ, He J, Philosophe B, Cameron AM, Wolfgang CL, Pawlik TM, Ghiaur G, Ewald AJ, Mezey E, Bader JS, Selaru FM. Human primary liver cancer organoids reveal intratumor and interpatient drug response heterogeneity. JCI Insight. 2019;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 164] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 23. | Boj SF, Hwang CI, Baker LA, Chio II, Engle DD, Corbo V, Jager M, Ponz-Sarvise M, Tiriac H, Spector MS, Gracanin A, Oni T, Yu KH, van Boxtel R, Huch M, Rivera KD, Wilson JP, Feigin ME, Öhlund D, Handly-Santana A, Ardito-Abraham CM, Ludwig M, Elyada E, Alagesan B, Biffi G, Yordanov GN, Delcuze B, Creighton B, Wright K, Park Y, Morsink FH, Molenaar IQ, Borel Rinkes IH, Cuppen E, Hao Y, Jin Y, Nijman IJ, Iacobuzio-Donahue C, Leach SD, Pappin DJ, Hammell M, Klimstra DS, Basturk O, Hruban RH, Offerhaus GJ, Vries RG, Clevers H, Tuveson DA. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160:324-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 1670] [Article Influence: 139.2] [Reference Citation Analysis (0)] |
| 24. | van de Wetering M, Francies HE, Francis JM, Bounova G, Iorio F, Pronk A, van Houdt W, van Gorp J, Taylor-Weiner A, Kester L, McLaren-Douglas A, Blokker J, Jaksani S, Bartfeld S, Volckman R, van Sluis P, Li VS, Seepo S, Sekhar Pedamallu C, Cibulskis K, Carter SL, McKenna A, Lawrence MS, Lichtenstein L, Stewart C, Koster J, Versteeg R, van Oudenaarden A, Saez-Rodriguez J, Vries RG, Getz G, Wessels L, Stratton MR, McDermott U, Meyerson M, Garnett MJ, Clevers H. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161:933-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1313] [Cited by in RCA: 1835] [Article Influence: 183.5] [Reference Citation Analysis (0)] |
| 25. | Jensen LH, Rogatto SR, Lindebjerg J, Havelund B, Abildgaard C, do Canto LM, Vagn-Hansen C, Dam C, Rafaelsen S, Hansen TF. Precision medicine applied to metastatic colorectal cancer using tumor-derived organoids and in-vitro sensitivity testing: a phase 2, single-center, open-label, and non-comparative study. J Exp Clin Cancer Res. 2023;42:115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 26. | Wang Z, Jin Y, Guo Y, Tan Z, Zhang X, Ye D, Yu Y, Peng S, Zheng L, Li J. Conversion Therapy of Intrahepatic Cholangiocarcinoma Is Associated with Improved Prognosis and Verified by a Case of Patient-Derived Organoid. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/