Published online Sep 15, 2023. doi: 10.4251/wjgo.v15.i9.1636
Peer-review started: July 4, 2023
First decision: July 19, 2023
Revised: July 25, 2023
Accepted: August 8, 2023
Article in press: August 8, 2023
Published online: September 15, 2023
Processing time: 70 Days and 23.4 Hours
Recurrence is the major challenge facing endoscopic submucosal dissection (ESD)-based treatment therapies for early gastric cancer (EGC). Urgent development of simple and easy surveillance approaches will enhance clinical treatment of the disease.
To explore the role of computed tomography (CT) recurrence in evaluating EGC after ESD treatment.
We retrospectively recruited patients from our endoscopy department, between January 2002 and December 2015, and analyzed their basic characteristics, including symptoms, CT results, and results of endoscopy with biopsy, among others.
Among a total of 2150 patients EGC patients surveyed, 1362 met our inclusion and exclusion criteria and were therefore enrolled in our study. The cohort’s sensitivity of CT for recurrent GC and specificity were 44.22% and 43.86%, respectively, with negative and positive predictive values of 40.15% (275/685) and 48.01% (325/677), respectively. The area under the curve of arterial and venous CT values for recurrent EGC were 0.545, and 0.604, respectively. Receiver operating characteristic curve revealed no statistically significant differences between arterial and venous CT values for recurrent EGC.
Enhanced CT has superior diagnostic efficacy, but less accuracy, compared to gold standard techniques in patients with recurrent EGC.
Core Tip: Development of a simple and easy approach to detect recurrence of early gastric cancer (EGC) treated with endoscopic mucosal exfoliation is imperative to effective clinical therapy. Here, we report the feasibility of multi-slice spiral computed tomography (CT), a quick and convenient auxiliary examination with sensitivity and specificity values of 44.22% and 43.86%, respectively, in evaluating arterial and venous CT values for recurrent EGC. Area under the curve value of arterial and venous CT values for recurrent EGC respectively were 0.55 and 0.60, indicating that enhanced CT can accurately predict EGC, although with low accuracy.
- Citation: Yin JJ, Hu X, Hu S, Sheng GH. Efficacy of multi-slice spiral computed tomography in evaluating gastric cancer recurrence after endoscopic submucosal dissection. World J Gastrointest Oncol 2023; 15(9): 1636-1643
- URL: https://www.wjgnet.com/1948-5204/full/v15/i9/1636.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i9.1636
Gastric cancer (GC), a disease ranked 5th and 4th with regards to incidence and mortality rates, respectively worldwide as of 2020, is associated with a heavy economic burden[1]. In some Asian countries, such as Korea, China, and Japan, GC is the most prevalent type of cancer and the leading cause of cancer-related deaths[2]. Early detection and treatment is considered the most effective way for reducing GC-related mortalities. Early GC (EGC) is a key stage of GC, characterized by invasion of cancer cells no deeper than the submucosa, regardless of lymph node metastasis. Studies have shown that the 5-year survival rate of EGC operative treatment is approximately 90%[3].
Various operative therapies, such as subtotal gastrectomy, endoscopic submucosal dissection (ESD), and endoscopic mucosal resection (EMR), have been developed for treatment of GC. Among them, ESD, which was developed by Japan in the late 1990s, is the current standard technique in most East Asian countries[4]. ESD, which is based on EMR, is a minimally invasive procedure that has been used for treatment of early gastrointestinal swelling tumor for long. Although this technique is minimally invasive, it has a lower recurrence rate, lower risk, and faster recovery compared to traditional surgical procedures[5,6]. Tanabe et al[7] conducted a long-term multicenter collaborative study, and found that ESD was efficacious against EGC. However, ESD application is significantly limited by recurrence, with studies reporting local recurrence rates was between 2.8% and 38.5% based on studies with a median follow-up of 15-36 mo[8,9]. Therefore, urgent development of simple and easy approaches to detect recurrence of EGC is imperative to effective treatment of the disease. Several "gold standard" diagnostic techniques for GC have been developed and applied, including endoscopy with biopsy, computed tomography (CT), endoscopic ultrasonography and sometimes diagnostic laparoscopy[10]. Based on patient acceptance, CT has shown excellent promise, whereas enhanced spiral CT scan has a high resolution thus can provide a basis for the identification of GC lesions. To date, however, the role of enhanced spiral CT scan in recurrence assessment of GC patients after ESD remains unclear.
In the present study, we explored this role with the aim of generating insights to guide future development of diagnostic and treatment approaches.
This retrospective study was approved by the department of Radiology, Huangshi Maternity and Children's health Hospital, Affiliated Maternity. This study was approved by the Ethics committee of our hospital. And all patients signed an informed consent form prior to inclusion in the study.
We searched our endoscopy department database for patients who were diagnosed with EGS and received ESD treatment between January 2000 and December 2015. The inclusion criteria were as follows: (1) Patients with EGC who received ESD treatment; (2) The patient has been returned regularly for more than 5 years; (3) No other cancer disease occurred after ESD treatment; (4) Signed informed consent to participate in our study; and (5) CT and endoscopy with biopsy was chosen as the tool to detect EGC recurrence. Patients who met the following criteria were excluded from the study: (1) EGS was diagnosed as other type of cancer; and (2) Had missing data.
This was a retrospective cohort study. We searched for eligible patients in the database at our endoscopy department from January 2002 to December 2015. In cases where CT or the patients’ symptoms indicated there was any chance of EGC recurrence, then endoscopy with biopsy were checked for definitive diagnosis. We collected each patient’s basic characteristics, including their symptoms, CT results, and results of endoscopy with biopsy, among others.
All subjects were asked to fast for 8 h, then given water to fill their stomachs and bowel. The patients were placed in supine position and scanned using the Siemens 64-slice spiral CT system under the following parameters: Tube current 250 mA, voltage 120 kV, layer thickness 5-10 mm, layer distance 0.5 mm, and pitch 1. Briefly, a plain scan was first performed from the top of diaphragm to the iliac crest. Next, 300 mg/mL of the contrast agent iodophenyl (plant Home: Shanghai Yuanye Biotechnology Co., LTD), injection rate was 3.5 mL/s, Gastrointestinal arterial phase (delay time 20-25 s), portal venous phase (delay time 40-45 s), delay period (delay time 120-180 s) to implement enhanced scanning CT values of the arterial and portal venous phases were described and measured.
We conducted gastroscopic biopsies to procure 1362 specimens from patients diagnosed with recurrent EGC. These specimens were then meticulously processed by fixing them in formaldehyde, embedding them in paraffin, and finally, sectioning and staining them with hematoxylin and eosin. Visible dilated lymphatic vessels were distributed in the lamina propria of the intestinal mucosa, submucosa, muscular layer, and the serosal layer. The stained sections were evaluated by two physicians with wide experience in pathological diagnosis.
Data were statistically analyzed using packages implemented in R 4.1.0 software, unless otherwise indicated. Descriptive statistics were used to report patients, patients’ symptoms, CT results, and results of endoscopy with biopsy. Continuous variables were presented as means and SD. Diagnostic efficacy was based on receiver operating characteristic curve (ROC), and the roc.test function in pROC package used to compare CT values between the arterial stage and portal stage groups during EGC.
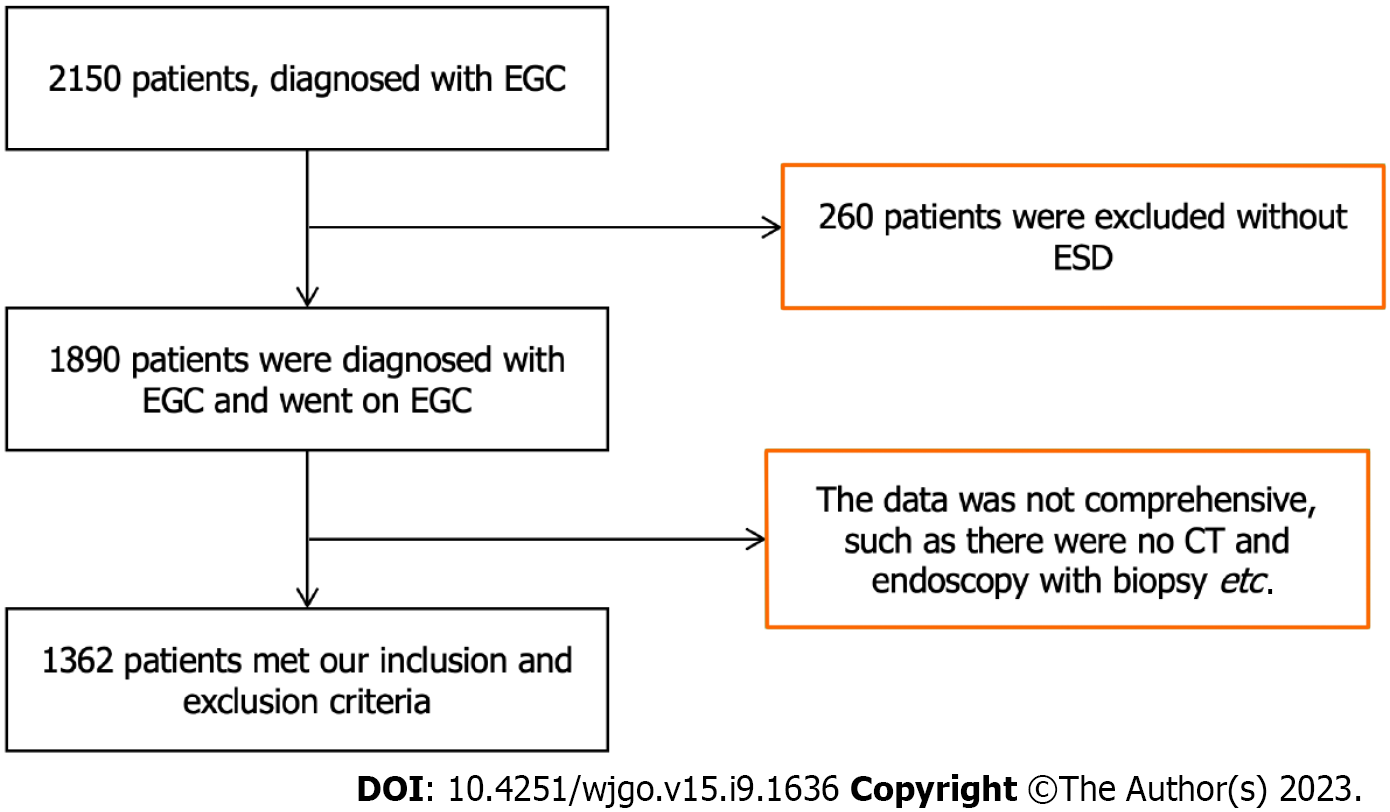
We initially recruited a total of 2150 patients, who were diagnosed with EGC at our department, of which 1890 underwent ESD. A total of 1362 patients met our inclusion criteria and therefore included in the final analysis (Figure 1). Patient characteristics and recurrent EGC parameters are presented in Table 1. In summary, 49.71% (677/1362) of the patients exhibited EGC recurrence, with the condition found to be highly occur in the lower place of stomach. The most TNM stage of recurrent EGC was T1b.
| Variables | No. (%) |
| Relapsed patients | 735 (53.96) |
| Non-relapsed patients | 627 (46.04) |
| Sex | |
| Male | 695 (51.03) |
| Female | 667 (48.98) |
| Age (year), mean ± SD | 64.7 ± 7.7 |
| Location | |
| Upper | 66 (9.75) |
| Middle | 13 (1.92) |
| Lower | 598 (88.33) |
| Size (cm), mean ± SD | 2.8 ± 0.5 |
| Tumor depth | |
| T1a | 65 (9.60) |
| T1b | 612 (90.40) |
| Lymphovascular invasion | |
| Absent | 636 (93.94) |
| Present | 41 (6.06) |
CT sensitivity and specificity for recurrent GC were 44.22% (325/735), and 43.86% (275/627), respectively, with negative and positive predictive values of 40.15% (275/685), and 48.01% (325/677), respectively, which was seen in Table 2. The Youden’s index was -0.12.
| CT | Tissue pathology (Gold standard) | Total | |
| Positive | Negative | ||
| Positive | 325 | 352 | 677 |
| Negative | 410 | 275 | 685 |
| Total | 735 | 627 | 1362 |
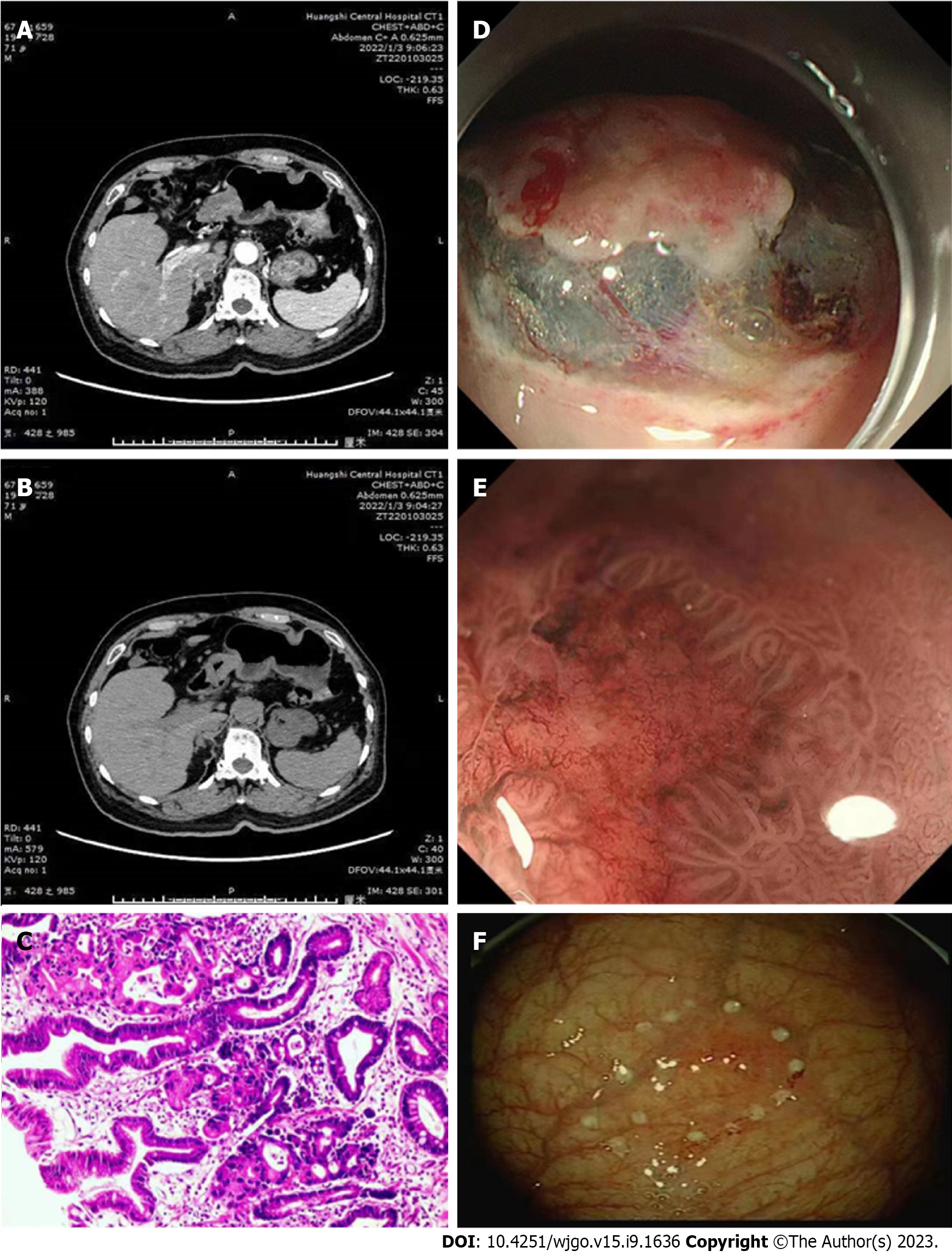
CT, gastroscopic, and histopathological examination results for patients with recurrent EGC are presented in Figure 2. Notably, patients without recurrent EGC had a mean arterial CT and venous CT values of 60.77, and 42.67, respectively. Patients with recurrent EGC had a mean arterial CT and venous CT values of 69.52, and 62.21, respectively. Predictive efficacy of arterial and venous CT values for EGC are summarized in Figure 2. A total of 473 (69.87%) and 204 (30.13%) cases exhibited obvious enhancement in the arterial and portal vein phase of the lesions, respectively. The enhancement ranged between 40-70 hu. The enhanced lesions had a slightly rough surface, which could also be accompanied by mild nodular or indentation changes. The gastric wall was also slightly stiff.
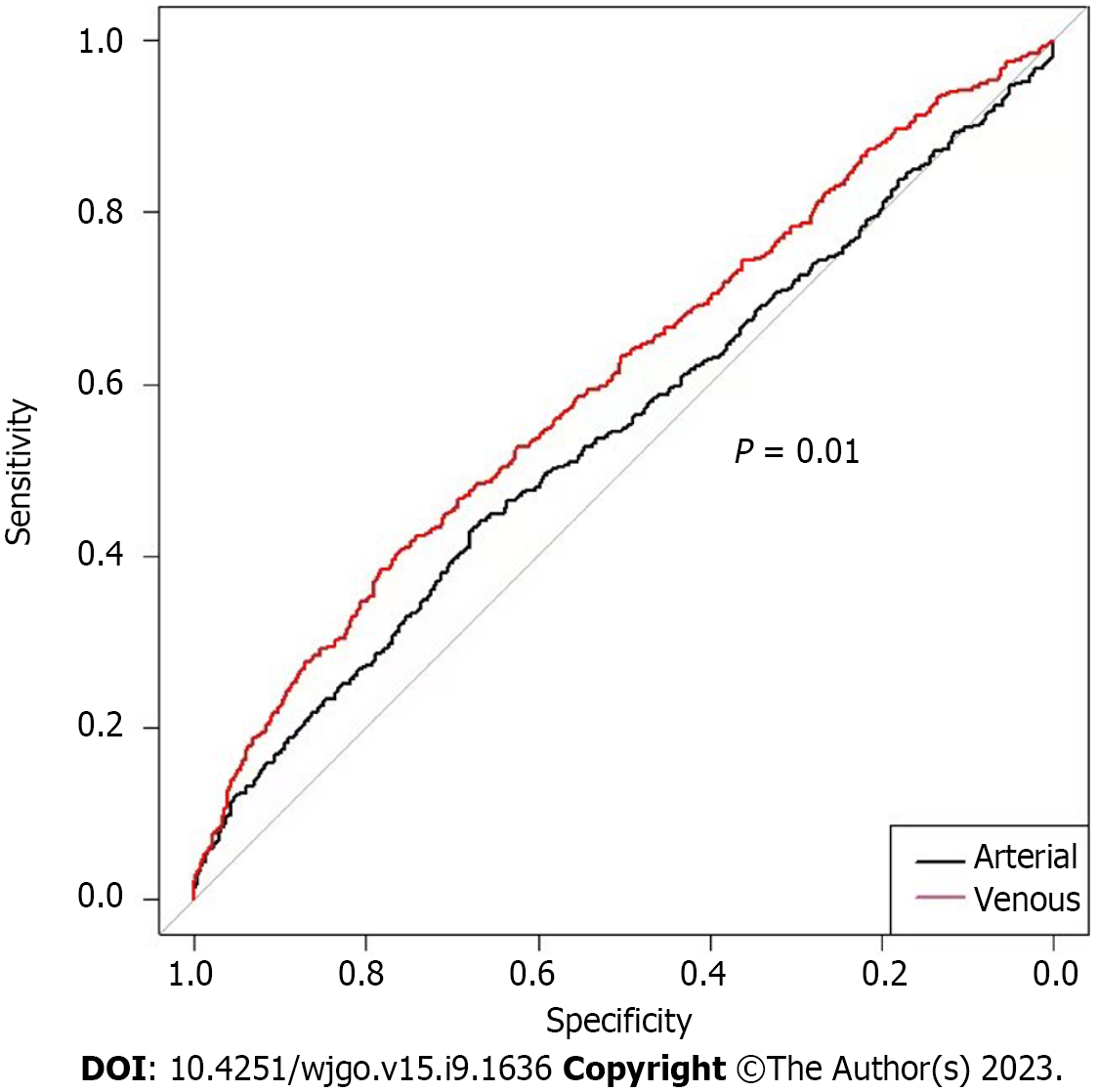
The predictive efficacy of arterial and venous CT values for patients with recurrent EGC is presented in Figure 3. The area under the curve (AUC) values for arterial and venous CT values for recurrent EGC were 0.545, and 0.604, respectively. Resulting ROC curves revealed no statistically significant differences between arterial and venous CT values for recurrent EGC (P = 0.001).
The symptoms of EGC are nonspecific, easy to be confused with other benign lesions, and often have entered the late stage when diagnosed, a phenomenon that leads to poor clinical treatment effect. Therefore, early screening and diagnosis of GC is imperative to prolonging the life of patients. Studies have shown that EGC patients who were treated with ESD are at risk of recurrence, thus should be subjected to early screening[11-13]. Although gastroscopy biopsy is the gold standard technique for GC diagnosis, it has poor acceptability among patients due to various shortcomings, key among them complicated examination and painful procedures, as well as high costs[14].
Enhanced spiral CT scan generates high resolution images, thus can provide a basis for identification of GC lesions. Studies have shown that application of phase III enhanced multi-slice spiral CT and window technique can increase its diagnostic efficacy in patients with EGC[15-17]. In this study, we found that Multi-layer helical CT enhanced imaging with narrow window + raised window has reduced image layers, with less display content, but revealed clear details, which offers critical advantages in the discovery and detection of subtle lesions. In addition, the lesions exhibited morphology and enhancement characteristics that were in sharp contrast with those of the adjacent normal gastric wall. In cases where the lesion exhibited a single-layer structure, we observed a noteworthy enhancement in the non-permeability of the gastric wall. This enhancement was characterized by either focal thickening of the gastric wall or a significant increase in enhancement without accompanying thickening of the gastric wall. When the lesion showed a multi-layer structure, the gastric wall was thickened and significantly enhanced without sudden disappearance of the middle and outer layers. At the lesion site, mucosal enhancement was obvious in arterial and portal vein stages, and basically subsided at the equilibrium stage. Some studies have reported the value of enhanced CT in the diagnosis of gastrointestinal neoplasms[18-21]. The standard window setting is suitable for general diagnostic purposes. However, a narrow window width offers advantages such as reduced layering and display content, which results in clearer details, increased image contrast, and improved resolution of both lesions and surrounding tissues. Additionally, using a narrow window width can enhance the darkening of images displayed on the window screen, further aiding in the diagnostic process. Moreover, enhanced lesion tissues appear on a good background due to the high CT value, whereas obvious superior substandard can be shown for local subtle enhanced lesions in the stomach wall.
In this study, most positive patients exhibited enhancement in both arterial and venous phases. However, there were still cases of missed diagnosis (55.78%). The technique used herein had sensitivity and specificity rates of 44.22% and 43.86%, respectively, which are far from satisfactory. However, the AUC of arterial and venous CT values for recurrent EGC was greater than 0.5, indicating that enhanced CT can predict EGC, albeit with low accuracy.
This study had some shortcomings. Firstly, this was a retrospective study. Secondly, some patients' data records were not detailed, which necessitated their elimination from the study, thus affecting the sample size.
Enhanced CT has superior diagnostic efficacy but lower accuracy in patients with recurrent EGC, compared to gold-standard techniques. Application value of CT in recurrent GC needs more extensive research.
There is an urgent need to develop a simple and easy approach for screening for early gastric cancer (EGC) recurrence in patients treated with endoscopic submucosal dissection (ESD).
Multi-slice spiral computed tomography (CT) is a quick, convenient and promising auxiliary examination. Enhanced spiral CT scan generates high resolution images, thus can provide a basis for the identification of gastric cancer lesions.
To explore the role of CT recurrence assessment in EGC patients who were treated with ESD.
This retrospective study recruited patients from the endoscopy department between January 2002 and December 2015. Basic characteristics, symptoms, CT results, and endoscopy with biopsy findings were analyzed. Sensitivity, specificity, negative and positive predictive values of CT for recurrent gastric cancer were calculated. Arterial and venous CT values were evaluated using area under the curve (AUC) analysis, and receiver operating characteristic curve analysis compared their performance for detecting recurrent EGC. The diagnostic efficacy and accuracy of enhanced CT were assessed in comparison to gold standard techniques for detecting recurrent EGC.
The approach had sensitivity and specificity rates of 44.22% and 43.86%, respectively, which are far from satisfactory. AUC value of arterial and venous CT values for recurrent EGC was greater than 0.5, indicating that enhanced CT can predict EGC, albeit at low accuracy.
Enhanced CT has superior diagnostic efficacy but lower accuracy than gold standard techniques in patients with recurrent EGC.
Multi-slice spiral CT is valuable in EGC screening.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68689] [Article Influence: 13737.8] [Reference Citation Analysis (201)] |
| 2. | Xia C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N, Chen W. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135:584-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1790] [Cited by in RCA: 2505] [Article Influence: 626.3] [Reference Citation Analysis (2)] |
| 3. | Yang K, Lu L, Liu H, Wang X, Gao Y, Yang L, Li Y, Su M, Jin M, Khan S. A comprehensive update on early gastric cancer: defining terms, etiology, and alarming risk factors. Expert Rev Gastroenterol Hepatol. 2021;15:255-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 4. | Landin MD, Guerrón AD. Endoscopic Mucosal Resection and Endoscopic Submucosal Dissection. Surg Clin North Am. 2020;100:1069-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Gong SD, Li H, Xie YB, Wang XH. Construction and analysis of an ulcer risk prediction model after endoscopic submucosal dissection for early gastric cancer. World J Gastrointest Oncol. 2022;14:1823-1832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 6. | Ning B, Abdelfatah MM, Othman MO. Endoscopic submucosal dissection and endoscopic mucosal resection for early stage esophageal cancer. Ann Cardiothorac Surg. 2017;6:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Tanabe S, Ishido K, Matsumoto T, Kosaka T, Oda I, Suzuki H, Fujisaki J, Ono H, Kawata N, Oyama T, Takahashi A, Doyama H, Kobayashi M, Uedo N, Hamada K, Toyonaga T, Kawara F, Tanaka S, Yoshifuku Y. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a multicenter collaborative study. Gastric Cancer. 2017;20:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Domper Arnal MJ, Ferrández Arenas Á, Lanas Arbeloa Á. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol. 2015;21:7933-7943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 554] [Cited by in RCA: 788] [Article Influence: 71.6] [Reference Citation Analysis (24)] |
| 9. | Ono H, Yao K, Fujishiro M, Oda I, Uedo N, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Fujimoto K. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig Endosc. 2021;33:4-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 363] [Article Influence: 72.6] [Reference Citation Analysis (1)] |
| 10. | Lim JS, Kim MJ, Yun MJ, Oh YT, Kim JH, Hwang HS, Park MS, Cha SW, Lee JD, Noh SH, Yoo HS, Kim KW. Comparison of CT and 18F-FDG pet for detecting peritoneal metastasis on the preoperative evaluation for gastric carcinoma. Korean J Radiol. 2006;7:249-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Douda L, Cyrany J, Tachecí I. Early gastric cancer. Vnitr Lek. 2022;68:371-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Gillen S. Advancing early gastric cancer detection. FEBS Open Bio. 2021;11:1812-1813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Shimizu S, Tada M, Kawai K. Early gastric cancer: its surveillance and natural course. Endoscopy. 1995;27:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 107] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1159] [Cited by in RCA: 1383] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 15. | Wani AH, Parry AH, Feroz I, Choh NA. Preoperative Staging of Gastric Cancer Using Computed Tomography and Its Correlation with Histopathology with Emphasis on Multi-planar Reformations and Virtual Gastroscopy. J Gastrointest Cancer. 2021;52:606-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Wang J, Zhong L, Zhou X, Chen D, Li R. Value of multiphase contrast-enhanced CT with three-dimensional reconstruction in detecting depth of infiltration, lymph node metastasis, and extramural vascular invasion of gastric cancer. J Gastrointest Oncol. 2021;12:1351-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Li CF, Zheng J, Xue YW. The value of contrast-enhanced computed tomography in predicting gastric cancer recurrence and metastasis. Cancer Biomark. 2017;19:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Kim EY, Lee WJ, Choi D, Lee SJ, Choi JY, Kim BT, Kim HS. The value of PET/CT for preoperative staging of advanced gastric cancer: comparison with contrast-enhanced CT. Eur J Radiol. 2011;79:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Cheng J, Wu J, Ye Y, Zhang C, Zhang Y, Wang Y. The prognostic significance of extramural venous invasion detected by multiple-row detector computed tomography in stage III gastric cancer. Abdom Radiol (NY). 2016;41:1219-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Lee IJ, Lee JM, Kim SH, Chang S, Han JK, Choi BI, Lee HJ, Yang HK, Lee KU. Helical CT evaluation of the preoperative staging of gastric cancer in the remnant stomach. AJR Am J Roentgenol. 2009;192:902-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Wang D, Zhang GB, Yan L, Wei XE, Zhang YZ, Li WB. CT and enhanced CT in diagnosis of gastrointestinal neuroendocrine carcinomas. Abdom Imaging. 2012;37:738-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Banach M, Poland; Trapani D, Italy S-Editor: Fan JR L-Editor: A P-Editor: Zhang XD