Published online Jul 15, 2023. doi: 10.4251/wjgo.v15.i7.1253
Peer-review started: February 6, 2023
First decision: March 15, 2023
Revised: March 16, 2023
Accepted: May 6, 2023
Article in press: May 6, 2023
Published online: July 15, 2023
Processing time: 156 Days and 5.7 Hours
Bones are one of the most common target organs for cancer metastasis. Early evaluation of bone metastasis (BM) status is clinically significant. Cancer patients often experience a hypercoagulable state.
To evaluate the correlation between coagulation indicators and the burden of BM in gastric cancer (GC).
We conducted a single-center retrospective study and enrolled 454 patients. Clinical information including routine blood examination and coagulation markers were collected before any treatment. Patients were grouped according to the status of BM. Receiver operating characteristic curves were used to assess diagnostic performance and determine the optimal cutoff values of the above indicators. Cutoff values, sensitivity and specificity were based on the maximum Youden index. Univariate and multivariate logistic regression analyses were used to evaluate the relationships between biomarkers and BM.
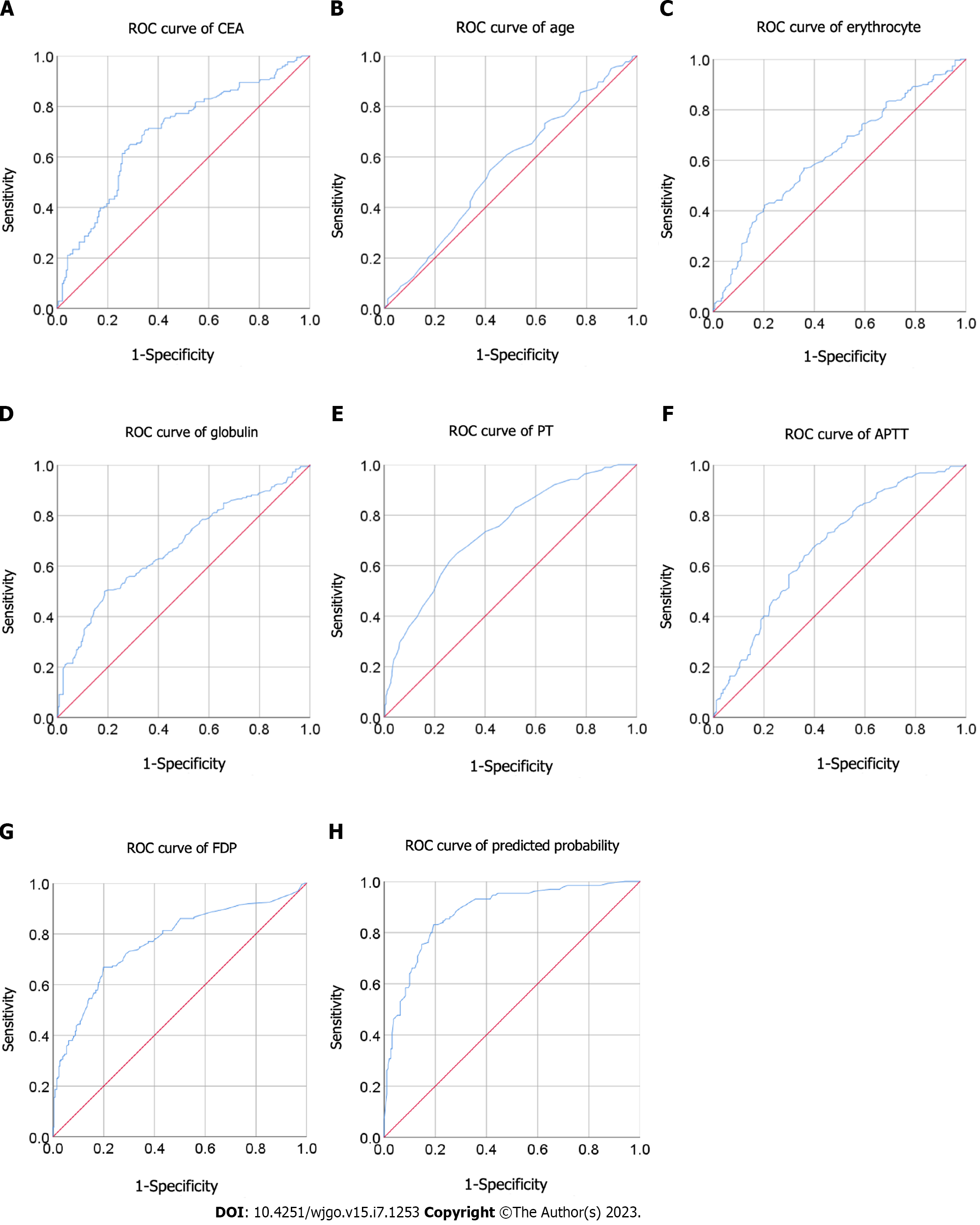
Of the 454 enrolled patients, 191 patients were diagnosed with BM. The receiver operating characteristic curve analysis suggested that prothrombin time (PT) [cutoff: 13.25; sensitivity: 0.651; specificity: 0.709; area under receiver operating characteristic curve (AUC) = 0.738], activated partial thromboplastin time (aPTT) (cutoff: 35.15; sensitivity: 0.640; specificity: 0.640; AUC = 0.678) and fibrin degradation products (FDP) (cutoff: 2.75; sensitivity: 0.668; specificity: 0.801; AUC = 0.768) act as novel predictors for BM. Based on multivariate logistic regression analysis, the results showed the independent correlation between PT [odds ratio (OR): 3.16; 95% confidence interval (CI): 1.612-6.194; P = 0.001], aPTT (OR: 2.234; 95%CI: 1.157-4.313; P = 0.017) and FDP (OR: 3.17; 95%CI: 1.637-6.139; P = 0.001) and BM in patients with GC. Moreover, age, carcinoembryonic antigen, erythrocyte and globulin were found to be significantly associated with BM.
Coagulation markers, namely PT, aPTT and FDP, might be potential predictors for screening BM in patients with GC.
Core Tip: Bones are one of the most common organs involved in cancer metastasis. Early evaluation of bone metastasis (BM) status is clinically significant. In this study, we confirmed that coagulation markers (prothrombin time, activated partial thromboplastin time and fibrin degradation products), carcinoembryonic antigen and globulin are independent risk factors for BM in patients with gastric cancer. Patients with these risk factors should be screened early for BM, which may significantly decrease mortality rates related to BM in patients with gastric cancer.
- Citation: Wang X, Wang JY, Chen M, Ren J, Zhang X. Clinical association between coagulation indicators and bone metastasis in patients with gastric cancer. World J Gastrointest Oncol 2023; 15(7): 1253-1261
- URL: https://www.wjgnet.com/1948-5204/full/v15/i7/1253.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i7.1253
Gastric cancer (GC) is one of the most malignant neoplasms worldwide. According to GLOBOCAN’s 2020 statistics, there were approximately 1.089 million new GC cases and 769000 GC deaths worldwide. GC has the fifth highest incidence rate and the fourth highest mortality rate of all cancers[1].
Common metastatic sites of GC are the liver, lungs, and peritoneum. Bone metastasis (BM) is relatively rare, ranging from 0.9% to 3.8%[2,3]. However, this incidence has been as high as 13.4% in autopsies[4]. The majority of patients with BM have several symptoms including bone pain, mobility disorders, hypercalcemia, pathological fractures and spinal cord compression, which seriously affects their quality of life. Unfortunately, BM is often underdiagnosed because sensitive diagnostic tests are recommended only after the onset of clinical symptoms. In addition, the median survival time for patients with GC-related BM is only 3-6 mo[3,5].
Imaging is currently the most important diagnostic method for BM. Elevated serum tumor markers such as carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9) and bone-associated alkaline phosphatase (ALP) provide additional diagnostic significance[6,7]. Computed tomography (CT) or enhanced CT is not a routine test for BM screening. It is only recommended when the patient is symptomatic, which leads to asymptomatic BM in patients with GC being largely undetected[4]. Previous studies have found that fibrinogen, activated partial thromboplastin time (aPTT) and D-dimer are independent risk factors for BM in non-small cell lung cancer[8]. However, there has been little research on multiple risk factors, such as a combination of clinical data and laboratory indicators, for BM in patients with GC. This study explored risk factors for BM from GC through multivariate analysis based on laboratory tests.
We retrospectively collected data on patients diagnosed with GC at the First Affiliated Hospital of Xi’an Jiaotong University from January 2014 to January 2019. The inclusion criteria were no distant metastases or BM. Exclusion criteria included: (1) A history of thrombotic disease, anticoagulant therapy or antiplatelet therapy; (2) Acute infection or disseminated intravascular coagulation; and (3) Lack of pretreatment laboratory data. In total, 454 patients were enrolled in this study. Data evaluated included sex, age at diagnosis, preoperative routine blood examination (erythrocyte, hemoglobin, leukocyte, neutrophil, lymphocyte, monocyte and platelet), glucose, albumin, globulin, CEA, CA19-9, CA72-4 and coagulation markers including prothrombin time (PT), prothrombin ratio (PTR), international normalized ratio (INR), aPTT, thrombin time (TT), fibrinogen, D-dimer and fibrin degradation products (FDP). Laboratory indicators were collected before any treatment. Blood parameters were those closest to the time of treatment. This study was approved by the Ethics Committee of First Affiliated Hospital of Xi’an Jiaotong University.
Cases were grouped according to BM status. Categorical variables were expressed as frequency (percentage) and compared using the χ2 test. Continuous variables were expressed as mean and standard deviation or median and interquartile range depending on whether they were normally distributed. Normally distributed continuous variables were compared using the Student’s t-test. Continuous variables that were not normally distributed were compared using the Mann-Whitney U test. The parameters with significant differences between the control group and the BM group were selected for receiver operating characteristic (ROC) analysis. The optimal cutoff values for parameters were obtained by ROC analyses based on the Youden index. The prediction probability (PP) of combined ROC curve was obtained by binary logistic regression. Multivariate logistic regression was performed to assess the relationship between laboratory variables and BM status. Statistical analyses and data plotting were performed with SPSS Statistics (version 20.0; IBM Corp., Armonk, NY, United States). A two-sided P value < 0.05 was considered statistically significant.
We collected data from 454 patients with GC and grouped them according to the method described previously. As shown in Table 1, there were 191 cases in the BM group. The median age of patients was 61 years, and males comprised the majority of patients (73.8%). Patients with BM had higher levels of GC markers (CEA, CA19-9 and CA72-4), neutrophils, glucose, globulin and most coagulation parameters (PT, PTR, INR, aPTT, fibrinogen, D-dimer and FDP) (all P < 0.001). Moreover, erythrocyte, lymphocyte and platelet levels were significantly lower in the BM group (all P <0.05) (Table 1).
| Characteristic | Overall, n = 454 | No bone metastasis, n = 263 | Bone metastasis, n = 191 | P value |
| Male sex | 335 (73.8) | 196 (74.5) | 139 (72.8) | 0.676 |
| Age, yr | 59 (50-67) | 61 (51-67) | 57 (49-66) | 0.046 |
| CEA, ng/mL | 3.53 (1.73-14.65) | 2.43 (1.35-4.89) | 5.29 (2.90-38.31) | < 0.001 |
| CA19-9, U/mL | 12.25 (6.30-46.88) | 9.89 (4.92-20.20) | 22.62 (9.73-113.35) | < 0.001 |
| CA72-4, U/mL | 3.39 (1.60-12.00) | 2.58 (1.57-6.78) | 7.09 (1.96-21.68) | < 0.001 |
| Erythrocyte, × 1012/L | 4.14 (3.65-4.57) | 4.27 (3.78-4.68) | 4.00 (3.50-4.39) | < 0.001 |
| Hemoglobin, g/L | 124 (106-139) | 127 (109-143) | 119 (103-133) | < 0.001 |
| Leukocyte, × 109/L | 5.69 (4.51-7.12) | 5.51 (4.43-6.76) | 6.20 (4.69-7.86) | 0.002 |
| Neutrophil, × 109/L | 3.54 (2.64-4.88) | 3.23 (2.51-4.33) | 4.04 (2.83-5.97) | < 0.001 |
| Lymphocyte, × 109/L | 1.37 (1.08-1.81) | 1.48 (1.12-1.95) | 1.30 (1.02-1.63) | 0.001 |
| Platelet, × 109/L | 196 (150-253) | 204 (160-260) | 180 (140-235) | 0.001 |
| Monocyte, × 109/L | 0.41 (0.30-0.54) | 0.42 (0.31-0.54) | 0.40 (0.30-0.55) | 0.713 |
| Glucose, mmol/L | 4.54 (4.17-5.07) | 4.40 (4.04-4.83) | 4.84 (4.39-5.55) | < 0.001 |
| Albumin, g/L | 38.11 ± 4.96 | 37.80 ± 4.71 | 38.55 ± 5.26 | 0.113 |
| Globulin, g/L | 26.4 (23.7-29.8) | 25.5 (22.8-28.2) | 28.8 (25.1-31.6) | < 0.001 |
| PT, s | 13.1 (12.5-13.8) | 12.8 (12.3-13.4) | 13.5 (13.0-14.4) | < 0.001 |
| PTR | 1.05 (0.99-1.10) | 1.03 (0.98-1.08) | 1.06 (1.01-1.12) | < 0.001 |
| INR | 1.05 (0.99-1.10) | 1.03 (0.98-1.08) | 1.07 (1.01-1.13) | < 0.001 |
| aPTT, s | 35.0 (31.5-38.1) | 33.5 (30.8-36.8) | 36.3 (33.7-39.1) | < 0.001 |
| TT, s | 16.3 (15.6-16.9) | 16.4 (15.7-17.0) | 16.0 (15.3-16.8) | < 0.001 |
| FIB, g/L | 3.32 (2.76-4.16) | 3.21 (2.62-3.82) | 3.63 (2.93-4.59) | < 0.001 |
| D-dimer, mg/L | 0.9 (0.3-2.5) | 0.5 (0.1-1.1) | 2.0 (0.8-6.6) | < 0.001 |
| FDP, mg/L | 1.9 (0.9-4.9) | 1.2 (0.7-2.5) | 4.5 (1.7-16.2) | < 0.001 |
We performed ROC analysis to assess the efficacy of parameters to predict BM in patients with GC and obtained a series of cutoff values. The optimal cutoff values (sensitivity and specificity) were: age, 59.5 (54.6% and 58.3%); CEA, 3.97 (64.9% and 71.1%); CA19-9, 12.81 (65.5% and 64.9%); CA72-4, 6.71 (51.8% and 74.7%); erythrocyte level, 4.43 (42.3% and 79.7%); hemoglobin, 133.5 (42.7% and 75.9%); leukocyte level, 6.28 (49.2% and 68.5%); neutrophil level, 4.23 (48.4% and 74.9%); lymphocyte level, 1.43 (55.2% and 64.2%); platelet level, 167.5 (73.1% and 44.9%); glucose, 4.82 (52.4% and 74.8%); globulin, 28.75 (50.0% and 81.4%); PT, 13.25 (65.1% and 70.9%); PTR, 1.09 (40.7% and 78.5%); INR, 1.1 (37.6% and 81.9%); aPTT, 35.15 (64.0% and 64.0%); TT, 15.95 (69.7% and 48.7%); fibrinogen, 4.06 (42.3% and 82.0%); D-dimer, 1.03 (69.0% and 72.8%); FDP, 2.75 (66.8% and 80.1%) (Figure 1, Supplementary Figures 1 and 2). The area under ROC curves and 95% confidence intervals (CI) were: CEA, 0.694 (0.639-0.748); CA19-9, 0.673 (0.617-0.729); CA72-4, 0.624 (0.560-0.688); PT, 0.738 (0.692-0.784); aPTT, 0.678 (0.629-0.727); and FDP, 0.768 (0.722-0.814) (Table 2, Figure 1).
| Parameter | AUC | 95%CI | Cutoff | Sen | Spe | Youden index | PPV | NPV | P value |
| Age | 0.558 | 0.501-0.616 | 59.5 | 0.546 | 0.583 | 0.129 | 0.433 | 0.546 | 0.046 |
| CEA | 0.694 | 0.639-0.748 | 3.97 | 0.649 | 0.711 | 0.36 | 0.665 | 0.711 | < 0.001 |
| CA19-9 | 0.673 | 0.617-0.729 | 12.81 | 0.655 | 0.649 | 0.304 | 0.614 | 0.649 | < 0.001 |
| CA72-4 | 0.624 | 0.560-0.688 | 6.71 | 0.518 | 0.747 | 0.265 | 0.603 | 0.747 | < 0.001 |
| Erythrocyte | 0.623 | 0.571-0.675 | 4.43 | 0.423 | 0.797 | 0.220 | 0.498 | 0.423 | < 0.001 |
| Hemoglobin | 0.599 | 0.547-0.651 | 133.50 | 0.427 | 0.759 | 0.186 | 0.488 | 0.427 | < 0.001 |
| Leukocyte | 0.587 | 0.532-0.641 | 6.28 | 0.492 | 0.685 | 0.177 | 0.529 | 0.685 | 0.002 |
| Neutrophil | 0.63 | 0.576-0.683 | 4.23 | 0.484 | 0.749 | 0.233 | 0.581 | 0.749 | < 0.001 |
| Lymphocyte | 0.591 | 0.539-0.644 | 1.43 | 0.552 | 0.642 | 0.194 | 0.508 | 0.552 | 0.001 |
| Platelet | 0.591 | 0.538-0.645 | 167.50 | 0.731 | 0.449 | 0.180 | 0.545 | 0.731 | 0.001 |
| Glucose | 0.664 | 0.613-0.716 | 4.82 | 0.524 | 0.748 | 0.272 | 0.595 | 0.748 | < 0.001 |
| Globulin | 0.675 | 0.624-0.726 | 28.75 | 0.500 | 0.814 | 0.314 | 0.655 | 0.814 | < 0.001 |
| PT | 0.738 | 0.692-0.784 | 13.25 | 0.651 | 0.709 | 0.360 | 0.618 | 0.709 | < 0.001 |
| PTR | 0.622 | 0.570-0.675 | 1.09 | 0.407 | 0.785 | 0.193 | 0.579 | 0.785 | < 0.001 |
| INR | 0.627 | 0.574-0.680 | 1.10 | 0.376 | 0.819 | 0.195 | 0.602 | 0.819 | < 0.001 |
| aPTT | 0.678 | 0.629-0.727 | 35.15 | 0.640 | 0.640 | 0.280 | 0.563 | 0.640 | < 0.001 |
| TT | 0.598 | 0.545-0.652 | 15.95 | 0.697 | 0.487 | 0.184 | 0.538 | 0.816 | < 0.001 |
| FIB | 0.616 | 0.562-0.669 | 4.06 | 0.423 | 0.820 | 0.243 | 0.630 | 0.820 | < 0.001 |
| D-dimer | 0.756 | 0.710-0.801 | 1.03 | 0.690 | 0.728 | 0.418 | 0.645 | 0.728 | < 0.001 |
| FDP | 0.768 | 0.722-0.814 | 2.75 | 0.668 | 0.801 | 0.469 | 0.706 | 0.801 | < 0.001 |
| PP | 0.879 | 0.841-0.917 | 0.364 | 0.831 | 0.806 | 0.637 | 0.745 | 0.806 | < 0.001 |
Parameters were grouped by aforementioned cutoff values. Multivariate logistic regression analysis showed that higher PT [odds ratio (OR): 3.16; 95%CI: 1.612-6.194; P = 0.001), higher aPTT (OR: 2.234; 95%CI: 1.157-4.313; P = 0.017) and elevated FDP (OR: 3.17; 95%CI: 1.637-6.139; P = 0.001) were independent risk factors for BM in patients with GC. In addition, higher CEA and globulin as well as lower age and red blood cell count were also independent risk factors for BM with an OR (95%CI) of 2.847 (1.496-5.418), 4.253 (2.114-8.558), 0.392 (0.203-0.756), and 0.482 (0.24-0.966), respectively (all P < 0.05) (Table 3). The area under ROC curve (95%CI) of PP was 0.879 (0.841-0.917) with a sensitivity of 0.831 and a specificity of 0.806 (Table 2, Figure 1).
| Univariate analysis | Multivariate analysis | |||||
| Parameter | Odds ratio | 95%CI | P value | Odds ratio | 95%CI | P value |
| Age | 0.594 | 0.398-0.887 | 0.011 | 0.392 | 0.203-0.756 | 0.005 |
| CEA | 4.559 | 2.931-7.090 | < 0.001 | 2.847 | 1.496-5.418 | 0.001 |
| CA19-9 | 3.511 | 2.271-5.429 | < 0.001 | 0.352 | ||
| CA72-4 | 3.176 | 1.995-5.056 | < 0.001 | 0.086 | ||
| Erythrocyte | 0.348 | 0.226-0.536 | < 0.001 | 0.482 | 0.240-0.966 | 0.040 |
| Hemoglobin | 0.425 | 0.281-0.645 | < 0.001 | 0.852 | ||
| Leukocyte | 2.102 | 1.426-3.099 | < 0.001 | 0.693 | ||
| Neutrophil | 2.798 | 1.872-4.183 | < 0.001 | 0.601 | ||
| Lymphocyte | 0.453 | 0.308-0.667 | < 0.001 | 0.575 | ||
| Platelet | 0.452 | 0.304-0.672 | < 0.001 | 0.066 | ||
| Glucose | 3.273 | 2.191-4.890 | < 0.001 | 0.087 | ||
| Globulin | 4.367 | 2.861-6.667 | < 0.001 | 4.253 | 2.114-8.558 | < 0.001 |
| PT | 4.536 | 3.038-6.774 | < 0.001 | 3.16 | 1.612-6.194 | 0.001 |
| PTR | 2.517 | 1.663-3.808 | < 0.001 | 0.145 | ||
| INR | 2.727 | 1.771-4.199 | < 0.001 | 0.072 | ||
| aPTT | 3.161 | 2.140-4.669 | < 0.001 | 2.234 | 1.157-4.313 | 0.017 |
| TT | 0.535 | 0.344-0.833 | 0.006 | 0.842 | ||
| FIB | 3.342 | 2.179-5.125 | < 0.001 | 0.193 | ||
| D-dimer | 5.952 | 3.939-8.993 | < 0.001 | 0.956 | ||
| FDP | 8.103 | 5.271-12.457 | < 0.001 | 3.17 | 1.637-6.139 | 0.001 |
BM is a common complication of certain cancers, including breast cancer and prostate cancer[9], whereas BM due to GC is less frequent[10]. The common metastatic sites of GC are the liver, lungs and peritoneum. Most patients with BM due to GC have multiple metastases, and most metastases are difficult to resect surgically[11]. Once tumors have metastasized to the bone, they are virtually incurable and cause severe morbidity before the patient dies. BM leads to pain, pathological fractures, nerve compression syndrome and hypercalcemia. According to relevant research reports, the proportion of patients suspected of BM due to GC found by bone scan screening was as high as 25.0%-45.3%[12].
Several factors have been shown to have predictive value for BM due to GC. BM is a dynamic process of osteolytic and osteogenesis mediated by osteoclasts that disrupts normal bone homeostasis. Bone ALP is an indicator of osteoblast metabolism and a relatively specific osteogenic marker, which has predictive value in patients with BM due to GC[6]. Bone screening is recommended for cancer types with a high incidence of BM, such as prostate cancer, breast cancer, small cell lung cancer and renal cell carcinoma. A variety of imaging studies are available, including plain X-rays, bone scintigraphy, CT scans, magnetic resonance imaging, positron emission tomography and positron emission tomography/CT, to assess bone involvement. However, bone screening has not been routinely recommended by the Chinese Society of Clinical Oncology for patients with GC[13]. Excessive X-rays and CT imaging are expensive and put patients at risk of unnecessary radiation exposure and/or invasive procedures due to false positive results. Therefore, it is necessary to evaluate BM through a combination of imaging and analyzing hematological parameters and patient symptoms.
In this study, we screened possible risk factors for BM by comparing baseline data between the control group and the BM group. Through multivariate logistic regression analysis of candidate tumor markers, routine blood counts, coagulation indicators, albumin and globulin, we found that elevated CEA, globulin, PT, aPTT and FDP and younger age and lower red blood cells were independent risk factors for BM due to GC. CEA is a classic GC marker and has been shown to be a risk factor for distant metastasis and lymph node metastasis[14,15]. Globulin was identified as an independent predictor of occult metastasis in the neck of oral squamous cell carcinoma[16]. In GC, a high level of globulin is a valuable predictor of tumor progression[17].
Tumors are often accompanied by a state of coagulation activation[18]. Fibrinogen, aPTT and D-dimer were found to be risk factors for BM in non-small cell lung carcinoma patients[19]. Our study confirmed that PT, aPTT and FDP, as coagulation indicators, are independent predictors of BM in GC patients. In fact, tumor cells often express tissue factor or other procoagulants that can initiate coagulation[20]. There is considerable evidence that inhibiting coagulation can inhibit tumor metastasis[21,22]. BM due to GC can develop regardless of the tumor stage, although the proportion of patients with stage IV GC with BM exceeds the proportion of patients with stages I-III combined. It was found that even after radical gastrectomy, BM recurred in 1.8% of patients[23]. This indicates that the risk of BM should be considered when these indicators are abnormally elevated in patients with GC, especially when they are higher than the cutoff values in Table 2. Furthermore, the cutoff values of the BM risk factors indicated in this study are different from their respective upper limits of clinical normality. In other words, elevated coagulation indicators may indicate BM risk even within the range of clinically normal reference values.
A hypercoagulable state represents a heterogeneous group of disorders that cover a variety of risk factors such as thrombosis, obesity, pregnancy, cancer and its treatment, antiphospholipid antibody syndrome, heparin-induced thrombocytopenia and myeloproliferative disorders[24]. This suggests that in order to improve the specificity of coagulation factors in assessing BM due to GC, other factors that may affect their levels need to be excluded. Although we discovered independent risk factors for BM due to GC, we did not explore whether they were specific to BM or due to other metastatic sites’ GC.
Because we retrospectively collected data from patients with GC, bone-associated ALP was not routinely tested and was not included in the analysis. There is evidence that tumor-induced hypercoagulability and fibrin formation are required for tumor angiogenesis, metastasis and invasion because cross-linked fibrin in the extracellular matrix may be the framework for tumor cell migration during invasion. Based on this, circulating tumor cells and micrometastases are considered early events in the process of tumor cell metastasis[25]. Bone ALP is a specific marker of osteoblast metabolism and is significantly associated with the presence and degree of bone involvement in metastatic tumors. Bone-associated ALP has been shown to be an important predictor of BM in patients with breast and prostate cancer[26]. More than half of patients with BM due to GC have elevated ALP and tumor markers[3,6]. Coagulation indexes and bone ALP reflect the two stages of tumor hematogenous metastasis and BM, respectively. In this study, we demonstrated that the PP obtained by combination ROC had a higher diagnostic efficacy than any single risk factor. The combination of coagulation indexes, globulins, tumor markers and bone ALP may greatly improve the diagnostic efficiency of BM due to GC.
Overall, coagulation markers (PT, aPTT and FDP), CEA and globulin are independent risk factors for BM due to GC. Patients with these risk factors should be screened for BM early, which could lead to significantly decreased mortality in patients with GC and BM.
Bones are one of the most common targets for cancer metastasis. However, bone metastasis (BM) is often underdiagnosed because sensitive diagnostic imaging methods are recommended only after the onset of clinical symptoms. Patients with gastric cancer (GC), especially in advanced stages, are often in a hypercoagulable state.
The purpose of this study was to explore the predictive value of blood indicators on the risk of BM due to GC and to improve the diagnostic efficacy of BM due to GC by screening effective risk factors.
The purpose of this study was to explore whether coagulation indicators can be used as independent risk factors for predicting BM due to GC, thus promoting the early diagnosis and treatment of BM.
We conducted a retrospective study and enrolled 454 patients in this study. Receiver operating characteristic (ROC) curves were used to assess diagnostic performance. Univariate and multivariate logistic regression analyses were used to evaluate the relationship between biomarkers and BM.
ROC curve analysis indicated that coagulation markers have similar or better diagnostic efficacy than traditional GC markers. Based on multivariate logistic regression analysis, prothrombin time, activated partial thromboplastin time and fibrin degradation products were independently associated with BM due to GC. Moreover, age, carcinoembryonic antigen, erythrocyte level and globulin were found to be risk factors of BM. Combining these indicators could improve the effectiveness of diagnosing BM.
Coagulation markers (prothrombin time, activated partial thromboplastin time and fibrin degradation products), carcinoembryonic antigen and globulin were independent risk factors for BM due to GC. Patients with these risk factors should be screened early to detect BM due to GC and prevent bone-related events.
Future research will explore the relationship and molecular mechanism between coagulation and tumor metastasis and explore new targets to block the process of tumor metastasis.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68357] [Article Influence: 13671.4] [Reference Citation Analysis (201)] |
| 2. | Mikami J, Kimura Y, Makari Y, Fujita J, Kishimoto T, Sawada G, Nakahira S, Nakata K, Tsujie M, Ohzato H. Clinical outcomes and prognostic factors for gastric cancer patients with bone metastasis. World J Surg Oncol. 2017;15:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Imura Y, Tateiwa D, Sugimoto N, Inoue A, Wakamatsu T, Outani H, Tanaka T, Tamiya H, Yagi T, Naka N, Okawa S, Tabuchi T, Takenaka S. Prognostic factors and skeletal-related events in patients with bone metastasis from gastric cancer. Mol Clin Oncol. 2020;13:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Turkoz FP, Solak M, Kilickap S, Ulas A, Esbah O, Oksuzoglu B, Yalcin S. Bone metastasis from gastric cancer: the incidence, clinicopathological features, and influence on survival. J Gastric Cancer. 2014;14:164-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Qiu MZ, Shi SM, Chen ZH, Yu HE, Sheng H, Jin Y, Wang DS, Wang FH, Li YH, Xie D, Zhou ZW, Yang DJ, Xu RH. Frequency and clinicopathological features of metastasis to liver, lung, bone, and brain from gastric cancer: A SEER-based study. Cancer Med. 2018;7:3662-3672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 6. | Lim SM, Kim YN, Park KH, Kang B, Chon HJ, Kim C, Kim JH, Rha SY. Bone alkaline phosphatase as a surrogate marker of bone metastasis in gastric cancer patients. BMC Cancer. 2016;16:385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Wu C, Lin X, Li Z, Chen Z, Xie W, Zhang X, Wang X. A Diagnostic Nomogram Based on (18)F-FDG PET/CT for Bone Metastasis of Gastric Cancer. Front Cell Dev Biol. 2021;9:783466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 8. | Li Y, Xu C, Yu Q. Risk factor analysis of bone metastasis in patients with non-small cell lung cancer. Am J Transl Res. 2022;14:6696-6702. [PubMed] |
| 9. | Yin JJ, Pollock CB, Kelly K. Mechanisms of cancer metastasis to the bone. Cell Res. 2005;15:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 273] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 10. | Xiaobin C, Zhaojun X, Tao L, Tianzeng D, Xuemei H, Fan Z, Chunyin H, Jianqiang H, Chen L. Analysis of Related Risk Factors and Prognostic Factors of Gastric Cancer with Bone Metastasis: A SEER-Based Study. J Immunol Res. 2022;2022:3251051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Nakanishi H, Araki N, Kuratsu S, Narahara H, Ishikawa O, Yoshikawa H. Skeletal metastasis in patients with gastric cancer. Clin Orthop Relat Res. 2004;208-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Choi CW, Lee DS, Chung JK, Lee MC, Kim NK, Choi KW, Koh CS. Evaluation of bone metastases by Tc-99m MDP imaging in patients with stomach cancer. Clin Nucl Med. 1995;20:310-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Wen L, Li YZ, Zhang J, Zhou C, Yang HN, Chen XZ, Xu LW, Kong SN, Wang XW, Zhang HM. Clinical analysis of bone metastasis of gastric cancer: incidence, clinicopathological features and survival. Future Oncol. 2019;15:2241-2249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Wang K, Jiang X, Ren Y, Ma Z, Cheng X, Li F, Xiao J, Yu Z, Jiao Z. The significance of preoperative serum carcinoembryonic antigen levels in the prediction of lymph node metastasis and prognosis in locally advanced gastric cancer: a retrospective analysis. BMC Gastroenterol. 2020;20:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Ikeda Y, Mori M, Adachi Y, Matsushima T, Sugimachi K, Saku M. Carcinoembryonic antigen (CEA) in stage IV gastric cancer as a risk factor for liver metastasis: a univariate and multivariate analysis. J Surg Oncol. 1993;53:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Jin W, Zhu M, Zheng Y, Wu Y, Ding X, Wu H, Ye J, Zhu Z, Song X. Perineural invasion, lactate dehydrogenase, globulin, and serum sodium predicting occult metastasis in oral cancer. Oral Dis. 2022;28:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Chen J, Zhou Y, Xu Y, Zhu HY, Shi YQ. Low pretreatment serum globulin may predict favorable prognosis for gastric cancer patients. Tumour Biol. 2016;37:3905-3911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Falanga A, Schieppati F, Russo D. Cancer Tissue Procoagulant Mechanisms and the Hypercoagulable State of Patients with Cancer. Semin Thromb Hemost. 2015;41:756-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 19. | Yao Y, Zhou Y, Yang Z, Shen H. [Risk Factors of Non-small Cell Lung Cancer with Bone Metastasis after Therapy]. Zhongguo Fei Ai Za Zhi. 2018;21:476-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 20. | Gale AJ, Gordon SG. Update on tumor cell procoagulant factors. Acta Haematol. 2001;106:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Gil-Bernabé AM, Lucotti S, Muschel RJ. Coagulation and metastasis: what does the experimental literature tell us? Br J Haematol. 2013;162:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Palumbo JS, Degen JL. Mechanisms linking tumor cell-associated procoagulant function to tumor metastasis. Thromb Res. 2007;120 Suppl 2:S22-S28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Park JM, Song KY, O JH, Kim WC, Choi MG, Park CH. Bone recurrence after curative resection of gastric cancer. Gastric Cancer. 2013;16:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Anderson JA, Weitz JI. Hypercoagulable states. Crit Care Clin. 2011;27:933-952, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Jiang HG, Li J, Shi SB, Chen P, Ge LP, Jiang Q, Tang XP. Value of fibrinogen and D-dimer in predicting recurrence and metastasis after radical surgery for non-small cell lung cancer. Med Oncol. 2014;31:22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Tankó LB, Karsdal MA, Christiansen C, Leeming DJ. Biochemical approach to the detection and monitoring of metastatic bone disease: What do we know and what questions need answers? Cancer Metastasis Rev. 2006;25:659-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Seretis C, Greece; Tanabe S, Japan S-Editor: Yan JP L-Editor: A P-Editor: Zhang XD