Published online Jun 15, 2023. doi: 10.4251/wjgo.v15.i6.979
Peer-review started: March 28, 2023
First decision: April 11, 2023
Revised: April 17, 2023
Accepted: May 12, 2023
Article in press: May 12, 2023
Published online: June 15, 2023
Processing time: 78 Days and 20.2 Hours
Autophagy is a physiological mechanism in which cells degrade themselves and quickly recover the degraded cell components. Recent studies have shown that autophagy plays an important role in the occurrence, development, treatment, and prognosis of colorectal cancer. In the early stages of colorectal cancer, auto
Core Tip: In the early stages of colon cancer, autophagy can inhibit the production and development of tumors through multiple mechanisms such as maintaining DNA stability, inducing tumor death, and enhancing immune surveillance. However, as colorectal cancer progresses, autophagy may mediate tumor resistance, enhance tumor metabolism, and other pathways to promote tumor development. Therefore, intervening in autophagy at the appropriate time has broad clinical application prospects.
- Citation: Ma TF, Fan YR, Zhao YH, Liu B. Emerging role of autophagy in colorectal cancer: Progress and prospects for clinical intervention. World J Gastrointest Oncol 2023; 15(6): 979-987
- URL: https://www.wjgnet.com/1948-5204/full/v15/i6/979.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i6.979
Colorectal cancer (CRC) refers to malignant epithelial tumors of the colon, rectum, and anal canal. In 2020, CRC was the third most common malignancy and the second most deadly cancer worldwide, with an estimated 1.88 million new cases (9.8%) and 910000 deaths (9.2%)[1]. Great progress has been made in CRC diagnosis and treatment with the availability of routine health check-ups and new techniques. At present, surgery remains the mainstay of treatment for CRC, while chemotherapy, targeted therapy, and immunotherapy have also been applied in clinical settings. However, due to its insidious onset, CRC is mostly diagnosed in advanced stages and becomes resistant to chemotherapy and targeted therapy[2]. Therefore, the prognosis of CRC is poor. Autophagy plays a critical role in regulating cancer development, and autophagy-based clinical interventions may address this clinical dilemma. This article reviews the recent progress in the research on the association between autophagy and CRC.
Autophagy is a highly conserved eukaryotic macromolecular degradation process that degrades and recycles macromolecular substances such as damaged organelles and sirtuins inside the cells to quickly replenish the substances required for normal physiological activities of cells[3]. Autophagy is strictly regulated by a variety of autophagy-related genes (ATGs). Under stressful conditions such as organelle damage, production of abnormal proteins, and nutrient deficiency, autophagosomes are formed, which fuse with intracellular lysosomes to form autophagolysosomes by wrapping or binding to the components to be degraded, thus initiating the degradation and recycling process[4]. In both normal and malignant cells, autophagy may be a response to cellular stresses including nutrient deficiencies, hypoxia, and toxin accumulation[5]. Nevertheless, the impact of autophagy on cells can be multi-faceted: It may be a protective factor that promotes cell survival, but can also lead to growth arrest and/or apoptosis.
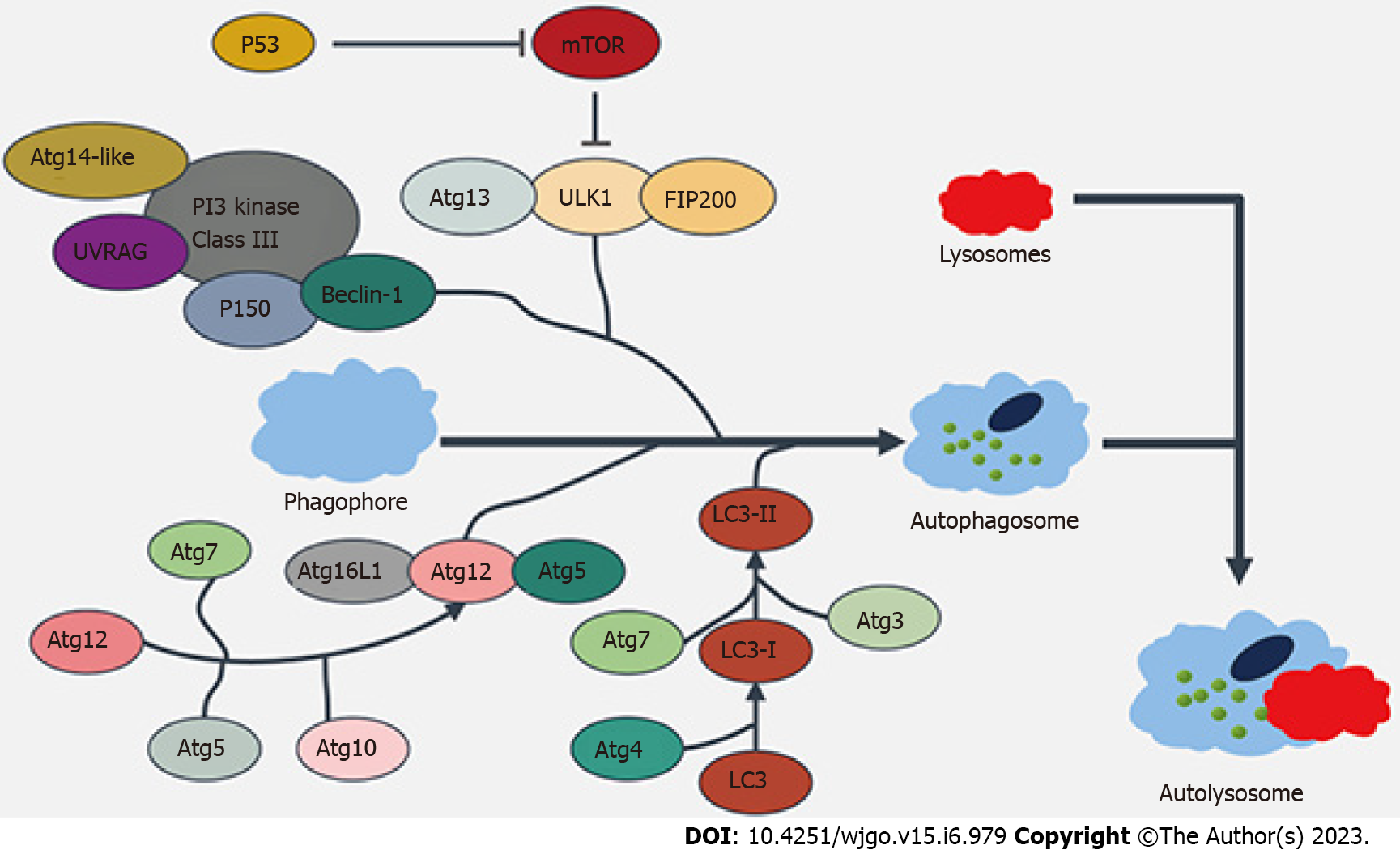
Autophagy, as a metabolic regulatory mechanism widely present within cells, can lead to various diseases such as neurodegenerative diseases and tumors once dysregulated. As shown in Figure 1, the molecular mechanism via which autophagy is regulated mainly includes the following three aspects: (1) The adenosine monophosphate-activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR) pathway: The mTOR kinase, as a receptor for amino acids, energy, and hormones in cells, can regulate autophagy in a negative feedback manner[6]; (2) The phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathway: Different types of PI3K play different roles in autophagy regulation, among which type I PI3K can inhibit autophagy after binding to AKT, whereas type III PI3K can induce the enhancement of autophagy by binding to the ultraviolet resistance-associated gene (UURAG) product; and (3) The negative feedback signaling pathway of G protein subunit Gai3 and amino acids: GTP can bind to Gai3 intracellularly and is an inhibitory signal of autophagy; GDP binds to Gai3 protein to activate autophagy; and, as the end products of protein degradation, amino acids negatively regulate the autophagy[7-8].
Autophagy can be divided into three types depending on the mechanism by which intracellular materials are delivered into the lysosome for degradation: Macroautophagy, microautophagy, and chaperone-mediated autophagy (CMA)[9]. Macroautophagy is a selective autophagy process that occurs mainly in macrophages. They can form phagosomes that ingest cytoplasmic proteins and damaged organelles and then further mature into double-membrane binding vesicles (known as autophagosomes). These autophagosomes are transported to lysosomes, where they degrade the damaged mitochondria, the invading microorganisms, and other components, thus maintaining the homeostasis of cells during the stress response. During macroautophagy, autophagy is mainly regulated via the AMPK/mTOR signaling pathway[10]. Microautophagy occurs through the invagination of lysosomes or endosomal membranes, which directly phagocytosize the substances to be degraded, during which the proteases in lysosomes play a degrading role. Microautophagy occurs in almost all normal cells and therefore is a widely existing intracellular energy and material circulation mechanism[11-12]. Notably, no direct link between microautophagy and tumorigenesis has been found.
Unlike macroautophagy and microautophagy, CMA is a selective lysosome-dependent protein-degrading mechanism[13]. CMA exists in most cells but exerts only basal activities under physiological conditions. Once cell stress occurs, the activity of CMA is rapidly enhanced, which facilitates the rapid circulation of intracellular proteins under stress conditions and minimizes the need for new protein degradation and production, thereby maximizing energy savings and improving cell survival. Compared to normal cells, tumor cells have increased CMA activity. Blocking CMA significantly inhibited tumor growth and induced regression of lung cancer or melanoma xenografts in mouse models[14]. Therefore, intervening in the CMA pathways may have broad antitumor activity. In the form of autophagy, all the proteins targeted by CMA contain KFERQ-like pentapeptide motifs, which can be recognized by heat shock protein family A (Hsp70) member 8 (HSPA8/HSC70) and form a complex with lysosome-associated membrane protein type 2a (LAMP-2A)[15]. No vesicle structure will be formed during this process; rather, the proteins to be degraded pass directly through the lysosomal membrane to enter the lysosomal cavity for degradation.
In addition to autophagy's important regulatory role in energy and material metabolism, the endosomal protein sorting nexin 5 (SNX5) interacts with beclin 1 and ATG14-containing class III phosphatidylinositol-3-kinase (PI3KC3) complex 1 (PI3KC3-C1), increases the lipid kinase activity of purified PI3KC3-C1, and is required for the endosomal generation of phosphatidylinositol-3-phosphate (PI3P) and recruitment of the PI3P-binding protein WIPI2 to virion-containing endosomes, thus mediating virus-induced autophagy[16]. Therefore, autophagy can also play an immune- and host-protecting role when cells are invaded by viruses.
As a regulatory mechanism of intracellular substance and energy metabolism, autophagy is deeply involved in a variety of biological behaviors such as cell repair, transformation, proliferation, senescence, and apoptosis[17]. With increasing in-depth research, the roles of autophagy in the pathogenesis, drug resistance, and therapeutic options of CRC have been revealed. For CRC, autophagy is a "double-edged sword": On the one hand, autophagy can significantly suppress proliferation and induce apoptosis in CRC cells; on the other hand, it can also provide CRC cells with additional energy to promote their abnormal proliferation and can reduce the response of CRC to various treatment measures[18].
As a housekeeping mechanism in normal cells, autophagy can scavenge and repair DNA damage, abnormal folding of proteins or abnormal accumulation of normal proteins, accumulation of oxygen free radicals, and damage to organelles (e.g. mitochondria) during normal physiological processes and under stress, which is extremely important for maintaining cell stability and avoiding carcinogenesis[3,19-21]. Therefore, as an early cancer-suppressing mechanism, autophagy can protect the human body through multiple pathways.
SRC kinases are non-receptor tyrosine kinases that mediate carcinogenesis. An abnormal elevation of SRC can be detected in about 80% of CRC patients, and sorting nexin 10 (SNX10), an endosomal protein, is negatively correlated with SRC expression. The downregulated expression of SNX10 is significantly associated with SRC activation, tumor differentiation, tumor metastasis, and patient survival. SNX10 regulates the fusion between autophagy and lysosomes. SNX10 deficiency can lead to impaired autophagic degradation of SRC, which ultimately promotes the occurrence and development of CRC. Interfering with the SRC autophagy pathway can achieve the regulation of tumor growth[22].
The level of the imprinted gene pleckstrin homology like domain family A member 2 (PHLDA2) can also be up-regulated in CRC tissues. Knockdown of PHLDA2 inhibited cellular proliferation, invasion, migration, and epithelial-mesenchymal transition (EMT) in vitro by activating the autophagy of CRC cells through the PI3K/AKT/mTOR and PI3K/AKT/GSK-3β signaling pathways[23]. Decreased or absent expressions of ATGs BeclinI and ATG5 can also lead to CRC progression and are significantly associated with poor prognosis[24,25]. Therefore, ATGs can exert a cancer-suppressing effect by inducing autophagy. In addition to ATGs that directly regulate the biological behaviors of tumor cells, epigenetic regulation of autophagy can also be a molecular basis for the body to inhibit tumorigenesis by regulating autophagy. BRG1, the ATPase subunit of the SWI/SNF chromatin remodeling complex, is required for maintaining the homeostasis of intestinal epithelial cells (IECs) to prevent inflammation and tumorigenesis. BRG1 is a key regulator that directly regulates Atg16 L1, Ambra1, Atg7, and Wipi2 transcription, which is important for autophagosome biogenesis. Defective autophagy in BRG1-deficient IECs results in excess reactive oxygen species (ROS), which leads to defects in barrier integrity and thus causes the occurrence of CRC[26].
Compared with normal tissue cells, tumor cells have unlimited self-replication ability and ultra-high metabolic level. The bulky growth of CRC creates a unique environment featured by hypoxia, low pH, and high metabolites within the tumor tissue[27]. On the one hand, it makes the energy acquisition of tumor cells more dependent on glycolytic pathways and autophagy[28-30]; on the other hand, the enhanced autophagy can further promote tumor progression by affecting the expressions of tumor suppressor genes (e.g., p53), the degradation of major histocompatibility complex I (MHC-I), and the infiltration of various immune cells[31,32]. In solid tumors, the autophagosome content receptor NBR1 mainly regulates the localization of MHC-I on the tumor cell surface, autophagosomes, and lysosomes, whereas MHC-I is closely related to the antigen presentation and anti-tumor activity of immune cells. Intervening with NBR1 can regulate autophagy, thereby affecting the expression of MHC-I and the immune status of the body[33], suggesting that autophagy may promote tumor progression via immune mechanisms.
The increased activity of autophagy in solid tumors is also closely related to the activation of intracellular oncogenes such as RAS[34]. The activation of RAS helps CRC cells maintain their energy and material supply under stress conditions[35]. Hu et al[36] have found that IL-6 accumulates in the tumor microenvironment, which can activate autophagy through the IL-6/JAK2/BECN1 pathway and promote the chemotherapy resistance of CRC cells. BECN1 Y333 phosphorylation is a predictive marker of poor prognosis and chemotherapy resistance in CRC. Under hypoxic conditions, tumor-initiating cells (TICs) can maintain their tumor initiation capacity and stemness through the autophagy-related PRKC/PKC-EZR pathway[37], thereby regulating the progression of CRC. CRC stem cells can also maintain the expressions of stemness markers Oct4, SOX2, and Nanog through autophagy-related proteins ATG5 and ATG7, and intervention in ATG5 and ATG7 can reduce stem cell proliferation and promote cell senescence and apoptosis[38,39]. E3 ubiquitin ligase TRAF6 interacts with MAP1LC3B/LC3B in colonic epithelial cells through its LC3 interaction region "YxxL" and catalyzes K63-linked LC3B polyubiquitination to trigger selective CTNNB1 degradation by autophagy, thereby playing an inhibitory role on epithelial mesenchymal transition (EMT) and CRC metastasis[40] (Table 1).
| Effects | Action cells | Targets | Pathways and mechanisms |
| As a tumor suppressor | CRC | SNX10 | SRC-STAT3 and SRC-TNNB1 |
| CRC | PHLDA2 | PI3K/AKT/GSK-3β | |
| CRC | BRG1 | Defective autophagy results in excess reactive oxygen species | |
| CRC | BeclinI | PI3K/AKT/mTOR | |
| As a tumor promoter | CRC and macrophages | NBR1 | After being regulated, MHC-I can affect the immune system and lead to immune evasion |
| CRC | RAS | MEK/ERK | |
| Regulatory T cells (Treg) | Atg7 | Down-regulation of the immune-suppressive protein FOXP3 promotes immune evasion | |
| CSC | ATG5, ATG7 | Affects the expressions of stemness markers Oct4, SOX2 and Nanog | |
| Tumor-initiating cells | PRKC | PRKC/PKC-EZR | |
| CRC | IL-6 | IL-6/JAK2/BECN1 | |
| Normal Colonic epithelial cells | TRAF6 | MAP1LC3B/LC3B ubiquitination |
Since autophagy plays an important role in the physiological activities of CRC cells, regulating key elements of the autophagy pathway may be a promising strategy for CRC treatment. For different tumors, autophagy can either suppress or promote tumorigenesis; therefore, the treatment strategies should be tailored (i.e., up-regulation or inhibition of autophagy according to different tumor types).
Mycobacterium tuberculosis and Bacillus Calmette–Guérin (BCG) vaccine can induce the death of autophagic cells through the TLR2 and TLR4 signaling pathways, thereby enhancing radiosensitization in CRC cell lines. In vivo evidence further supports that BCG-mediated radiosensitization is an autophagy-dependent pathway[41]. The combination of BCG and ionizing radiation can induce autophagy, providing a potential strategy to enhance the radiotherapeutic effect in CRC cells. As an antimalarial drug, chloroquine has recently been found to prevent autophagosome-lysosome fusion in tumor cells; thus, it may act as an autophagy inhibitor on the autophagy pathway of tumor cells[42].
CRC cells can develop autophagy-dependent chemotherapy resistance by activating autophagy to combat 5-fluorouracil (5-FU). Clinically, chloroquine is used in combination with chemotherapy drugs such as 5-FU[43] or trifluorothymidine (TFT)[44] to enhance the cytotoxicity of chemotherapy drugs against tumor cells. Preoperative use of chloroquine in CRC patients significantly increases the sensitivity of CRC to 5-FU and radiotherapy; in addition, it enhances intracellular ROS production in tumor cells, further promoting tumor cell death[45-46]. As a widely used antitumor drug, temsirolimus (TEM) can inhibit CRC cells by inducing G1 cell cycle arrest and reducing HIF1A and VEGF levels[47]. Meanwhile, as an mTOR inhibitor, TEM also has the function of autophagy inhibitors[48]. When used in combination with chloroquine, TEM can significantly increase the apoptosis level of CRC cells and increase the BAX: BCL2 ratio. Thus, TEM and chloroquine have synergistic anti-tumor effects, which sheds new light on the treatment of CRC. Fu et al[49] discovered an autophagy-targeting small molecule S130 by integrating into silico screening and in vitro assays. S130 binds to ATG4B with strong affinity and specifically suppresses the activity of ATG4B, thereby limiting the autophagic activity of CRC cells. In vitro and in vivo experiments confirmed that S130 could significantly inhibit the growth of CRC cells, suggesting the potential clinical value of small-molecule autophagy inhibitors.
Housekeeping and regulatory immune factors [e.g., macrophages and regulatory T lymphocytes (Tregs) in the tumor microenvironment] are also involved in the occurrence and development of CRC[50-52]. Akbari-Birgani et al[53] found that autophagy targeting Tregs and tumor cells can improve the therapeutic effect against CRC, possibly due to the fact that specific deletion of the Atg7 gene in Treg cells is associated with the increase of apoptosis and the downregulation of the transcription factor FOXP3. The loss of autophagy leads to the upregulation of metabolic mediators (such as MTORC1 and MYC), thereby removing the negative regulatory effect of Tregs on autoimmunity and improving the body's anti-tumor ability[54-56]. In addition, downregulating the autophagy activity of tumor cells and macrophages by chloroquine or other autophagy inhibitors[57,58] can avoid the downregulation of MHC-I expression on their surfaces; meanwhile, it can also enhance the presentation of tumor-associated antigens by antigen-presenting cells (APCs) and induce immune cells to exert their antitumor effects[33].
In addition to the direct use of autophagy inhibitors and the use of autophagy's regulatory role in immune cells for treating CRC, photodynamic therapy (PDT) combined with proteasome inhibitors (e.g., bortezomib) may also enhance tumor sensitivity to PDT through the autophagy pathway[59]. Protoporphyrin IX mediates PDT to induce autophagy in CRC stem cells. The inhibition of PDT-induced autophagy by gene knockout or pharmacological means can trigger apoptosis of tumor stem cells and decrease the ability of colonosphere formation in vitro and tumorigenicity in vivo[60].
In general, artificial intervention in tumor biological behavior can be achieved by directly targeting the autophagy mechanism using autophagy modulators in CRC experimental models. Autophagy modulators, chemotherapy drugs, radiotherapy, and immunotherapy have synergistic anti-tumor effects, and their combined use can enhance the efficacy of existing therapies. In fact, autophagy-based treatments have broad applications in CRC treatments.
Autophagy is an extremely potential therapeutic target for the treatment of rectal cancer, but appropriate interventions should be selected according to the different stages of colorectal cancer. Autophagy can inhibit tumorigenesis in the early stages of CRC by preventing DNA damage, maintaining genomic stability, and inducing apoptosis. However, with the progression of tumors, autophagy can promote CRC growth by enhancing energy metabolism in tumor cells, by mediating drug resistance, and by avoiding tumor cell death. Therefore, autophagy-based CRC treatment strategies should be tailored according to the specific CRC type, tumor stage, and tumor metabolic characteristics. The combination of multiple therapeutic methods can enhance the inhibitory effect of autophagy on tumors and weaken its role as a tumor promoter, therefore playing a key role in CRC treatment.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68447] [Article Influence: 13689.4] [Reference Citation Analysis (201)] |
| 2. | Aparicio C, Belver M, Enríquez L, Espeso F, Núñez L, Sánchez A, de la Fuente MÁ, González-Vallinas M. Cell Therapy for Colorectal Cancer: The Promise of Chimeric Antigen Receptor (CAR)-T Cells. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 3. | Galluzzi L, Pietrocola F, Bravo-San Pedro JM, Amaravadi RK, Baehrecke EH, Cecconi F, Codogno P, Debnath J, Gewirtz DA, Karantza V, Kimmelman A, Kumar S, Levine B, Maiuri MC, Martin SJ, Penninger J, Piacentini M, Rubinsztein DC, Simon HU, Simonsen A, Thorburn AM, Velasco G, Ryan KM, Kroemer G. Autophagy in malignant transformation and cancer progression. EMBO J. 2015;34:856-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 905] [Cited by in RCA: 972] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 4. | Cao W, Li J, Yang K, Cao D. An overview of autophagy: Mechanism, regulation and research progress. Bull Cancer. 2021;108:304-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 290] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 5. | Ferro F, Servais S, Besson P, Roger S, Dumas JF, Brisson L. Autophagy and mitophagy in cancer metabolic remodelling. Semin Cell Dev Biol. 2020;98:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 204] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 6. | Yamazaki T, Bravo-San Pedro JM, Galluzzi L, Kroemer G, Pietrocola F. Autophagy in the cancer-immunity dialogue. Adv Drug Deliv Rev. 2021;169:40-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 7. | Wang Y, Zhang H. Regulation of Autophagy by mTOR Signaling Pathway. Adv Exp Med Biol. 2019;1206:67-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 265] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 8. | Klionsky DJ, Petroni G, Amaravadi RK, Baehrecke EH, Ballabio A, Boya P, Bravo-San Pedro JM, Cadwell K, Cecconi F, Choi AMK, Choi ME, Chu CT, Codogno P, Colombo MI, Cuervo AM, Deretic V, Dikic I, Elazar Z, Eskelinen EL, Fimia GM, Gewirtz DA, Green DR, Hansen M, Jäättelä M, Johansen T, Juhász G, Karantza V, Kraft C, Kroemer G, Ktistakis NT, Kumar S, Lopez-Otin C, Macleod KF, Madeo F, Martinez J, Meléndez A, Mizushima N, Münz C, Penninger JM, Perera RM, Piacentini M, Reggiori F, Rubinsztein DC, Ryan KM, Sadoshima J, Santambrogio L, Scorrano L, Simon HU, Simon AK, Simonsen A, Stolz A, Tavernarakis N, Tooze SA, Yoshimori T, Yuan J, Yue Z, Zhong Q, Galluzzi L, Pietrocola F. Autophagy in major human diseases. EMBO J. 2021;40:e108863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 555] [Cited by in RCA: 1150] [Article Influence: 230.0] [Reference Citation Analysis (0)] |
| 9. | Li X, He S, Ma B. Autophagy and autophagy-related proteins in cancer. Mol Cancer. 2020;19:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 1199] [Article Influence: 199.8] [Reference Citation Analysis (0)] |
| 10. | Hardie DG. AMPK: positive and negative regulation, and its role in whole-body energy homeostasis. Curr Opin Cell Biol. 2015;33:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 366] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 11. | Deretic V. Autophagy in inflammation, infection, and immunometabolism. Immunity. 2021;54:437-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 592] [Article Influence: 118.4] [Reference Citation Analysis (0)] |
| 12. | Takamura A, Komatsu M, Hara T, Sakamoto A, Kishi C, Waguri S, Eishi Y, Hino O, Tanaka K, Mizushima N. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 2011;25:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 923] [Cited by in RCA: 1088] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 13. | Dong S, Wang Q, Kao YR, Diaz A, Tasset I, Kaushik S, Thiruthuvanathan V, Zintiridou A, Nieves E, Dzieciatkowska M, Reisz JA, Gavathiotis E, D'Alessandro A, Will B, Cuervo AM. Chaperone-mediated autophagy sustains haematopoietic stem-cell function. Nature. 2021;591:117-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 215] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 14. | Qiao L, Ma J, Zhang Z, Sui W, Zhai C, Xu D, Wang Z, Lu H, Zhang M, Zhang C, Chen W, Zhang Y. Deficient Chaperone-Mediated Autophagy Promotes Inflammation and Atherosclerosis. Circ Res. 2021;129:1141-1157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 15. | Anding AL, Baehrecke EH. Cleaning House: Selective Autophagy of Organelles. Dev Cell. 2017;41:10-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 475] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 16. | Dong X, Yang Y, Zou Z, Zhao Y, Ci B, Zhong L, Bhave M, Wang L, Kuo YC, Zang X, Zhong R, Aguilera ER, Richardson RB, Simonetti B, Schoggins JW, Pfeiffer JK, Yu L, Zhang X, Xie Y, Schmid SL, Xiao G, Gleeson PA, Ktistakis NT, Cullen PJ, Xavier RJ, Levine B. Sorting nexin 5 mediates virus-induced autophagy and immunity. Nature. 2021;589:456-461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 17. | Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221:3-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2980] [Cited by in RCA: 2934] [Article Influence: 183.4] [Reference Citation Analysis (0)] |
| 18. | Long J, He Q, Yin Y, Lei X, Li Z, Zhu W. The effect of miRNA and autophagy on colorectal cancer. Cell Prolif. 2020;53:e12900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 19. | Li W, He P, Huang Y, Li YF, Lu J, Li M, Kurihara H, Luo Z, Meng T, Onishi M, Ma C, Jiang L, Hu Y, Gong Q, Zhu D, Xu Y, Liu R, Liu L, Yi C, Zhu Y, Ma N, Okamoto K, Xie Z, Liu J, He RR, Feng D. Selective autophagy of intracellular organelles: recent research advances. Theranostics. 2021;11:222-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 337] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 20. | Liu K, Wang L, Zhao T. Autophagy in Normal Stem Cells and Specialized Cells. Adv Exp Med Biol. 2019;1206:489-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | White E, Mehnert JM, Chan CS. Autophagy, Metabolism, and Cancer. Clin Cancer Res. 2015;21:5037-5046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 535] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 22. | Zhang S, Yang Z, Bao W, Liu L, You Y, Wang X, Shao L, Fu W, Kou X, Shen W, Yuan C, Hu B, Dang W, Nandakumar KS, Jiang H, Zheng M, Shen X. SNX10 (sorting nexin 10) inhibits colorectal cancer initiation and progression by controlling autophagic degradation of SRC. Autophagy. 2020;16:735-749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 23. | Ma Z, Lou S, Jiang Z. PHLDA2 regulates EMT and autophagy in colorectal cancer via the PI3K/AKT signaling pathway. Aging (Albany NY). 2020;12:7985-8000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 24. | Zhao Y, Yang J, Liao W, Liu X, Zhang H, Wang S, Wang D, Feng J, Yu L, Zhu WG. Cytosolic FoxO1 is essential for the induction of autophagy and tumour suppressor activity. Nat Cell Biol. 2010;12:665-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 507] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 25. | Peng X, Wang Y, Li H, Fan J, Shen J, Yu X, Zhou Y, Mao H. ATG5-mediated autophagy suppresses NF-κB signaling to limit epithelial inflammatory response to kidney injury. Cell Death Dis. 2019;10:253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 26. | Liu M, Sun T, Li N, Peng J, Fu D, Li W, Li L, Gao WQ. BRG1 attenuates colonic inflammation and tumorigenesis through autophagy-dependent oxidative stress sequestration. Nat Commun. 2019;10:4614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 27. | Hinshaw DC, Shevde LA. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019;79:4557-4566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 750] [Cited by in RCA: 2323] [Article Influence: 331.9] [Reference Citation Analysis (2)] |
| 28. | Ganapathy-Kanniappan S, Geschwind JF. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013;12:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 519] [Cited by in RCA: 1021] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 29. | Paul S, Ghosh S, Kumar S. Tumor glycolysis, an essential sweet tooth of tumor cells. Semin Cancer Biol. 2022;86:1216-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 291] [Reference Citation Analysis (0)] |
| 30. | Li CH, Liao CC. The Metabolism Reprogramming of microRNA Let-7-Mediated Glycolysis Contributes to Autophagy and Tumor Progression. Int J Mol Sci. 2021;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Mizushima N, Levine B. Autophagy in Human Diseases. N Engl J Med. 2020;383:1564-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 829] [Article Influence: 138.2] [Reference Citation Analysis (0)] |
| 32. | White E. Autophagy and p53. Cold Spring Harb Perspect Med. 2016;6:a026120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 256] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 33. | Yamamoto K, Venida A, Yano J, Biancur DE, Kakiuchi M, Gupta S, Sohn ASW, Mukhopadhyay S, Lin EY, Parker SJ, Banh RS, Paulo JA, Wen KW, Debnath J, Kim GE, Mancias JD, Fearon DT, Perera RM, Kimmelman AC. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature. 2020;581:100-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 927] [Article Influence: 154.5] [Reference Citation Analysis (0)] |
| 34. | Guo JY, Chen HY, Mathew R, Fan J, Strohecker AM, Karsli-Uzunbas G, Kamphorst JJ, Chen G, Lemons JM, Karantza V, Coller HA, Dipaola RS, Gelinas C, Rabinowitz JD, White E. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 2011;25:460-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1090] [Cited by in RCA: 1082] [Article Influence: 72.1] [Reference Citation Analysis (1)] |
| 35. | Wang Y, Xiong H, Liu D, Hill C, Ertay A, Li J, Zou Y, Miller P, White E, Downward J, Goldin RD, Yuan X, Lu X. Autophagy inhibition specifically promotes epithelial-mesenchymal transition and invasion in RAS-mutated cancer cells. Autophagy. 2019;15:886-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 36. | Hu F, Song D, Yan Y, Huang C, Shen C, Lan J, Chen Y, Liu A, Wu Q, Sun L, Xu F, Hu F, Chen L, Luo X, Feng Y, Huang S, Hu J, Wang G. IL-6 regulates autophagy and chemotherapy resistance by promoting BECN1 phosphorylation. Nat Commun. 2021;12:3651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 182] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 37. | Qureshi-Baig K, Kuhn D, Viry E, Pozdeev VI, Schmitz M, Rodriguez F, Ullmann P, Koncina E, Nurmik M, Frasquilho S, Nazarov PV, Zuegel N, Boulmont M, Karapetyan Y, Antunes L, Val D, Mittelbronn M, Janji B, Haan S, Letellier E. Hypoxia-induced autophagy drives colorectal cancer initiation and progression by activating the PRKC/PKC-EZR (ezrin) pathway. Autophagy. 2020;16:1436-1452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 38. | Sharif T, Martell E, Dai C, Kennedy BE, Murphy P, Clements DR, Kim Y, Lee PW, Gujar SA. Autophagic homeostasis is required for the pluripotency of cancer stem cells. Autophagy. 2017;13:264-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 39. | Ma Y, Qi M, An Y, Zhang L, Yang R, Doro DH, Liu W, Jin Y. Autophagy controls mesenchymal stem cell properties and senescence during bone aging. Aging Cell. 2018;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 275] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 40. | Wu H, Lu XX, Wang JR, Yang TY, Li XM, He XS, Li Y, Ye WL, Wu Y, Gan WJ, Guo PD, Li JM. TRAF6 inhibits colorectal cancer metastasis through regulating selective autophagic CTNNB1/β-catenin degradation and is targeted for GSK3B/GSK3β-mediated phosphorylation and degradation. Autophagy. 2019;15:1506-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 41. | Yuk JM, Shin DM, Song KS, Lim K, Kim KH, Lee SH, Kim JM, Lee JS, Paik TH, Kim JS, Jo EK. Bacillus calmette-guerin cell wall cytoskeleton enhances colon cancer radiosensitivity through autophagy. Autophagy. 2010;6:46-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 42. | Ferreira PMP, Sousa RWR, Ferreira JRO, Militão GCG, Bezerra DP. Chloroquine and hydroxychloroquine in antitumor therapies based on autophagy-related mechanisms. Pharmacol Res. 2021;168:105582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 43. | Bijnsdorp IV, Peters GJ, Temmink OH, Fukushima M, Kruyt FA. Differential activation of cell death and autophagy results in an increased cytotoxic potential for trifluorothymidine compared to 5-fluorouracil in colon cancer cells. Int J Cancer. 2010;126:2457-2468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Jia HJ, Zhou M, Vashisth MK, Xia J, Hua H, Dai QL, Bai SR, Zhao Q, Wang XB, Shi YL. Trifluridine induces HUVECs senescence by inhibiting mTOR-dependent autophagy. Biochem Biophys Res Commun. 2022;610:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 45. | Kimura T, Takabatake Y, Takahashi A, Isaka Y. Chloroquine in cancer therapy: a double-edged sword of autophagy. Cancer Res. 2013;73:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 407] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 46. | Xu R, Ji Z, Xu C, Zhu J. The clinical value of using chloroquine or hydroxychloroquine as autophagy inhibitors in the treatment of cancers: A systematic review and meta-analysis. Medicine (Baltimore). 2018;97:e12912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 47. | Kaneko M, Nozawa H, Hiyoshi M, Tada N, Murono K, Nirei T, Emoto S, Kishikawa J, Iida Y, Sunami E, Tsuno NH, Kitayama J, Takahashi K, Watanabe T. Temsirolimus and chloroquine cooperatively exhibit a potent antitumor effect against colorectal cancer cells. J Cancer Res Clin Oncol. 2014;140:769-781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | Yurube T, Ito M, Kakiuchi Y, Kuroda R, Kakutani K. Autophagy and mTOR signaling during intervertebral disc aging and degeneration. JOR Spine. 2020;3:e1082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 49. | Fu Y, Hong L, Xu J, Zhong G, Gu Q, Guan Y, Zheng X, Dai Q, Luo X, Liu C, Huang Z, Yin XM, Liu P, Li M. Discovery of a small molecule targeting autophagy via ATG4B inhibition and cell death of colorectal cancer cells in vitro and in vivo. Autophagy. 2019;15:295-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 50. | Toor SM, Murshed K, Al-Dhaheri M, Khawar M, Abu Nada M, Elkord E. Immune Checkpoints in Circulating and Tumor-Infiltrating CD4(+) T Cell Subsets in Colorectal Cancer Patients. Front Immunol. 2019;10:2936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 51. | Liu P, Zhu H, Zhang X, Feng A, Zhu X, Sun Y. Predicting Survival for Hepatic Arterial Infusion Chemotherapy of Unresectable Colorectal Liver Metastases: Radiomics Analysis of Pretreatment Computed Tomography. J Transl Int Med. 2022;10:56-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 52. | Gao Z, Jiang J, Hou L, Zhang B. Dysregulation of MiR-144-5p/RNF187 Axis Contributes To the Progression of Colorectal Cancer. J Transl Int Med. 2022;10:65-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 53. | Akbari-Birgani S, Paranjothy T, Zuse A, Janikowski T, Cieślar-Pobuda A, Likus W, Urasińska E, Schweizer F, Ghavami S, Klonisch T, Łos MJ. Cancer stem cells, cancer-initiating cells and methods for their detection. Drug Discov Today. 2016;21:836-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 54. | Kato H, Perl A. Blockade of Treg Cell Differentiation and Function by the Interleukin-21-Mechanistic Target of Rapamycin Axis Via Suppression of Autophagy in Patients With Systemic Lupus Erythematosus. Arthritis Rheumatol. 2018;70:427-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 55. | Rabanal-Ruiz Y, Otten EG, Korolchuk VI. mTORC1 as the main gateway to autophagy. Essays Biochem. 2017;61:565-584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 442] [Article Influence: 49.1] [Reference Citation Analysis (13)] |
| 56. | Jahangiri L, Pucci P, Ishola T, Trigg RM, Williams JA, Pereira J, Cavanagh ML, Turner SD, Gkoutos GV, Tsaprouni L. The Contribution of Autophagy and LncRNAs to MYC-Driven Gene Regulatory Networks in Cancers. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 57. | Oh DS, Lee HK. Autophagy protein ATG5 regulates CD36 expression and anti-tumor MHC class II antigen presentation in dendritic cells. Autophagy. 2019;15:2091-2106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 58. | Shao BZ, Han BZ, Zeng YX, Su DF, Liu C. The roles of macrophage autophagy in atherosclerosis. Acta Pharmacol Sin. 2016;37:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 190] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 59. | Szokalska A, Makowski M, Nowis D, Wilczynski GM, Kujawa M, Wójcik C, Mlynarczuk-Bialy I, Salwa P, Bil J, Janowska S, Agostinis P, Verfaillie T, Bugajski M, Gietka J, Issat T, Glodkowska E, Mrówka P, Stoklosa T, Hamblin MR, Mróz P, Jakóbisiak M, Golab J. Proteasome inhibition potentiates antitumor effects of photodynamic therapy in mice through induction of endoplasmic reticulum stress and unfolded protein response. Cancer Res. 2009;69:4235-4243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 60. | Wei MF, Chen MW, Chen KC, Lou PJ, Lin SY, Hung SC, Hsiao M, Yao CJ, Shieh MJ. Autophagy promotes resistance to photodynamic therapy-induced apoptosis selectively in colorectal cancer stem-like cells. Autophagy. 2014;10:1179-1192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 175] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alonso C, Spain; Bottalico L, Italy S-Editor: Li L L-Editor: A P-Editor: Li L