Published online Jun 15, 2023. doi: 10.4251/wjgo.v15.i6.1036
Peer-review started: February 11, 2023
First decision: April 10, 2023
Revised: April 18, 2023
Accepted: May 4, 2023
Article in press: May 4, 2023
Published online: June 15, 2023
Processing time: 123 Days and 17.8 Hours
Perihilar cholangiocarcinoma (pCCA) has a poor prognosis and urgently needs a better predictive method. The predictive value of the age-adjusted Charlson comorbidity index (ACCI) for the long-term prognosis of patients with multiple malignancies was recently reported. However, pCCA is one of the most surgically difficult gastrointestinal tumors with the poorest prognosis, and the value of the ACCI for the prognosis of pCCA patients after curative resection is unclear.
To evaluate the prognostic value of the ACCI and to design an online clinical model for pCCA patients.
Consecutive pCCA patients after curative resection between 2010 and 2019 were enrolled from a multicenter database. The patients were randomly assigned 3:1 to training and validation cohorts. In the training and validation cohorts, all patients were divided into low-, moderate-, and high-ACCI groups. Kaplan-Meier curves were used to determine the impact of the ACCI on overall survival (OS) for pCCA patients, and multivariate Cox regression analysis was used to determine the independent risk factors affecting OS. An online clinical model based on the ACCI was developed and validated. The concordance index (C-index), calibration curve, and receiver operating characteristic (ROC) curve were used to evaluate the predictive performance and fit of this model.
A total of 325 patients were included. There were 244 patients in the training cohort and 81 patients in the validation cohort. In the training cohort, 116, 91 and 37 patients were classified into the low-, moderate- and high-ACCI groups. The Kaplan-Meier curves showed that patients in the moderate- and high-ACCI groups had worse survival rates than those in the low-ACCI group. Multivariable analysis revealed that moderate and high ACCI scores were independently associated with OS in pCCA patients after curative resection. In addition, an online clinical model was developed that had ideal C-indexes of 0.725 and 0.675 for predicting OS in the training and validation cohorts. The calibration curve and ROC curve indicated that the model had a good fit and prediction performance.
A high ACCI score may predict poor long-term survival in pCCA patients after curative resection. High-risk patients screened by the ACCI-based model should be given more clinical attention in terms of the management of comorbidities and postoperative follow-up.
Core Tip: Our study assessed the prognostic value of the age-adjusted Charlson comorbidity index (ACCI) and designed an online clinical model for perihilar cholangiocarcinoma (pCCA). We retrospectively evaluated 496 pCCA patients from multiple centers who underwent radical resection. This study proposed that the ACCI is an independent predictor of pCCA prognosis, and a nomogram based on the ACCI is a promising predictive model for overall survival in pCCA patients.
- Citation: Pan Y, Liu ZP, Dai HS, Chen WY, Luo Y, Wang YZ, Gao SY, Wang ZR, Dong JL, Liu YH, Yin XY, Liu XC, Fan HN, Bai J, Jiang Y, Cheng JJ, Zhang YQ, Chen ZY. Development of a model based on the age-adjusted Charlson comorbidity index to predict survival for resected perihilar cholangiocarcinoma. World J Gastrointest Oncol 2023; 15(6): 1036-1050
- URL: https://www.wjgnet.com/1948-5204/full/v15/i6/1036.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i6.1036
Cholangiocarcinoma (CCA) is the most common biliary malignancy and the second most common hepatic malignancy after hepatocellular carcinoma (HCC)[1]. Perihilar CCA (pCCA), arising at the site of biliary fusion or in the right or left hepatic duct, represents 60% of CCA cases[2,3]. The overall incidence of pCCA has increased progressively worldwide over the past four decades[4-6]. Curative resection provides a possible cure for eligible patients with pCCA[7]. However, even after successful curative resection, the prognosis of most pCCA patients remains unsatisfactory, with a five-year survival rate of approximately 20%[8]. Therefore, the accurate identification of important factors affecting long-term prognosis and screening of patients with a high survival risk is essential to improve long-term survival. However, the specificity and complexity of the anatomical location of pCCA greatly increases the difficulty of surgery. The relationship between whether a patient is "strong" enough to withstand the shock of surgery and long-term prognosis may be overlooked in existing forecasting models.
Comorbidity is defined as the “coexistence of disorders in addition to a primary disease of interest”[9]. The coexistence of cancer and other chronic diseases has significant implications for cancer treatment decisions and outcomes[10-12]. Recent studies indicated the substantial influence of comorbidities on postoperative survival in different kinds of solid neoplasms, including breast, vulvar and colorectal cancers[13,14]. Regrettably, most cancer treatment guidelines do not consider the complex interrelationships between cancer and comorbidities and instead adopt a “single-disease” approach to management. Currently, most clinicians also judge prognosis based on tumor-related information alone, ignoring the patient's own disease status. Although some previous studies have taken comorbidities into account, the simple classification into the presence/absence of comorbidities is not comprehensive[13].
At present, the most frequently used system for evaluating the grade of patients’ comorbidity burden is the Charlson comorbidity index (CCI). The CCI has excellent clinical efficacy in predicting patient prognosis by assessing the number of certain comorbidities and their severity[15]. Since age had been determined to affect prognosis, Charlson et al developed an additional age-adjusted CCI (ACCI) to correct the final score of the CCI[16]. Recently, the predictive value of the ACCI for long-term prognosis in patients with multiple malignancies, such as prostate cancer, pancreatic cancer, colorectal cancer and HCC, has been determined[17-20]. Nevertheless, pCCA is one of the most surgically difficult gastrointestinal tumors with the poorest prognosis, and the relationship between the ACCI and the prognosis of pCCA has not been studied.
Therefore, a multicenter database was utilized to assess the impact of the ACCI on the long-term prognosis of patients with pCCA after curative resection. Furthermore, to help surgeons make better clinical decisions, a prognostic model to predict the overall survival (OS) of pCCA patients after curative resection was developed in this study based on the ACCI and tumor-related indicators.
This study retrospectively enrolled newly diagnosed pCCA patients who underwent curative resection between January 2010 and December 2019 at three institutions (Southwest Hospital, Sichuan Provincial People's Hospital and the Affiliated Hospital of Qinghai University) in China. Computer-generated random numbers were used to assign three-quarters of the patients to the training cohort and the remaining one-quarter to the validation cohort. Drawing on the previous methods, the patients in the training and validation cohorts were categorized into three groups by the ACCI score: Low-ACCI (ACCI = 0-1), moderate-ACCI (ACCI = 2-3) and high-ACCI (ACCI ≥ 4) groups[20,21]. The patients were classified by the CCI into low- and high-risk groups according to zero and nonzero scores. All tumors originated from the left or right hepatic ducts, biliary confluence, or common hepatic duct, which were confirmed by postoperative histological examination. All patients underwent hepatectomy, bile duct resection, locoregional lymphadenectomy and choledochojejunostomy. Hepatectomy-pancreaticoduodenectomy and revascularization were performed when necessary. Curative resection was defined as a clear-cut edge without tumor cells under macroscopy and microscopy. The exclusion criteria included the following: (1) Recurrent pCCA; (2) death within 30 d after resection; (3) incomplete medical records; and (4) loss to follow-up.
The study followed the ethical guidelines of the World Medical Association and Declaration of Helsinki. Approval for the present study was obtained from the Ethics Committee of Southwest Hospital (approval number: KY2021129). An informed consent form was signed by all patients prior to surgery.
The multicenter database was prospectively created and dynamically maintained, and data were retrospectively collected. Demographic information included sex, age, American Society of Anesthesiologists score, various comorbidities and preoperative percutaneous transhepatic cholangial drainage. Preoperative laboratory variables included alanine aminotransferase, aspartate transaminase, platelet count, albumin, total bilirubin, international normalized ratio, and carbohydrate antigen 19-9 (CA19-9). Surgical variables included extent of hepatectomy, intraoperative blood loss and perioperative blood transfusion. Pathological variables included cirrhosis, maximum tumor size, macrovascular invasion, microvascular invasion, peripheral nerve invasion, tumor differentiation, lymphoid metastasis, 8th American Joint Committee on Cancer (AJCC) stage[22] and Bismuth classification[23].
Major hepatectomy was defined as three or more resected Couinaud liver segments, while minor hepatectomy was defined as two or fewer resected Couinaud liver segments. All pathological variables were confirmed by postoperative pathological examination.
The patients' preoperative comorbidities were rigorously assessed based on the disease definition[15]. The comorbidity severity was assessed by the CCI and ACCI[16]. The CCI incorporates nineteen common preoperative comorbidities, with each weighing from 1 to 6 points. On the basis of the CCI, the ACCI considers the influence of age on prognosis. As shown in Table 1, the risk increases by 1 point for each decade of age over 40 years (50-59 years, 1 point; 60-69 years, 2 points; 70-79 years, 3 points; and > 80 years, 4 points), and the points for age are added to the total ACCI score.
| Conditions | Training cohort (n = 244) | Validation cohort (n = 81) | Total patients (n = 325) |
| 1 point per decade for age > 40 (0 to 4 points) | |||
| < 50 | 88 (36.1) | 27 (33.3) | 115 (35.4) |
| 50-59 | 64 (26.2) | 25 (30.9) | 89 (27.4) |
| 60-69 | 55 (22.5) | 19 (23.5) | 74 (22.8) |
| 70-79 | 27 (11.1) | 7 (8.6) | 34 (10.5) |
| ≥ 80 | 10 (4.1) | 3 (3.7) | 13 (4.0) |
| 1 point | |||
| Mild liver disease | 42 (17.2) | 12 (14.8) | 54 (16.6) |
| Peptic ulcer disease | 11 (4.5) | 4 (4.9) | 15 (4.6) |
| Congestive heart failure | 9 (3.6) | 2 (2.4) | 11 (3.4) |
| Peripheral vascular disease | 9 (3.6) | 5 (6.1) | 14 (4.3) |
| Cerebrovascular disease | 6 (2.4) | 1 (1.2) | 7 (2.2) |
| Chronic pulmonary disease | 6 (2.4) | 1 (1.2) | 7 (2.2) |
| Connective tissue disease | 4 (1.6) | 3 (3.7) | 7 (2.2) |
| Myocardial infarction | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dementia | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Diabetes without end-organ damage | 42 (17.2) | 12 (14.8) | 54 (16.6) |
| 2 points | |||
| Diabetes with end-organ damage | 10 (4.1) | 4 (4.9) | 14 (4.3) |
| Moderate/severe renal disease | 8 (3.2) | 1 (1.2) | 9 (2.8) |
| Other tumor | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Leukemia | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hemiplegia/paraplegia | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Malignant lymphoma | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 points | |||
| Moderate/severe liver disease | 3 (1.2) | 0 (0.0) | 3 (0.9) |
| 6 points | |||
| Metastatic solid tumor | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| AIDS | 0 (0.0) | 0 (0.0) | 0 (0.0) |
All patients were followed up in the participating hospitals after discharge. A standardized follow-up protocol was strictly followed, which included a physical examination, laboratory tests (tumor biomarkers and liver function) and imaging examinations. Imaging examinations included abdominal contrast-enhanced ultrasound (CEUS), contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI). Imaging examinations were performed at least once every 2 mo in the first year after resection and then every 3 mo from the second year on. Recurrence was defined as the appearance of a new lesion or multiple new lesions on CEUS, contrast-enhanced CT or MRI. In the case of recurrence, conservative treatment, systemic chemotherapy, and repeat surgical resection were available options, and the treatment strategy was determined considering the doctor's advice and the patient's own wishes. The endpoint was OS after pCCA resection, which was defined as the interval between the date of surgery and the date of patient death or the last follow-up. The last follow-up date for all patients was September 2022.
Continuous variables with a normal distribution are expressed as the mean ± SD or median (range), and Student’s t test or the Mann-Whitney U test was used as appropriate. Categorical variables are expressed as numbers and percentages, and the χ2 test or Fisher’s exact test was used as appropriate. According to our previous studies, the included continuous variables were transformed into categorical variables[24,25]. The Kaplan-Meier method was used to calculate the OS of patients. The log-rank test was used to compare OS between the low- and moderate-ACCI groups and between the low- and high-ACCI groups. Multivariate Cox regression analysis was then performed to determine independent risk factors associated with reduced OS after curative resection of pCCA. The hazard ratio (HR) and its 95% confidence interval (CI) were estimated in univariate and multivariate Cox regression analyses. In particular, variables with a significant P value < 0.10 in the univariate analysis were included in the multivariate Cox regression analysis.
The nomogram factors were selected based on the independent variables associated with OS in multivariate Cox regression analysis to construct the nomogram model. Calibration curves and Harrell’s concordance index (C-index) were applied to evaluate the fit and accuracy of the nomogram. Furthermore, the discriminative power of the model was assessed by a receiver operating characteristic (ROC) curve through the "survivalROC" package in R. The comparison between the nomogram and the 8th AJCC staging system was achieved using decision curve analysis (DCA) through the "rmda" package in R. For the validation cohort, the performance evaluation of the model was performed using the same approach as that in the training cohort. According to the ROC curve for the prediction of 1-year OS, the optimal cutoff value of the nomogram score was calculated, and all patients were divided into high- and low-risk groups. Using the Kaplan-Meier method and the log-rank test, OS rates were compared between the low- and high-risk groups.
Statistical analysis was performed using SPSS 26.0 (SPSS, Chicago, IL, United States) and R software (version 4.1.3. https://www.r-project.org/wDyn). An internet browser calculator based on the model was constructed by using the “DynNom” package in R. Statistical significance was set at P < 0.05 for all analyses.
Of 496 pCCA patients who underwent radical resection during the study period, 171 patients were excluded according to the exclusion criteria, and 325 pCCA patients were finally included in this study. Of these, 244 patients were assigned to the training cohort, and the remaining 81 patients were assigned to the validation cohort, as shown in Supplementary Figure 1. In the training cohort, the low-, mode
| Patient demographics | Total (n = 244) | ACCI = 0-1 (n = 116) | ACCI = 2-3 (n = 91) | ACCI ≥ 4 (n = 37) | P value |
| Sex, Female/Male | 102/142 (41.8/58.2) | 48/68 (41.4/58.6) | 38/53 (41.8/58.2) | 16/21 (43.2/56.8) | 0.980 |
| Age (years), ≤ 70/> 70 | 207/37 (84.8/15.2) | 116/0 (100.0/0) | 91/0 (100.0/0) | 0/37 (0/100.0) | < 0.001 |
| CCI, Low/High | 96/148 (39.3/60.7) | 57/59 (49.1/50.9) | 34/57 (37.4/62.6) | 5/22 (13.5/86.5) | 0.001 |
| Diabetes, No/Yes | 224/20 (91.8/8.2) | 106/10 (91.4/8.6) | 85/6 (93.4/6.6) | 33/4 (89.2/10.8) | 0.714 |
| Cirrhosis, No/Yes | 222/22 (91.0/9.0) | 109/7 (94.0/6.0) | 82/9 (90.1/9.9) | 31/6 (83.8/16.2) | 0.159 |
| ALT (U/L), ≤ 40/> 40 | 64/180 (26.2/73.8) | 28/88 (24.1/75.9) | 29/62 (31.9/68.1) | 7/30 (18.9/81.1) | 0.249 |
| AST (U/L), ≤ 40/> 40 | 63/181 (25.8/74.2) | 35/81 (30.2/69.8) | 21/70 (23.1/76.9) | 7/30 (18.9/81.1) | 0.297 |
| PLT (× 109/L), ≥ 100/< 100 | 11/233 (4.5/95.5) | 6/110 (5.2/94.8) | 4/87 (4.4/95.6) | 1/36 (2.7/97.3) | 0.818 |
| ALB (g/L), ≥ 35/< 35 | 161/83 (66.0/34.0) | 80/38 (69.0/31.0) | 60/31 (65.9/34.1) | 21/16 (56.8/43.2) | 0.394 |
| TB (mg/dL), ≤ 1/> 1 | 51/193 (20.9/79.1) | 26/90 (22.4/77.6) | 20/71 (22.0/78.0) | 5/32 (13.5/86.5) | 0.485 |
| INR, ≤ 1.25/> 1.25 | 211/33 (86.5/13.5) | 102/14 (87.9/12.1) | 78/13 (85.7/14.3) | 31/6 (83.8/16.2) | 0.785 |
| CA19-9 (U/L), ≤ 150/> 150 | 111/133 (45.5/54.5) | 57/59 (49.1/50.9) | 37/54 (40.7/59.3) | 17/20 (45.9/54.1) | 0.477 |
| Preoperative PTCD, No/Yes | 168/76 (68.9/31.1) | 82/34 (70.7/29.3) | 60/31 (65.9/34.1) | 26/11 (70.3/29.7) | 0.749 |
| Maximum tumor size (cm), < 3/3-5/> 5 | 101/117/26 (41.4/48.0/10.7) | 55/49/12 (47.4/42.2/10.3) | 35/46/10 (38.5/50.5/11.0) | 11/22/4 (29.7/59.5/10.8) | 0.357 |
| Macrovascular invasion, No/Yes | 183/61 (75.0/25.0) | 89/27 (76.7./23.3) | 66/25 (72.5/27.5) | 28/9 (75.7/24.3) | 0.783 |
| Microvascular invasion, No/Yes | 199/45 (81.6/18.4) | 99/17 (85.3/14.7) | 69/22 (75.8/24.2) | 31/6 (83.8/16.2) | 0.200 |
| Perineural infiltration, No/Yes | 196/48 (80.3/19.7) | 96/20 (82.8/17.2) | 70/21 (76.9/23.1) | 30/7 (81.1/18.9) | 0.573 |
| Tumor differentiation, well/(moderate/poor) | 202/42 (82.8/17.2) | 98/18 (84.5/15.5) | 72/19 (79.1/20.9) | 32/5 (86.5/13.5) | 0.485 |
| Extent of resection, Minor/Major | 62/182 (25.4/74.6) | 34/82 (29.3/70.7) | 21/70 (23.1/76.9) | 7/30 (18.9/81.1) | 0.365 |
| 8th AJCC staging system, I-II/III/IV | 134/99/11 (54.9/40.6/4.5) | 67/45/4 (57.8/38.8/3.4) | 52/35/4 (57.1/38.5/4.4) | 15/19/3 (40.5/51.4/8.1) | 0.373 |
| Bismuth classification, I-II/III/IV | 55/51/138 (22.5/20.9/56.6) | 25/23/68 (21.6/19.8/58.6) | 21/22/48 (23.1/24.2/52.7) | 9/6/22 (24.3/16.2/59.5) | 0.843 |
| Lymphoid metastasis, No (ELN > 4)/No (ELN ≤ 4)/Yes | 85/91/68 (34.8/37.3/27.9) | 44/40/32 (37.9/34.5/27.6) | 31/37/23 (34.1/40.7/25.3) | 10/14/13 (27.0/37.8/35.1) | 0.657 |
| Intraoperative blood loss (mL), ≤ 500/> 500 | 91/153 (37.3/62.7) | 42/74 (36.2/63.8) | 37/54 (40.7/59.3) | 12/25 (32.4/67.6) | 0.646 |
| Perioperative blood transfusion, No/Yes | 85/159 (34.8/65.2) | 42/74 (36.2/63.8) | 30/61 (33.0/67.0) | 13/24 (35.1/64.9) | 0.888 |
| Period of follow-up, months1 | 25.7 ± 22.7 | 32.7 ± 25.4 | 20.9 ± 18.9 | 15.7 ± 14.5 | 0.222 |
| Recurrence during follow-up | 183 (75.0) | 81 (69.8) | 69 (75.8) | 33 (89.2) | 0.059 |
| Death during follow-up | 166 (68.0) | 69 (59.5) | 66 (72.5) | 31 (83.8) | 0.011 |
| OS, months2 | 23.0 (19.1-26.9) | 34.0 (27.1-40.9) | 18.0 (12.9-23.1) | 11.0 (9.1-12.9) | < 0.001 |
| 1-yr OS rate, % | 72.7 | 91.4 | 74.6 | 39.3 | |
| 3-yr OS rate, % | 32.4 | 45.6 | 19.8 | 14.6 | |
| 5-yr OS rate, % | 22.3 | 31.1 | 11.9 | 6.0 |
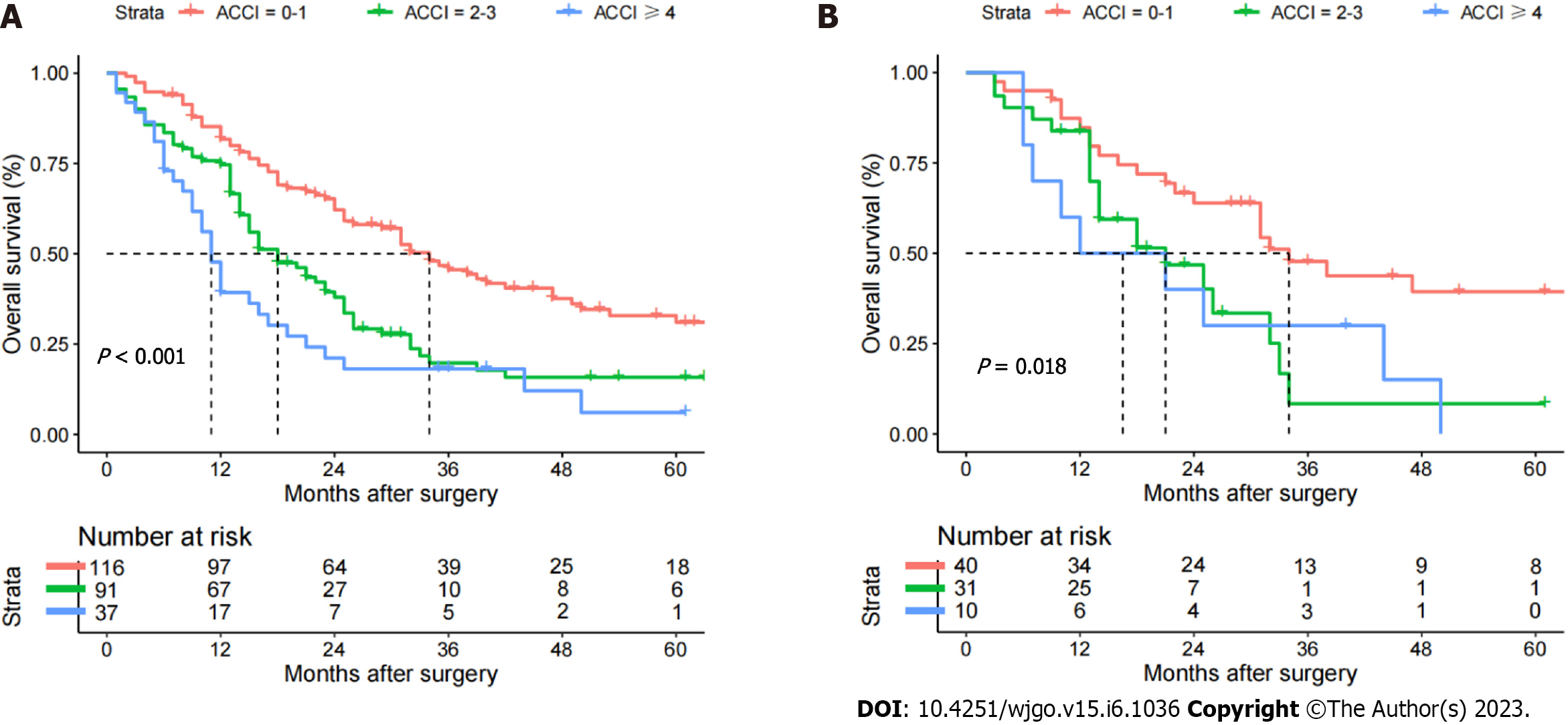
The median follow-up time was 24.0 (21.2-26.8) mo in the whole dataset. In the training cohort, 75.0% of the patients (183/244) developed recurrence, and 68.0% of the patients (166/244) died during follow-up. The 1-, 3- and 5-year OS rates were 72.7%, 32.4% and 22.9%, respectively. The 1-, 3-, and 5-year OS rates were 81.7%, 45.6%, and 31.1% in the low-ACCI group; 74.7%, 19.8%, and 15.8% in the moderate-ACCI group; and 39.9%, 14.6%, and 6.0% in the high-ACCI group, as shown in Table 2. The survival rate was lowest in the high-ACCI group and the highest in the low-ACCI group, with a significant difference in survival rates among the three groups (P < 0.001), as shown in Figure 1A. In the validation cohort, 73.8% of the patients (59/81) developed recurrence, and 63.0% of the patients (51/81) died during follow-up. The 1-, 3- and 5-year OS rates were 80.1%, 34.7% and 25.2%, respectively, as shown in Supple
The results of univariate and multivariate analyses of OS for pCCA patients after curative resection are shown in Table 3. Considering the effect of covariance between covariates on the results, age was excluded from the Cox regression model. Finally, seven variables were found to be independently associated with the OS of pCCA, as shown in Table 3: ACCI (2-3 vs 0-1) (HR: 1.605, 95%CI: 1.133-2.273, P = 0.008); ACCI (≥ 4 vs 0-1) (HR: 2.498, 95%CI: 1.614-3.866, P < 0.001); CA19-9 (> 150 vs ≤ 150 U/L) (HR: 1.471, 95%CI: 1.059-2.043, P = 0.021); maximum tumor size (> 5 vs < 3 cm) (HR: 1.990, 95%CI: 1.166-3.396, P = 0.011); macrovascular invasion (yes vs no) (HR: 1.700, 95%CI: 1.198-2.412, P = 0.003); microvascular invasion (yes vs no) (HR: 1.752, 95%CI: 1.166-2.634, P = 0.007); tumor differentiation (poor vs well/moderate) (HR: 1.550, 95%CI: 1.042-2.305, P = 0.030); and lymphoid metastasis [yes vs no (ELN > 4)] (HR: 2.549, 95%CI: 1.684-3.859, P < 0.001).
| Variable | R comparison | Univariable Cox regression | Multivariable Cox regression | ||
| HR (95%CI) | P value | HR (95%CI) | P value1 | ||
| Age | > 70 vs ≤ 70 yr | 1.793 (1.314-2.447) | < 0.001 | ||
| Sex | Male vs Female | 1.141 (0.838-1.555) | 0.402 | ||
| Diabetes | Yes vs No | 1.203 (0.718-2.017) | 0.482 | ||
| Cirrhosis | Yes vs No | 1.220 (0.738-2.016) | 0.438 | ||
| PLT | > 100 vs ≤ 100 × 109/L | 1.538 (0.719-3.290) | 0.267 | ||
| Albumin | < 35 vs ≥ 35 | 1.131 (0.823-1.555) | 0.447 | ||
| ALT | > 40 vs ≤ 40 U/L | 1.202 (0.848-1.704) | 0.302 | ||
| AST | > 40 vs ≤ 40 U/L | 1.155 (0.815-1.638) | 0.418 | ||
| TB | > 1 vs ≤ 1 mg/dL | 1.204 (0.813-1.785) | 0.354 | ||
| INR | > 1.25 vs ≤ 1.25 | 1.217 (0.795-1.863) | 0.365 | ||
| CA19-9 | > 150 vs ≤ 150 U/L | 1.768 (1.289-2.426) | < 0.001 | 1.471 (1.059-2.043) | 0.021 |
| Preoperative PTCD | Yes vs No | 1.172 (0.848-1.620) | 0.336 | ||
| Maximum tumor size | 3-5 vs < 3 cm | 1.777 (1.269-2.488) | 0.001 | 1.236 (0.858-1.779) | 0.255 |
| > 5 vs < 3 cm | 2.289 (1.377-3.803) | 0.001 | 1.990 (1.166-3.396) | 0.011 | |
| Macrovascular invasion | Yes vs No | 2.165 (1.539-3.045) | < 0.001 | 1.700 (1.198-2.412) | 0.003 |
| Microvascular invasion | Yes vs No | 2.212 (1.526-3.205) | < 0.001 | 1.752 (1.166-2.634) | 0.007 |
| Perineural infiltration | Yes vs No | 1.267 (0.878-1.827) | 0.205 | ||
| Tumor differentiation | Poor vs Well/moderate | 1.616 (1.102-2.369) | 0.014 | 1.550 (1.042-2.305) | 0.030 |
| Extent of resection | Major vs Minor | 1.348 (0.941-1.931) | 0.104 | ||
| Intraoperative blood loss | > 500 vs ≤ 500 mL | 1.128 (0.821-1.550) | 0.457 | ||
| Perioperative blood transfusion | Yes vs No | 1.069 (0.773-1.477) | 0.688 | ||
| Lymphoid metastasis | No (ELN ≤ 4) vs No (ELN > 4) | 1.673 (1.146-2.441) | 0.008 | 1.454 (0.987-2.141) | 0.058 |
| Yes vs No (ELN > 4) | 2.403 (1.618-3.567) | < 0.001 | 2.549 (1.684-3.859) | < 0.001 | |
| CCI | High vs Low | 1.239 (0.901-1.703) | 0.187 | ||
| ACCI | Moderate vs Low | 1.818 (1.292-2.558) | 0.001 | 1.605 (1.133-2.273) | 0.008 |
| High vs Low | 2.791 (1.818-4.287) | < 0.001 | 2.498 (1.614-3.866) | < 0.001 | |
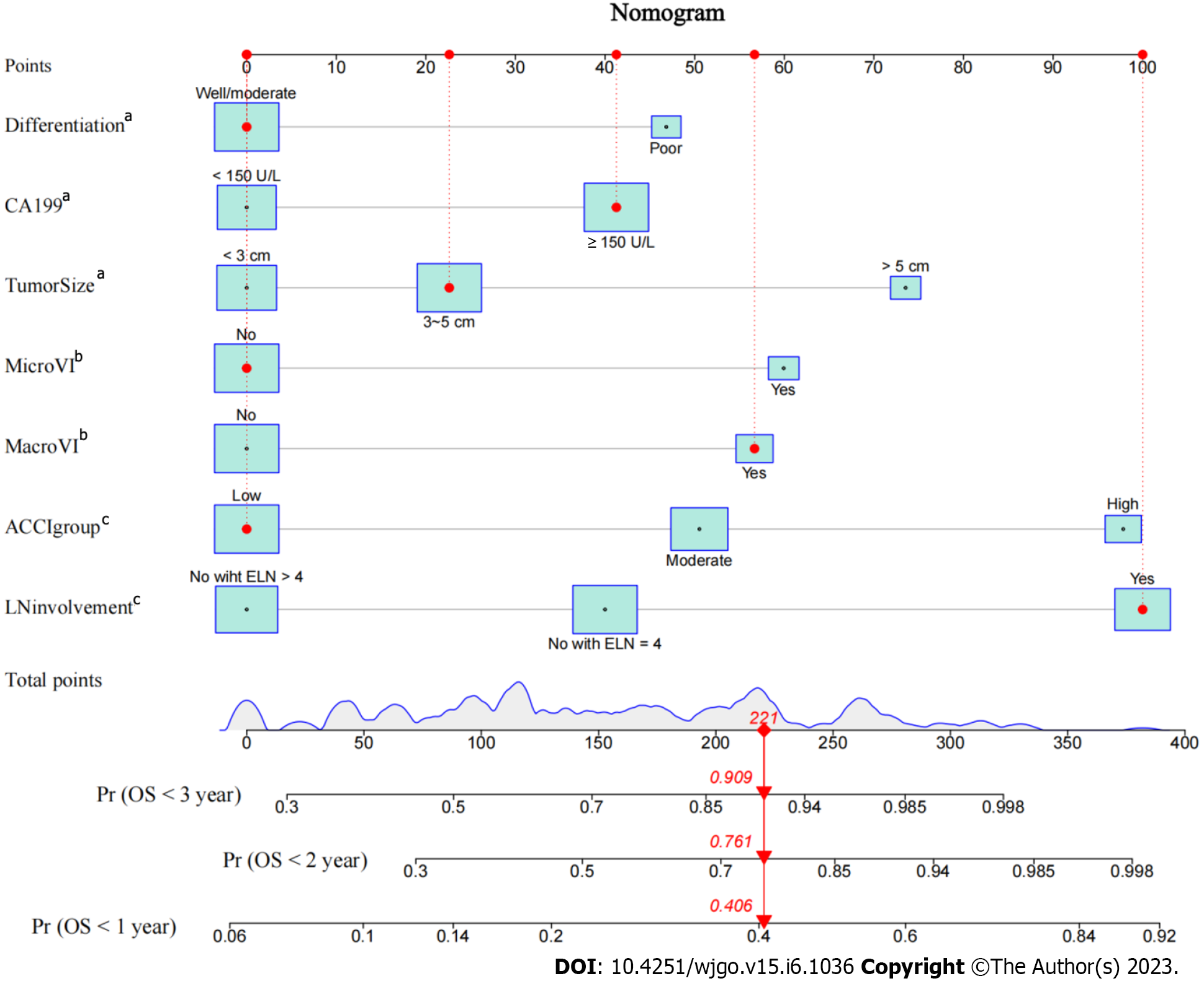
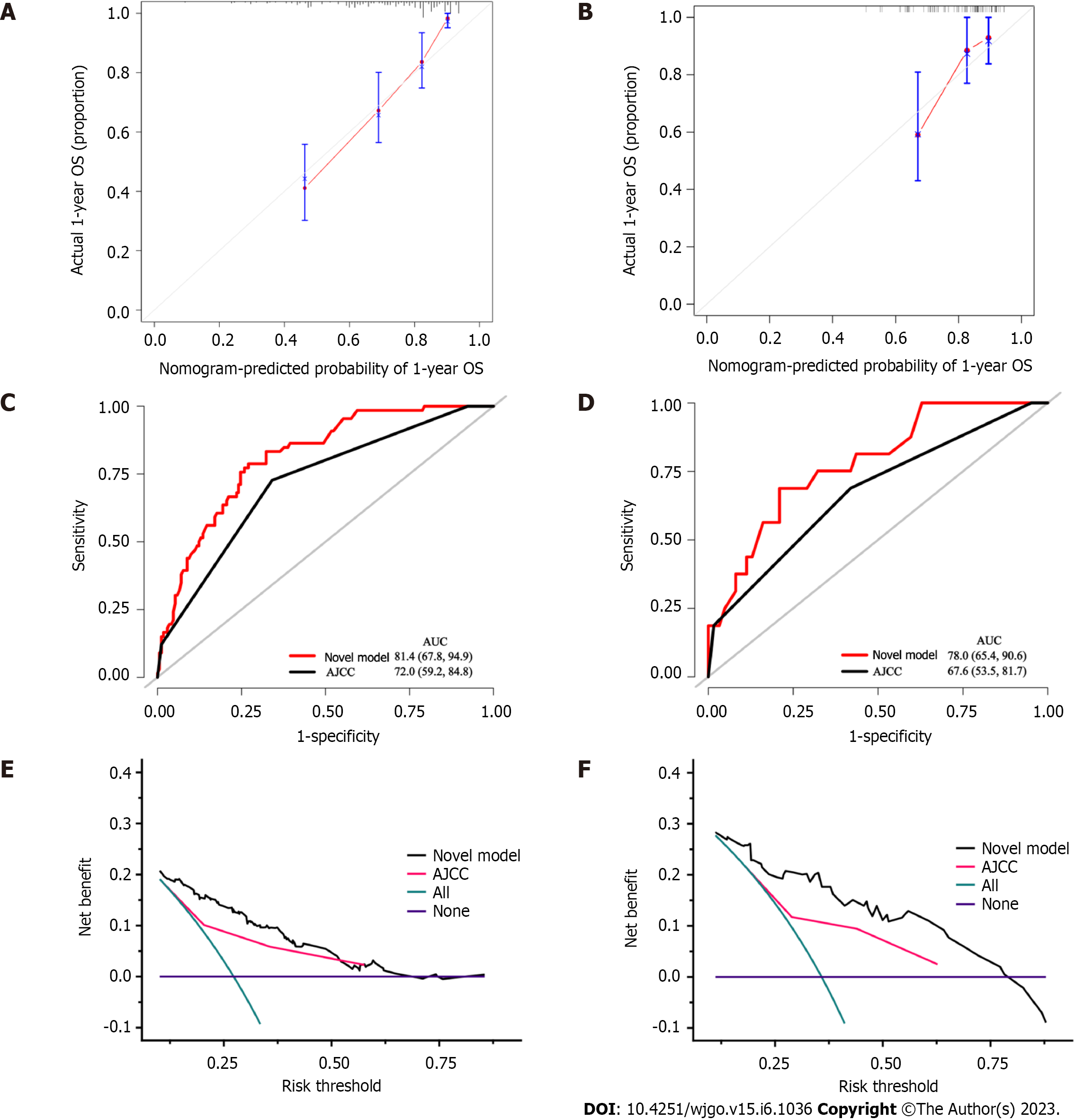
Using the variables from multivariate analysis, a nomogram to assess the OS of patients after curative resection was constructed based on the clinically relevant factors, as shown in Figure 2. To optimize its practicality, the nomogram was also transformed into an internet browser calculator (https://acci.shinyapps.io/newDynNomapp/). The relevant information of patients can be input, and information on the postoperative survival of patients could be obtained. The C-indexes of the prognostic nomogram for predicting OS were 0.725 (95%CI: 0.706-0.744) and 0.675 (95%CI: 0.635-0.715) in the training and validation cohorts, respectively. The calibration curves for the probability of 1-year OS in the training and validation cohorts were plotted, and the results revealed optimal accordance between the nomogram predictions and actual observations in both cohorts, as shown in Figure 3A and B.
The ROC curves for the training and validation cohorts suggested that the nomogram performed better than the 8th AJCC staging system in predicting OS within 1 year after curative resection, as shown in Figure 3C and D. Furthermore, the nomogram was compared with the 8th AJCC staging system by utilizing DCA. As shown in Figure 3E and F, the nomogram demonstrated superior net benefits with a wider range of threshold probabilities compared to the 8th AJCC staging system in predicting the OS of patients in both the training and validation cohorts. All these results indicated that this nomogram was an excellent predictive model for predicting the long-term outcomes of pCCA patients following curative resection.
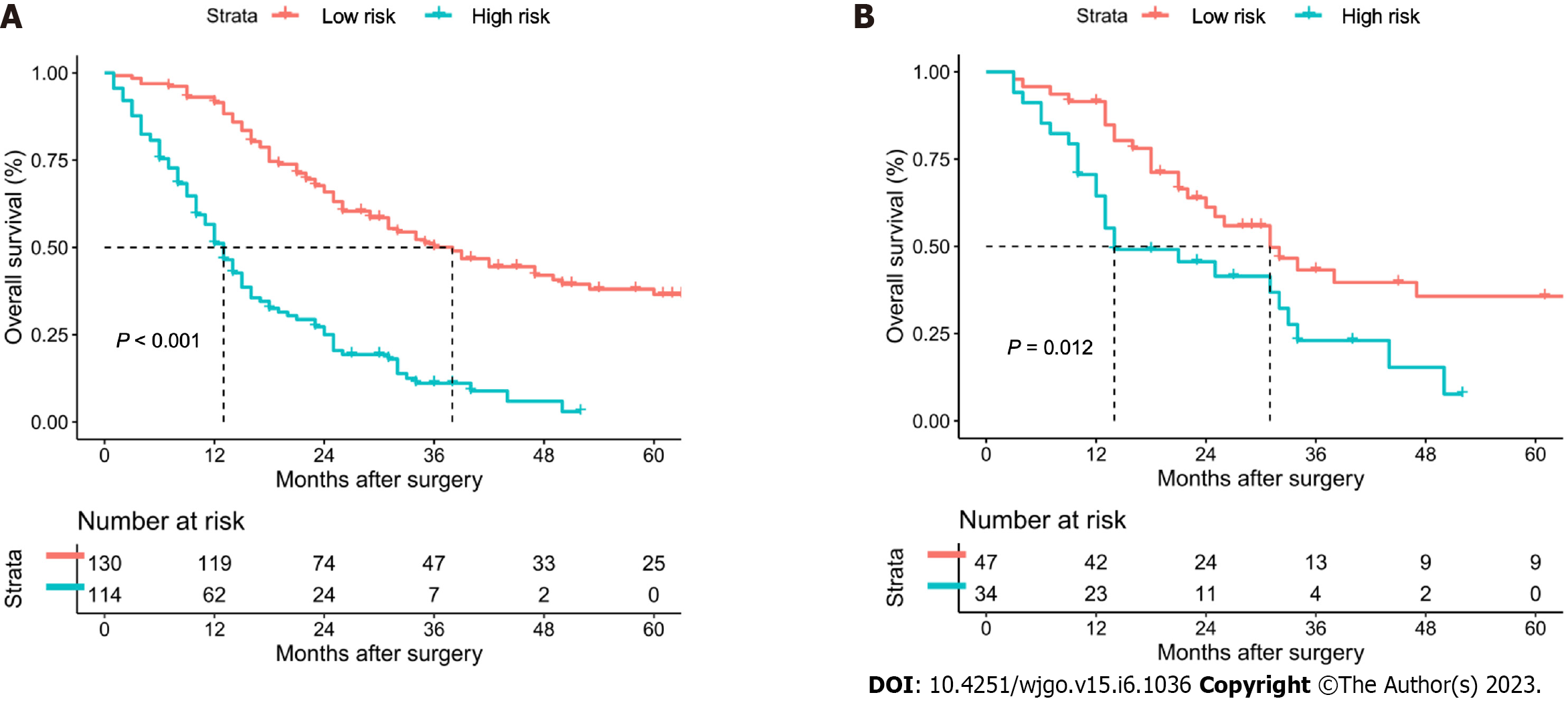
According to the ROC curve for the prediction of 1-year OS, the optimal cutoff value of the nomogram score was 156. Therefore, all patients were effectively separated into low- and high-risk groups. In the training cohort, patients in the high-risk group had 1-, 3-, and 5-year OS rates of 51.1%, 11.1%, and 0%, and patients in the low-risk group had 1-, 3-, and 5-year OS rates of 91.5%, 50.1%, and 36.5%, as shown in Figure 4A. The high-risk group had a significantly lower survival rate than the low-risk group (P < 0.001). In the validation cohort, patients in the high-risk group had 1-, 3-, and 5-year OS rates of 64.5%, 23.0%, and 0%, and patients in the low-risk group had 1-, 3-, and 5-year OS rates of 91.5%, 61.2%, and 35.7%, as shown in Figure 4B. Similarly, the survival rate was found to be significantly lower in the high-risk group than in the low-risk group (P = 0.012).
Comorbidities are common in cancer patients and are becoming more prevalent as the population ages[26]. An increasing number of studies have demonstrated that comorbidities potentially affect the development, diagnosis, treatment and prognosis of patients with cancer[11,12,27]. The ACCI is an excellent indicator that combines age and comorbidities. A higher ACCI implies a more complex preoperative situation, lower tolerance for complicated surgery, more difficult postoperative care and longer postoperative recovery. These conditions will directly impact the patient's perioperative safety and long-term prognosis. There is evidence that patient comorbidities can directly affect the choice of patient treatment modality[28]. Recently, the impact of the ACCI on the long-term prognosis of patients with various gastrointestinal carcinomas, such as gastric, colorectal, and pancreatic cancers, has been demonstrated[18,29,30]. Nevertheless, pCCA is one of the most surgically difficult gastrointestinal tumors with a poor prognosis, but the relationship between the ACCI and the prognosis of pCCA has not been studied. Therefore, our team conducted the first multicenter study to explore the impact of the ACCI on the long-term prognosis of patients after curative resection for pCCA.
In this study, we investigated for the first time the comorbidity distribution of 325 pCCA patients from multiple centers who underwent curative resection. The ACCI was used to assess comorbidity status, and drawing on previous methods, the patients were categorized into three groups by the ACCI score: Low-ACCI (ACCI = 0-1), moderate-ACCI (ACCI = 2-3) and high-ACCI (ACCI ≥ 4) groups. Multivariable analysis revealed that moderate and high ACCI scores were independently associated with reduced OS after curative resection for pCCA. To enhance guidance on treatment strategies, a clinical prediction model for the OS of pCCA patients after curative resection was constructed based on the ACCI and other independent risk factors associated with worse OS and validated. The satisfactory predictive performance of the model and its ability to identify patients with a high-risk prognosis allows it to guide clinical decision making.
In the long-term survival analysis, the univariate analysis results indicated that CCI did not significantly affect the long-term prognosis of pCCA, whereas ACCI was ultimately proven to be an independent prognostic factor for pCCA. This result suggests that the ACCI, a composite of age and comorbidity, provides a better prognostic assessment for patients. Multivariate Cox regression analysis revealed that moderate and high ACCI scores were independently associated with reduced OS in patients with pCCA after curative resection. This exciting and interesting result might be explained by the following findings.
Advanced age is not a contraindication to hepatobiliary surgery[31], nor is it a comorbidity[32]. However, elderly patients with comorbidities have a slow metabolism and poor recovery. The ACCI is a composite of age and comorbidities, and a high ACCI score indicates that the patient is elderly and/or has one or more comorbidities. Preoperative comorbidities, including diabetes, respiratory disease, and cardiovascular disease, are more common in older patients. Organ reserve function is reduced, and the long-term use of multiple medications can lead to further liver damage. Some pCCA patients may have prolonged obstructive jaundice prior to admission, which leads to a further decline in liver function. Moreover, pCCA patients may require hemihepatectomy or more extensive liver resection to achieve radical resection, further increasing the risk of perioperative liver failure. In addition, patients with high ACCI scores have worse nutritional status[33], and gastrointestinal diseases such as pCCA often lead to a reduction in the nutritional intake of patients, resulting in a substantially increased incidence of perioperative malnutrition. The combination of these factors leads to a significant increase in the perioperative risk of patients with high ACCI scores.
The National Comprehensive Cancer Network guidelines recommend that adjuvant therapy be considered after pCCA resection, especially for patients at high risk of recurrence with lymphatic metastases or R1 resection[34]. Cisplatin and S1 are two key drugs used in the postoperative adjuvant treatment of CCA, and their combination with gemcitabine significantly prolongs survival in patients with bile duct cancer[35]. However, some elderly patients with comorbidities cannot tolerate this treatment, resulting in the need for dose adjustment or contraindication[35,36]. Indeed, age and comorbidity burden led to lower rates of introduction of first-line combination chemotherapy and second-line chemotherapy[37]. In addition, various reasons, such as damage to liver and kidney function after adjuvant therapy, have forced patients to discontinue adjuvant therapy midway, resulting in a worse prognosis for the patient. Hence, reduced intensity or discontinuation of postoperative adjuvant therapy in elderly patients with comorbidities may be associated with poor prognosis.
In our opinion, patients with high ACCI scores should undergo a more careful multidisciplinary evaluation in terms of both the choice of the surgical procedure and the choice of postoperative adjuvant treatment.
In addition to the ACCI, a number of other independent risk factors for reduced OS were identified in the present study. These risk factors included CA19-9 (> 150 U/L), maximum tumor size (> 5 cm), lymphoid metastasis (yes), macrovascular invasion, microvascular invasion, and tumor differentiation. All these risk factors have been reported previously[38-40]. We constructed a nomogram using the above independent risk factors.
Nomograms are a visual tool for predicting the prognosis of patients with various cancers and are widely recognized in clinical practice for their applicability and accuracy[41]. Thus, based on the ACCI and these independent risk factors, a clinical prediction model to assess the OS of pCCA patients after curative resection was constructed and validated. To optimize its practicality, the nomogram was also further transformed into an internet browser calculator. According to the nomogram, we were able to identify high-risk patients (nomogram score > 156), who had a worse OS.
The ROC curves and DCA results for both the training and validation cohorts showed that the nomogram performed better than the 8th AJCC staging system in terms of its ability to predict OS after curative resection and its superior net clinical benefits. The TNM staging system has been promoted in abdominal surgery for a long time. With the continuous optimization of the staging system, the prediction of prognosis for many gastrointestinal tumors, such as gastric and colon cancers, has become increasingly accurate[42]. However, for parenchymal organs, whether pancreatic or liver tumors, the predictive accuracy of TNM staging is greatly reduced. For HCC, the clinical significance of N stage may be overestimated by the TNM staging system due to the exceptionally small probability of lymphatic metastasis. For pCCA, in addition to N stage, MVI and degree of differentiation are also critical in predicting prognosis. Thus, our model not only incorporates more comprehensive oncological information, including a highly specific serum tumor marker of pCCA, CA19-9, but also takes into account the patient’s comorbidity status. This allows our model to obtain a better predictive performance than TNM staging and to better guide clinical decisions.
Nevertheless, this study has several limitations. First, this was a retrospective study, and bias in data collection was inevitable. However, we included consecutive patients, so this study was closer to the real world than a randomized controlled trial. Second, although this was a multicenter study, there was a dearth of patient data from Western countries. We tried external validation using data from public databases such as surveillance, epidemiology, and end results but ultimately failed because only CCA but not pCCA could be identified in the database. Third, this study lacks data on postoperative adjuvant therapy. The patients in this study were recruited between 2010 and 2019. Due to the uncertainty of the efficacy, we did not record the adjuvant treatment in detail and will add these data in the future[43].
In conclusion, this multicenter study showed that a high ACCI score was independently associated with worse OS following curative resection for pCCA. The nomogram based on the ACCI provides a good prediction of OS, which can help surgeons make better clinical decisions.
Curative resection provides a possible cure for eligible patients with perihilar cholangiocarcinoma (pCCA). The predictive value of the age-adjusted Charlson comorbidity index (ACCI) for the long-term prognosis of patients with multiple malignancies was recently reported. However, pCCA is one of the most surgically difficult gastrointestinal tumors with the poorest prognosis, and the value of the ACCI for the prognosis of pCCA patients after curative resection is unclear.
The present study attempted to evaluate the prognostic value of the ACCI and to design an online clinical model to predict the overall survival (OS) of pCCA patients after curative resection.
This study aimed to identify the prognostic value of the ACCI in pCCA patients and to construct an online clinical model to predict the OS of pCCA patients after curative resection.
Consecutive pCCA patients after curative resection between 2010 and 2019 were enrolled from a multicenter database. The patients were randomly assigned 3:1 to training and validation cohorts. In the training and validation cohorts, all patients were divided into low-, moderate-, and high-ACCI groups. Kaplan-Meier curves were used to determine the impact of the ACCI on OS for pCCA patients, and multivariate Cox regression analysis was used to determine the independent risk factors affecting OS. An online clinical model based on the ACCI was developed and validated. The concordance index (C-index), calibration curve, and receiver operating characteristic (ROC) curve were used to evaluate the predictive performance and fit of this model.
Mild liver disease and diabetes were the most common comorbidities in pCCA patients undergoing radical surgery. The Kaplan-Meier curves showed that patients in the moderate- and high-ACCI groups had worse survival rates than those in the low-ACCI group. Multivariable analysis revealed that moderate and high ACCI scores were independently associated with OS in pCCA patients after curative resection. In addition, an online clinical model was developed that had ideal C-indexes of 0.725 and 0.675 for predicting OS in the training and validation cohorts, respectively. The calibration curve and ROC curve indicated that the model had a good fit and prediction performance.
A high ACCI score may predict poor long-term survival in pCCA patients after curative resection. High-risk patients screened by the ACCI-based model should be given more clinical attention in terms of the management of comorbidities and postoperative follow-up.
Although our multicenter study identified the prognostic value of the ACCI in pCCA patients after curative resection, future prospective studies with larger samples should be conducted to further explore the association between the ACCI and the prognosis of pCCA patients and the guidance of the ACCI on treatment allocation.
| 1. | Welzel TM, McGlynn KA, Hsing AW, O'Brien TR, Pfeiffer RM. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst. 2006;98:873-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 270] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 2. | Cardinale V. Classifications and misclassification in cholangiocarcinoma. Liver Int. 2019;39:260-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Klatskin G. Adenocarcinoma of the hepatic duct at its bifurcation within the PORTA hepatis. An unusual tumor with distinctive clinical and pathological features. Am J Med. 1965;38:241-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 502] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 4. | Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist. 2016;21:594-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 595] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 5. | Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37:806-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 424] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 6. | Taylor-Robinson SD, Toledano MB, Arora S, Keegan TJ, Hargreaves S, Beck A, Khan SA, Elliott P, Thomas HC. Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968-1998. Gut. 2001;48:816-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 313] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 7. | van Keulen AM, Franssen S, van der Geest LG, de Boer MT, Coenraad M, van Driel LMJW, Erdmann JI, Haj Mohammad N, Heij L, Klümpen HJ, Tjwa E, Valkenburg-van Iersel L, Verheij J, Groot Koerkamp B, Olthof PB; Dutch Hepatocellular & Cholangiocarcinoma Group (DHCG). Nationwide treatment and outcomes of perihilar cholangiocarcinoma. Liver Int. 2021;41:1945-1953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 8. | DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, Choti MA, Yeo CJ, Schulick RD. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 882] [Cited by in RCA: 1037] [Article Influence: 54.6] [Reference Citation Analysis (1)] |
| 9. | Feinstein AR. The pre-therapeutic classification of co-morbidity in chronic disease. J Chronic Dis. 1970;23:455-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 846] [Article Influence: 76.9] [Reference Citation Analysis (0)] |
| 10. | Extermann M. Measurement and impact of comorbidity in older cancer patients. Crit Rev Oncol Hematol. 2000;35:181-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 205] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 11. | Satariano WA, Silliman RA. Comorbidity: implications for research and practice in geriatric oncology. Crit Rev Oncol Hematol. 2003;48:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Liu ZP, Chen WY, Zhang YQ, Jiang Y, Bai J, Pan Y, Zhong SY, Zhong YP, Chen ZY, Dai HS. Postoperative morbidity adversely impacts oncological prognosis after curative resection for hilar cholangiocarcinoma. World J Gastroenterol. 2022;28:948-960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Boakye D, Rillmann B, Walter V, Jansen L, Hoffmeister M, Brenner H. Impact of comorbidity and frailty on prognosis in colorectal cancer patients: A systematic review and meta-analysis. Cancer Treat Rev. 2018;64:30-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 14. | Di Donato V, Page Z, Bracchi C, Tomao F, Musella A, Perniola G, Panici PB. The age-adjusted Charlson comorbidity index as a predictor of survival in surgically treated vulvar cancer patients. J Gynecol Oncol. 2019;30:e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32099] [Cited by in RCA: 39598] [Article Influence: 1015.3] [Reference Citation Analysis (0)] |
| 16. | Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4325] [Cited by in RCA: 5281] [Article Influence: 165.0] [Reference Citation Analysis (0)] |
| 17. | Lee JY, Kang HW, Rha KH, Cho NH, Choi YD, Hong SJ, Cho KS. Age-adjusted Charlson comorbidity index is a significant prognostic factor for long-term survival of patients with high-risk prostate cancer after radical prostatectomy: a Bayesian model averaging approach. J Cancer Res Clin Oncol. 2016;142:849-858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Dias-Santos D, Ferrone CR, Zheng H, Lillemoe KD, Fernández-Del Castillo C. The Charlson age comorbidity index predicts early mortality after surgery for pancreatic cancer. Surgery. 2015;157:881-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Wu CC, Hsu TW, Chang CM, Yu CH, Lee CC. Age-adjusted Charlson comorbidity index scores as predictor of survival in colorectal cancer patients who underwent surgical resection and chemoradiation. Medicine (Baltimore). 2015;94:e431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Shinkawa H, Tanaka S, Takemura S, Amano R, Kimura K, Nishioka T, Miyazaki T, Kubo S. Predictive Value of the Age-Adjusted Charlson Comorbidity Index for Outcomes After Hepatic Resection of Hepatocellular Carcinoma. World J Surg. 2020;44:3901-3914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Kahl A, du Bois A, Harter P, Prader S, Schneider S, Heitz F, Traut A, Alesina PF, Meier B, Walz M, Brueckner A, Groeben HT, Brunkhorst V, Heikaus S, Ataseven B. Prognostic Value of the Age-Adjusted Charlson Comorbidity Index (ACCI) on Short- and Long-Term Outcome in Patients with Advanced Primary Epithelial Ovarian Cancer. Ann Surg Oncol. 2017;24:3692-3699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann Surg Oncol. 2018;25:845-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 578] [Article Influence: 64.2] [Reference Citation Analysis (1)] |
| 23. | Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet. 1975;140:170-178. [PubMed] |
| 24. | Liu ZP, Cheng ZJ, Dai HS, Zhong SY, Zhao DC, Gong Y, Zuo JH, Che XY, Chen WY, Wang ZR, Yu T, Cheng JJ, Liu XC, Bai J, Jiang Y, Zhang YQ, Lau WY, Deng SQ, Chen ZY. Impact of perioperative blood transfusion on long-term survival in patients with different stages of perihilar cholangiocarcinoma treated with curative resection: A multicentre propensity score matching study. Front Oncol. 2022;12:1059581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 25. | Chen C, Liu ZP, Chen WY, Wang X, Liu YH, Wang Y, Liu XC, Fan HN, Bai J, Jiang Y. Anatomical hepatectomy for achieving textbook outcome for perihilar cholangiocarcinoma treated with curative-intent resection A multicenter study. iLIVER. 2022;1:7. |
| 26. | Williams GR, Deal AM, Lund JL, Chang Y, Muss HB, Pergolotti M, Guerard EJ, Shachar SS, Wang Y, Kenzik K, Sanoff HK. Patient-Reported Comorbidity and Survival in Older Adults with Cancer. Oncologist. 2018;23:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Lee L, Cheung WY, Atkinson E, Krzyzanowska MK. Impact of comorbidity on chemotherapy use and outcomes in solid tumors: a systematic review. J Clin Oncol. 2011;29:106-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 28. | Gross CP, McAvay GJ, Guo Z, Tinetti ME. The impact of chronic illnesses on the use and effectiveness of adjuvant chemotherapy for colon cancer. Cancer. 2007;109:2410-2419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Kellokumpu I, Kairaluoma M, Mecklin JP, Kellokumpu H, Väyrynen V, Wirta EV, Sihvo E, Kuopio T, Seppälä TT. Impact of Age and Comorbidity on Multimodal Management and Survival from Colorectal Cancer: A Population-Based Study. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Lin JX, Huang YQ, Xie JW, Wang JB, Lu J, Chen QY, Cao LL, Lin M, Tu R, Huang ZN, Lin JL, Zheng CH, Huang CM, Li P. Association of the age-adjusted Charlson Comorbidity Index and systemic inflammation with survival in gastric cancer patients after radical gastrectomy. Eur J Surg Oncol. 2019;45:2465-2472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Fong Y, Blumgart LH, Fortner JG, Brennan MF. Pancreatic or liver resection for malignancy is safe and effective for the elderly. Ann Surg. 1995;222:426-34; discussion 434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 194] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 32. | Cucchetti A, Ercolani G, Vivarelli M, Cescon M, Ravaioli M, Ramacciato G, Grazi GL, Pinna AD. Is portal hypertension a contraindication to hepatic resection? Ann Surg. 2009;250:922-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 33. | Takada Y, Kawashima H, Ohno E, Ishikawa T, Mizutani Y, Iida T, Yamamura T, Kakushima N, Furukawa K, Nakamura M, Honda T, Ishigami M, Ito A, Hirooka Y. The impact of the age-adjusted Charlson comorbidity index as a prognostic factor for endoscopic papillectomy in ampullary tumors. J Gastroenterol. 2022;57:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, Bachini M, Borad M, Brown D, Burgoyne A, Chahal P, Chang DT, Cloyd J, Covey AM, Glazer ES, Goyal L, Hawkins WG, Iyer R, Jacob R, Kelley RK, Kim R, Levine M, Palta M, Park JO, Raman S, Reddy S, Sahai V, Schefter T, Singh G, Stein S, Vauthey JN, Venook AP, Yopp A, McMillian NR, Hochstetler C, Darlow SD. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:541-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 643] [Article Influence: 128.6] [Reference Citation Analysis (2)] |
| 35. | Morizane C, Okusaka T, Mizusawa J, Katayama H, Ueno M, Ikeda M, Ozaka M, Okano N, Sugimori K, Fukutomi A, Hara H, Mizuno N, Yanagimoto H, Wada K, Tobimatsu K, Yane K, Nakamori S, Yamaguchi H, Asagi A, Yukisawa S, Kojima Y, Kawabe K, Kawamoto Y, Sugimoto R, Iwai T, Nakamura K, Miyakawa H, Yamashita T, Hosokawa A, Ioka T, Kato N, Shioji K, Shimizu K, Nakagohri T, Kamata K, Ishii H, Furuse J; members of the Hepatobiliary and Pancreatic Oncology Group of the Japan Clinical Oncology Group (JCOG-HBPOG). Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: the FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann Oncol. 2019;30:1950-1958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 235] [Article Influence: 33.6] [Reference Citation Analysis (1)] |
| 36. | Takahara N, Isayama H, Nakai Y, Sasaki T, Ishigaki K, Saito K, Akiyama D, Uchino R, Mizuno S, Yagioka H, Kogure H, Togawa O, Matsubara S, Ito Y, Toda N, Tada M, Koike K. Gemcitabine and S-1 versus gemcitabine and cisplatin treatment in patients with advanced biliary tract cancer: a multicenter retrospective study. Invest New Drugs. 2017;35:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Takahara N, Nakai Y, Saito K, Sasaki T, Suzuki Y, Inokuma A, Oyama H, Kanai S, Suzuki T, Sato T, Hakuta R, Ishigaki K, Saito T, Hamada T, Mizuno S, Kogure H, Tada M, Isayama H, Koike K. The impact of age and comorbidity in advanced or recurrent biliary tract cancer receiving palliative chemotherapy. J Gastroenterol Hepatol. 2020;35:1828-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Liu ZP, Zhang QY, Chen WY, Huang YY, Zhang YQ, Gong Y, Jiang Y, Bai J, Chen ZY, Dai HS. Evaluation of Four Lymph Node Classifications for the Prediction of Survival in Hilar Cholangiocarcinoma. J Gastrointest Surg. 2022;26:1030-1040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Ercolani G, Dazzi A, Giovinazzo F, Ruzzenente A, Bassi C, Guglielmi A, Scarpa A, D'Errico A, Pinna AD. Intrahepatic, peri-hilar and distal cholangiocarcinoma: Three different locations of the same tumor or three different tumors? Eur J Surg Oncol. 2015;41:1162-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 40. | Fabris L, Alvaro D. The prognosis of perihilar cholangiocarcinoma after radical treatments. Hepatology. 2012;56:800-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173-e180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1119] [Cited by in RCA: 2571] [Article Influence: 233.7] [Reference Citation Analysis (0)] |
| 42. | Oh SE, An JY, Choi MG, Lee JH, Sohn TS, Bae JM. Comparisons of remnant primary, residual, and recurrent gastric cancer and applicability of the 8th AJCC TNM classification for remnant gastric cancer staging. Eur J Surg Oncol. 2020;46:2236-2242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | Mallick S, Benson R, Haresh KP, Julka PK, Rath GK. Adjuvant radiotherapy in the treatment of gall bladder carcinoma: What is the current evidence. J Egypt Natl Canc Inst. 2016;28:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Broering DC, Saudi Arabia; Yildiz K, Turkey S-Editor: Fan JR L-Editor: A P-Editor: Zhang XD