Published online May 15, 2023. doi: 10.4251/wjgo.v15.i5.843
Peer-review started: November 5, 2022
First decision: February 2, 2023
Revised: February 16, 2023
Accepted: March 15, 2023
Article in press: March 15, 2023
Published online: May 15, 2023
Processing time: 188 Days and 4.4 Hours
Intraductal papillary neoplasm of the bile duct (IPNB) is a rare distinct subtype of precursor lesions of biliary carcinoma. IPNB is considered to originate from luminal biliary epithelial cells, typically displays mucin-hypersecretion or a papillary growth pattern, and results in cystic dilatation[1]. IPNB develops anywhere in the intrahepatic and extrahepatic biliary tracts, and can occur in various pathological stages from low-grade dysplasia to invasive carcinoma. IPNBs have similar phenotypic changes in the occurrence and development of all subtypes, and the prognosis is significantly better than that of traditional (non-papillary) cholangiocarcinoma.
To evaluate the clinicopathological features of IPNB to provide evidence-based guidance for treatment.
Invasive IPNB, invasive intraductal papillary mucinous neoplasm of the pancreas (IPMN), and traditional cholangiocarcinoma data for affected individuals from 1975 to 2016 were obtained from the Surveillance, Epidemiology, and End Results (SEER) database. Annual percentage changes (APCs) in the incidence and incidence-based (IB) mortality were calculated. We identified the independent predictors of overall survival (OS) and cancer-specific survival (CSS) in indivi
The incidence and IB mortality of invasive IPNB showed sustained decreases, with an APC of -4.5% (95%CI: -5.1% to -3.8%) and -3.3% (95%CI: -4.1% to -2.6%) (P < 0.001), respectively. Similar decreases in incidence and IB mortality were seen for invasive IPMN but not for traditional cholangiocarcinoma. Both OS and CSS for invasive IPNB were better than for invasive IPMN and traditional cholangiocarcinoma. A total of 1635 individuals with invasive IPNB were included in our prognosis analysis. The most common tumor sites were the pancreaticobiliary ampulla (47.9%) and perihilar tract (36.7%), but the mucin-related subtype of invasive IPNB was the main type, intrahepatically (approximately 90%). In the univariate and multivariate Cox regression analysis, age, tumor site, grade and stage, subtype, surgery, and chemotherapy were associated with OS and CSS (P < 0.05).
Incidence and IB mortality of invasive IPNB trended steadily downward. The heterogeneity of IPNB comprises site and the tumor’s mucin-producing status.
Core Tip: Intraductal papillary neoplasms of the bile duct (IPNB) is a rare subtype of biliary cholangiocarcinoma, and also considered as a counterpart of intraductal papillary mucinous neoplasm of the pancreas (IPMN). Current management decisions are based on anecdotal evidence and small case series. There have been no large-sample multicenter studies of IPNB. This manuscript aimed to evaluate the clinicopathological features of IPNB to provide evidence-based guidance for treatment.
- Citation: Wu RS, Liao WJ, Ma JS, Wang JK, Wu LQ, Hou P. Epidemiology and outcome of individuals with intraductal papillary neoplasms of the bile duct. World J Gastrointest Oncol 2023; 15(5): 843-858
- URL: https://www.wjgnet.com/1948-5204/full/v15/i5/843.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i5.843
Intraductal papillary neoplasm of the bile duct (IPNB) is a rare distinct subtype of precursor lesions of biliary carcinoma. IPNB is considered to originate from luminal biliary epithelial cells, typically displays mucin-hypersecretion or a papillary growth pattern, and results in cystic dilatation[1]. IPNB develops anywhere in the intrahepatic and extrahepatic biliary tracts, and can occur in various pathological stages from low-grade dysplasia to invasive carcinoma. IPNBs have similar phenotypic changes in the occurrence and development of all subtypes, and the prognosis is significantly better than that of traditional (non-papillary) cholangiocarcinoma[2]. Based on these characteristics, IPNBs have been called mucinous cholangiocarcinoma, biliary papillomatosis, biliary intraductal papillary neoplasm, mucin-hypersecreting intrahepatic biliary neoplasm, and intraductal papillary neoplasm of the liver[3,4].
According to the 2010 World Health Organization (WHO) classification of tumors in the digestive system, IPNB is defined as the biliary counterpart of intraductal papillary mucinous neoplasm of the pancreas (IPMN) and has identical histopathologic pancreaticobiliary, gastric, intestinal, and oncocytic features[5,6]. Because of the anatomical proximity of the pancreas and the bile duct, the simultaneous development of the foregut endoderm, the peribiliary gland containing multipotent stem cells in biliary tract can differentiate into cholangiocytes as well as hepatocytes or pancreatocytes[7]. However, several important differences between IPMN and IPNB exist, such as the incidence and prognosis of invasive cancer, frequency of each tumor subtype, frequency of mucin production, and the presence of known high risk factors, such as choledocholithiasis and parasitic infection, and gene mutation, such as CTNNB1, TP53, SMAD4 and PIK3CA[8-10].Compared with traditional cholangiocarcinoma, IPNB has also been described anecdotally as a tumor type with limited invasive potential, typically involving only cellular atypia and at most, carcinoma in situ[5].
Unlike invasive IPMN and traditional cholangiocarcinoma in the biliary tract, very little is known about the clinicopathological features and prognostic variables of invasive IPNB. Previous studies were based solely on single-center case series, for example, Wu et al[11] reported that IPNB occured mainly in patients of advanced age. In addition, a multicenter study indicated that IPNB shown a better long-term prognosis than traditional cholangiocarcinoma, and were relatively invasive features in extrahepatic lesions[12]. Therefore, the epidemiology, tumor characteristics, treatment strategy, and long-term results of invasive IPNB are limited because of the relatively low case numbers. We conducted a Surveillance, Epidemiology, and End Results (SEER) database evaluation of invasive IPNB to address these shortcomings, and to further elucidate the epidemiological and clinical trends to guide treatment decision-making and to identify further clinical and scientific research areas.
The SEER database is an authoritative source of information about cancer incidence and survival rates in the United States. SEER currently collects and publishes data on cancer incidence and survival from population-based cancer registries covering approximately 28% of the United States population, and the database is maintained by the National Cancer Institute (NCI). The SEER Program is the only comprehensive population-based source of information in the United States for cancer stage during diagnosis, incidence, and survival data. Mortality data reported by SEER are provided by the National Center for Health Statistics. Population data used to calculate cancer incidence have passed appropriate standards before abstraction.
This was a retrospective cohort study using data from SEER databases submitted up to November 2018. Data from 1975 to 2016 are available from 18 SEER registries (with additional and treatment fields): Alaska Natives, Atlanta, California (excluding San Francisco/San José Monterey/Los Angeles), Connecticut, Detroit (metropolitan), Greater Georgia, Hawaii, Iowa, Kentucky, Los Angeles, Louisiana, New Jersey, New Mexico, Rural Georgia, San Francisco-Oakland standard metropolitan statistical area, San José Monterey, Seattle, and Utah. The data are adjusted for areas affected by hurricanes Katrina and Rita.
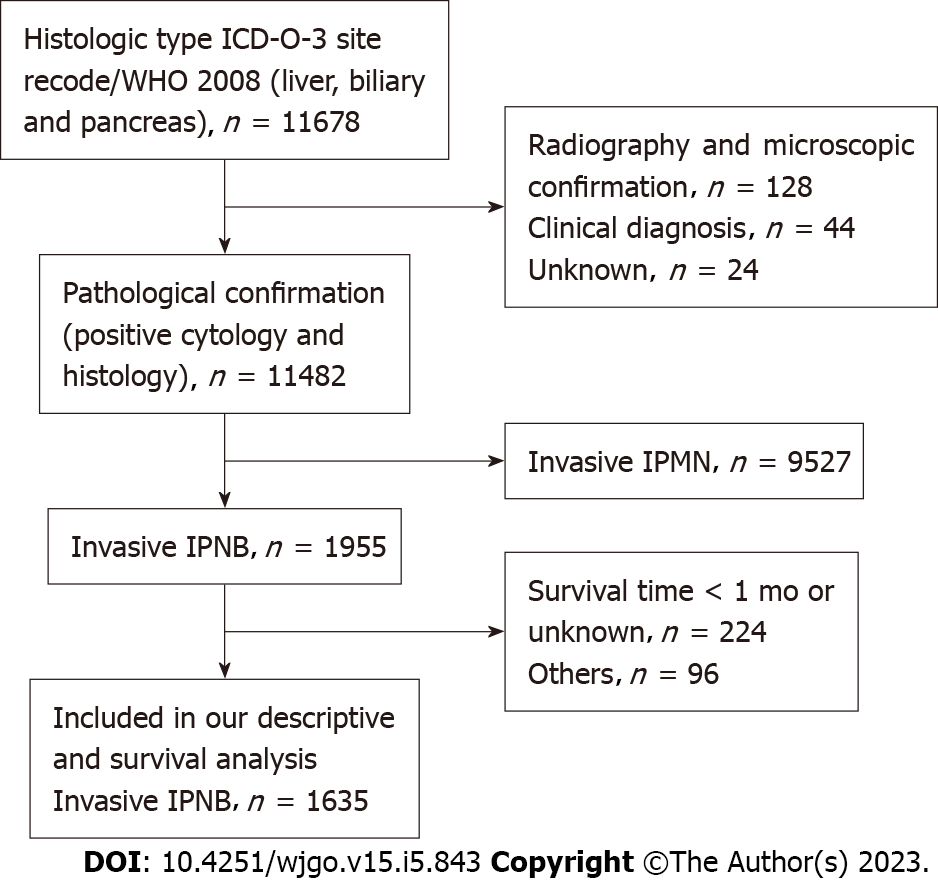
The database was queried by selecting biliary tract [including other biliary (C240–C249), intrahepatic bile duct (C221), and liver (C220) and pancreas (C250-C259)] as the disease sites [sites recoded according to the international classification of disease (ICD) O–3/WHO 2008)] and using the following codes from the ICD for oncology (ICD-O) 3rd edition: 8050/3, 8260/3, 8450/3, 8453/3, 8471/3, 8480/3, 8481/3, and 8503/3 for invasive IPNB and invasive IPMN[13-16] and 8140/3 and 8160/3 for data on individuals with traditional adenocarcinoma and cholangiocarcinoma of the biliary tract, for comparison[17]. We included 34972 patients with pathological evidence of traditional adenocarcinoma or cholangiocarcinoma of the biliary tract and 9527 patients with invasive IPMN. Invasive IPNBs were categorized as the mucinous subtype of invasive IPNBs (ICD-O-3rd: 8453/3, 8471/3, 8480/3, 8481/3) and the non-mucinous subtype of invasive IPNBs (ICD-O-3rd: 8050/3 8260/3 8450/3, 8503/3). All individuals in our study cohort had pathologically-confirmed diagnoses. In addition, because the smallest unit of survival was months rather than days, the data for individuals who died within 1 mo after diagnosis were excluded to avoid analyzing the situation where the survival time was zero. If individuals did not have complete demographic or clinical pathology and follow-up information, they were excluded (Figure 1).
We recorded the following demographic and clinicopathological variables: sex, age at diagnosis, year of diagnosis, race, detailed tumor site according to the tumor, node and metastasis (TNM) 7 and cancer staging schema, version 0204, histological subtype, SEER historic stage, grade, surgery, radiotherapy, chemotherapy, survival (in months), vital status recode, and cause-specific death. The SEER stage classification provides consistent time rather than the American Joint Committee on Cancer stage classification, which may have changed during the study period. Field descriptions of the TNM7/CSv0204 + schema information was collected under the specifications of a particular schema according to site and histology, such as site recode: 003 am pull avatar bile (defined pancreaticobiliary ampulla); 006 bile ducts distal; 007 bile ducts intrahepatic; 008 bile ducts perihilar; 009 biliary other; and 062 liver. The SEER historic stages are divided into four stages: localized stage (confined to the primary site), regional stage (spread to regional lymph nodes), distant stage (cancer had metastasized), and unstaged or unknown. The types of surgical treatment include defined radical surgery, non-defined radical surgery, and palliative surgery. The defined radical surgery includes: radical surgery (partial/total removal of primary site plus partial or total removal of other organs); radical surgery; partial/simple removal of primary site with dissection of lymph nodes; wedge resection, nitric oxide synthase (NOS) segmental resection, lobectomy; extended lobectomy: resection of a single lobe plus a segment of another lobe. The undefined radical surgery includes surgery, NOS; partial/simple removal of primary site without dissection of lymph nodes; surgery of regional and/or distant site(s)/node(s) only. Palliative surgery included: excisional biopsy; polypectomy; excision of lesion and photodynamic therapy. The primary outcomes of our cohort study were the overall survival (OS) rate and the cancer-specific survival (CSS) rate. OS was calculated as the time from diagnosis to death (from any cause), and CSS was calculated as the time from diagnosis to death (attributable to the cancer).
The incidence rates of invasive IPNB, invasive IPMN, and cholangiocarcinoma were calculated per 1000000 persons, and results were age-adjusted to the 2000 United States standard population using SEER*Stat (version 8.3.8). Annual percentage changes (APCs) of incidence and incidence-based (IB) mortality were calculated using the NCI joinpoint regression analysis program (version 4.8.0.1). APC is a method of describing incidence or mortality trends over time by showing slope gradients or directions for each straight segment. Therefore, tumor incidence or mortality rate is considered to change by a constant percentage from the previous year. We used linear-by-linear association tests to evaluate the trends in the ordinal data, which provides a meaningful measure of ordinal variables; the SEER*Stat software calculates 95%CIs. The Kaplan- Meier method and the log-rank test were used to calculate the cumulative survival rate and survival curves. We used a Cox proportional hazard regression model in the multivariable analysis using IBM SPSS Statistics software (version 26; IBM Corp., Armonk, NY). The multi-variables analysed included patient age, site, tumour grade, stage, mucin classification and treatment. P values were two-sided, and P values < 0.05 were considered statistically significant.
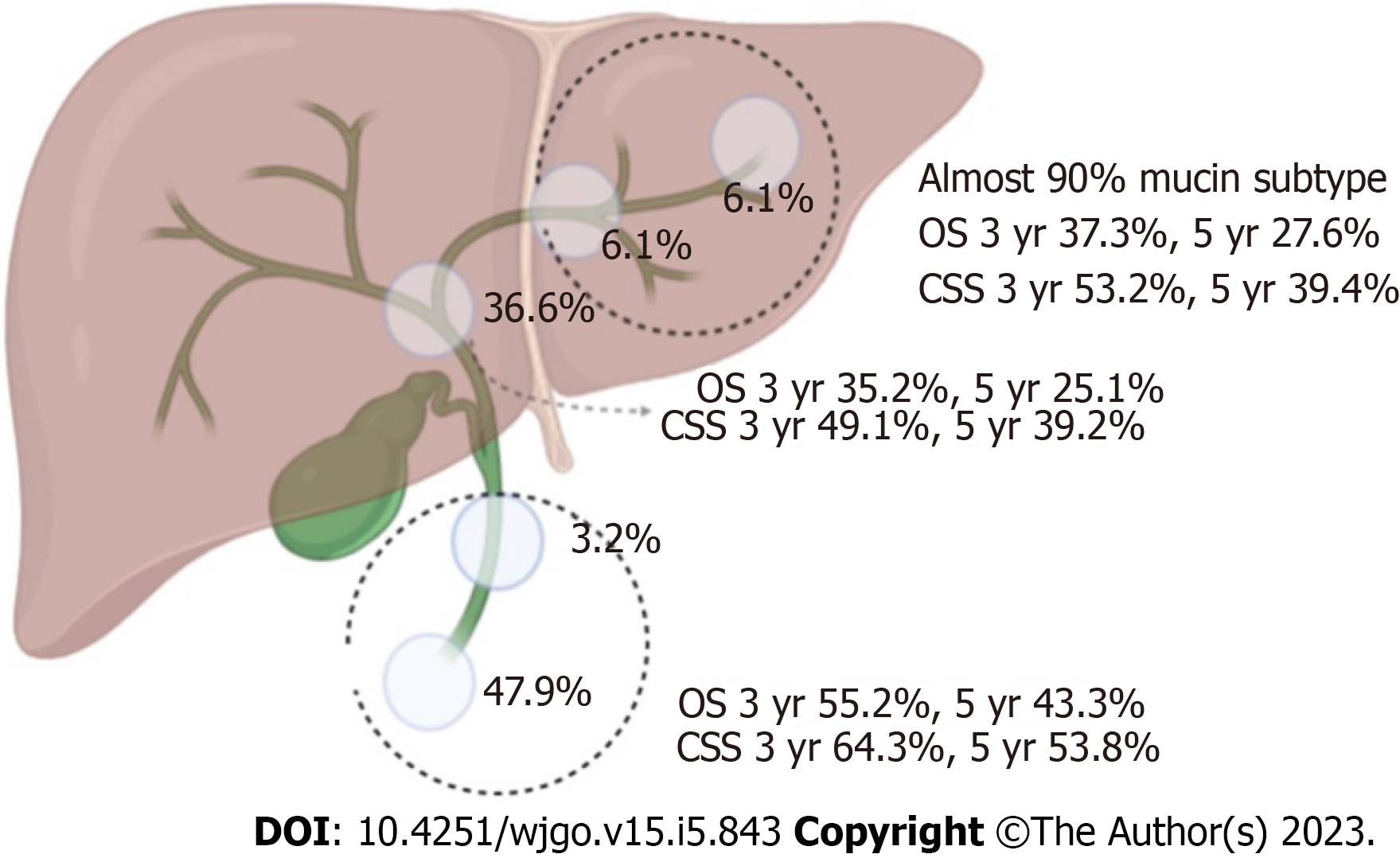
A total of 11678 individuals were diagnosed with invasive IPNB and invasive IPMN in the pancreaticobiliary duct system from 1975 to 2016. Of these individuals, 1635 individuals met our study criteria (Figure 1). The percentages of the mucin-related subtype and non-mucin subtype of invasive IPNBs were 56.6% (n = 926) and 43.4% (n = 709), respectively. The mean age and standard deviation in the total cohort was 69 ± 12.2 years, and the median age of the overall cohort was 68 years (range: 27-97 years). Individuals aged ≥ 68 and < 68 years accounted for 51.3% and 48.7% of the individuals, respectively. The proportions of men and women in the total cohort were 54.8% (n = 896) and 45.2% (n = 739), respectively. The vast majority of invasive IPNB patients were white (n = 1278, 78.2%). The tumor sites differed significantly and were most commonly the pancreaticobiliary ampulla (n = 783, 47.9%) and the perihilar tract (n = 600, 36.7%), followed by the liver (n = 100, 6.1%), intrahepatic biliary tract (n = 99, 6.1%), and distal tract (n = 53, 3.2%). The mucin-related subtype of invasive IPNB was the main type in the intrahepatic biliary duct system. The percentages of the locations of the mucin-related subtype were both nearly 90% (liver: 90/100, intrahepatic bile duct: 87/99). Most individuals had SEER historic stage regional tumors (n = 814, 49.8%), followed by the localized stage (n = 401, 24.5%), distant stage (n = 317, 19.4%), and unstaged or unknown (n = 103, 6.3%). Tumors were categorized by pathological grade as follows: well-differentiated, grade I (n = 373, 22.8%); moderately-differentiated, grade II (n = 532, 32.5%); poorly-differentiated, grade III (n = 227, 13.9%); and undifferentiated grade (n = 22, 1.3%) and unknown grade (n = 481, 29.4%). In the total cohort, 60.1% (n = 982) of individuals with invasive IPNB underwent surgery, and 35.4% (n = 579) received defined radical surgery, 22.9% (n = 375) received undefined radical surgery, 1.8% (n = 28) received palliative surgery, while only 17.5% (n = 286) received radiotherapy, and 28.0% (n = 458) received chemotherapy (Table 1). Approximately 11.5% (n = 188) of the individuals underwent combined radiotherapy, and chemotherapy, while 15.0% (n = 245) of the individuals underwent combined surgery and chemotherapy; 11.4% (n = 187) underwent combined surgery and radiotherapy. Only 8.1% (n = 133) of the individuals received triple therapy (chemotherapy, radiotherapy, and surgery).
| Variable | Total (n = 1635) | Subtype 1 (mucin) | Subtype 2 (non-mucin) |
| Age at diagnosis, yr | n = 926 | n = 709 | |
| < 68 | 796 (48.7) | 464 (50.1) | 332 (46.8) |
| ≥ 68 | 839 (51.3) | 462 (49.9) | 377 (53.2) |
| Sex | |||
| Male | 896 (54.8) | 504 (56.3) | 392 (55.3) |
| Female | 739 (45.2) | 422 (45.6) | 317 (44.7) |
| Race | |||
| White | 1278 (78.2) | 741 (80.0) | 537 (75.7) |
| Black | 116 (7.1) | 67 (7.2) | 49 (7.1) |
| Others | 241 (14.7) | 118 (12.7) | 123 (17.3) |
| THM 7/CSv0204+ schema | |||
| Liver | 100 (6.1) | 90 (9.7) | 10 (1.4) |
| Intrahepatic bile | 99 (6.1) | 87 (9.4) | 12 (1.7) |
| Perihilar bile | 600 (36.6) | 304 (32.8) | 296 (41.7) |
| Distal bile | 53 (3.2) | 27 (2.9) | 26 (3.7) |
| Pancreaticobiliaryampulla | 783 (47.9) | 418 (45.1) | 365 (51.5) |
| SEER historic stage | |||
| Localized | 401 (24.5) | 130 (14) | 271 (38.2) |
| Regional | 814 (49.8) | 482 (52.1) | 332 (46.8) |
| Distant | 317 (19.4) | 259 (28) | 58 (8.2) |
| Unstaged | 103 (6.3) | 55 (5.9) | 48 (6.8) |
| Grade | |||
| Well (I) | 373 (22.8) | 119 (12.9) | 254 (35.8) |
| Moderately (II) | 532 (32.5) | 310 (33.5) | 222 (31.3) |
| Poorly (III) | 227 (13.8) | 176 (19.0) | 51 (7.2) |
| Undifferentiated (IV) | 22 (1.3) | 11 (1.2) | 11 (1.6) |
| Unknown | 481 (29.4) | 310 (33.5) | 171 (24.1) |
| Surgery | |||
| Performed | 982 (60.1) | 483 (52.2) | 489 (70.4) |
| Defined radical surgery | 579 (35.4) | 336 (36.3) | 243 (35.0) |
| Undefined radical surgery | 375 (22.9) | 130 (14.1) | 235 (33.4) |
| Palliative surgery | 28 (1.8) | 17 (1.8) | 11 (2.0) |
| Non | 653 (39.9) | 443 (47.8) | 210 (29.6) |
| Radiatherapy | |||
| Performed | 286 (17.5) | 173 (18.7) | 113 (15.9) |
| Non | 1349 (82.5) | 753 (81.3) | 596 (84.1) |
| Chemotherapy | |||
| Performed | 458 (28.0) | 339 (36.6) | 119 (16.8) |
| Non | 1177 (72.0) | 587 (63.4) | 590 (83.2) |
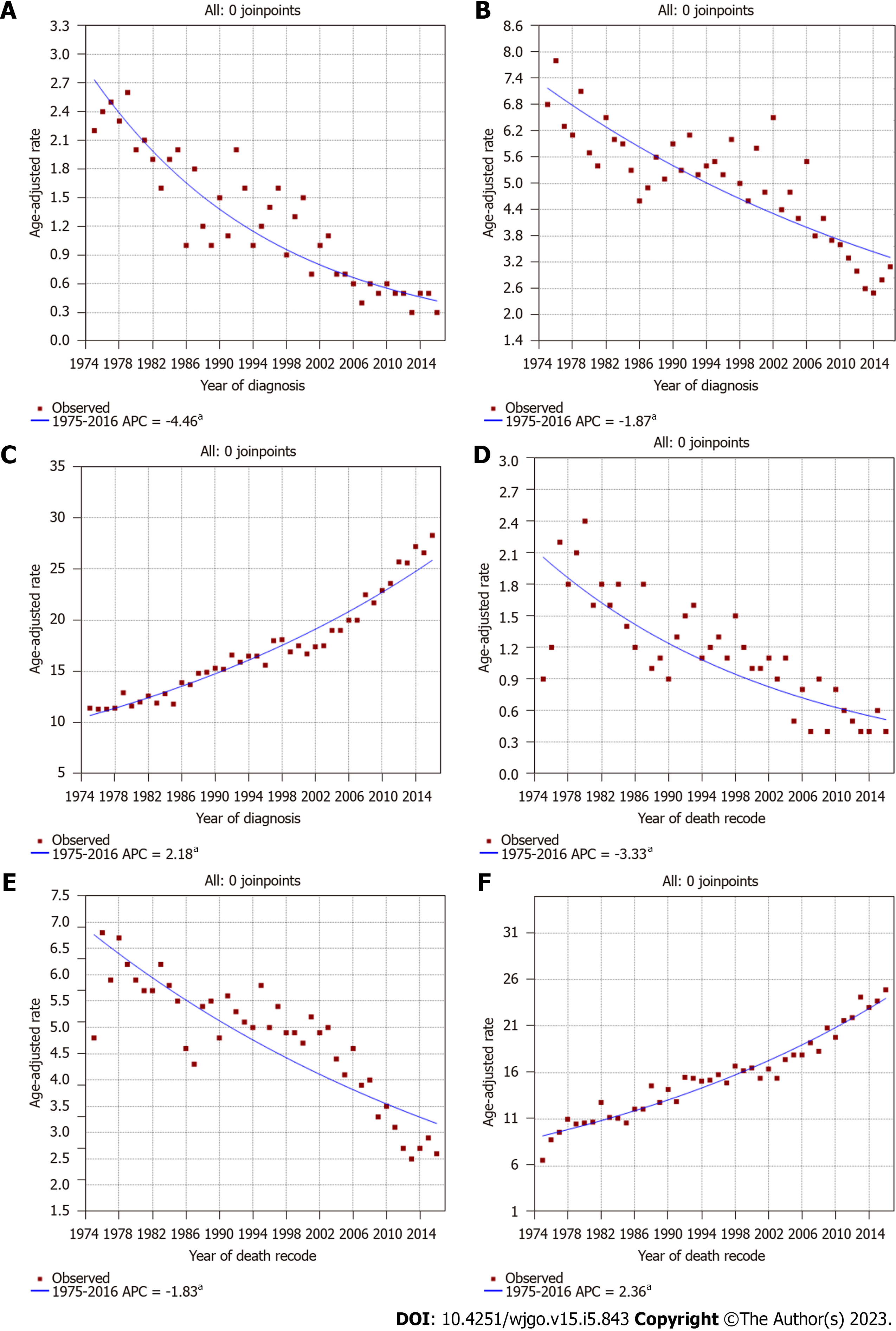
During the study period, the incidence of invasive IPNB decreased steadily (Figure 2A), as with invasive IPMN; however, the incidence of traditional cholangiocarcinoma increased steadily (Figure 2B and C). The incidence of invasive IPNB was 2.2 cases per 1000000 individuals in 1975 and 0.3 cases per 1000000 individuals in 2016. The APC over this period was -4.5% (95%CI: -5.1% to -3.8%; P < 0.05). While the slope of the decrease increased near 1999, the APC (i.e., the extent of the decrease) for the incidence of invasive IPNB from 1999 to 2016 was -6.6% per year (95%CI: -8.8% to -4.4%; P < 0.05), whereas from 1975 to 1999, the change was -3.2% per year (Supplementary Figure 1A). Similarly, the incidence of invasive IPMN also showed a sustained decrease (Figure 2B). The incidence of invasive IPMN was 6.8 cases per 1000000 individuals in 1975 and 3.1 cases per 1000000 individuals in 2016. The APC of invasive IPMN over this period was -1.9% (95%CI: -2.3% to -1.5%; P < 0.05). Conversely, the incidence of traditional cholangiocarcinoma in the biliary tract increased steadily. The incidence of traditional cholangiocarcinoma was 11.4 cases per 1000000 individuals in 1975 and 28.3 cases per 1000000 individuals in 2016, and the APC was 2.18% (95%CI: 2.0%-2.3%; P < 0.05) (Figure 2C).
The IB mortality of invasive IPNB also showed a steady decrease over the study period (Figure 2D), with an APC of -3.3% (95%CI: -4.1% to -2.6%; P < 0.05), and a decrease from 2.1 cases per 1000000 individuals in 1975 to 0.5 cases per 1000000 individuals in 2016 (Figure 2D). While the slope of the decrease increased near 1999, the APC for the IB mortality of invasive IPNB from 1999 to 2016 was -5.7% per year (95%CI: -7.9% to -3.3%; P < 0.05), whereas from 1977 to 1999, the change was -2.5% per year (95%CI: -4.2% to -0.8%; P < 0.05) (Supplementary Figure 1B). The changes were similar for IPMN, which also showed decreased IB mortality over the study period, with an APC of -1.83% (95%CI: -2.2% to -0.8%; P < 0.05) (Figure 2E). Conversely, the IB mortality for traditional cholangiocarcinoma increased during the study period, from 6.6 cases per 1000000 individuals in 1975 to 24.9 cases per 1000000 individuals in 2016; the APC was 2.36% (95%CI: 2.1%-2.6%; P < 0.05) (Figure 2F).
Regarding the incidence by sex, we found a steady decreasing trend in invasive IPNB incidence in males from 1975 to 2016; the APC was -4.8 % (95%CI: -5.7 to -3.8; P < 0.01). The incidence in males was 3.6 per 1000000 individuals in 1975 and 0.5 per 1000000 individuals in 2016 (Supplementary Figure 2A). The incidence of invasive IPNB in females followed a similar pattern. The APC was -4.5% (95%CI: -5.4 to -3.7; P < 0.01), and 2.2 per 1000000 individuals in 1975 and 0.3 per 1000000 individuals in 2016 (Supplementary Figure 2B). In both males and females, the IB mortality rate of invasive IPNB also decreased during 1975-2016. The APCs were -3.39% (95%CI: -4.4 to -2.4; P < 0.01) and -3.43% (95%CI: -4.2 to -2.7; P < 0.01), respectively (Supplementary Figure 2C and D).
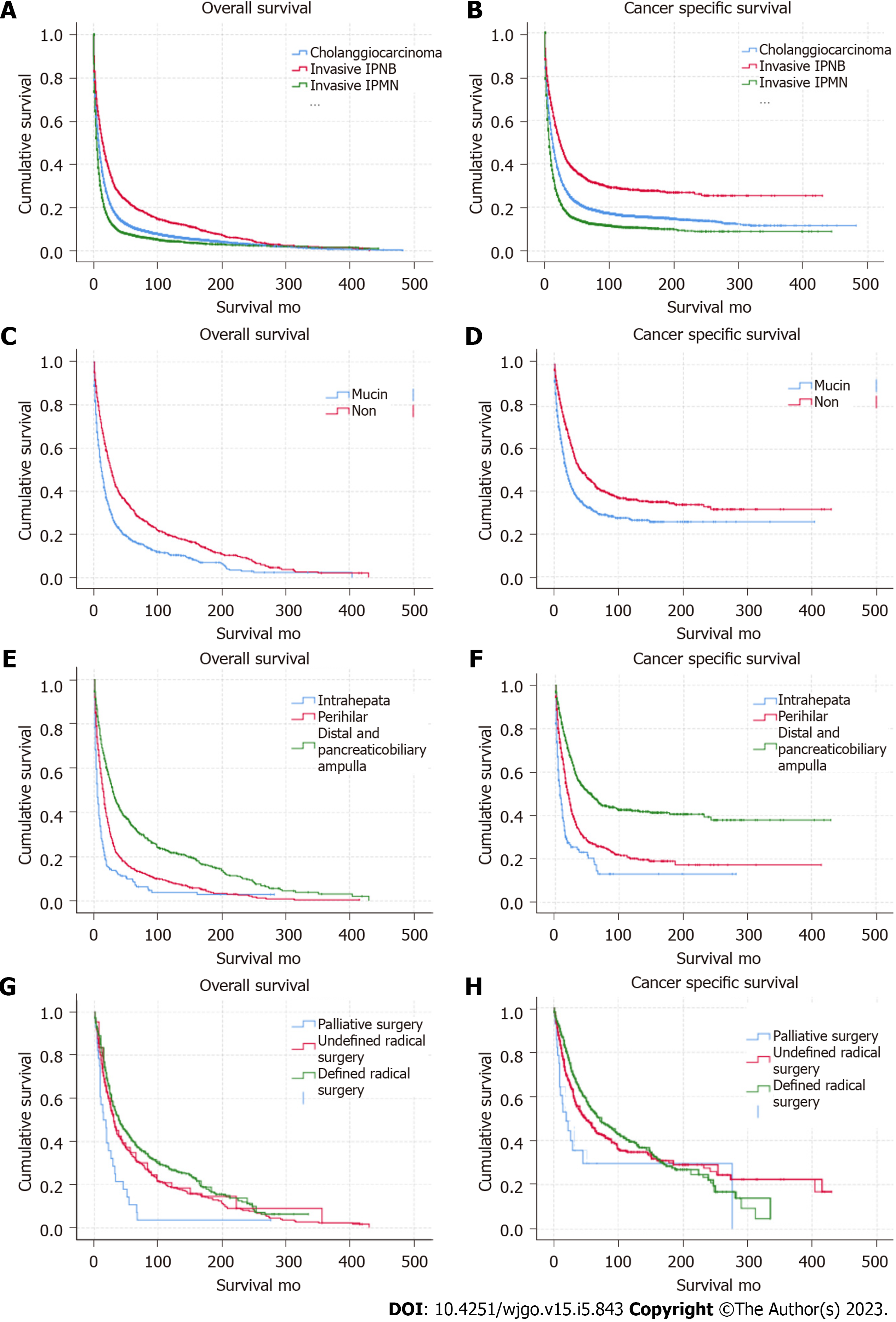
Both OS and CSS for invasive IPNB improved (Supplementary Figure 3A and B) during 1975-1985, 1986-1995, 1996-2005, and 2005-2016. The Kaplan-Meier OS and CSS analyses showed that invasive IPNB had better survival than invasive IPMN and traditional cholangiocarcinoma (Figure 3A and B; log-rank P < 0.001). The median OS and CSS of individuals with invasive IPNB in this cohort was 17 mo (95%CI: 15-18 mo) and 27 mo (95%CI: 24-29 mo), respectively, and the 1-, 3-, and 5-year OS and CSS rates were 58.2% and 68.6%, 31.5% and 43.5%, and 23.2% and 36.4%, respectively. However, the median OS and CSS of individuals with invasive IPMN was the worst at only 6 mo (95%CI: 5.8-6.2 mo) and 9 mo (95%CI: 8.6-9.4 mo), respectively, and the 1-, 3-, and 5-year OS and CSS rates were 30.3% and 39.5%, 11.5% and 18.8%, and 8.4% and 15%, respectively. The median OS and CSS of individuals with traditional cholangiocarcinoma was 10 mo (95%CI: 9.7-10.2 mo) and 15 mo (95%CI: 14.6-15 mo), respectively; and 1-, 3- and 5-year OS rates were 43.9% and 54.1%, 18.3% and 28.6%, and 12.4% and 22.2%, respectively.
The OS and CSS for invasive IPNB differed for different tumor locations and subtypes. Kaplan-Meier and long-rank analyses showed that the non-mucin subtype had better OS and CSS than for the mucin subtype, and invasive IPNB located in the distal tract and at the ampulla had the best prognosis (Figure 3C-F; log-rank P < 0.001). Interestingly, both the OS and CSS of the mucin and non-mucin subtypes of invasive IPMN were also statistically significant (Supplementary Figure 3C and D; log-rank P < 0.001). The median OS and CSS of individuals with the non-mucin subtype of invasive IPNB were 27 mo (95%CI: 24-30 mo) and 42 mo (95%CI: 32-52 mo), respectively, and the 1-, 3-, and 5-year OS and CSS rates were 69.4% and 77.8%, 41.7% and 53.1%, and 31.4% and 43.9%, respectively. The median OS and CSS of individuals with the mucin subtype of invasive IPNB were 12 mo (95%CI: 10-13 mo) and 19 mo (95%CI: 16-21 mo), respectively, and the 1-, 3-, and 5-year OS and CSS rates were 49.2% and 61.2%, 23.7% and 36.9%, and 17.1% and 31.2%, respectively. The median OS and CSS of individuals with invasive IPNB in the distal tract and the pancreaticobiliary ampulla were 29 mo (95%CI: 26-33 mo) and 54 mo (95%CI: 42-66 mo), and the 1-, 3-, and 5-year OS and CSS rates were 69.1% and 78.9%, 43.9% and 56%, and 33.7% and 48.3%, respectively. In intrahepatic locations, namely the liver and intrahepatic biliary tract, the median OS and CSS were 5 mo (95%CI: 4-6 mo) and 7 mo (95%CI: 4-10 mo), and the 1-, 3-, and 5-year OS and CSS rates were 27.1% and 37.4%, 12.5% and 23.0%, and 9.2% and 18.8%, respectively. In the perihilar location, the median OS and CSS were 14 mo (95%CI: 12-16 mo) and 20 mo (95%CI: 17-23 mo), and the 1-, 3-, and 5-year OS and CSS rates were 52.9% and 63.8%, 21.2% and 33.6%, and 33.7% and 48.30%, respectively.
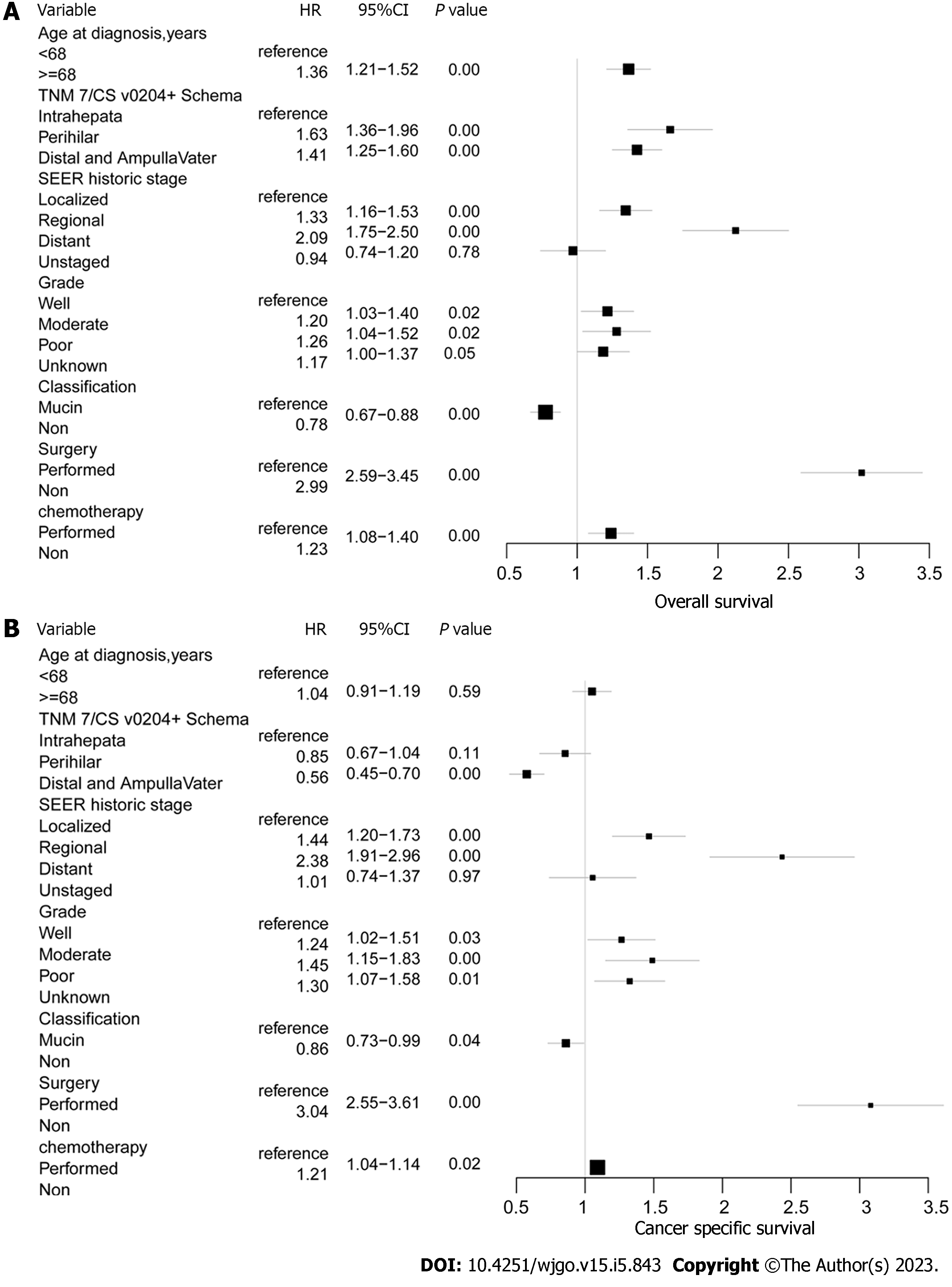
In addition to tumor location and subtype, univariable Kaplan-Meier analysis and the log rank test for OS and CSS in individuals with invasive IPNBs also depended on age, tumor grade, SEER historic stage, and treatment. Kaplan–Meier and log-rank analysis of variance indicated that age ≥ 68 years, tumor grade (moderately-differentiated, poorly-differentiated, and undifferentiated or unknown grade), SEER historic stage (regional, distant, and unknown stage), and not undergoing surgery and chemotherapy were associated with higher mortality (P < 0.05) (Table 2).
| Variable | Univariate (P value) | |
| Overall survival | Cancer specific survival | |
| Age at diagnosis, yr | 0.000 | 0.030 |
| < 68 | ||
| ≥ 68 | ||
| Sex | 0.785 | 0.355 |
| Male | ||
| Female | ||
| Race | 0.062 | 0.689 |
| White | ||
| Black | ||
| Others | ||
| TNM7/CSv0204+ schema | 0.000 | 0.000 |
| Intrahepata and liver | ||
| Perihilar | ||
| Distal and pancreaticobiliary ampulla | ||
| SEER historic stage | 0.000 | 0.000 |
| Localized | ||
| Regional | ||
| Distant | ||
| Unstaged | ||
| Grade | 0.000 | 0.000 |
| Well | ||
| Moderate | ||
| Poor and undifferentiated | ||
| Unknown | ||
| Classification | 0.000 | 0.000 |
| Mucin | ||
| Non | ||
| Surgery | 0.000 | 0.000 |
| Defined radical surgery | ||
| Undefined radical surgery | ||
| Palliative surgery | ||
| Non | ||
| Radiatheray | 0.909 | 0.222 |
| Performed | ||
| Non | ||
| Chemotherapy | 0.075 | 0.040 |
| Performed | ||
| Non | ||
The median OS and CSS for the total cohort with invasive IPNB in the surgical group was 34 mo (95%CI: 30-37 mo) and 64 mo (95%CI: 48-80 mo), respectively, and the 1-, 3-, and 5-year survival rates were 77.0% and 84.3%, 48.2% and 59.6%, and 36.9% and 50.8%, respectively. In both the surgical group and non-surgical group, individuals with the non-mucin subtype of invasive IPNB had better OS and CSS compared with the mucin subtype (P = 0.000) (Supplementary Figure 4A-D). The median OS and CSS of patients with the mucin subtype of invasive IPNB was 27 mo (95%CI: 23-31 mo) and 52 mo (95%CI: 36-68 mo), respectively, compared with 43 mo (95%CI: 35-51 mo) and 72 mo (95%CI: 45-97 mo), respectively. For the mucin subtype of invasive IPNB, the 1-, 3-, and 5-year OS and CSS rates were 74.0% and 83.1%, 41.6% and 54.3%, and 31.3% and 47.6%, respectively. For the non-mucin subtype of invasive IPNB, the 1-, 3-, and 5-year OS and CSS rates were 79.9% and 85.5%, 54.3% and 63.3%, and 42%and 53.8%, respectively. Meanwhile, the OS and CSS of individuals with resected invasive IPNB in the distal tract and the pancreaticobiliary ampulla had the best prognosis compared with individuals with tumors in other sites. The median OS and CSS of patients with invasive IPNB in the distal tract and the pancreaticobiliary ampulla was 44 mo (95%CI: 36-52 mo) and 72 mo (95%CI: 55-88 mo), respectively, and the 1-, 3-, and 5-year survival rates were 80.9% and 86.9%, 55.2% and 64.7%, and 43.3% and 56.7%, respectively. In particular, the 3-, 5-, and 10-year OS and CSS rates of patients with radical resected invasive IPNB in the distal tract and the pancreaticobiliary ampulla were 93.9% and 95.4%, 89.2% and 91.6%, and 73.4% and 81.5%, respectively (Figures 3G, H and 4).
According to the multivariable Cox regression analysis of OS and CSS adjusted for the results of the univariable analysis (P < 0.05), both OS and CSS were statistically significantly different when comparing surgery and chemotherapy, as follows: surgery not performed vs performed [hazard ratio (HR) = 2.99; 95%CI: 2.59-3.45; P < 0.001) and (HR = 3.04, 95%CI: 2.55-3.61; P < 0.001), respectively; chemotherapy not performed vs performed (HR = 1.23, 95%CI: 1.09-1.40; P < 0.001) and (HR = 1.21, 95%CI: 1.04-1.14; P < 0.02), respectively. Tumor subtype was another important factor related to OS and CSS. For the non-mucin subtype of invasive IPNB vs the mucin subtype, OS HR = 0.78; 95%CI: 0.67-0.88; P < 0.001 and CSS HR = 0.86; 95%CI: 0.73-0.99; P < 0.04. Individuals aged ≥ 68 years (HR = 1.36, 95%CI: 1.21-1.52; P < 0.001) had unfavorable OS compared with individuals aged < 68 years. Tumors in the perihilar location (OS: HR = 1.63; 95%CI: 1.36-1.96; P < 0.001) and tumors located in the distal tract and at the pancreaticobiliary ampulla (OS HR = 1.41; 95%CI: 1.25-1.60; P < 0.001) had better OS vs tumor in the intrahepatic location. Likewise, a relatively favorable OS was observed in individuals with well-differentiated grade and localized stage lymph node metastasis compared with moderately- or poorly-differentiated grades and regional and distant lymph node metastasis (P < 0.01). The related OS values were as follows: SEER historic regional stage HR = 1.33, 95%CI: 1.16-1.53, P < 0.001 and distant stage OS HR = 2.09, 95%CI: 1.75-2.50, P < 0.001 vs the localized stage; moderately-differentiated tumors (grade II) HR = 1.20, 95%CI: 1.03-1.40, P < 0.02 and poorly-differentiated tumors (grade III and IV) HR = 1.26, 95%CI: 1.03-1.52, P < 0.02) vs well-differentiated (grade I) (Figure 5).
To our knowledge, our study is the first to use SEER data to identify the rare incidence of invasive IPNB occurring throughout the biliary tract. We evaluated the incidence and IB mortality associated with invasive IPMN of the pancreas, which may represent a carcinogenic pathway different from the traditional carcinogenic pathway of cholangiocarcinoma caused by flat atypical hyperplasia[18]. The incidence and IB mortality of invasive IPNB and invasive IPMN showed steady decreases in the United States population over the study period, but an increasing trend for traditional cholangiocarcinoma in the biliary tract. The continued decline in the APC of invasive IPNB and IPMN over the study period may be associated with the fact that preventive measures have improved greatly according to the etiology, treatment, and management in recent years. Conversely, treatment for traditional cholangiocarcinoma may still have severe challenges. Hence, more resources should be devoted to traditional cholangiocarcinoma, and efforts should be made to develop improved prevention and treatment strategies.
The prognoses of individuals with invasive IPNB were better than for individuals with invasive IPMN and traditional cholangiocarcinoma. The median OS and CSS in individuals with invasive IPNBs was higher than that of individuals with invasive IPMN and traditional cholangiocarcinoma in the biliary tract. As reported previously, the prognosis of invasive IPNB is much better than that of traditional cholangiocarcinoma in the biliary tract[19]. It is not clear whether this is because of inherent biological characteristics of these tumors or the growth pattern of IPNB, which grows mainly in the intra-bile ducts, and which may contribute to the early diagnosis of biliary obstruction before it invades the surrounding tissue. However, previous studies consistently indicated that invasive IPNB had a higher degree of malignancy and a worse prognosis compared with invasive IPMN[20]. We found that ampullary invasive IPNB accounted for 47.9% of the individuals in our study cohort, and the prognosis for ampullary tumors was much better than that of individuals with tumors in other pancreaticobiliary ducts.
Our analysis also identified several important clinicopathological features and prognosis findings related to invasive IPNB. Outcomes after surgery for IPNB were generally not well-reported and were hampered by the fact that verifying outcome measurements were used. Gordon-Weeks et al[21] reported a 5-year OS rate of 65%, with a range of 24%-84% across seven studies. Our study indicated that HRs for both OS and CSS for invasive IPNB in individuals who did not undergo surgery were three times higher than for individuals who underwent surgery, and the 5-year OS and CSS rates for individuals with invasive IPNB treated with surgery were 36.9% and 50.8%, respectively. These results appear to be more persuasive because, in our cohort, we ruled out benign IPNB and carcinoma in situ, and evaluated only invasive IPNB (ICD-O, 3rd/3). Furthermore, for the first time, to our knowledge, we provided clear evidence that adjuvant chemotherapy can improve OS and CSS rates in individuals with invasive IPNB. In individuals with resected invasive IPMNs, the counterpart to invasive IPNB, adjuvant chemotherapy and radiotherapy were associated with significantly improved OS in the presence of nodal metastases[22-24]. Conversely, radiotherapy had no statistically significant effect on OS and CSS in individuals with invasive IPNB.
In addition to therapeutic factors, our study also indicated that age, tumor grade, SEER lymph node metastasis, tumor site, and mucin-related subtypes also affected the prognosis of individuals with invasive IPNB. In fact, IPNB is a heterogeneous disease, and invasive IPNB belongs to mainly pancreaticobiliary and intestinal type tumors, with invasive colloid carcinoma[25-27]. In our study, most of the mucin-related subtypes belonged to the colloid intestinal-type IPNB, which was always associated with KRAS, GNAS, and RNF43 mutations[9]. Our published study have shown that mucus production is also associated with GNAS mutation in highly malignancy hepatic mucoepidermoid carcinoma[28]. Individuals with the mucin subtype of invasive IPNB suffered a much worse prognosis following resection compared with individuals with the non-mucin subtype, which was inconsistent with the results of the study by Kim et al[27] mainly because the study included mild and carcinoma in situ intestinal types. Meanwhile, the prognosis of invasive IPNB located in the distal bile duct and ampulla was significantly better than that of intrahepatic and perihilar invasive IPNB. On the one hand, intrahepatic invasive IPNB constituted more than 90% mucin-related subtype, which indicated worse prognosis. On the other hand, in individuals with invasive IPNB located in the distal bile duct or ampulla, the clinical symptoms often appeared earlier. Pancreaticoduodenectomy is the main choice of surgical methods for tumors in these sites, which can maximize the chance of radical cure. In addition, the majority of the individuals in this study cohort were white (78.2%), and there is no significant statistic comparing other races for OS and CSS. Because the high-risk factors for IPNB are related to endemic clonorchiasis infections and hepatolithiasis in Asian races compared with the risk factors in Western countries[20].
This study has limitations. First, studies using the SEER database involve a retrospective design, and the registries contain data for individuals from different institutions and time periods. According to 2019 WHO proposal, intraductal papillary neoplasm of ampulla are not included in IPNB[29]. In fact, the peribiliary glands are attracted attention as a potential origin of IPNB, and predominantly occur at branching points of the biliary tree and are most numerous at the hepatopancreatic ampulla[30,31]. Additionally, the database lacks central reviews by professional pathologists. Second, the study cohort lacked detailed information regarding tumor recurrence, and palliative surgical methods and chemotherapy regimens, which have considerable OS and CSS impact. Furthermore, we used the ICD-O code for IPMN as the reference for IPNB. A group of pathologists in Japan and South Korea suggested that IPNB should be divided into two types; type 1 is the histological counterpart of IPMN, and type 2 has a more complex histological structure[32]. Despite these limitations, some interesting observations were identified. First, in our study cohort, the prognosis between mucin and non-mucin subtypes differed significantly, which also indicated that the expression of mucin is related to the subtypes of IPMN and IPNB[9]. Mucus secretion was mainly immunohistochemically positive for MUC1, which was always associated with the invasive phenotype and individuals’ prognosis, similar to previous published reports[21]. Importantly, we also detected correlations between tumor type and location. The minority of invasive IPNBs (6.1%) occurred in the liver, and the majority occurred in the perihilar region (36.6%) and the pancreaticobiliary ampulla (49.7%). This is because IPNB may originate from biliary stem/progenitor cells, which are located mainly in the peribiliary gland of the perihilum and the hepatopancreatic ampulla; however, biliary stem/progenitor cells also can originate from the canals of Hering and large intrahepatic biliary ducts[30,33-35].
In conclusion, the current population-based study revealed a gradual decrease in the incidence and IB mortality rates of invasive IPNB in the United States population during 1975-2016, which was similar to findings for invasive IPMN, but in contrast to the rates for traditional cholangiocarcinoma in the biliary tract. The majority of invasive IPNBs occurred in the perihilum and pancreaticobiliary ampulla. The prognosis of invasive IPNB was not only regarding tumor grade and SEER historic stage, but also for different sites and tumor subtypes. Surgery and chemotherapy are associated with improved invasive IPNB outcomes; individuals who do not undergo surgery have the highest risk of death.
Intraductal papillary neoplasm of the bile duct (IPNB) is a rare distinct subtype of precursor lesions of biliary carcinoma. IPNB is considered to originate from luminal biliary epithelial cells, typically displays mucin-hypersecretion or a papillary growth pattern, and results in cystic dilatation. According to the 2010 World Health Organization classification of tumors in the digestive system, IPNB is defined as the biliary counterpart of intraductal papillary mucinous neoplasm of the pancreas (IPMN) and has identical histopathologic pancreaticobiliary, gastric, intestinal, and oncocytic features. There are still several important differences between IPMN and IPNB exist, unlike invasive IPMN and traditional cholangiocarcinoma in the biliary tract, very little is known about the clinicopathological features and prognostic variables of invasive IPNB.
The epidemiology, tumor characteristics, treatment strategy, and long-term results of invasive IPNB are limited because of the relatively low case numbers.
We conducted a Surveillance, Epidemiology, and End Results (SEER) database evaluation of invasive IPNB to address these shortcomings, and to further elucidate the epidemiological and clinical trends to guide treatment decision-making and to identify further clinical and scientific research areas.
Invasive IPNB, IPMN, and traditional cholangiocarcinoma data for affected individuals from 1975 to 2016 were obtained from the SEER database. Annual percentage changes in the incidence and incidence-based (IB) mortality were calculated.
The incidence and IB mortality of invasive IPNB showed sustained decreases. Similar decreases in incidence and IB mortality were seen for invasive IPMN but not for traditional cholangiocarcinoma. Both overall survival (OS) and cancer-specific survival (CSS) for invasive IPNB were better than for invasive IPMN and traditional cholangiocarcinoma. The most common tumor sites were the pancreaticobiliary ampulla (47.9%) and perihilar tract (36.7%), but the mucin-related subtype of invasive IPNB was the main type, intrahepatically (approximately 90%). In the univariate and multivariate Cox regression analysis, age, tumor site, grade and stage, subtype, surgery, and chemotherapy were associated with OS and CSS (P < 0.05).
Current population-based study revealed a gradual decrease in the incidence and IB mortality rates of invasive IPNB in the United States population during 1975-2016. The prognosis of invasive IPNB was not only regarding tumor grade and SEER historic stage, but also for different sites and tumor subtypes. Surgery and chemotherapy are associated with improved invasive IPNB outcomes; individuals who do not undergo surgery have the highest risk of death.
The inspiration of this article is that we found a rare case of hepatic mucoidepidermoid carcinoma (HMEC) in our cancer research center, and found that the malignancy mucinous carcinoma in liver including IPNB, mucinous cystadenocarcinoma and adenosquamous carcinoma. So in our future research direction, we will analyze IPNB cases’ tissues from our center by the next generation sequencing, combined with our published article to analyze the relationship between IPNB and HMEC.
We thank Peng Huang, PhD, from the center for Evidence-Base Medicine, School of Public Health, Nanchang University for professional knowledge guidance in statistics.
| 1. | Chen TC, Nakanuma Y, Zen Y, Chen MF, Jan YY, Yeh TS, Chiu CT, Kuo TT, Kamiya J, Oda K, Hamaguchi M, Ohno Y, Hsieh LL, Nimura Y. Intraductal papillary neoplasia of the liver associated with hepatolithiasis. Hepatology. 2001;34:651-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 193] [Article Influence: 7.7] [Reference Citation Analysis (2)] |
| 2. | Zen Y, Fujii T, Itatsu K, Nakamura K, Minato H, Kasashima S, Kurumaya H, Katayanagi K, Kawashima A, Masuda S, Niwa H, Mitsui T, Asada Y, Miura S, Ohta T, Nakanuma Y. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology. 2006;44:1333-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 290] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 3. | Lee SS, Kim MH, Lee SK, Jang SJ, Song MH, Kim KP, Kim HJ, Seo DW, Song DE, Yu E, Lee SG, Min YI. Clinicopathologic review of 58 patients with biliary papillomatosis. Cancer. 2004;100:783-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 169] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 4. | Shibahara H, Tamada S, Goto M, Oda K, Nagino M, Nagasaka T, Batra SK, Hollingsworth MA, Imai K, Nimura Y, Yonezawa S. Pathologic features of mucin-producing bile duct tumors: two histopathologic categories as counterparts of pancreatic intraductal papillary-mucinous neoplasms. Am J Surg Pathol. 2004;28:327-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Nakanuma Y, Miyata T, Uchida T. Latest advances in the pathological understanding of cholangiocarcinomas. Expert Rev Gastroenterol Hepatol. 2016;10:113-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4th ed. Lyon, France: IARC, 2010. |
| 7. | Eberhard D, Tosh D, Slack JM. Origin of pancreatic endocrine cells from biliary duct epithelium. Cell Mol Life Sci. 2008;65:3467-3480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 8. | Fukumura Y, Nakanuma Y, Kakuda Y, Takase M, Yao T. Clinicopathological features of intraductal papillary neoplasms of the bile duct: a comparison with intraductal papillary mucinous neoplasm of the pancreas with reference to subtypes. Virchows Arch. 2017;471:65-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Ohtsuka M, Kimura F, Shimizu H, Yoshidome H, Kato A, Yoshitomi H, Furukawa K, Takeuchi D, Takayashiki T, Suda K, Takano S, Kondo Y, Miyazaki M. Similarities and differences between intraductal papillary tumors of the bile duct with and without macroscopically visible mucin secretion. Am J Surg Pathol. 2011;35:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Nakanuma Y, Uesaka K, Kakuda Y, Sugino T, Kubota K, Furukawa T, Fukumura Y, Isayama H, Terada T. Intraductal Papillary Neoplasm of Bile Duct: Updated Clinicopathological Characteristics and Molecular and Genetic Alterations. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 11. | Wu X, Li B, Zheng C, Chang X, Zhang T, He X, Zhao Y. Intraductal papillary neoplasm of the bile duct: a single-center retrospective study. J Int Med Res. 2018;46:4258-4268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Kim JR, Jang KT, Jang JY, Lee K, Kim JH, Kim H, Kim SW, Kwon W, Choi DW, Heo J, Han IW, Hwang S, Kim WJ, Hong SM, Kim DS, Yu YD, Kim JY, Nah YW, Park HW, Choi HJ, Han HS, Yoon YS, Park SJ, Hong EK, Seo HI, Park DY, Kang KJ, Kang YN, Yu HC, Moon WS, Lim CS, Bae JM, Jo S, Lee W, Roh YH, Jeong JS, Jeong CY, Lee JS, Song IS, Kim KH, Kim HG, Cho CH, Joo SH, Won KY, Kim HJ, Choi JH, Chu CW, Lee JH, Park IY, Lee H, Lee SE, Kim HS, Lee HK, Cho MS, Han KM. Clinicopathologic analysis of intraductal papillary neoplasm of bile duct: Korean multicenter cohort study. HPB (Oxford). 2020;22:1139-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Gaitanidis A, Alevizakos M, Tsaroucha A, Tsalikidis C, Simopoulos C, Pitiakoudis M. Conditional survival analysis for patients with intraductal papillary mucinous neoplasms (IPMNs) undergoing curative resection. Eur J Surg Oncol. 2018;44:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Worni M, Akushevich I, Gloor B, Scarborough J, Chino JP, Jacobs DO, Hahn SM, Clary BM, Pietrobon R, Shah A. Adjuvant radiotherapy in the treatment of invasive intraductal papillary mucinous neoplasm of the pancreas: an analysis of the surveillance, epidemiology, and end results registry. Ann Surg Oncol. 2012;19:1316-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Kargozaran H, Vu V, Ray P, Bagaria S, Steen S, Ye X, Gagandeep S. Invasive IPMN and MCN: same organ, different outcomes? Ann Surg Oncol. 2011;18:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Wasif N, Bentrem DJ, Farrell JJ, Ko CY, Hines OJ, Reber HA, Tomlinson JS. Invasive intraductal papillary mucinous neoplasm versus sporadic pancreatic adenocarcinoma: a stage-matched comparison of outcomes. Cancer. 2010;116:3369-3377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Khan SA, Emadossadaty S, Ladep NG, Thomas HC, Elliott P, Taylor-Robinson SD, Toledano MB. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol. 2012;56:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 18. | Rocha FG, Lee H, Katabi N, DeMatteo RP, Fong Y, D'Angelica MI, Allen PJ, Klimstra DS, Jarnagin WR. Intraductal papillary neoplasm of the bile duct: a biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology. 2012;56:1352-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 189] [Article Influence: 13.5] [Reference Citation Analysis (2)] |
| 19. | Fujikura K, Fukumoto T, Ajiki T, Otani K, Kanzawa M, Akita M, Kido M, Ku Y, Itoh T, Zen Y. Comparative clinicopathological study of biliary intraductal papillary neoplasms and papillary cholangiocarcinomas. Histopathology. 2016;69:950-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Zen Y, Jang KT, Ahn S, Kim DH, Choi DW, Choi SH, Heo JS, Yeh MM. Intraductal papillary neoplasms and mucinous cystic neoplasms of the hepatobiliary system: demographic differences between Asian and Western populations, and comparison with pancreatic counterparts. Histopathology. 2014;65:164-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Gordon-Weeks AN, Jones K, Harriss E, Smith A, Silva M. Systematic Review and Meta-analysis of Current Experience in Treating IPNB: Clinical and Pathological Correlates. Ann Surg. 2016;263:656-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Mungo B, Croce C, Oba A, Ahrendt S, Gleisner A, Friedman C, Schulick RD, Del Chiaro M. Controversial Role of Adjuvant Therapy in Node-Negative Invasive Intraductal Papillary Mucinous Neoplasm. Ann Surg Oncol. 2021;28:1533-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Wu JY, Wang YF, Ma H, Li SS, Miao HL. Nomograms predicting long-term survival in patients with invasive intraductal papillary mucinous neoplasms of the pancreas: A population-based study. World J Gastroenterol. 2020;26:535-549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Aronsson L, Marinko S, Ansari D, Andersson R. Adjuvant therapy in invasive intraductal papillary mucinous neoplasm (IPMN) of the pancreas: a systematic review. Ann Transl Med. 2019;7:689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Aoki Y, Mizuma M, Hata T, Aoki T, Omori Y, Ono Y, Mizukami Y, Unno M, Furukawa T. Intraductal papillary neoplasms of the bile duct consist of two distinct types specifically associated with clinicopathological features and molecular phenotypes. J Pathol. 2020;251:38-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 26. | Choi SC, Lee JK, Jung JH, Lee JS, Lee KH, Lee KT, Rhee JC, Jang KT, Choi SH, Heo JS, Choi DW, Lim JH. The clinicopathological features of biliary intraductal papillary neoplasms according to the location of tumors. J Gastroenterol Hepatol. 2010;25:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Kim KM, Lee JK, Shin JU, Lee KH, Lee KT, Sung JY, Jang KT, Heo JS, Choi SH, Choi DW, Lim JH. Clinicopathologic features of intraductal papillary neoplasm of the bile duct according to histologic subtype. Am J Gastroenterol. 2012;107:118-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 28. | Hou P, Su X, Cao W, Xu L, Zhang R, Huang Z, Wang J, Li L, Wu L, Liao W. Whole-exome sequencing reveals the etiology of the rare primary hepatic mucoepidermoid carcinoma. Diagn Pathol. 2021;16:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2730] [Article Influence: 455.0] [Reference Citation Analysis (3)] |
| 30. | Cardinale V, Wang Y, Carpino G, Reid LM, Gaudio E, Alvaro D. Mucin-producing cholangiocarcinoma might derive from biliary tree stem/progenitor cells located in peribiliary glands. Hepatology. 2012;55:2041-2042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Nakagawa H, Hayata Y, Yamada T, Kawamura S, Suzuki N, Koike K. Peribiliary Glands as the Cellular Origin of Biliary Tract Cancer. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Nakanuma Y, Jang KT, Fukushima N, Furukawa T, Hong SM, Kim H, Lee KB, Zen Y, Jang JY, Kubota K. A statement by the Japan-Korea expert pathologists for future clinicopathological and molecular analyses toward consensus building of intraductal papillary neoplasm of the bile duct through several opinions at the present stage. J Hepatobiliary Pancreat Sci. 2018;25:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 33. | Carpino G, Cardinale V, Onori P, Franchitto A, Berloco PB, Rossi M, Wang Y, Semeraro R, Anceschi M, Brunelli R, Alvaro D, Reid LM, Gaudio E. Biliary tree stem/progenitor cells in glands of extrahepatic and intraheptic bile ducts: an anatomical in situ study yielding evidence of maturational lineages. J Anat. 2012;220:186-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 34. | Nakanuma Y, Kurumaya H, Ohta G. Multiple cysts in the hepatic hilum and their pathogenesis. A suggestion of periductal gland origin. Virchows Arch A Pathol Anat Histopathol. 1984;404:341-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 72] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Turner R, Lozoya O, Wang Y, Cardinale V, Gaudio E, Alpini G, Mendel G, Wauthier E, Barbier C, Alvaro D, Reid LM. Human hepatic stem cell and maturational liver lineage biology. Hepatology. 2011;53:1035-1045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kitamura K, Japan; Šarenac TM, Serbia; Tuysuz U, Turkey; Zamani M, Iran S-Editor: Zhang H L-Editor: A P-Editor: Zhang H