Published online May 15, 2023. doi: 10.4251/wjgo.v15.i5.828
Peer-review started: January 25, 2023
First decision: February 28, 2023
Revised: March 3, 2023
Accepted: April 19, 2023
Article in press: April 19, 2023
Published online: May 15, 2023
Processing time: 106 Days and 20.8 Hours
Liver cancer is among the top five most common cancers globally. Lipid-lowering drugs such as statins can lower the risk of liver cancer, but may also cause liver damage. LipoCol Forte capsules (LFC), a red yeast rice product, have de
To evaluate whether LFC lowers the risk of liver cancer in adults in this propensity score-matched, nationwide, population-based cohort study.
We used data from Taiwan’s National Health Insurance Research Database, which includes electronic medical records for up to 99.99% of Taiwan’s population. LFC users and LFC non-users were matched 1:1 by propensity scores between January 2010 and December 2017. All had follow-up data for at least 1 year. Statistical analyses compared demographic distributions including sex, age, comorbidities, and prescribed medications. Cox regression analyses estimated adjusted hazard ratios (aHRs) after adjusting for potential confounders.
We enrolled 33231 LFC users and 33231 non-LFC users (controls). No significant differences between the study cohorts were identified regarding comorbidities and medications [standardized mean difference (SMD) < 0.05]. At follow-up, the overall incidence of liver cancer was significantly lower in the LFC cohort compared with controls [aHR 0.91; 95% confidence interval (CI): 0.86-0.95; P < 0.001]. The risk of liver cancer was significantly reduced in both females (aHR 0.87; 95%CI: 0.8-0.94; P < 0.001) and males (aHR 0.93; 95%CI: 0.87-0.98; P < 0.01) in the LFC cohort compared with their counterparts in the non-LFC cohort. The antitumor protective effects applied to patients with comorbidities (including hypertension, ischemic stroke, diabetes mellitus, hyperlipidemia, hepatitis B infection and hepatitis C infection). Those using LFC for more than 84 drug days had a 0.64-fold lower risk of liver cancer compared with controls (P < 0.001). Compared with controls, the risk of developing liver cancer in the LFC cohort progressively decreased over time; the lowest incidence of liver cancer occurred in LFC users followed-up for more than 6 years (27.44 vs 31.49 per 1,000 person-years; aHR 0.75; 95%CI: 0.68-0.82; P < 0.001).
This retrospective cohort study indicates that LFC has a significantly protective effect on lowering the risk of liver cancer, in a dose-dependent and time-dependent manner.
Core Tip: LipoCol Forte capsules (LFC), a red yeast rice product, have lipid-lowering effects and good safety reports. Lipid-lowering therapies such as statins can lower the risk of liver cancer, but may also cause liver damage. We evaluated whether LFC lowers the risk of liver cancer in adults in this propensity score-matched, nationwide, population-based cohort study. The LFC cohort had a 9% lower incidence of liver cancer compared with controls; this lower risk was dose-dependent and time-dependent, with a 0.64-fold lower risk found in those using LFC for more than 84 drug days. The lowest incidence of liver cancer occurred in LFC users followed-up for more than 6 years.
- Citation: Lai HC, Lin HJ, Shih YH, Chou JW, Lin KW, Jeng LB, Huang ST. LipoCol Forte capsules reduce the risk of liver cancer: A propensity score-matched, nationwide, population-based cohort study. World J Gastrointest Oncol 2023; 15(5): 828-842
- URL: https://www.wjgnet.com/1948-5204/full/v15/i5/828.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i5.828
LipoCol Forte capsules (LFC) are a product of red yeast rice, which is made by fermenting rice with yeasts, mainly Monascus purpureus[1]. Asian countries and territories, including China, Japan and Taiwan, have traditionally used red yeast to make rice wine, increase the intensity of food flavoring and as a food coloring. Traditional Chinese medicine uses red yeast rice as a digestive aid, to promote blood circulation and alleviate dampness. This fermented rice contains several types of monacolins, gamma-aminobutyric acid, flavonoids, pigments (e.g., rubropunctamine and monascorubramine), polyketides, and dimerumic acid[2,3]. Monacolins are known for their lipid-lowering qualities. In particular, monacolin K lowers cholesterol levels by inhibiting hydroxymethyl glutaryl coenzyme A reductase (HMG-CoA), the rate-controlling enzyme of the cholesterol synthesis pathway[1]. The renowned lipid-lowering drug, lovastatin, is mainly monacolin K. LFC has received approval from the Taiwan Food and Drug Administration for the indication of antihyperlipidemia[4]. Each 600 mg capsule of LFC contains the equivalent of 5.76 mg of lovastatin and the recommended oral dose is twice daily[4]. In a Taiwanese study involving 79 patients with hyperlipidemia, twice-daily dosing with Monascus purpureus Went rice therapy (600 mg) LFC significantly reduced levels of low-density lipoprotein (LDL) cholesterol, total cholesterol, triglycerides and apolipoprotein B levels after 4 and 8 weeks compared with placebo therapy, without any major side effects[5]. In another study involving 1530 elderly patients with hypertension and a history of myocardial infarction enrolled in the Chinese Coronary Secondary Prevention Study, a partial extract of red yeast rice reduced the incidence of cardiovascular events and all-cause mortality by lowering LDL and total cholesterol[6].
The Global Burden of Diseases, Injuries, and Risk Factors Study 2019 reported that liver cancer was among the leading five cancers globally by disability-adjusted life years[7]. Risk factors for liver cancer include viral hepatitis (e.g., hepatitis B and hepatitis C), parasitic infestation, alcohol, toxins (e.g., aflatoxin, pesticides) and insulin resistance[8]. In East Asia, hepatitis B and C infections are major contributors to the development of liver cancer[8]. Nonalcoholic fatty liver disease (NAFLD) has been reported by several studies to be an important risk factor for liver cancer[9]. Metabolic dysfunction related to oxidative stress and lipotoxicity promote the development of chronic liver inflammation and fibrosis, and consequently increase the risk of NAFLD-related hepatocellular carcinoma (HCC)[9]. Recently, lipid-lowering therapies such as statins have been linked to a lower risk for HCC[10]. However, these drugs are associated with unwanted side effects such as elevated liver enzymes, myalgia and diabetogenic effects[11]. The risk of adverse drug reactions can increase when statins are co-administered with cytochrome P450 3A4 inhibitors, so some patients discontinue statins in order to decrease the risk of myopathy and other drug-related toxicities[11].
Similar lipid-lowering effects have been reported with red yeast rice products, with a safety advantage[12]. Up until now, no research has reported the preventive effects of red yeast rice on the risk for liver cancer. We are the first to propose that LFC, a red yeast rice extract, decreases the incidence of liver cancer via lipid-lowering benefits. In view of the time-consuming nature of cancer development, we decided to conduct a population-based retrospective cohort study using data from the Taiwan National Health Insurance Research Database (NHIRD) for this investigation into the association between LFC use and liver cancer occurrence.
The data analyzed in this study were extracted from Taiwan’s NHIRD, which was established in 1995 and now includes up to 99.99% of Taiwan’s population with their electronic medical records. The database includes demographic data, comprehensive inpatient and outpatient health care information, diagnostic codes, and prescription details for each beneficiary. Prior to 2016, diagnoses in the NHIRD used the International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM); since 2016, the Tenth Edition (ICD-10) has been used. This study was approved by the Central Regional Research Ethics Committee of China Medical University, Taichung, Taiwan [CMUH109-REC2-031(CR-2)]. The encrypted nature of all individual information contained in the NHIRD meant that informed patient consent could be waived.
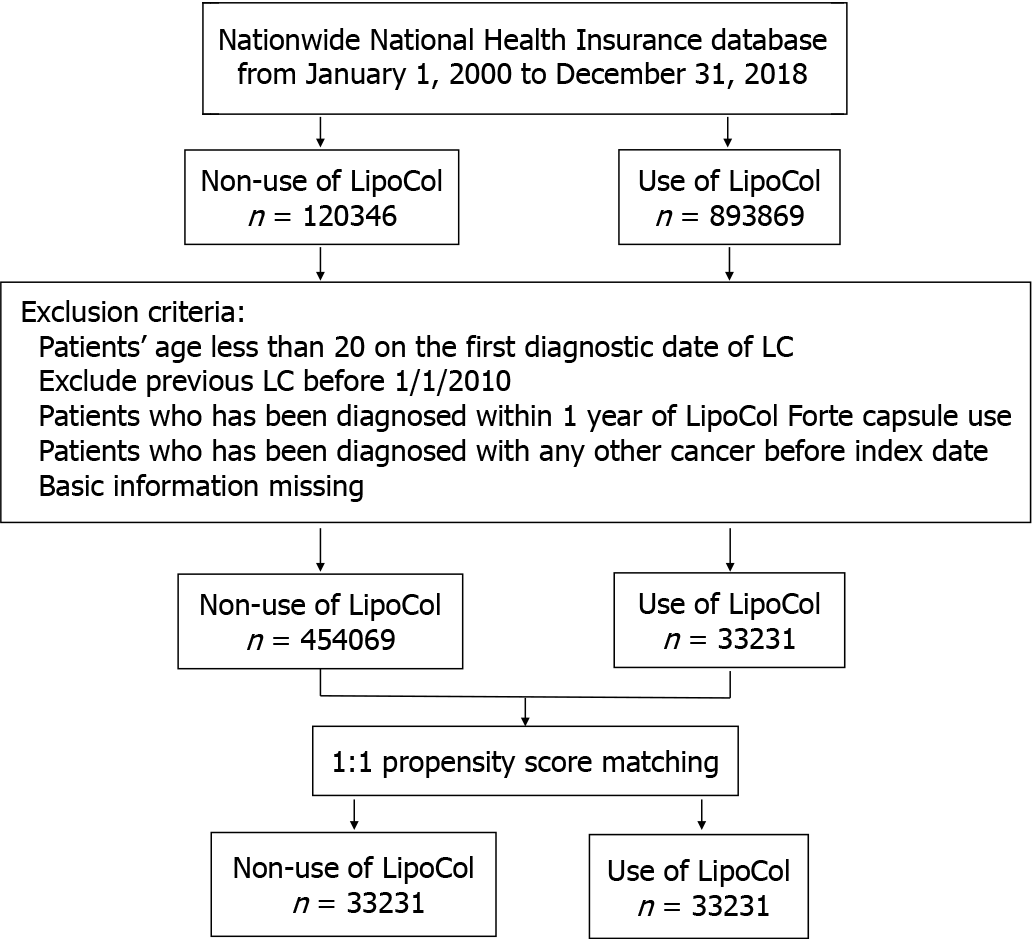
The case cohort consisted of the LFC users (ATC: A047152) during the period from January 2010 through December 2017. For the case cohort, the index date was defined as the first date with a prescription of LFC, whereas for the LFC non-users the index date was a random date within the study period. Patients aged less than 20 years, who had been diagnosed with liver cancer or any other cancer before the index date, or diagnosed with liver cancer within 1 year of LFC use and withdrew from the insurance program before the index date were excluded. Each patient in the case cohort was frequency-matched with the controls (randomly selected from all NHI beneficiaries aged 20 years and more) at a 1:1 ratio by sex, age (every 5 years span), baseline comorbidities, medicine and the index year (Figure 1).
The main outcome of this cohort study was liver cancer (ICD-9-CM codes 155.0, 155.1, 155.2; ICD-10-CM codes C220, C221, C228, C229). The end date of this study was the date when the patients were diagnosed with liver cancer, were lost to follow-up due to withdrawal from the NHIRD or death, or until December 31, 2017. All disease codes including main outcomes and baseline comorbidities were defined as at least 2 clinic visits or 1 inpatient admission. Comorbidities included hypertension (ICD-9-CM codes 401-405; ICD-10-CM codes I10, I11.0, I11.9, I12.0, I12.9, I13.0, I13.10, I13.11, I15.0, I15.1, I15.8, I15.9), coronary heart disease (ICD-9-CM codes 410-414; ICD-10-CM codes I20.0, I20.1, I20.8, I20.9, I21. I22, I24.1, I24.8, I24.9, I25.1, I25.2), ischemic stroke (ICD-9-CM codes 433, 434, 436, 437; ICD-10-CM codes I63, I65, I66, I67, I68, G46.3-G46.8), hemorrhagic stroke (ICD-9-CM codes 430, 431, 432; ICD-10-CM codes I60-I62), diabetes mellitus (ICD-9-CM code 250; ICD-10-CM codes E08-E13), hyperlipidemia (ICD-9-CM code 272; ICD-10-CM code E78), renal insufficiency (ICD-9-CM codes 585, 586, 588.8, 588.9; ICD-10-CM codes N18, N19, N25.8, N25.9), cirrhosis (ICD-9-CM codes 571.2, 571.5, 571.6; ICD-10-CM codes K70.2, K70.30, K70.31, K74.0, K74.1, K74.2, K74.3, K74.4, K74.5, K74.60, K74.69), alcoholic liver damage (ICD-9-CM codes 571.0, 571.1, 571.3; ICD-10-CM codes K70.0, K70.10, K70.11, K70.40, K70.41, K70.0), NAFLD (ICD-9-CM code 571.8; ICD-10-CM codes K74.4, K75.81, K76.0, K76.89), hepatitis B virus (HBV) infection (ICD-9-CM codes V02.61, 070.20, 070.22, 070.30, 070.32; ICD-10-CM codes Z22.51, B16.2, B16.9, B18.1, B19.10, B19.11) and hepatitis C virus (HCV) infection (ICD-9-CM codes V02.62, 070.41, 070.44, 070.51, 070.54; ICD-10-CM codes Z22.52, B17.10, B17.11, B18.2, B19.20, B19.21) were matched. We also compared medication use between the study groups for statins (simvastatin, lovastatin, fluvastatin, atorvastatin, pravastatin, and rosuvastatin), non-statin lipid-lowering drugs (cholestyramine, colestipol, colesevelam, nicolar, lipo-nicin, acipimox, probucol, gemfibrozil, bezafibrate, etofibrate, fenofibrate, and ezetimibe), aspirin, HBV treatments (lamivudine, adefovir, entecavir, telbivudine, tenofovir and peg-interferon α-2a) and HCV treatments (Harvoni, Sovaldi, Zepatier, Maviret, Epclusa, Viekirax plus Exviera, Daklinza, Daklinza plus Sunvepra and Interferon plus Ribavirin), metformin and thiazolidinedione (TZD) (Pioglitazone and Rosiglitazone).
We used the Chi-square test to compare baseline demographic characteristics, comorbidities and medication status between the LFC and non-LFC cohorts. Categorical variables are listed as counts and percentages; the differences in continuous variables are presented as the means and standard deviations, and were evaluated using the unpaired Student’s t-test. The standardized mean difference (SMD) was calculated to assess the difference of each variable between the LFC users and non-LFC users. An SMD value of less than 0.05 indicated a negligible difference between the two cohorts. In this study, we calculated the hazard ratios (HRs) and 95% confidence intervals (CIs) in univariate and multivariate Cox proportional hazard regression models. Multivariate analysis adjusted for the variables of age, sex, comorbidities and medications. The Kaplan–Meier method was used to estimate the cumulative incidence of liver cancer; the cumulative incidence curve was plotted by R software. SAS statistical software version 9.4 (SAS Institute Inc., Cary, NC, United States) was used for all statistical analyses. Statistical significance was set as a P value of less than 0.05.
Baseline demographics and comorbidities of the study population are shown in Table 1. We enrolled 33231 patients in the LFC cohort and 33231 controls in the non-LFC cohort. Similar proportions in the LFC cohort and non-LFC cohort were male (52.46% and 52.25%, respectively); corresponding mean ages were 62.75 ± 13.62 years and 63.22 ± 13.60 years, respectively. The study subjects were predominantly aged 50 years and over. No significant differences between the study cohorts were observed for the distributions of comorbidities and medications (SMD < 0.05).
| Variables | Non-LFC users (n = 33231) | LFC users (n = 33231) | SMD | ||
| n | % | n | % | ||
| Sex | |||||
| Female | 15869 | 47.75 | 15798 | 47.54 | 0.004 |
| Male | 17362 | 52.25 | 17433 | 52.46 | 0.004 |
| Age (yr) | |||||
| 20-29 | 300 | 0.90 | 319 | 0.96 | 0.006 |
| 30-39 | 1434 | 4.32 | 1545 | 4.65 | 0.016 |
| 40-49 | 3677 | 11.07 | 3816 | 11.48 | 0.013 |
| > 50 | 27820 | 83.72 | 27551 | 82.91 | 0.022 |
| mean (SD) | 63.22 | 13.60 | 62.75 | 13.62 | 0.035 |
| Comorbidities | |||||
| Hypertension | 21195 | 63.78 | 20825 | 62.67 | 0.023 |
| Coronary heart disease | 11255 | 33.87 | 10972 | 33.02 | 0.018 |
| Ischemic stroke | 6902 | 20.77 | 6784 | 20.41 | 0.009 |
| Hemorrhagic stroke | 816 | 2.46 | 939 | 2.83 | 0.023 |
| Diabetes mellitus | 12348 | 37.16 | 12172 | 36.63 | 0.011 |
| Hyperlipidemia | 16793 | 50.53 | 16196 | 48.74 | 0.036 |
| Renal insufficiency | 4028 | 12.12 | 4007 | 12.06 | 0.002 |
| Cirrhosis | 2585 | 7.78 | 2643 | 7.95 | 0.007 |
| Alcoholic liver damage | 2554 | 7.69 | 2606 | 7.84 | 0.006 |
| Nonalcoholic fatty liver disease | 1648 | 4.96 | 1825 | 5.49 | 0.024 |
| HBV infection | 3239 | 9.75 | 3342 | 10.06 | 0.010 |
| HCV infection | 2061 | 6.20 | 2196 | 6.61 | 0.017 |
| Medications | |||||
| Statin | 12008 | 36.13 | 11666 | 35.11 | 0.022 |
| Non-statin lipid-lowering drug | 6339 | 19.08 | 6161 | 18.54 | 0.014 |
| Aspirin | 15564 | 46.84 | 15330 | 46.13 | 0.014 |
| HBV treatment | 1531 | 4.61 | 1572 | 4.73 | 0.006 |
| HCV treatment | 7 | 0.02 | 10 | 0.03 | 0.006 |
| Metformin | 8674 | 26.10 | 8469 | 25.49 | 0.014 |
| Thiazolidinediones | 2533 | 7.62 | 2483 | 7.47 | 0.006 |
Analyses stratified for demographic characteristics, comorbidities and medications in the patients with liver cancer are shown in Table 2. In analyses adjusting for age, sex, comorbidities and medications, the overall incidence of liver cancer was significantly lower in the LFC cohort than in the non-LFC cohort (19.26 vs 20.62 per 1000 person-years; aHR 0.91; 95%CI: 0.86-0.95; P < 0.001). The risk of liver cancer was significantly reduced in both females (aHR 0.87; 95%CI: 0.8-0.94; P < 0.001) and males (aHR 0.93; 95%CI: 0.87-0.98; P < 0.01) in the LFC cohort compared with their counterparts in the non-LFC cohort. In the subgroup aged over 50 years, LFC users had a significantly lower risk of liver cancer compared with LFC non-users (aHR 0.91; 95%CI: 0.87-0.95; P < 0.001). In comorbidity-specific analysis, LFC users with hypertension (aHR 0.89; 95%CI: 0.84-0.94; P < 0.001), ischemic stroke (aHR 0.9; 95%CI: 0.81-0.99; P < 0.05), diabetes mellitus (aHR 0.92; 95%CI: 0.86-0.98; P = 0.01), hyperlipidemia (aHR 0.93; 95%CI: 0.87-1; P < 0.05), HBV infection (aHR 0.91; 95%CI: 0.84-0.99; P < 0.05), or HCV infection (aHR 0.9; 95%CI: 0.82-0.98; P < 0.05) were significantly less likely to develop liver cancer compared with their counterparts in the non-LFC cohort. Among patients with LFC using other medications, those on aspirin (aHR 0.93; 95%CI: 0.87-1; P < 0.05) or metformin (aHR 0.92; 95%CI: 0.85-0.99; P < 0.05) had a significantly reduced risk of liver cancer compared with patients on aspirin or metformin in the non-LFC cohort.
| Non-LFC users | LFC users | Crude | Adjusted | |||||||||
| Variable | Event | Person-years | IR | Event | Person-years | IR | cHR | 95%CI | P value | aHR1 | 95%CI | P value |
| Overall | 3848 | 186604 | 20.62 | 3700 | 192122 | 19.26 | 0.89 | (0.85, 0.94)c | < 0.001 | 0.91 | (0.86, 0.95)c | < 0.001 |
| Sex | ||||||||||||
| Female | 1416 | 91487 | 15.48 | 1267 | 94190 | 13.45 | 0.83 | (0.77, 0.9)c | < 0.001 | 0.87 | (0.8, 0.94)c | < 0.001 |
| Male | 2432 | 95117 | 25.57 | 2433 | 97932 | 24.84 | 0.93 | (0.88, 0.99)a | 0.014 | 0.93 | (0.87, 0.98)b | 0.008 |
| Age (yr) | ||||||||||||
| 20-29 | 11 | 1884 | 5.84 | 9 | 2054 | 4.38 | 0.67 | (0.27, 1.68) | 0.396 | 0.61 | (0.24, 1.59) | 0.313 |
| 30-39 | 68 | 8881 | 7.66 | 77 | 9854 | 7.81 | 0.96 | (0.69, 1.34) | 0.827 | 0.79 | (0.56, 1.11) | 0.178 |
| 40-49 | 285 | 22392 | 12.73 | 290 | 23617 | 12.28 | 0.95 | (0.8, 1.12) | 0.508 | 0.91 | (0.77, 1.07) | 0.249 |
| > 50 | 3484 | 153447 | 22.71 | 3324 | 156597 | 21.23 | 0.89 | (0.85, 0.94)c | < 0.001 | 0.91 | (0.87, 0.95)c | < 0.001 |
| Comorbidities | ||||||||||||
| Hypertension | ||||||||||||
| No | 1169 | 69398 | 16.85 | 1281 | 73107 | 17.52 | 1 | (0.92, 1.08) | 0.910 | 0.93 | (0.86, 1.01) | 0.090 |
| Yes | 2679 | 117206 | 22.86 | 2419 | 119015 | 20.33 | 0.85 | (0.81, 0.9)c | < 0.001 | 0.89 | (0.84, 0.94)c | < 0.001 |
| Coronary heart disease | ||||||||||||
| No | 2455 | 124923 | 19.65 | 2413 | 130037 | 18.56 | 0.9 | (0.85, 0.95)c | < 0.001 | 0.88 | (0.84, 0.94)c | < 0.001 |
| Yes | 1393 | 61681 | 22.58 | 1287 | 62085 | 20.73 | 0.89 | (0.83, 0.96)b | 0.004 | 0.94 | (0.87, 1.02) | 0.143 |
| Ischemic stroke | ||||||||||||
| No | 3002 | 149577 | 20.07 | 2983 | 154633 | 19.29 | 0.92 | (0.87, 0.97)b | 0.001 | 0.91 | (0.86, 0.96)c | < 0.001 |
| Yes | 846 | 37027 | 22.85 | 717 | 37489 | 19.13 | 0.8 | (0.73, 0.89)c | < 0.001 | 0.9 | (0.81, 0.99)a | 0.033 |
| Hemorrhagic stroke | ||||||||||||
| No | 3738 | 182382 | 20.50 | 3586 | 187046 | 19.17 | 0.89 | (0.85, 0.94)c | < 0.001 | 0.9 | (0.86, 0.95)c | < 0.001 |
| Yes | 110 | 4222 | 26.05 | 114 | 5076 | 22.46 | 0.86 | (0.66, 1.12) | 0.265 | 1 | (0.76, 1.32) | 0.992 |
| Diabetes mellitus | ||||||||||||
| No | 2023 | 119363 | 16.95 | 1972 | 124172 | 15.88 | 0.9 | (0.84, 0.96)c | < 0.001 | 0.89 | (0.84, 0.95)c | < 0.001 |
| Yes | 1825 | 67241 | 27.14 | 1728 | 67950 | 25.43 | 0.9 | (0.84, 0.96)b | 0.001 | 0.92 | (0.86, 0.98)a | 0.010 |
| Hyperlipidemia | ||||||||||||
| No | 1986 | 92314 | 21.51 | 1980 | 98628 | 20.08 | 0.9 | (0.84, 0.96)c | < 0.001 | 0.89 | (0.83, 0.94)c | < 0.001 |
| Yes | 1862 | 94290 | 19.75 | 1720 | 93494 | 18.40 | 0.89 | (0.83, 0.95)c | < 0.001 | 0.93 | (0.87, 1)a | 0.040 |
| Renal insufficiency | ||||||||||||
| No | 3319 | 165852 | 20.01 | 3221 | 170964 | 18.84 | 0.9 | (0.86, 0.95)c | < 0.001 | 0.9 | (0.85, 0.94)c | < 0.001 |
| Yes | 529 | 20752 | 25.49 | 479 | 21158 | 22.64 | 0.86 | (0.76, 0.97)a | 0.016 | 0.96 | (0.85, 1.1) | 0.580 |
| Cirrhosis | ||||||||||||
| No | 2514 | 174893 | 14.38 | 2314 | 180074 | 12.85 | 0.84 | (0.8, 0.89)c | < 0.001 | 0.83 | (0.79, 0.88)c | < 0.001 |
| Yes | 1334 | 11711 | 113.91 | 1386 | 12048 | 115.04 | 0.98 | (0.91, 1.06) | 0.606 | 1 | (0.93, 1.08) | 0.921 |
| Alcoholic liver damage | ||||||||||||
| No | 3297 | 173575 | 19.00 | 3164 | 178842 | 17.69 | 0.89 | (0.84, 0.93)c | < 0.001 | 0.88 | (0.84, 0.93)c | < 0.001 |
| Yes | 551 | 13029 | 42.29 | 536 | 13281 | 40.36 | 0.95 | (0.85, 1.07) | 0.432 | 1 | (0.88, 1.13) | 0.970 |
| Nonalcoholic fatty liver disease | ||||||||||||
| No | 3553 | 177753 | 19.99 | 3404 | 182155 | 18.69 | 0.89 | (0.85, 0.94)c | < 0.001 | 0.9 | (0.86, 0.94)c | < 0.001 |
| Yes | 295 | 8851 | 33.33 | 296 | 9967 | 29.70 | 0.86 | (0.73, 1.01) | 0.06 | 0.92 | (0.78, 1.09) | 0.322 |
| HBV infection | ||||||||||||
| No | 2604 | 170246 | 15.30 | 2501 | 175001 | 14.29 | 0.89 | (0.84, 0.94)c | < 0.001 | 0.88 | (0.83, 0.93)c | < 0.001 |
| Yes | 1244 | 16359 | 76.05 | 1199 | 17121 | 70.03 | 0.89 | (0.82, 0.96)b | 0.003 | 0.91 | (0.84, 0.99)a | 0.025 |
| HCV infection | ||||||||||||
| No | 2784 | 176772 | 15.75 | 2658 | 181323 | 14.66 | 0.89 | (0.84, 0.94)c | < 0.001 | 0.88 | (0.83, 0.93)c | < 0.001 |
| Yes | 1064 | 9832 | 108.22 | 1042 | 10799 | 96.49 | 0.85 | (0.78, 0.92)c | < 0.001 | 0.9 | (0.82, 0.98)a | 0.016 |
| Medication | ||||||||||||
| Statins | ||||||||||||
| No | 2693 | 120440 | 22.36 | 2681 | 125954 | 21.29 | 0.91 | (0.87, 0.97)b | 0.001 | 0.91 | (0.86, 0.96)c | < 0.001 |
| Yes | 1155 | 66164 | 17.46 | 1019 | 66168 | 15.40 | 0.84 | (0.77, 0.92)c | < 0.001 | 0.92 | (0.84, 1) | 0.058 |
| Non-statin lipid-lowering drugs | ||||||||||||
| No | 3203 | 151343 | 21.16 | 3080 | 157120 | 19.60 | 0.89 | (0.85, 0.93)c | < 0.001 | 0.9 | (0.86, 0.95)c | < 0.001 |
| Yes | 645 | 35261 | 18.29 | 620 | 35003 | 17.71 | 0.92 | (0.82, 1.03) | 0.135 | 0.94 | (0.84, 1.05) | 0.295 |
| Aspirin | ||||||||||||
| No | 1947 | 101738 | 19.14 | 1935 | 105708 | 18.31 | 0.91 | (0.86, 0.97)b | 0.004 | 0.88 | (0.83, 0.94)c | < 0.001 |
| Yes | 1901 | 84866 | 22.40 | 1765 | 86414 | 20.43 | 0.88 | (0.82, 0.94)c | < 0.001 | 0.93 | (0.87, 1)a | 0.043 |
| HBV treatment | ||||||||||||
| No | 2971 | 179610 | 16.54 | 2788 | 184920 | 15.08 | 0.87 | (0.82, 0.91)c | < 0.001 | 0.83 | (0.79, 0.88)c | < 0.001 |
| Yes | 877 | 6994 | 125.40 | 912 | 7203 | 126.62 | 0.97 | (0.89, 1.07) | 0.592 | 0.99 | (0.9, 1.09) | 0.807 |
| HCV treatment | ||||||||||||
| No | 3843 | 186594 | 20.60 | 3697 | 192084 | 19.25 | 0.89 | (0.86, 0.94)c | < 0.001 | 0.91 | (0.87, 0.95)c | < 0.001 |
| Yes | 5 | 10 | 487.00 | 3 | 39 | 77.83 | 0.21 | (0.04, 1.07) | 0.060 | NA | NA | 1 |
| Metformin | ||||||||||||
| No | 2471 | 140106 | 17.64 | 2413 | 145474 | 16.59 | 0.9 | (0.85, 0.96)c | < 0.001 | 0.9 | (0.85, 0.95)c | < 0.001 |
| Yes | 1377 | 46498 | 29.61 | 1287 | 46648 | 27.59 | 0.89 | (0.82, 0.96)b | 0.002 | 0.92 | (0.85, 0.99)a | 0.033 |
| Thiazolidinediones | ||||||||||||
| No | 3453 | 173105 | 19.95 | 3331 | 178541 | 18.66 | 0.89 | (0.85, 0.94)c | < 0.001 | 0.91 | (0.87, 0.95)c | < 0.001 |
| Yes | 395 | 13499 | 29.26 | 369 | 13581 | 27.17 | 0.9 | (0.78, 1.04) | 0.141 | 0.89 | (0.77, 1.03) | 0.110 |
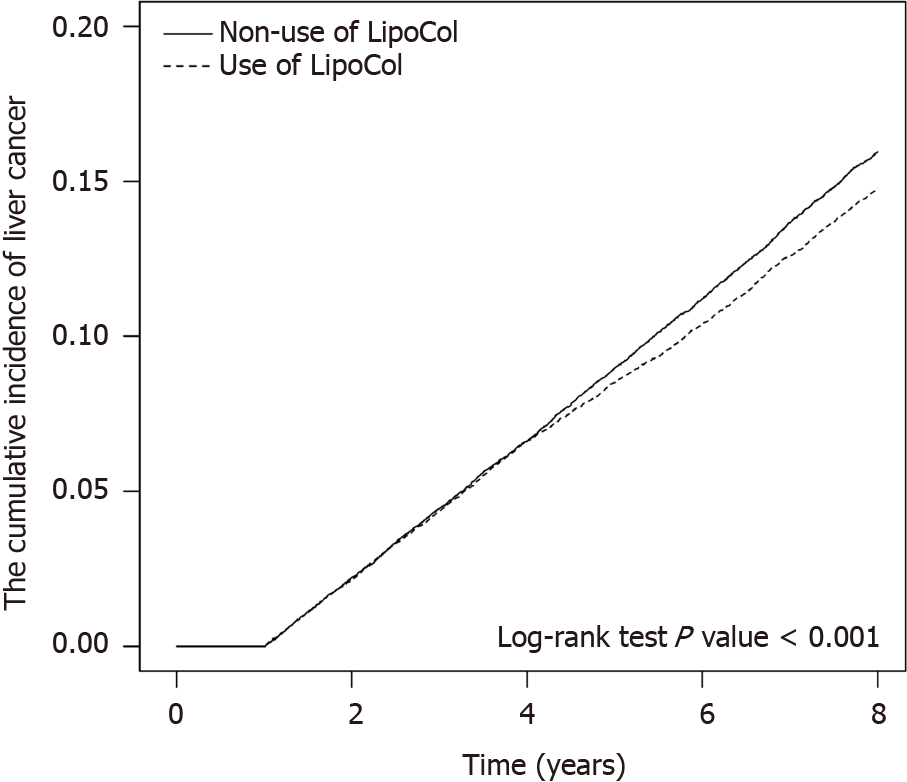
As shown in Table 3, when analyses assessed the risk of developing liver cancer stratified by days of LFC use and adjusted for demographic factors, comorbidities and medications, the risk of liver cancer was 0.94-fold lower among patients using LFC for fewer than 28 drug days; 0.79-fold lower among those using LFC for any time between 28 and 84 drug days and 0.64-fold lower among those using LFC for more than 84 drug days with medication consumption. After adjusting for age, sex, all comorbidities and medications listed, stratified with each dose of LFC treatment, we found that a higher cumulative dosage of LFC and longer duration had the most protective effects against the development of liver cancer (aHR 0.46; 95%CI: 0.39-0.55) (Table 4). When we further stratified the patients by duration of follow-up into three groups including 2-3 years, 4-6 years and beyond 6 years (Table 5), the risk of developing liver cancer in the LFC cohort progressively decreased over time compared with the risk in the non-LFC cohort; the lowest incidence of liver cancer occurred in LFC users followed-up for more than 6 years (27.44 vs 31.49 per 1000 person-years; aHR 0.75; 95%CI: 0.68-0.82; P < 0.001). Figure 2 shows the significantly lower cumulative incidence of liver cancer in the LFC cohort compared with the non-LFC cohort after 8 years of follow-up (P < 0.001).
| Variable | n | PY | IR | cHR | 95%CI | P value | aHR1 | 95%CI | P value |
| Non-use of LipoCol Forte capsules as reference | 3848 | 186604 | 20.621 | 1.00 | Reference | 1.00 | Reference | ||
| LipoCol Forte capsules | |||||||||
| < 28 d | 3115 | 161794 | 19.253 | 0.9 | (0.86, 0.94)c | < 0.001 | 0.94 | (0.89, 0.98)b | 0.006 |
| 28-84 d | 533 | 27871 | 19.124 | 0.87 | (0.79, 0.95)b | 0.002 | 0.79 | (0.72, 0.87)c | < 0.001 |
| > 84 d | 52 | 2457 | 21.165 | 0.99 | (0.75, 1.3) | 0.925 | 0.64 | (0.48, 0.84)b | 0.001 |
| Variable | n | PY | IR | cHR | 95%CI | P value | aHR1 | 95%CI | P value |
| Non-use of LFC as reference | 3848 | 186604.2 | 20.6212 | 1.00 | (Reference) | - | 1.00 | (Reference) | - |
| LFC dose (g) | |||||||||
| < 91 | 3182 | 158379 | 20.09 | 0.94 | (0.9, 0.98)b | 0.0089 | 0.98 | (0.94, 1.03) | 0.4435 |
| 91-179 | 366 | 21568 | 16.97 | 0.77 | (0.69, 0.86)c | <0.001 | 0.69 | (0.62, 0.77)c | < 0.001 |
| > 179 | 152 | 12175 | 12.48 | 0.55 | (0.47, 0.65)c | <0.001 | 0.46 | (0.39, 0.55)c | < 0.001 |
Although previous studies have shown a benefit with statins in reducing the risk of HCC, this is the first study using a population-based database to show that LFC use significantly decreased the risk of liver cancer by 9% (aHR 0.91) in analyses adjusted for sex, age, comorbidities and medication use. Furthermore, the protective effect of LFC use was dose-dependent, with a progressively lower risk of liver cancer seen with prolonged LFC use.
LFC is a product of red yeast rice. Red yeast rice is a traditional Chinese food that is created by fermenting a red yeast strain (most commonly Monascus purpureus) with rice. The major active component in red yeast rice is monacolin K (lovastatin), which has demonstrated good oral bioavailability in red yeast rice products, including LFC[13], and has proven efficacy in the management of dyslipidemia and prevention of steatohepatitis[14,15]. The ability of LFC to prevent metabolic dysfunction suggests that this product may reduce oxidative stress, chronic inflammation and lipid toxicities, and thus prevent liver cancer development[9]. Other research has also suggested that red yeast rice helps to prevent coronary heart disease, diabetes mellitus and cancer[16]. Rice fermented with Monascus purpureus reportedly inhibits prostate cancer by decreasing gene expression of androgen-synthesizing enzymes and inducing autophagy[17,18]. Other research also claims beneficial effects of red yeast rice in colon cancer, breast cancer and liver cancers[19-21]. In another study, ankaflavin extracted from Monascus-fermented red rice inhibited the growth of human cancer cell lines Hep G2 and A549 by cell cycle arrest and appeared to induce apoptosis[21]. Monascus purpureus CWT715 fermented extract has demonstrated antioxidation activity in the BNL cell line (mouse liver cancer) and antimigratory, antiinvasive activities in SK-Hep-1 human hepatocarcinoma cells by inducing nm23-H1 (non-metastasis protein 23-H1) protein expression[22,23]. Rubropunctamine and monascorubramine, the red Monascus pigments, reportedly induce antimitotic effects on immortalized human kidney epithelial cells[24]. Interestingly, azaphilone compounds extracted from rice fermented with Monascus purpureus have shown selective cytotoxicity in human cancer cells and not in normal cells at equivalent concentrations[25,26]. Dysbiosis is correlated to liver carcinogenesis. A higher Firmicutes/Bacteroidetes ratio might be associated with a higher liver cancer risk and lower response rate to nivolumab treatment[27]. Red yeast rice can modulate gut microbiota by decreasing Firmicutes, Bacteroidetes, and Clostridium species and increasing Lactobacillus and Ruminococcacea[28-31]. This amelioration of gut microbiota composition shows that red yeast rice has the potential to prevent liver cancer occurrence. Thus, we hypothesized that LFC can prevent liver cancer not only by lowering cholesterol levels, but also via direct antitumor effects with possible mechanisms including cell cycle arrest, antimitotic and gut microbiota modulation.
In subgroup analysis, the benefit of LFC use was significant in both males and females, although LFC appeared to be more protective in females (aHR 0.87) than in males (aHR 0.93). This might be due to sex differences in liver cancer, as for instance is the case with inflammation-driven HCC, which occurs more often in males than in females[32]. Moreover, gender differences exist in the association between metabolic factors and HCC risk[33]. However, we observed significant benefits with LFC treatment only in the over-50-year-old age group, reflected by the larger numbers of cases diagnosed with liver cancers in older-aged patients. Our analyses adjusted for important confounding factors including all lipid-lowering drugs, aspirin, metformin and TZD. Statins have been shown in previous studies to reduce the occurrence of liver cancer, with HRs ranging from 0.4 to 0.72[10,34-36]. A 2013 population-based, case-control study conducted in Taiwan using NHIRD data revealed that statin use reduced the likelihood of HCC by 28% (aHR 0.72)[36]. The same study also identified that the individual statins lovastatin, simvastatin and atorvastatin all significantly lowered the risk of HCC[36]. In our study, the fact that LFC shares a similar pharmacological pathway to that of statins meant that LFC use protected against the development of liver cancer in patients with comorbidities including hypertension, coronary heart disease, ischemic stroke, hemorrhagic stroke, diabetes mellitus, HBV and HCV infection. In patients without major liver cancer risks such as cirrhosis, alcoholic liver damage, NAFLD, HBV or HCV infection, LFC showed protective effects against liver cancer (aHRs 0.83-0.9). Our results suggest that LFC use is also appropriate for patients who are considered to be at “low risk” of liver cancer. LFC use was beneficial in users of both statin and non-statin lipid-lowering drugs. However, statistical significance was achieved only by the non-users (aHR 0.91 in the statin cohort and aHR 0.9 in the non-statin lipid-lowering drug cohort), due to limited case numbers or fewer synergistic effects because of similar mechanisms between the different classes of lipid-lowering agents. Aspirin has previously been reported to reduce the risk of HCC with increasing dose and duration[37], which is similar to what we observed, with aHRs ranging from 0.61 to 0.73. Notably, patients not receiving HBV or HCV treatment still derived significant benefit from LFC use (aHR 0.83 in the HBV non-treatment cohort and aHR 0.91 in the HCV non-treatment cohort). However, the HBV and HCV treatment groups did not reach statistical significance, which is likely due to the treatment of HBV and HCV reducing the progression of liver cancer and potentially masking the LFC-induced protective effect. Moreover, Taiwan’s NHIRD did not cover direct-acting antiviral agents in HCV treatment until 2016. Consequently, we only enrolled 8 cases in our cohort study and are therefore unable to formulate any meaningful conclusion. Studies have reported that metformin and TZD lower the risk of HCC, with aHRs ranging from 0.49 to 0.72[38-41]. Thus, we included these drugs in our analyses of confounding factors, to exclude the possibility of an interaction. We observed a significant dose-dependent association between LFC use and the incidence of liver cancer, with aHRs of 0.94, 0.79 and 0.64, respectively, for patients who used LFC for up to 28 d, 28-84 d, or more than 84 d. Our result is similar to reports from other drug-HCC prevention investigations[36,38]. We also report progressively lower cumulative incidence values of liver cancer among LFC users compared with non-LFC users in the 4-6-year subgroup (aHR 0.92; P < 0.05) and in the over 6 years subgroup (aHR 0.75; P < 0.001). These findings indicate that LFC use reduces the risk of liver cancer development in the long-term.
Taiwan’s NHI is a universal healthcare system that covers nearly all of the country’s population. The large database enhances the possibility of producing conclusive patient data, with adjustment for sex, age, comorbidities and medication use. However, several limitations must be noted with this study. First, we used the ICD-9-CM (from 2010 to 2015) and the ICD-10-CM (from 2016 to 2017) algorithms to define diseases diagnosed by clinical physicians. We included only patients with correct ICD-9-CM or ICD-10-CM coding after a single inpatient admission, or after two outpatient clinical visits, to increase the validity and accuracy of comorbidity diagnoses. The major outcome of liver cancer diagnosis was double-checked using the Registry for Catastrophic Illness Patient Database. Second, the NHIRD data lack important information on potential confounding factors, including body mass index, cirrhosis severity, hepatitis viral load, alcohol consumption, environmental/chemical exposure, and family history. Furthermore, biochemical data, abdominal ultrasound reports, computed tomography reports, grading and staging of liver cancer, cannot be defined in Taiwan’s NHI database studies. The demographic characteristics of our patients, the proportions with cirrhosis, alcoholic liver damage or HBV/HCV infection, were not significantly different between the groups. Thus, the background risk of liver cancer occurrence was likely similar for each group. However, by highlighting potential confounding factors, especially the aspect of drug interactions, our analysis is more advanced than previous NHIRD studies. Third, although we took all potential confounding factors into account, a causal relationship between LFC and liver cancer risk could not be directly inferred owing to the observational nature of this study. Thus, we excluded liver cancers diagnosed within 1 year of study commencement. We also considered potential mechanisms in the management of dyslipidemia, direct antitumor effects and microbiota theories as explanations of our findings, as mentioned earlier. Longer-term, prospective clinical studies are needed to confirm our findings.
This is the first study to show that LFC use significantly decreases the risk of liver cancer by 9% in analyses adjusted for sex, age, comorbidities, and medication use. The protective effect of LFC was dose-dependent. Thus, our results of this cohort study suggest that LFC therapy may be associated with reducing risk of liver cancer over an 8-year follow-up. However, long-term studies are needed to confirm our findings. Since LFC is a cheap and commonly used product, prospective clinical trials are feasible and necessary to confirm its beneficial effects on the prevention of liver cancer.
Liver cancer is among the top five most common cancers globally. Anti-lipid therapies such as statins lowered risk of liver cancer. Lipid-lowering drugs such as statins can lower the risk of liver cancer, but may also cause liver damage. LipoCol Forte capsules (LFC), a red yeast rice product, have demonstrated significant antihypercholesterolemic effects and a good safety profile in clinical studies.
We evaluated whether using LFC lowers the risk of liver cancer.
The objective of this study was to evaluate whether LFC lowers the risk of liver cancer in adults, by analyzing data from Taiwan’s National Health Insurance Research Database (NHIRD) in a propensity score-matched, nationwide, population-based cohort study.
Patients using LFC and those not using LFC (controls) between January 2010 and December 2017 were selected from Taiwan’s NHIRD and matched 1:1 by propensity scores. Statistical analyses assessed between-group demographic differences by sex, age, comorbidities, and prescribed medications.
We enrolled 33231 patients in the LFC cohort and 33231 controls. The overall incidence of liver cancer was significantly lower in the LFC cohort compared with controls (aHR 0.91; P < 0.001). The risk of liver cancer was significantly reduced in both females and males in the LFC cohort compared with their counterparts in the non-LFC cohort. There was a 0.64-fold lower liver cancer risk among those using LFC for more than 84 drug days. The risk of developing liver cancer in the LFC cohort progressively decreased over time; the lowest incidence of liver cancer occurred in LFC users followed-up for more than 6 years.
This retrospective cohort study indicates that LFC has a significantly protective effect against the development of liver cancer, in a dose-dependent and time-dependent manner.
Since LFC is a cheap and commonly used product, prospective clinical trials are feasible and necessary to confirm its beneficial effects in the prevention of liver cancer.
The authors would like to thank MacDonald IJ (China Medical University) for the critical reading and revision of our manuscript.
| 1. | Cicero AFG, Fogacci F, Banach M. Red Yeast Rice for Hypercholesterolemia. Methodist Debakey Cardiovasc J. 2019;15:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Wang TJ, Lien AS, Chen JL, Lin CH, Yang YS, Yang SH. A Randomized Clinical Efficacy Trial of Red Yeast Rice (Monascus pilosus) Against Hyperlipidemia. Am J Chin Med. 2019;47:323-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Liu BY, Xu F, Bai J, Yan DJ, Zhang L, Zhang D, Hu YC. Six new monacolin analogs from red yeast rice. Chin J Nat Med. 2019;17:394-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Chen TL, Yeh CC, Lin CS, Shih CC, Liao CC. Effects of red yeast rice prescription (LipoCol Forte) on adverse outcomes of surgery. QJM. 2019;112:253-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Lin CC, Li TC, Lai MM. Efficacy and safety of Monascus purpureus Went rice in subjects with hyperlipidemia. Eur J Endocrinol. 2005;153:679-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Li JJ, Lu ZL, Kou WR, Chen Z, Wu YF, Yu XH, Zhao YC; Chinese Coronary Secondary Prevention Study Group. Beneficial impact of Xuezhikang on cardiovascular events and mortality in elderly hypertensive patients with previous myocardial infarction from the China Coronary Secondary Prevention Study (CCSPS). J Clin Pharmacol. 2009;49:947-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Global Burden of Disease 2019 Cancer Collaboration, Kocarnik JM, Compton K, Dean FE, Fu W, Gaw BL, Harvey JD, Henrikson HJ, Lu D, Pennini A, Xu R, Ababneh E, Abbasi-Kangevari M, Abbastabar H, Abd-Elsalam SM, Abdoli A, Abedi A, Abidi H, Abolhassani H, Adedeji IA, Adnani QES, Advani SM, Afzal MS, Aghaali M, Ahinkorah BO, Ahmad S, Ahmad T, Ahmadi A, Ahmadi S, Ahmed Rashid T, Ahmed Salih Y, Akalu GT, Aklilu A, Akram T, Akunna CJ, Al Hamad H, Alahdab F, Al-Aly Z, Ali S, Alimohamadi Y, Alipour V, Aljunid SM, Alkhayyat M, Almasi-Hashiani A, Almasri NA, Al-Maweri SAA, Almustanyir S, Alonso N, Alvis-Guzman N, Amu H, Anbesu EW, Ancuceanu R, Ansari F, Ansari-Moghaddam A, Antwi MH, Anvari D, Anyasodor AE, Aqeel M, Arabloo J, Arab-Zozani M, Aremu O, Ariffin H, Aripov T, Arshad M, Artaman A, Arulappan J, Asemi Z, Asghari Jafarabadi M, Ashraf T, Atorkey P, Aujayeb A, Ausloos M, Awedew AF, Ayala Quintanilla BP, Ayenew T, Azab MA, Azadnajafabad S, Azari Jafari A, Azarian G, Azzam AY, Badiye AD, Bahadory S, Baig AA, Baker JL, Balakrishnan S, Banach M, Bärnighausen TW, Barone-Adesi F, Barra F, Barrow A, Behzadifar M, Belgaumi UI, Bezabhe WMM, Bezabih YM, Bhagat DS, Bhagavathula AS, Bhardwaj N, Bhardwaj P, Bhaskar S, Bhattacharyya K, Bhojaraja VS, Bibi S, Bijani A, Biondi A, Bisignano C, Bjørge T, Bleyer A, Blyuss O, Bolarinwa OA, Bolla SR, Braithwaite D, Brar A, Brenner H, Bustamante-Teixeira MT, Butt NS, Butt ZA, Caetano Dos Santos FL, Cao Y, Carreras G, Catalá-López F, Cembranel F, Cerin E, Cernigliaro A, Chakinala RC, Chattu SK, Chattu VK, Chaturvedi P, Chimed-Ochir O, Cho DY, Christopher DJ, Chu DT, Chung MT, Conde J, Cortés S, Cortesi PA, Costa VM, Cunha AR, Dadras O, Dagnew AB, Dahlawi SMA, Dai X, Dandona L, Dandona R, Darwesh AM, das Neves J, De la Hoz FP, Demis AB, Denova-Gutiérrez E, Dhamnetiya D, Dhimal ML, Dhimal M, Dianatinasab M, Diaz D, Djalalinia S, Do HP, Doaei S, Dorostkar F, Dos Santos Figueiredo FW, Driscoll TR, Ebrahimi H, Eftekharzadeh S, El Tantawi M, El-Abid H, Elbarazi I, Elhabashy HR, Elhadi M, El-Jaafary SI, Eshrati B, Eskandarieh S, Esmaeilzadeh F, Etemadi A, Ezzikouri S, Faisaluddin M, Faraon EJA, Fares J, Farzadfar F, Feroze AH, Ferrero S, Ferro Desideri L, Filip I, Fischer F, Fisher JL, Foroutan M, Fukumoto T, Gaal PA, Gad MM, Gadanya MA, Gallus S, Gaspar Fonseca M, Getachew Obsa A, Ghafourifard M, Ghashghaee A, Ghith N, Gholamalizadeh M, Gilani SA, Ginindza TG, Gizaw ATT, Glasbey JC, Golechha M, Goleij P, Gomez RS, Gopalani SV, Gorini G, Goudarzi H, Grosso G, Gubari MIM, Guerra MR, Guha A, Gunasekera DS, Gupta B, Gupta VB, Gupta VK, Gutiérrez RA, Hafezi-Nejad N, Haider MR, Haj-Mirzaian A, Halwani R, Hamadeh RR, Hameed S, Hamidi S, Hanif A, Haque S, Harlianto NI, Haro JM, Hasaballah AI, Hassanipour S, Hay RJ, Hay SI, Hayat K, Heidari G, Heidari M, Herrera-Serna BY, Herteliu C, Hezam K, Holla R, Hossain MM, Hossain MBH, Hosseini MS, Hosseini M, Hosseinzadeh M, Hostiuc M, Hostiuc S, Househ M, Hsairi M, Huang J, Hugo FN, Hussain R, Hussein NR, Hwang BF, Iavicoli I, Ibitoye SE, Ida F, Ikuta KS, Ilesanmi OS, Ilic IM, Ilic MD, Irham LM, Islam JY, Islam RM, Islam SMS, Ismail NE, Isola G, Iwagami M, Jacob L, Jain V, Jakovljevic MB, Javaheri T, Jayaram S, Jazayeri SB, Jha RP, Jonas JB, Joo T, Joseph N, Joukar F, Jürisson M, Kabir A, Kahrizi D, Kalankesh LR, Kalhor R, Kaliyadan F, Kalkonde Y, Kamath A, Kameran Al-Salihi N, Kandel H, Kapoor N, Karch A, Kasa AS, Katikireddi SV, Kauppila JH, Kavetskyy T, Kebede SA, Keshavarz P, Keykhaei M, Khader YS, Khalilov R, Khan G, Khan M, Khan MN, Khan MAB, Khang YH, Khater AM, Khayamzadeh M, Kim GR, Kim YJ, Kisa A, Kisa S, Kissimova-Skarbek K, Kopec JA, Koteeswaran R, Koul PA, Koulmane Laxminarayana SL, Koyanagi A, Kucuk Bicer B, Kugbey N, Kumar GA, Kumar N, Kurmi OP, Kutluk T, La Vecchia C, Lami FH, Landires I, Lauriola P, Lee SW, Lee SWH, Lee WC, Lee YH, Leigh J, Leong E, Li J, Li MC, Liu X, Loureiro JA, Lunevicius R, Magdy Abd El Razek M, Majeed A, Makki A, Male S, Malik AA, Mansournia MA, Martini S, Masoumi SZ, Mathur P, McKee M, Mehrotra R, Mendoza W, Menezes RG, Mengesha EW, Mesregah MK, Mestrovic T, Miao Jonasson J, Miazgowski B, Miazgowski T, Michalek IM, Miller TR, Mirzaei H, Mirzaei HR, Misra S, Mithra P, Moghadaszadeh M, Mohammad KA, Mohammad Y, Mohammadi M, Mohammadi SM, Mohammadian-Hafshejani A, Mohammed S, Moka N, Mokdad AH, Molokhia M, Monasta L, Moni MA, Moosavi MA, Moradi Y, Moraga P, Morgado-da-Costa J, Morrison SD, Mosapour A, Mubarik S, Mwanri L, Nagarajan AJ, Nagaraju SP, Nagata C, Naimzada MD, Nangia V, Naqvi AA, Narasimha Swamy S, Ndejjo R, Nduaguba SO, Negoi I, Negru SM, Neupane Kandel S, Nguyen CT, Nguyen HLT, Niazi RK, Nnaji CA, Noor NM, Nuñez-Samudio V, Nzoputam CI, Oancea B, Ochir C, Odukoya OO, Ogbo FA, Olagunju AT, Olakunde BO, Omar E, Omar Bali A, Omonisi AEE, Ong S, Onwujekwe OE, Orru H, Ortega-Altamirano DV, Otstavnov N, Otstavnov SS, Owolabi MO, P A M, Padubidri JR, Pakshir K, Pana A, Panagiotakos D, Panda-Jonas S, Pardhan S, Park EC, Park EK, Pashazadeh Kan F, Patel HK, Patel JR, Pati S, Pattanshetty SM, Paudel U, Pereira DM, Pereira RB, Perianayagam A, Pillay JD, Pirouzpanah S, Pishgar F, Podder I, Postma MJ, Pourjafar H, Prashant A, Preotescu L, Rabiee M, Rabiee N, Radfar A, Radhakrishnan RA, Radhakrishnan V, Rafiee A, Rahim F, Rahimzadeh S, Rahman M, Rahman MA, Rahmani AM, Rajai N, Rajesh A, Rakovac I, Ram P, Ramezanzadeh K, Ranabhat K, Ranasinghe P, Rao CR, Rao SJ, Rawassizadeh R, Razeghinia MS, Renzaho AMN, Rezaei N, Rezapour A, Roberts TJ, Rodriguez JAB, Rohloff P, Romoli M, Ronfani L, Roshandel G, Rwegerera GM, S M, Sabour S, Saddik B, Saeed U, Sahebkar A, Sahoo H, Salehi S, Salem MR, Salimzadeh H, Samaei M, Samy AM, Sanabria J, Sankararaman S, Santric-Milicevic MM, Sardiwalla Y, Sarveazad A, Sathian B, Sawhney M, Saylan M, Schneider IJC, Sekerija M, Seylani A, Shafaat O, Shaghaghi Z, Shaikh MA, Shamsoddin E, Shannawaz M, Sharma R, Sheikh A, Sheikhbahaei S, Shetty A, Shetty JK, Shetty PH, Shibuya K, Shirkoohi R, Shivakumar KM, Shivarov V, Siabani S, Siddappa Malleshappa SK, Silva DAS, Singh JA, Sintayehu Y, Skryabin VY, Skryabina AA, Soeberg MJ, Sofi-Mahmudi A, Sotoudeh H, Steiropoulos P, Straif K, Subedi R, Sufiyan MB, Sultan I, Sultana S, Sur D, Szerencsés V, Szócska M, Tabarés-Seisdedos R, Tabuchi T, Tadbiri H, Taherkhani A, Takahashi K, Talaat IM, Tan KK, Tat VY, Tedla BAA, Tefera YG, Tehrani-Banihashemi A, Temsah MH, Tesfay FH, Tessema GA, Thapar R, Thavamani A, Thoguluva Chandrasekar V, Thomas N, Tohidinik HR, Touvier M, Tovani-Palone MR, Traini E, Tran BX, Tran KB, Tran MTN, Tripathy JP, Tusa BS, Ullah I, Ullah S, Umapathi KK, Unnikrishnan B, Upadhyay E, Vacante M, Vaezi M, Valadan Tahbaz S, Velazquez DZ, Veroux M, Violante FS, Vlassov V, Vo B, Volovici V, Vu GT, Waheed Y, Wamai RG, Ward P, Wen YF, Westerman R, Winkler AS, Yadav L, Yahyazadeh Jabbari SH, Yang L, Yaya S, Yazie TSY, Yeshaw Y, Yonemoto N, Younis MZ, Yousefi Z, Yu C, Yuce D, Yunusa I, Zadnik V, Zare F, Zastrozhin MS, Zastrozhina A, Zhang J, Zhong C, Zhou L, Zhu C, Ziapour A, Zimmermann IR, Fitzmaurice C, Murray CJL, Force LM. Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022;8:420-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1180] [Cited by in RCA: 1351] [Article Influence: 337.8] [Reference Citation Analysis (0)] |
| 8. | Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor-Robinson SD. Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J Gastroenterol. 2008;14:4300-4308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 456] [Cited by in RCA: 502] [Article Influence: 27.9] [Reference Citation Analysis (6)] |
| 9. | Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 1358] [Article Influence: 271.6] [Reference Citation Analysis (0)] |
| 10. | Wong YJ, Qiu TY, Ng GK, Zheng Q, Teo EK. Efficacy and Safety of Statin for Hepatocellular Carcinoma Prevention Among Chronic Liver Disease Patients: A Systematic Review and Meta-analysis. J Clin Gastroenterol. 2021;55:615-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Ramkumar S, Raghunath A, Raghunath S. Statin Therapy: Review of Safety and Potential Side Effects. Acta Cardiol Sin. 2016;32:631-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 129] [Reference Citation Analysis (0)] |
| 12. | Ong YC, Aziz Z. Systematic review of red yeast rice compared with simvastatin in dyslipidaemia. J Clin Pharm Ther. 2016;41:170-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Chen CH, Yang JC, Uang YS, Lin CJ. Improved dissolution rate and oral bioavailability of lovastatin in red yeast rice products. Int J Pharm. 2013;444:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Lee HS, Lee YJ, Chung YH, Nam Y, Kim ST, Park ES, Hong SM, Yang YK, Kim HC, Jeong JH. Beneficial Effects of Red Yeast Rice on High-Fat Diet-Induced Obesity, Hyperlipidemia, and Fatty Liver in Mice. J Med Food. 2015;18:1095-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Fujimoto M, Tsuneyama K, Chen SY, Nishida T, Chen JL, Chen YC, Fujimoto T, Imura J, Shimada Y. Study of the effects of monacolin k and other constituents of red yeast rice on obesity, insulin-resistance, hyperlipidemia, and nonalcoholic steatohepatitis using a mouse model of metabolic syndrome. Evid Based Complement Alternat Med. 2012;2012:892697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Yang CW, Mousa SA. The effect of red yeast rice (Monascus purpureus) in dyslipidemia and other disorders. Complement Ther Med. 2012;20:466-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Hong MY, Henning S, Moro A, Seeram NP, Zhang Y, Heber D. Chinese red yeast rice inhibition of prostate tumor growth in SCID mice. Cancer Prev Res (Phila). 2011;4:608-615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Chiu HW, Fang WH, Chen YL, Wu MD, Yuan GF, Ho SY, Wang YJ. Monascuspiloin enhances the radiation sensitivity of human prostate cancer cells by stimulating endoplasmic reticulum stress and inducing autophagy. PLoS One. 2012;7:e40462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Mahmoud AM, Aboul-Soud MA, Han J, Al-Sheikh YA, Al-Abd AM, El-Shemy HA. Transcriptional profiling of breast cancer cells in response to mevinolin: Evidence of cell cycle arrest, DNA degradation and apoptosis. Int J Oncol. 2016;48:1886-1894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Hong MY, Seeram NP, Zhang Y, Heber D. Anticancer effects of Chinese red yeast rice versus monacolin K alone on colon cancer cells. J Nutr Biochem. 2008;19:448-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Su NW, Lin YL, Lee MH, Ho CY. Ankaflavin from Monascus-fermented red rice exhibits selective cytotoxic effect and induces cell death on Hep G2 cells. J Agric Food Chem. 2005;53:1949-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Chang WT, Chuang CH, Lee WJ, Huang CS. Extract of Monascus purpureus CWT715 Fermented from Sorghum Liquor Biowaste Inhibits Migration and Invasion of SK-Hep-1 Human Hepatocarcinoma Cells. Molecules. 2016;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Huang CS, Hu HH, Tsai YM, Chang WT. In vitro effects of Monascus purpureus on antioxidation activity during fermentation of Kinmen sorghum liquor waste. J Biosci Bioeng. 2013;115:418-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Knecht A, Humpf HU. Cytotoxic and antimitotic effects of N-containing Monascus metabolites studied using immortalized human kidney epithelial cells. Mol Nutr Food Res. 2006;50:406-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Li JJ, Shang XY, Li LL, Liu MT, Zheng JQ, Jin ZL. New cytotoxic azaphilones from Monascus purpureus-fermented rice (red yeast rice). Molecules. 2010;15:1958-1966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Zhu L, Yau LF, Lu JG, Zhu GY, Wang JR, Han QB, Hsiao WL, Jiang ZH. Cytotoxic dehydromonacolins from red yeast rice. J Agric Food Chem. 2012;60:934-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Chung MW, Kim MJ, Won EJ, Lee YJ, Yun YW, Cho SB, Joo YE, Hwang JE, Bae WK, Chung IJ, Shin MG, Shin JH. Gut microbiome composition can predict the response to nivolumab in advanced hepatocellular carcinoma patients. World J Gastroenterol. 2021;27:7340-7349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (2)] |
| 28. | Zhou W, Guo R, Guo W, Hong J, Li L, Ni L, Sun J, Liu B, Rao P, Lv X. Monascus yellow, red and orange pigments from red yeast rice ameliorate lipid metabolic disorders and gut microbiota dysbiosis in Wistar rats fed on a high-fat diet. Food Funct. 2019;10:1073-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 29. | Dong Y, Cheng H, Liu Y, Xue M, Liang H. Red yeast rice ameliorates high-fat diet-induced atherosclerosis in Apoe(-/-) mice in association with improved inflammation and altered gut microbiota composition. Food Funct. 2019;10:3880-3889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 30. | Huang YP, Li P, Du T, Du XJ, Wang S. Protective effect and mechanism of Monascus-fermented red yeast rice against colitis caused by Salmonella enterica serotype Typhimurium ATCC 14028. Food Funct. 2020;11:6363-6375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Huang ZR, Chen M, Guo WL, Li TT, Liu B, Bai WD, Ai LZ, Rao PF, Ni L, Lv XC. Monascus purpureus-fermented common buckwheat protects against dyslipidemia and non-alcoholic fatty liver disease through the regulation of liver metabolome and intestinal microbiome. Food Res Int. 2020;136:109511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 32. | Zheng B, Zhu YJ, Wang HY, Chen L. Gender disparity in hepatocellular carcinoma (HCC): multiple underlying mechanisms. Sci China Life Sci. 2017;60:575-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 33. | Chen CL, Kuo MJ, Yen AM, Yang WS, Kao JH, Chen PJ, Chen HH. Gender Difference in the Association Between Metabolic Factors and Hepatocellular Carcinoma. JNCI Cancer Spectr. 2020;4:pkaa036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Vahedian-Azimi A, Shojaie S, Banach M, Heidari F, Cicero AFG, Khoshfetrat M, Jamialahmadi T, Sahebkar A. Statin therapy in chronic viral hepatitis: a systematic review and meta-analysis of nine studies with 195,602 participants. Ann Med. 2021;53:1227-1242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Yang SY, Wang CC, Chen KD, Liu YW, Lin CC, Chuang CH, Tsai YC, Yao CC, Yen YH, Hsiao CC, Hu TH, Tsai MC. Statin use is associated with a lower risk of recurrence after curative resection in BCLC stage 0-A hepatocellular carcinoma. BMC Cancer. 2021;21:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Lai SW, Liao KF, Lai HC, Muo CH, Sung FC, Chen PC. Statin use and risk of hepatocellular carcinoma. Eur J Epidemiol. 2013;28:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 37. | Memel ZN, Arvind A, Moninuola O, Philpotts L, Chung RT, Corey KE, Simon TG. Aspirin Use Is Associated with a Reduced Incidence of Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Hepatol Commun. 2021;5:133-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 38. | Tseng CH. Metformin and risk of hepatocellular carcinoma in patients with type 2 diabetes. Liver Int. 2018;38:2018-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 39. | Antwi SO, Li Z, Mody K, Roberts LR, Patel T. Independent and Joint Use of Statins and Metformin by Elderly Patients With Diabetes and Overall Survival Following HCC Diagnosis. J Clin Gastroenterol. 2020;54:468-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Yip TC, Wong VW, Chan HL, Tse YK, Hui VW, Liang LY, Lee HW, Lui GC, Kong AP, Wong GL. Thiazolidinediones reduce the risk of hepatocellular carcinoma and hepatic events in diabetic patients with chronic hepatitis B. J Viral Hepat. 2020;27:904-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Huang MY, Chung CH, Chang WK, Lin CS, Chen KW, Hsieh TY, Chien WC, Lin HH. The role of thiazolidinediones in hepatocellular carcinoma risk reduction: a population-based cohort study in Taiwan. Am J Cancer Res. 2017;7:1606-1616. [PubMed] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Deng Y, China; Gupta L, Indonesia S-Editor: Yan JP L-Editor: A P-Editor: Wu RR