Published online Apr 15, 2023. doi: 10.4251/wjgo.v15.i4.689
Peer-review started: October 26, 2022
First decision: December 14, 2022
Revised: December 16, 2022
Accepted: March 23, 2023
Article in press: March 23, 2023
Published online: April 15, 2023
Processing time: 167 Days and 23.7 Hours
Although immune checkpoint inhibitor (ICI) therapy has improved the prognosis of unresectable hepatocellular carcinoma (HCC), it has also resulted in unique immune-related adverse events (irAEs). The relationship between irAE and treatment outcomes in ICI-treated unresectable HCC patients remains unknown.
To elucidate the correlation between immune-related toxic effects and prognosis in patients with unresectable HCC treated with pembrolizumab.
From March 2019 to February 2021, a total of 190 unresectable HCC (Barcelona Clinic Liver Cancer C) patients receiving pembrolizumab treatment were retrospectively reviewed. Overall survival (OS) was the primary endpoint, while objective response rate (ORR), disease control rate (DCR), and time to progression (TTP) were secondary evaluation indexes. We assessed demographics, irAEs, and outcomes by retrospective review.
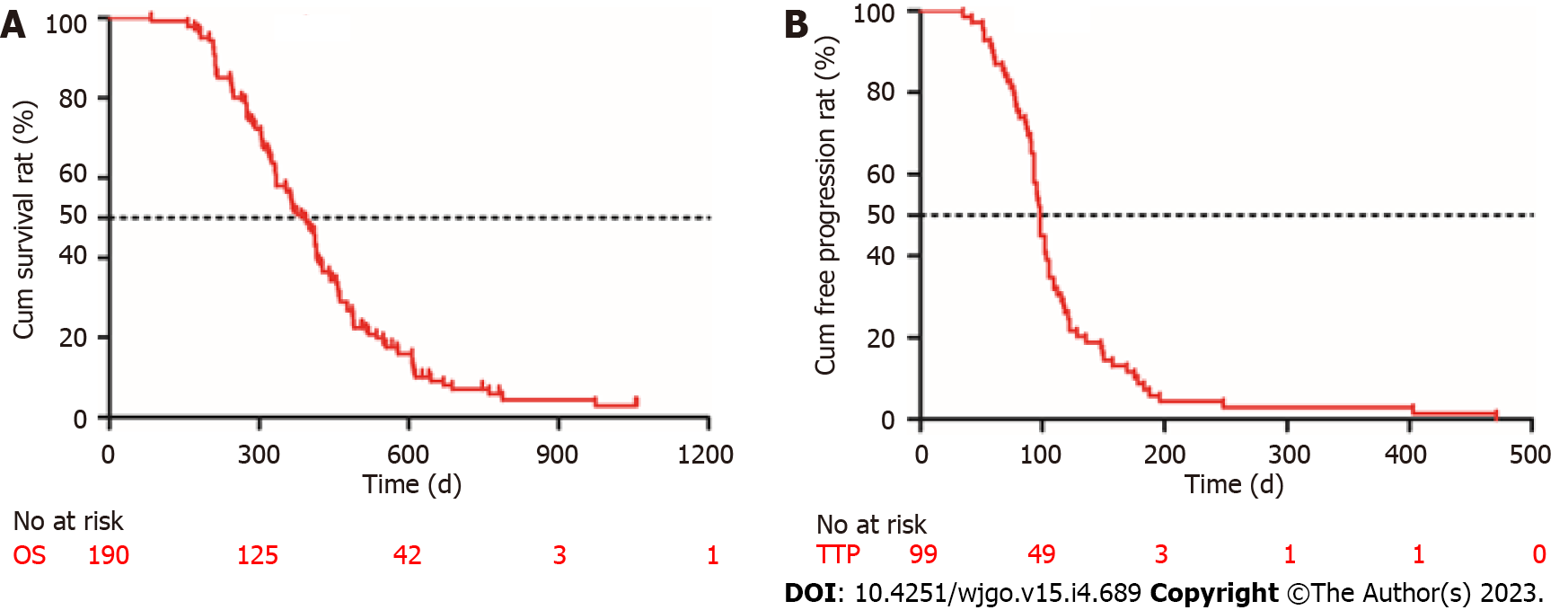
One hundred and forty-three males and 47 females were included in the study. The ORR and DCR were 12.1% (23/190) and 52.1% (99/190), respectively. The median OS was 376 d [95% confidence interval (CI): 340-411 d] and the median TTP was 98 d (95%CI: 75-124 d). The overall incidence of treatment-related adverse events was 72.6% (138/190) and 10.0% of them were severe irAEs (grade ≥ 3). Child-Pugh B class, portal vein tumor thrombus, extrahepatic metastasis, and hypothyroidism were the independent risk factors for survival. Patients with hypothyroidism showed a longer OS [517 d (95%CI: 423-562) vs 431 d (95%CI: 412-485), P = 0.011] and TTP [125 d (95%CI: 89-154) vs 87 d (95%CI: 61-98), P = 0.004] than those without irAEs.
Pembrolizumab-treated patients with unresectable HCC who experienced hypothyroidism have promising ORR and durable response. Hypothyroidism, an irAE, may be used as a clinical evaluation parameter of response to ICIs in unresectable HCC.
Core Tip: This is a retrospective study to elucidate the correlation between immune-related toxic effects and prognosis in patients with unresectable hepatocellular carcinoma (HCC) treated with pembrolizumab. One hundred and forty-three male patients and forty-seven female patients were included in the study. The overall incidence of treatment-related adverse events was 72.6% (138/190) and 10.0% of them were severe immune-related adverse events (irAEs) (grade ≥ 3). Patients with hypothyroidism were observed to have a longer overall survival and time to progression than those without irAEs. Unresectable HCC patients who experienced hypothyroidism had a better therapeutic effect.
- Citation: Zhou JM, Xiong HF, Chen XP, Zhang ZW, Zhu LP, Wu B. Correlation between immune-related adverse events and long-term outcomes in pembrolizumab-treated patients with unresectable hepatocellular carcinoma: A retrospective study. World J Gastrointest Oncol 2023; 15(4): 689-699
- URL: https://www.wjgnet.com/1948-5204/full/v15/i4/689.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i4.689
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death globally[1]. Because early-stage HCC is insidious and the tumors have a tendency to invade intrahepatic vessels and create distant metastases, 80% of patients are already in the advanced stage at diagnosis and lose the opportunity for hepatectomy. In addition, 70% of patients who receive radical liver resection will experience tumor recurrence[2]. Therefore, a large number of patients with unresectable HCC need comprehensive treatment. Locoregional therapy including transarterial chemoembolization or selective internal radiation therapy has been proven to prolong the median survival of patients with unresectable HCC[3]. Since sorafenib has been shown to improve the prognosis of unresectable HCC[4], tyrosine kinase inhibitors (TKIs) have become the first-line treatment for advanced HCC. However, the objective response rate (ORR) to TKIs is unsatisfactory, ranging from 6.5%-18.8%, and the prognosis improvement is not significant, with an average prolonged survival time of 2.8-5.8 mo[5,6]. Recent studies have shown that immune checkpoint inhibitors (ICIs) represented by pembrolizumab have become the second-line treatment for unresectable HCC due to their safety and long duration of response. KEYNOTE-224, a non-randomized, multi-center, open-label, phase 2 trial, has proven that pembrolizumab was effective and tolerable in patients with unresectable HCC who had previously been treated with sorafenib[7].
However, the response rate to both TKIs and ICIs was less than 30%. Moreover, adverse effects of the drugs may interfere with the judgment of curative effect and decrease compliance to the best treatment available. Therefore, determining patients who are mostly likely to benefit from TKIs or ICIs is very important. Researchers have been trying to find biomarkers that predict patient survival or response to drugs[8]. Previous studies have reported that unresectable HCC patients who developed hand-foot skin reaction possessed a prolonged time to progression (TTP) and better disease-control rate (DCR)[9]. Studies have suggested that vascular endothelial growth factor inhibition may rely on a stronger recruitment of inflammatory cells to take a more pronounced antitumor effect that is accompanied by stronger treatment-related adverse events[10]. Whether similar adverse events can be found to evaluate the response of tumor to ICIs remains to be investigated. Immune-related adverse events (irAEs) have been reported to include polymyalgia, colitis, skin lesions (rash, pruritus, and vitiligo), hypophysitis, hepatitis, thyroiditis, uveitis, Guillain-Barré syndrome, and immune-mediated cytopenia. It was reported that the overall incidence of all-grade irAEs was 60%-80% and the incidence of high-grade irAEs was 20%-30%[11,12]. Several studies have suggested that autoimmune-like toxic effects are thought to represent bystander effects from activated T-cells, accompanied with antitumor effects, and are consistent with the mechanism of action of ICIs[13,14]. Herein, we retrospectively studied whether immune-related toxic effects correlated with prognosis in patients with unresectable HCC treated with pembrolizumab.
Overall survival (OS) was defined as the time from the start of ICIs until death or until last follow-up. ORR was defined as the proportion of patients achieving complete response (CR) and partial response (PR). DCR was defined as the proportion of patients achieving CR, PR, and stable disease (SD). TTP was defined as the time from the start of ICIs to the radiological confirmation of tumor progression. Tumor burden score (TBS) was calculated as 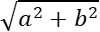 [a = maximum tumor diameter (cm), measuring the area of arterial enhancement and excluding the area of internal necrosis[15]; b = tumor number, the lesion is at least 1 cm in size][16].
[a = maximum tumor diameter (cm), measuring the area of arterial enhancement and excluding the area of internal necrosis[15]; b = tumor number, the lesion is at least 1 cm in size][16].
Between March 2019 and February 2021, a total of 190 patients who received ICI treatment were included in this retrospective study at Tongji Hospital of Tongji Medical College of Huazhong University of Science and Technology. All patients included in the study met the following criteria: (1) Child-Pugh A/B; (2) Advanced HCC [Barcelona Clinic Liver Cancer (BCLC) stage C]; and (3) Not receiving previous antitumor therapy. HCC was diagnosed by the European Association for the Study of Liver criteria and American Association for the Study of Liver Disease guidelines[17,18]. Demographics, including age, sex, etc. laboratory examination, including levels of alanine aminotransferase, aspartate aminotransferase, alpha fetoprotein (AFP), etc. imaging examination, and survival status were reviewed retrospectively. The study was approved by the Ethical Committee of Tongji Hospital of Tongji Medical College of Huazhong University of Science and Technology. All procedures performed in this study abided by the Declaration of Helsinki.
All of the patients received treatment with pembrolizumab (KEYTRUDA, Merck Sharp & Dohme Co., Inc.), 200 mg/time, every 3 wk, via intravenous infusion[7] according to the guidelines or expert consensus, with dose modification according to toxic effects, as needed.
An experienced radiologist blinded to patient information assessed tumor response according to modified response evaluation criteria in solid tumor criteria every 6 wk by contrast-enhanced computed tomography or magnetic resonance imaging examination. Common Terminology Criteria for Adverse Events (CTCAE) were used by two experienced hepatologists to independently assess irAEs. Corresponding examinations or imaging examinations were performed to diagnose irAEs and assess the grades according to CTCAE. Follow-up was terminated on April 24, 2022. The survival status was confirmed by governmental death registration or telephone. The flow diagram of the study is displayed in Figure 1.
Continuous variables are presented as the median and interquartile range (IQR) and categorical data as numbers and percentages. Survival analysis was carried out using the Kaplan-Meier method and log-rank test. Univariate and multivariate Cox proportional regression analyses were used to evaluate risk factors for OS or TTP. A two-sided P value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 19.0 for Windows (SPSS, Chicago, Illinois, United States). GraphPad Prism 7 software was used for all graphical drawings.
The baseline characteristics of 190 unresectable HCC (BCLC stage C) patients receiving pembrolizumab as an initial treatment are summarized in Table 1. One hundred and fifty-four (81.1%) of the patients were male, and the primary cause of disease was chronic hepatitis B virus infection (75.8%). One hundred and forty-six (76.8%) of the patients had Child-Pugh class A and 44 (23.2%) had Child-Pugh class B. All of the patients were at BCLC stage C.
| Clinical characteristic | No. of patients |
| Age (yr), median (IQR) | 51 (42-58) |
| Sex | |
| Male, n (%) | 154 (81.1) |
| Etiology | |
| Hepatitis B | 144 (75.8) |
| Hepatitis C | 4 (2.1) |
| Hepatitis B & C | 2 (1.1) |
| Non-hepatitis B, non-hepatitis C | 40 (21.0) |
| ALT (U/L), median (IQR) | 75 (51-254) |
| AST (U/L), median (IQR) | 57 (47-302) |
| TBiL (μmol/L), median (IQR) | 20.1 (11.2-31.2) |
| AFP (ng/mL), median (IQR) | 205 (19.2-4692) |
| Child-Pugh class | |
| A | 146 (76.8) |
| B | 44 (23.2) |
| Portal vein tumor thrombosis | 131 (68.9) |
| Extrahepatic metastasis | |
| Lung | 64 (33.7) |
| Bone | 31 (16.3) |
| Peritoneum | 25 (13.1) |
| Multiple locations | 15 (7.9) |
| BCLC stage | |
| C | 190 (100) |
| Tumor response | |
| Complete response | 0 (0) |
| Partial response | 23 (12.1) |
| Stable disease | 76 (40.0) |
| Progressive disease | 91 (47.9) |
| Follow-up time, median (range) | 748 (438-1146) d |
| TTP, median (95%CI) | 98 (75-124) d |
| OS, median (95%CI) | 376 (340-411) d |
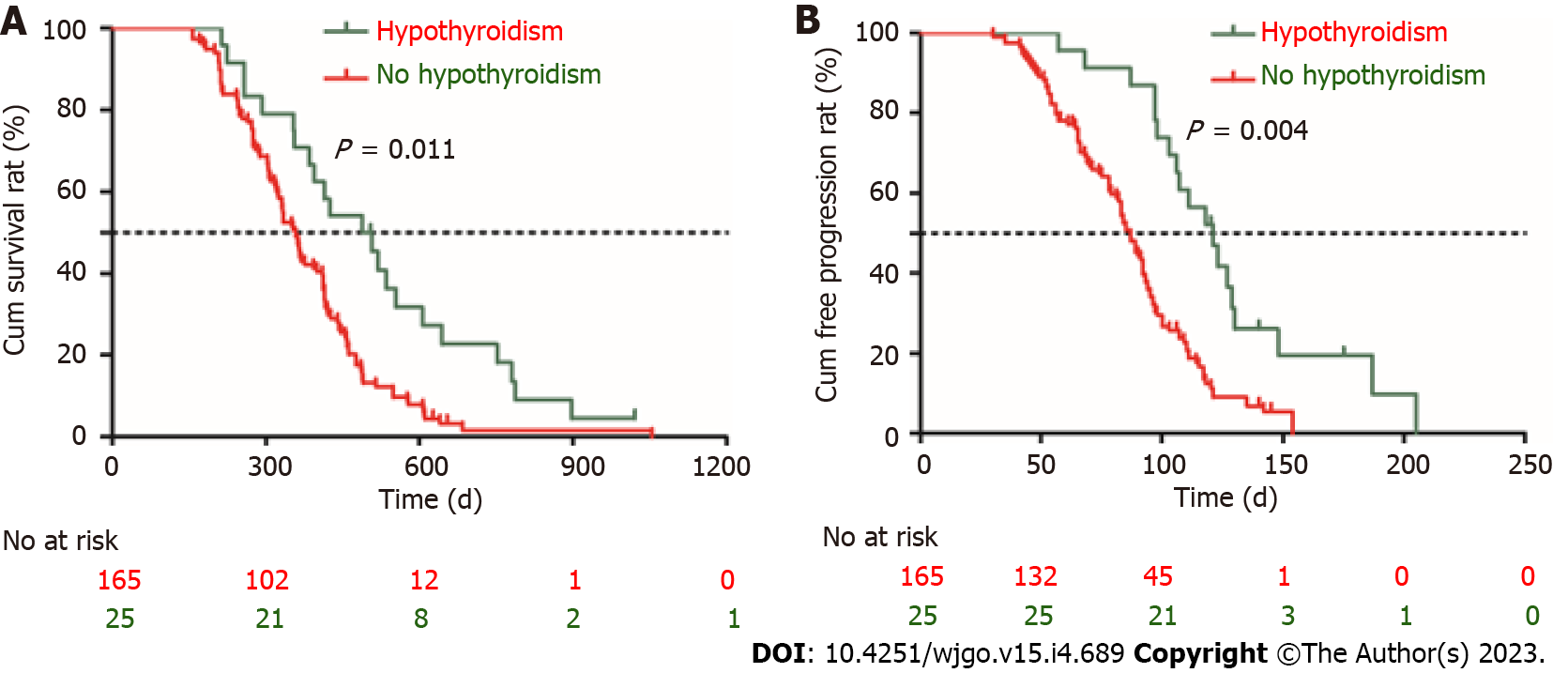
Follow-up was terminated on April 24, 2022. The mean follow-up time was 814 d (median, 748 d; range, 438-1146 d). No patients achieved CR. Twelve percent (23/190) of the patients achieved PR and 40% (76/190) had SD. The DCR was 52.1% (99/190), and 47.9% (91/190) of the patients had progressive disease (PD). All patients who experienced disease progression permanently discontinued ICI treatment; none of the patients discontinued ICIs because of adverse effects. At the end of the follow-up, 130 patients (68.4%) died. The median OS was 376 d [95% confidence interval (CI): 340-411 d] and the median TTP was 98 d (95%CI: 75-124 d) (Figure 2).
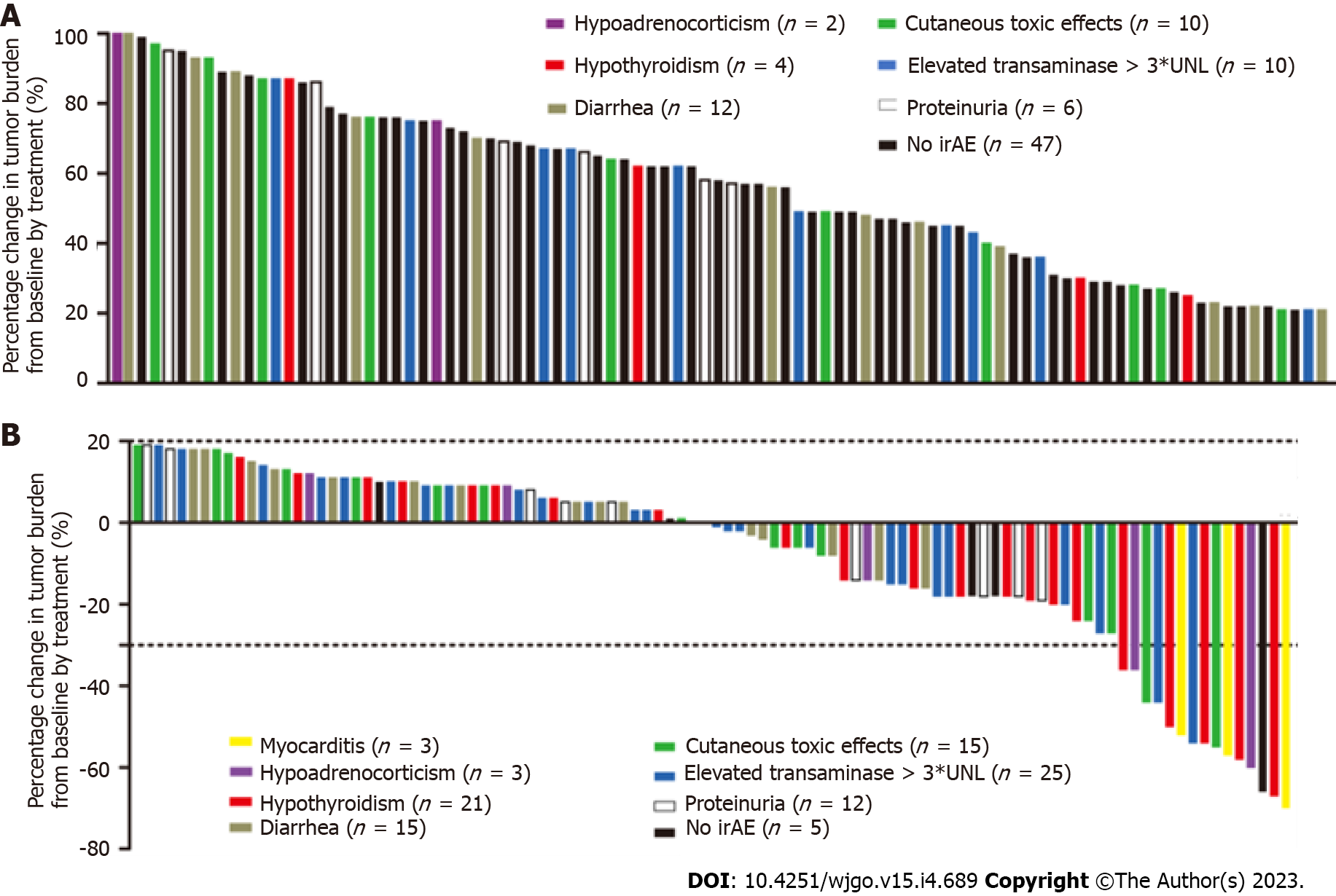
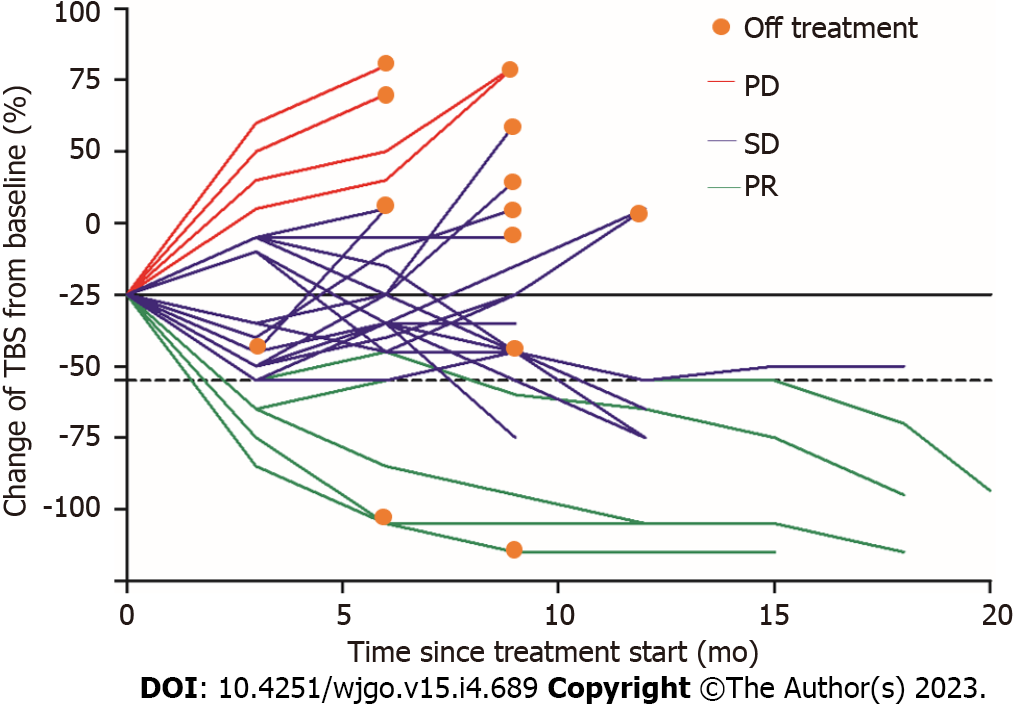
The overall incidence of irAEs was 72.6% (138/190) and 10.0% of them were severe irAEs (grade ≥ 3). Table 2 summarizes the observed representative irAEs. Elevated transaminase (> 3 times upper limit of normal) was the most common adverse reaction to ICIs seen in 69 (36.3%) patients, followed by diarrhea in 40 (21.1%), cutaneous toxic effects in 37 (19.5%), hypothyroidism in 25 (13.2%), and proteinuria in 24 (12.6%). Relatively rare adverse events were myocarditis in 3 (1.6%) patients and hypoadrenocorticism in 5 (2.6%). The elevated transaminase was the earliest irAE, and the median (IQR) time to onset was 4.8 (3.5-7.2) wk, followed by cutaneous toxic effects [5.9 (3.8-7.9) wk], proteinuria [6.8 (4.3-8.1) wk], diarrhea [7.1 (5.2-8.9) wk], hypoadrenocorticism [7.9 (6.7-9.5) wk], hypothyroidism [8.7 (6.9-10.9) wk], and myocarditis [10.2 (8.6-11.9) wk]. Figure 3A shows that the percentage of tumor burden score (TBS) changed from baseline by treatment of 91 patients who had PD. Figure 3B shows that the percentage of TBS changed from baseline by treatment of 99 patients who achieved CR, PR, or SD. We found that those patients who developed myocarditis or hypothyroidism tended to achieve PR and have a more significant decrease in tumor burden. In addition, patients without irAEs were more likely to enter a PD status. Figure 4 shows a spider plot which depicts the percentage change in TBS of 25 hypothyroidism patients from baseline by treatment over time. We observed substantial reductions in tumor burden and several responders exhibited deep responses.
| Adverse event | Any grade | Grade 3 | Grade 4 |
| Cutaneous toxic effects | 25 (13.2) | 3 (1.6) | 2 (1.1) |
| Mucositis | 8 (4.2) | 2 (1.1) | 1 (< 1) |
| Rash | 7 (3.7) | 1 (< 1) | 1 (< 1) |
| Pruritus | 10 (5.3) | 2 (1.1) | 0 (0) |
| Diarrhea | 27 (14.2) | 4 (2.1) | 1 (< 1) |
| Elevated transaminases | 35 (18.4) | 10 (5.3) | 2 (1.1) |
| Hypothyroidism | 25 (13.2) | 4 (2.1) | 2 (1.1) |
| Myocarditis | 3 (1.6) | 1 (< 1) | 2 (1.1) |
| Hypoadrenocorticism | 5 (2.6) | 1 (< 1) | 0 (0) |
| Proteinuria | 18 (9.5) | 5 (2.6) | 0 (0) |
| Overall incidence | 138 (72.6) | 12 (6.3) | 7 (3.7) |
In order to determine independent risk factors affecting OS, the clinical parameters including demographics, laboratory results, and adverse events were included in the univariate and multivariate Cox proportional regression analyses. We found that Child-Pugh class B [hazard ratio (HR) = 1.321; 95%CI: 1.112-1.711], portal vein tumor thrombus (PVTT) (HR = 3.125; 95%CI: 3.021-3.568), extrahepatic metastasis (HR = 2.871; 95%CI: 2.579-3.052), and hypothyroidism (HR = 0.641; 95%CI: 0.489-0.901) were the independent risk factors for survival. Similarly, AFP > 400 ng/mL (HR = 1.872; 95%CI: 1.357-2.135), PVTT (HR = 2.472; 95%CI: 2.243-2.891), extrahepatic metastasis (HR = 1.489; 95%CI: 1.246-1.574), and irAE of hypothyroidism (HR = 0.613; 95%CI: 0.362-0.886) were the independent risk factors for TTP (Table 3). The OS and TTP of patients who developed hypothyroidism (n = 25) were compared with those patients without irAE using the Kaplan-Meier method and a log-rank test. We found that the median OS was 517 d (95%CI: 423-562 d) in patients with hypothyroidism, which was longer than that of patients without irAE [431 d (95%CI: 412-485 d), P = 0.011] (Figure 5A). Similarly, the median TTP was 125 d (95%CI: 89-154) in patients with hypothyroidism, which was longer than that of patients without irAE [87 d (95%CI: 61-98 d), P = 0.004] (Figure 5B).
| Variable | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| OS | ||||
| Child-Pugh, class B | 1.446 (1.044-1.547) | 0.039 | 1.321 (1.112-1.711) | 0.021 |
| AFP > 400 ng/mL | 1.651 (1.351-1.782) | 0.021 | 1.446 (0.875-1.836) | 0.239 |
| PIVKA > 40 mAU/mL | 1.324 (1.023-1.472) | 0.043 | 1.568 (0.411-1.687) | 0.085 |
| PVTT, yes | 2.145 (1.897-3.587) | 0.003 | 3.125 (3.015-3.568) | 0.008 |
| Extrahepatic metastasis, yes | 1.784 (1.254-2.571) | 0.013 | 2.871 (2.581-3.052) | 0.028 |
| Cutaneous toxic effects, yes | 0.741 (0.654-0.968) | 0.025 | 0.845 (0.425-1.751) | 0.129 |
| Hypothyroidism, yes | 0.623 (0.487-0.912) | 0.034 | 0.641 (0.489-0.901) | 0.017 |
| TTP | ||||
| AFP > 400 ng/mL | 1.757 (1.271-1.972) | 0.023 | 1.872 (1.357-2.135) | 0.017 |
| PVTT, yes | 2.595 (1.377-3.889) | 0.003 | 2.472 (2.243-2.891) | 0.008 |
| Extrahepatic metastasis, yes | 1.536 (1.296-1.765) | 0.031 | 1.489 (1.246-1.574) | 0.012 |
| Cutaneous toxic effects, yes | 0.874 (0.621-0.925) | 0.036 | 0.785 (0.358-1.258) | 0.157 |
| Hypothyroidism, yes | 0.741 (0.514-0.870) | 0.024 | 0.613 (0.362-0.886) | 0.018 |
Previous study has reported that among the irAEs manifesting as endocrine dysfunctions, hypothyroidism (6.07%) and hyperthyroidism (2.82%) were most common[19]. Thyroid events occur in approximately 10% of patients treated with anti-programmed death 1 (PD-1)/programmed death-ligand 1 (PD-L1) monotherapy[20,21]. The median time to onset of thyroid dysfunction, most of which is hypothyroidism, is 6 wk after ICI initiation[22]. In our study, we found that patients who developed hypothyroidism had a longer OS and TTP than those without irAE. Multivariate analysis showed that hypothyroidism was an independent prognostic factor. In addition, we found that patients with hypothyroidism had a significant reduction in TBS from baseline by treatment, which was an intuitive manifestation of the effectiveness of immunotherapy. Many previous studies have also shown that patients who experienced irAEs had a superior progression free survival and OS compared to those who did not experience irAEs[23-25]. A study of 270 non-small cell lung cancer (NSCLC) patients treated with at least one dose of anti-PD-L1 or anti-PD-1 antibodies showed that patients who experienced thyroiditis had statistically significant improvements in OS compared to patients who did not (P = 0.01)[26]. A meta-analysis of 12 randomized controlled trials identified 3815 metastatic head & neck and lung cancer patients treated with ICIs and showed a significant correlation between endocrine irAEs and OS was observed (P = 0.019)[27]. In addition, a retrospective study reviewed 318 advanced melanoma patients and found that patients who experienced irAEs had a superior OS compared to those who did not[13].
Although the precise mechanisms by which irAEs occur have not been fully uncovered, they are thought to represent bystander effects from activated T-cells and are consistent with the mechanism of action of ICIs[14,28]. It is now generally accepted that the pathogenesis of ICI-induced dysthyroidism involves both immune and non-immune mechanisms[29]. It is traditionally believed that thyroperoxidase (TPO) and thyroglobulin (Tg) antibodies may play an important role in mediating thyroiditis. Maekura et al[30] considered that the presence of anti-thyroid antibodies such as TPO and Tg antibodies is a positive predictive factor for developing hypothyroidism in a Japanese cohort of 64 patients with advanced NSCLC treated with nivolumab. Recent study found that the mechanism of thyroid destruction by PD-1 antibodies may be mediated by T cells, natural killer (NK) cells, and monocytes[31-33]. Delivanis et al[31] showed that patients treated with anti-PD-1 therapy had an increasing circulating number of CD56+CD16+ NK cells and high HLA-DR surface expression in CD14+ CD16+ monocytes that mediate the inflammation. In addition, Das et al[34] considered that B lymphocytes also played a role in mediating dysthyroidism. They showed that patients with advanced melanoma treated by a combined checkpoint blockade who developed high-grade irAEs, compared to those who did not, had a decreased total peripheral B lymphocyte count with increased plasmablasts and a subset of B lymphocytes[34,35].
There are some limitations to our study. First, the study is retrospective and conducted only in one center; therefore, multiple centers should be evaluated in further studies. Second, the sample size was relatively small so that the sample of irAEs was small, resulting in large confidence intervals and imprecise results. Third, the effect of different ICI agents on adverse reactions and prognosis in patients was not strictly excluded in this study. Fourth, our study did not include those patients with autoimmune disease, meaning that the correlation between irAEs and prognosis in patients with autoimmune disease needs further exploration. Finally, some adverse events which tended to be ignored, such as fever, weakness, and anorexia, were not recorded, resulting in a lower incidence of irAEs than in previous studies.
In conclusion, dysthyroidism is the most common irAE related to a good prognosis and involves T and B-lymphocytes, multiple cytokines, and diverse factors. Further clinical and laboratory studies should be conducted to clarify the mechanism of ICI-related dysthyroidism. Additionally, the clinical diagnosis and management of thyroid irAEs should be enhanced to avoid life-threatening complications. In addition, the long-term effects of ICIs on dysthyroidism should be further researched to better understand thyroid irAEs and autoimmune thyroid diseases.
Unresectable hepatocellular carcinoma (HCC).
Immune-related adverse events (irAEs) have a high incidence in immune checkpoint inhibitor (ICI) treatment of unresectable HCC. The relationship between irAEs and treatment outcomes in ICI-treated unresectable HCC patients remains unknown.
A retrospective study was conducted to elucidate the correlation between immune-related toxic effects and prognosis in patients with unresectable HCC treated with pembrolizumab.
A total of 190 unresectable HCC (Barcelona Clinic Liver Cancer stage C) patients receiving pembrolizumab treatment were retrospectively reviewed. All irAEs were reviewed and the relationship between irAEs and prognosis was analyzed.
In our study, we found that the overall incidence of irAEs was 72.6% (138/190) and 10.0% of them were severe irAEs (grade ≥ 3); elevated transaminase (> 3 times upper limit of normal) was the most common adverse reaction to ICIs. Patients who developed myocarditis or hypothyroidism tended to achieve partial response and have a more significant decrease in tumor burden. In addition, patients without irAEs were more likely to have progressive disease. It suggested that irAEs are indeed closely related to antitumor effects. In addition, hypothyroidism was the independent risk factors for time to progression and overall survival.
irAEs, especially hypothyroidism, could be used as an indicator to evaluate the effect of immunotherapy.
The study could help doctors in identifying patients who are responding to immunotherapy. In general, the response rate to both tyrosine kinase inhibitors and ICIs is less than 30%. Serious adverse events may put patients at risk of death. Therefore, timely identification of the right patients can not only reduce the side effects of immunotherapy but also improve the effectiveness of treatment.
The authors would like to thank Chang Shu (Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology) for his assistance with statistical analysis.
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21466] [Article Influence: 1951.5] [Reference Citation Analysis (6)] |
| 2. | Li J, Huang L, Liu C, Qiu M, Yan J, Yan Y, Wei S. Risk factors and clinical outcomes of extrahepatic recurrence in patients with post-hepatectomy recurrent hepatocellular carcinoma. ANZ J Surg. 2021;91:1174-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3371] [Article Influence: 481.6] [Reference Citation Analysis (45)] |
| 4. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10533] [Article Influence: 585.2] [Reference Citation Analysis (9)] |
| 5. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 4116] [Article Influence: 514.5] [Reference Citation Analysis (5)] |
| 6. | Herzog TJ, Secord AA, Coleman RL, Naumann RW. European society of medical oncology (ESMO) 2019 meeting report features practice changing data in gynecologic malignancies. Gynecol Oncol. 2020;156:265-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 7. | Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M; KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1982] [Article Influence: 247.8] [Reference Citation Analysis (0)] |
| 8. | Otsuka T, Eguchi Y, Kawazoe S, Yanagita K, Ario K, Kitahara K, Kawasoe H, Kato H, Mizuta T; Saga Liver Cancer Study Group. Skin toxicities and survival in advanced hepatocellular carcinoma patients treated with sorafenib. Hepatol Res. 2012;42:879-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Vincenzi B, Santini D, Russo A, Addeo R, Giuliani F, Montella L, Rizzo S, Venditti O, Frezza AM, Caraglia M, Colucci G, Del Prete S, Tonini G. Early skin toxicity as a predictive factor for tumor control in hepatocellular carcinoma patients treated with sorafenib. Oncologist. 2010;15:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 144] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Pastore S, Mascia F, Mariotti F, Dattilo C, Mariani V, Girolomoni G. ERK1/2 regulates epidermal chemokine expression and skin inflammation. J Immunol. 2005;174:5047-5056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 153] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Darnell EP, Mooradian MJ, Baruch EN, Yilmaz M, Reynolds KL. Immune-Related Adverse Events (irAEs): Diagnosis, Management, and Clinical Pearls. Curr Oncol Rep. 2020;22:39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 289] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 12. | Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 511] [Cited by in RCA: 533] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 13. | Quach HT, Dewan AK, Davis EJ, Ancell KK, Fan R, Ye F, Johnson DB. Association of Anti-Programmed Cell Death 1 Cutaneous Toxic Effects With Outcomes in Patients With Advanced Melanoma. JAMA Oncol. 2019;5:906-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 14. | Passat T, Touchefeu Y, Gervois N, Jarry A, Bossard C, Bennouna J. [Physiopathological mechanisms of immune-related adverse events induced by anti-CTLA-4, anti-PD-1 and anti-PD-L1 antibodies in cancer treatment]. Bull Cancer. 2018;105:1033-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | Llovet JM, Lencioni R. mRECIST for HCC: Performance and novel refinements. J Hepatol. 2020;72:288-306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 503] [Cited by in RCA: 498] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 16. | Sasaki K, Morioka D, Conci S, Margonis GA, Sawada Y, Ruzzenente A, Kumamoto T, Iacono C, Andreatos N, Guglielmi A, Endo I, Pawlik TM. The Tumor Burden Score: A New "Metro-ticket" Prognostic Tool For Colorectal Liver Metastases Based on Tumor Size and Number of Tumors. Ann Surg. 2018;267:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 368] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 17. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6630] [Article Influence: 442.0] [Reference Citation Analysis (1)] |
| 18. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4563] [Article Influence: 325.9] [Reference Citation Analysis (5)] |
| 19. | Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X, Shen C, Duma N, Vera Aguilera J, Chintakuntlawar A, Price KA, Molina JR, Pagliaro LC, Halfdanarson TR, Grothey A, Markovic SN, Nowakowski GS, Ansell SM, Wang ML. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA Oncol. 2019;5:1008-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 665] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 20. | Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, Smylie M, Dummer R, Hill A, Hogg D, Haanen J, Carlino MS, Bechter O, Maio M, Marquez-Rodas I, Guidoboni M, McArthur G, Lebbé C, Ascierto PA, Long GV, Cebon J, Sosman J, Postow MA, Callahan MK, Walker D, Rollin L, Bhore R, Hodi FS, Larkin J. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017;377:1345-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2362] [Cited by in RCA: 2873] [Article Influence: 319.2] [Reference Citation Analysis (6)] |
| 21. | Kassi E, Angelousi A, Asonitis N, Diamantopoulos P, Anastasopoulou A, Papaxoinis G, Kokkinos M, Giovanopoulos I, Kyriakakis G, Petychaki F, Savelli A, Benopoulou O, Gogas H. Endocrine-related adverse events associated with immune-checkpoint inhibitors in patients with melanoma. Cancer Med. 2019;8:6585-6594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Tan MH, Iyengar R, Mizokami-Stout K, Yentz S, MacEachern MP, Shen LY, Redman B, Gianchandani R. Spectrum of immune checkpoint inhibitors-induced endocrinopathies in cancer patients: a scoping review of case reports. Clin Diabetes Endocrinol. 2019;5:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 23. | Grangeon M, Tomasini P, Chaleat S, Jeanson A, Souquet-Bressand M, Khobta N, Bermudez J, Trigui Y, Greillier L, Blanchon M, Boucekine M, Mascaux C, Barlesi F. Association Between Immune-related Adverse Events and Efficacy of Immune Checkpoint Inhibitors in Non-small-cell Lung Cancer. Clin Lung Cancer. 2019;20:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 169] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 24. | Sato K, Akamatsu H, Murakami E, Sasaki S, Kanai K, Hayata A, Tokudome N, Akamatsu K, Koh Y, Ueda H, Nakanishi M, Yamamoto N. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer. 2018;115:71-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 320] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 25. | Ricciuti B, Genova C, De Giglio A, Bassanelli M, Dal Bello MG, Metro G, Brambilla M, Baglivo S, Grossi F, Chiari R. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol. 2019;145:479-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 274] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 26. | Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 702] [Cited by in RCA: 867] [Article Influence: 123.9] [Reference Citation Analysis (1)] |
| 27. | Gomes-Lima CJ, Kwagyan J, King F, Fernandez SJ, Burman KD, Veytsman I. A comprehensive meta-analysis of endocrine immune-related adverse events of immune checkpoint inhibitors and outcomes in head and neck cancer and lung cancer. J Clin Oncol. 2019;37:e14096-e14096. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Yoest JM. Clinical features, predictive correlates, and pathophysiology of immune-related adverse events in immune checkpoint inhibitor treatments in cancer: a short review. Immunotargets Ther. 2017;6:73-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 134] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 29. | Alhusseini M, Samantray J. Hypothyroidism in Cancer Patients on Immune Checkpoint Inhibitors with anti-PD1 Agents: Insights on Underlying Mechanisms. Exp Clin Endocrinol Diabetes. 2017;125:267-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Maekura T, Naito M, Tahara M, Ikegami N, Kimura Y, Sonobe S, Kobayashi T, Tsuji T, Minomo S, Tamiya A, Atagi S. Predictive Factors of Nivolumab-induced Hypothyroidism in Patients with Non-small Cell Lung Cancer. In Vivo. 2017;31:1035-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Delivanis DA, Gustafson MP, Bornschlegl S, Merten MM, Kottschade L, Withers S, Dietz AB, Ryder M. Pembrolizumab-Induced Thyroiditis: Comprehensive Clinical Review and Insights Into Underlying Involved Mechanisms. J Clin Endocrinol Metab. 2017;102:2770-2780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 202] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 32. | Yamauchi I, Sakane Y, Fukuda Y, Fujii T, Taura D, Hirata M, Hirota K, Ueda Y, Kanai Y, Yamashita Y, Kondo E, Sone M, Yasoda A, Inagaki N. Clinical Features of Nivolumab-Induced Thyroiditis: A Case Series Study. Thyroid. 2017;27:894-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 33. | Neppl C, Kaderli RM, Trepp R, Schmitt AM, Berger MD, Wehrli M, Seiler CA, Langer R. Histology of Nivolumab-Induced Thyroiditis. Thyroid. 2018;28:1727-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Das R, Verma R, Sznol M, Boddupalli CS, Gettinger SN, Kluger H, Callahan M, Wolchok JD, Halaban R, Dhodapkar MV, Dhodapkar KM. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol. 2015;194:950-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 382] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 35. | Liudahl SM, Coussens LM. B cells as biomarkers: predicting immune checkpoint therapy adverse events. J Clin Invest. 2018;128:577-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Nakeep S, Egypt; Shomura M, Japan S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ