Published online Apr 15, 2023. doi: 10.4251/wjgo.v15.i4.632
Peer-review started: December 24, 2022
First decision: January 30, 2023
Revised: February 10, 2023
Accepted: March 15, 2023
Article in press: March 15, 2023
Published online: April 15, 2023
Processing time: 108 Days and 18.2 Hours
Despite recent improvements in the diagnosis and treatment of pancreatic cancer (PC), clinical outcomes remain dismal. Moreover, there are no effective prognostic or predictive biomarkers or options beyond carbohydrate antigen 19-9 for personalized and precise treatment. Circulating tumor cells (CTCs), as a member of the liquid biopsy family, could be a promising biomarker; however, the rarity of CTCs in peripheral venous blood limits their clinical use. Because the first venous drainage of PC is portal circulation, the portal vein can be a more suitable location for the detection of CTCs. Endoscopic ultrasound-guided portal venous sampling of CTCs is both feasible and safe. Several studies have suggested that the detection rate and number of CTCs may be higher in the portal blood than in the peripheral blood. CTC counts in the portal blood are highly associated with hepatic metastasis, recurrence after surgery, and survival. The phenotypic and genotypic properties measured in the captured portal CTCs can help us to understand tumor heterogeneity and predict the prognosis of PC. Small sample sizes and heterogeneous CTC detection methods limit the studies to date. Therefore, a large number of prospective studies are needed to corroborate portal CTCs as a valid biomarker in PC.
Core Tip: Circulating tumor cells (CTCs) are emerging minimally invasive biomarkers for evaluating tumor characteristics; however, limited CTCs are detected in the peripheral blood. Portal venous blood, which does not undergo hepatic filtration, can theoretically harbor a large number of CTCs and can be safely assessed using endoscopic ultrasound. The efficacy of CTCs in portal venous blood have shown encouraging results (i.e., higher detection rate and better prediction of prognosis). Here, we provide an overview of CTCs in portal venous blood in the clinical context and future perspectives to enhance the role of portal CTCs as a valid biomarker in pancreatic cancer.
- Citation: Ko SW, Yoon SB. Clinical implications and perspectives of portal venous circulating tumor cells in pancreatic cancer. World J Gastrointest Oncol 2023; 15(4): 632-643
- URL: https://www.wjgnet.com/1948-5204/full/v15/i4/632.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i4.632
In recent decades, improvements in screening methods, surgical techniques, and the development of novel chemotherapeutic drugs have improved the prognosis of various cancers. However, clinical outcomes of pancreatic cancer (PC) remain dismal, with a 5-year survival rate of approximately 8% in the United States[1]. Several factors, including the absence of symptoms in the early stages and notoriously aggressive cancer biology, act as hurdles for the early diagnosis of PC[2,3]. Therefore, at the time of diagnosis, only 20% or fewer patients with PC are eligible for surgical resection[4]. Furthermore, the lack of a standardized assessment of perioperative recurrence risk and treatment strategies also contributes to the decreased survival rate. In a retrospective study of 957 patients with PC undergoing curative resection, 51.5% reported recurrence within one year after surgery[5]. This result suggests the presence of undetectable micrometastases in resectable PC before surgery despite extensive evaluation. Several randomized controlled trials have evaluated the effectiveness of neoadjuvant chemotherapy in patients with resectable PC; however, its efficacy has been inconsistent between studies[4]. Therefore, there is a need to discover biomarkers that may enable more precise stratification for recurrence after surgery or to determine which patients will benefit from neoadjuvant chemotherapy. However, biomarkers to identify these patients are not currently available.
Serum carbohydrate antigen 19-9 is the most commonly utilized biomarker of PC. Its value for PC is usually confined to treatment response rather than early detection or prognosis prediction because the sensitivity (80%) and specificity (75%) are not sufficient to meet the needs of clinicians[6]. Circulating tumor cells (CTCs), as part of the liquid biopsy family, are regarded as precursors of metastases[7]. CTCs have been evaluated as minimally invasive biomarkers for assessing prognostic indicators, such as progression-free survival (PFS) and overall survival (OS) in various solid tumors[8-11]. However, detecting CTCs in peripheral blood is challenging because approximately one CTC exists per billion blood cells in patients with PC[12]. Particularly in the non-metastatic status, peripheral blood specimens may have a yield too low for clinical value. A previous study revealed that CTCs have dynamic, spatiotemporal localization according to the location of the tumor; therefore, specific targeting of vascular compartments may increase the yield of CTCs[13]. Because blood drainage bypasses the liver first via the portal system in PC, the portal vein may be the most suitable blood vessel for CTC evaluation. The aim of this review was to describe the clinical implications and perspectives of portal venous CTCs in patients with PC.
CTCs are shed from the primary tumor site and can enter the vascular system, ultimately leading to metastasis in distant organs. Various methods and technologies have been introduced for CTC enrichment, isolation, and identification[2,14,15]. These techniques use the unique properties of CTCs, which have different sizes, densities, and electrical charges compared with normal blood cells[16]. Among them, the CellSearch® system (Menarini Silicon Biosystems, Huntingdon Valley, PA, United States) is the only Food and Drug Administration (FDA) approved assay method for CTC detection[2]. It relies on capturing CTCs immunomagnetically using antibodies against epithelial cell adhesion molecules, which are commonly expressed in malignant epithelial cells.
Detecting CTCs in cancerous diseases allows for the identification of high-risk patients who may require more intensive surveillance and treatment. Specifically, CTCs could be a potential prognostic indicator of chemoradiotherapy in gastrointestinal malignancies[17,18]. As with many other solid tumors, the clinical usefulness of a prognostic predictor in PC has been demonstrated in previous studies. In a recent meta-analysis of 19 studies of over 1300 patients with PC, the presence of CTCs in the peripheral venous blood was associated with worse PFS and OS[19]. However, the paucity of CTCs in the peripheral blood considerably limits their use in various clinical settings. PC is one of the malignancies with the least number of CTCs detected by the CellSearch® method in comparison with other tumor entities[20]. Its detection rate was as low as 7%-48% at various stages of PC[2]. A recent study showed that the median number of CTCs was only 4 per milliliter in the peripheral blood of 46 patients with PC[21]. This low value has been attributed to the biophysical characteristics of CTCs and the venous drainage system of the pancreas. The average diameter of CTCs is approximately 25 μm, which is too large to allow them to enter capillary beds (8 μm in diameter)[20]. Furthermore, CTCs shed from the pancreas flow via the portal vein into the liver, and subsequent hepatic filtration could make the detection of CTCs in the peripheral blood very challenging[22,23]. Therefore, there is a need to discover blood sources that are abundant in CTCs theoretically.
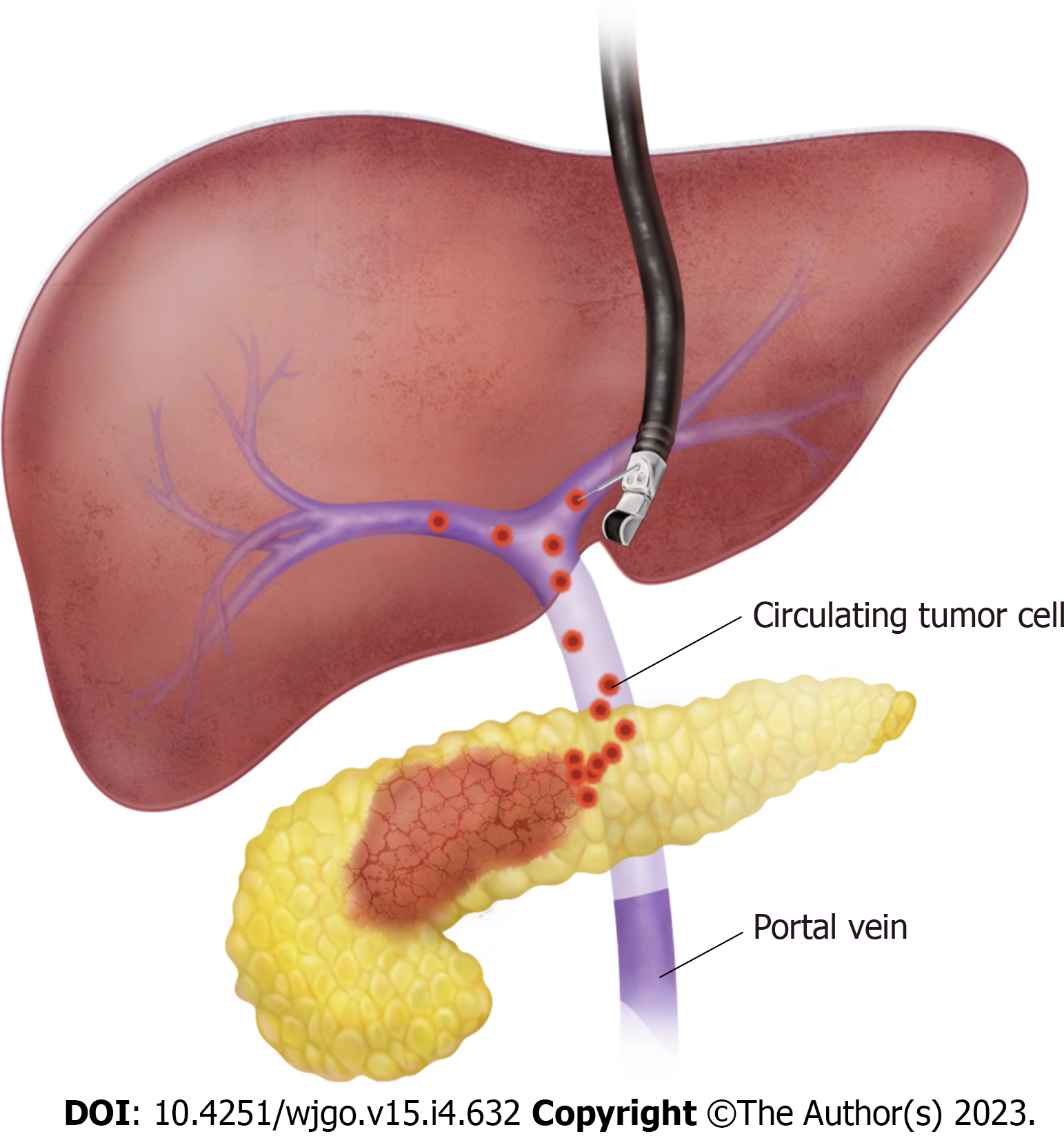
During surgery, portal venous blood can be collected by direct puncture of the extrahepatic portal vein with a syringe. However, non-surgical access to the portal vein is needed for individual risk stratification decision-making before neoadjuvant chemotherapy or surgery. Endoscopic ultrasonography (EUS) was initially introduced as a diagnostic imaging modality. However, the development of a linear echoendoscope in the early 1990s changed the landscape of EUS from a diagnostic to a therapeutic tool[24]. With the development of devices and accessories fit for echoendoscopes, various techniques for abdominal organs were introduced with minimal invasiveness, e.g., EUS-guided fine needle aspiration (FNA), transmural drainage of pancreatic pseudocysts, EUS-guided bile duct and gallbladder drainage, EUS-guided gastrojejunostomy, and EUS-guided celiac plexus/neurolysis[25]. Furthermore, applications of EUS are not limited to visceral organs, but have also been extended to the field of vascular interventions[26]. Owing to its unique proximity and accessibility to the portal vein, various applications for EUS-guided portal vein interventions have been introduced, including EUS-guided FNA of portal vein thrombosis in hepatocellular carcinoma, portal injection chemotherapy, and measurement of portal vein pressure[27]. Importantly, the role of EUS-guided portal venous blood sampling in the detection of CTCs has recently drawn attention. Catenacci et al[22] first reported the feasibility and safety of EUS-guided acquisition of portal venous CTCs in patients with pancreaticobiliary cancer. After verifying the blood flow signal using Doppler ultrasound, the EUS-FNA needle was advanced transhepatically into the portal vein and blood was aspirated safely (Figure 1). It is necessary to pay close attention to the hepatic artery and bile ducts because these structures course together and can potentially lead to complications or inaccurate sampling. It is recommended to use a wide bore needle, such as a 19-G needle, for EUS-guided portal venous sampling to prevent blood clotting and CTC damage[28]. The amount of blood required for CTC isolation and identification is generally between 5 and 10 mL.
The portal vein is the main drainage vessel of the pancreas, and the blood in the portal vein does not undergo hepatic filtration. Therefore, sampling from the portal vein might yield higher concentrations of CTCs than sampling from peripheral blood. Catenacci et al[22] first reported the feasibility and safety of EUS-guided acquisition of portal venous CTCs in 18 patients with pancreaticobiliary cancer. CTCs were detected in all portal vein samples (100%) but only 22% of peripheral blood samples. The median number of CTCs was significantly higher in samples from the portal vein than the peripheral blood (118.4 CTCs/7.5 mL vs 0.8 CTCs/7.5 mL, P < 0.01). These findings were validated in subsequent studies. In 41 patients with PC, the detection rate (58.5% vs 39.0%, P = 0.02) and number of CTCs (mean count, 313.4/3 mL vs 92.9/mL, P < 0.01) were significantly higher in samples from the portal vein than the peripheral blood[29]. Liu et al[23] also evaluated the detection rate and number of CTCs in the portal vein and peripheral blood of 29 patients with advanced or metastatic PC. CTCs were detected in all portal vein blood samples (100%), whereas CTCs were found in only 54% of peripheral blood samples. Furthermore, the mean count of CTCs in the portal venous blood was approximately 10 times higher than that in the peripheral blood (282.0/7.5 mL vs 21.0/7.5 mL, P < 0.01). Similar results were reported by Chapman et al[30]; portal venous blood demonstrated superior outcomes in both the detection rate (100% vs 23.5%) and enumeration (mean count, 118/7.5 mL vs 0.67/7.5 mL) in 17 patients with pancreaticobiliary cancers. Zhang et al[31] and Choi et al[32] reported that the number of CTCs in the portal venous blood was higher than that in the peripheral blood, whereas the detection rates were comparable between the portal and peripheral blood. In a meta-analysis by Pang et al[33] which included five studies that indicated patient-level data[22,23,34-36], the yield of CTCs was 7.7-fold (95%CI: 1.35-43.9) higher in the portal venous blood than in the peripheral blood.
Some studies, however, have presented contradictory results to those of previous studies. In a study of 32 patients with resectable PC, there was no difference in the detection rate between the portal and peripheral veins (62.5% vs 68.8%)[37]. The number of CTCs also did not differ between the two sampling sites in this study. A study by Padillo-Ruiz et al[38] also reported no differences between the portal and peripheral blood in terms of the detection rate (100% vs 100%) and number of CTCs (median 310 vs 405.7, P = 0.239). A comparison of the CTC detection rate and number between the portal vein and peripheral blood in patients with PC is summarized in Table 1.
| Ref. | Patients, N | Cancer stage | Blood source | PoV sample | CTC isolation method | Detection rate, % (n/N) | Number of CTCs (mean ± SD) | Main findings |
| Catenacci et al[22], 2015 | 18 | All | PoV, PV | EUS-guided | CellSearch | PoV: 100 (18/18), PV: 22.2 (4/18) | PoV: 118.4 ± 36.8/7.5 mL, PV: 0.8 ± 0.4/7.5 mL | Both the detection rate and number of CTCs were higher in the PoV than in the PV |
| Tien et al[29], 2016 | 41 | Resectable | PoV, PV | Intraoperative | CMx platform | PoV: 58.5 (24/41), PV: 39.0 (16/41) | PoV: 313.4/3 mL, PV: 92.9/3 mL | Both the detection rate and number of CTCs were higher in the PoV than in the PV |
| Liu et al[23], 2018 | 29 | Locally advanced, metastatic | PoV, PV | Transabdominal US-guided | ClearCell FX system | PoV: 100 (29/29), PV: 54 (8/14) | PoV: 282.0/7.5 mL, PV: 21.0/7.5 mL | Both the detection rate and number of CTCs were higher in the PoV than in the PV |
| Chapman et al[30], 2020 | 171 | All | PoV, PV | EUS-guided | CellSearch | PoV: 100 (17/17), PV: 23.5 (4/17) | PoV: 118.4 (1-516)/7.5 mL2, PV: 0.67 (0-7)/7.5 mL2 | Both the detection rate and number of CTCs were higher in the PoV than in the PV |
| Song et al[37], 2020 | 32 | Resectable | PoV, PV | Intraoperative | Microfabricated Filter | PoV: 62.5 (20/32), PV: 68.8 (22/32) | Not shown | No differences in detection rate and number of CTCs between the PoV and PV |
| Padillo-Ruiz et al[38], 2021 | 35 | Resectable | PoV, CV | Intraoperative | IsoFluxTM | PoV: 100 (35/35), CV: 100 (35/35) | PoV: 310 (132.1-446.0)/mL3, CV: 405.7 (130.7-533.8)/mL3 | No differences in detection rate and number of CTCs between the PoV and CV |
| White et al[40], 2021 | 34 | Resectable | PoV, PV | Intraoperative | CellSearch | PoV: 71 (22/31), PV: 50 (11/22) | Not shown | No differences in detection rate and number of CTCs between the PoV and PV |
| Zhang et al[31], 2021 | 31 | All | PoV, PV | EUS-guided | Cyttel detection kit | PoV: 97 (31/30), PV: 87 (27/31) | PoV: 10/5 mL4, PV: 6/5 mL4 | Number of CTCs was higher in the PoV than in the PV |
| Choi et al[32], 2022 | 33 | All | PoV, PV | Intraoperative | SMART | PoV: 75.8 (25/33), PB: 92.1 (23/28) | PoV: 2.5/7.5 mL4, PV: 1/7.5 mL4 | Number of CTCs was higher in the PoV than in the PV |
The correlation between the number of CTCs in the peripheral blood and tumor burden has been well established in various solid cancers[39]. However, only limited data are available on the correlation between portal CTCs and PC stages. Zhang et al[31] reported that the number of portal venous CTCs, especially the mesenchymal subtype, is positively correlated with advanced PC stages, including stages III and IV. In another study by Choi et al[32], a higher number of CTCs in the portal vein (≥ 3/7.5 mL) is positively correlated with advanced stages and lymph node metastasis. The association between CTCs and tumor differentiation was evaluated by Padillo-Ruiz et al[38] in 35 patients with resectable PC. Patients with poorly differentiated carcinoma had a larger number of CTC clusters than those with well or moderately differentiated types (median 41.0 vs 14.0), although the difference was not significant (P = 0.107).
Several studies have investigated the association between portal venous CTCs and liver metastasis in patients with PC. In a study by Bissolati et al[36] which included 20 patients who underwent surgery for PC, a greater risk of liver metastasis was observed in patients with CTCs in the portal venous blood than in those without CTCs (53% vs 8%, P = 0.038). Tien et al[29] reported a similar result; identification of CTCs in the portal venous blood was the only significant factor for the development of liver metastasis within 6 mo after surgery in 41 patients with resectable PC. A comparable result was also reported in a study that included locally advanced or metastatic PC[23]; patients with liver metastases demonstrated a higher mean number of CTCs than those without metastases (449.0/7.5 mL vs 126.0/7.5 mL, P < 0.01).
The role of portal venous CTCs as prognostic markers for PC has also been evaluated. According to a study by Liu et al[23] which included 29 patients with locally advanced or metastatic PC, OS was significantly shorter in patients with a CTC count ≥ 150/7.5 mL in portal venous blood compared to those with a CTC count ≤ 150/7.5 mL (median OS 9.2 wk vs 19.8 wk, P < 0.01). A similar result was reported by Chapman et al[30]; specifically, patients with portal venous CTCs ≥ 185/7.5 mL had significantly shorter PFS than patients with CTCs < 185/7.5 mL (mean PFS, 12.8 wk vs 43.3 wk, P < 0.01). OS was also unfavorable in patients with higher counts of CTCs; however, the difference was not significant (mean OS, 29.5 wk vs 75.4 wk, P = 0.07). Moreover, a Cox-proportional hazards regression model demonstrated that every 10-cell increase in CTCs in the portal venous blood was associated with an increased likelihood of progression (P = 0.03) and death (P = 0.01) by 5% and 4%, respectively. White et al[40] reported the superiority of portal venous CTCs over peripheral blood for predicting survival. Thirty-one and 22 samples from the portal and peripheral veins, respectively, were collected during PC operation in 34 patients. Patients with ≥ 1 portal venous CTC/7.5 mL had an OS rate of 70% at 18 mo, whereas no deaths were reported in the absence of portal venous CTCs (P < 0.01). However, no correlation was observed between CTCs in the peripheral blood and OS. Similar results were validated by Choi et al[32]: CTCs in the portal vein, but not CTCs in peripheral blood, were a significant predictor of shorter PFS and OS. Zhang et al[31] also reported the prognostic value of portal venous CTCs, indicating that patients with a higher number of CTCs in the portal vein had poorer OS. The studies that analyzed the impact of portal venous CTCs on prognosis of patients with PC are summarized in Table 2.
| Ref. | Patients, N | Cancer stage | Blood source | PoV sample | CTC isolation method | OS, median (95%CI) | PFS, median (95%CI) | Main findings |
| Bissolati et al[36], 2015 | 20 | Resectable | PoV, PV | Intraoperative | CellSearch | CTCs (-): 23.1 (15.1-31.1) mo, CTCs (+): 26.2 (18.7-33.8) mo | CTCs (-): 19.4 (10.9-27.8) mo, CTCs (+): 18.9 (10.4-27.3) mo | PoV CTC-positive patients had a higher rate of liver metastases than CTC-negative patients. |
| Tien et al[29], 2016 | 41 | Resectable | PoV, PV | Intraoperative | CMx platform | Not shown | Not shown | Number of CTCs in the PoV was the only significant risk factor of liver metastases within 6 mo after surgery. |
| Liu et al[23], 2018 | 29 | Locally advanced, metastatic | PoV, PV | Transabdominal US-guided | ClearCell FX system | CTCs < 150/7.5 mL: 19.8 (16.8-25.4) wk, CTCs ≥ 150/7.5 mL: 9.2 (7.8-11.8) wk | Not shown | Higher CTC count in the PoV was associated with liver metastases and shorter OS. |
| Chapman et al[30], 2020 | 14 | All | PoV, PV | EUS-guided | CellSearch | CTCs < 185/7.5 mL: 40.0 wk, CTCs ≥ 185/7.5 mL: 12.8 wk | CTCs < 185/7.5 mL: 75.4 wk, CTCs ≥ 185/7.5 mL: 29.5 wk | Every 10 CTC increase in the PoV was associated with a 5% and 4% increase in the likelihood of progression and death, respectively. |
| Padillo-Ruiz et al[38], 2021 | 35 | Resectable | PoV, CV | Intraoperative | IsoFluxTM | CTCs < 185/mL: 24.5 (19.6-29.4) mo, CTCs ≥ 185/mL: 10.0 (7.4-12.5) mo | Not shown | Higher number of CTCs in the PoV was associated with poorly differentiated cancer and shorter OS. |
| Pan et al[48], 2021 | 32 | Resectable | PoV, PV | Intraoperative | CanPatrolTM | Not shown | Not shown | Mesenchymal CTCs in the PoV ≥ 1/5 mL was a significant risk factor for metastasis, PFS, and OS. |
| White et al[40], 2021 | 34 | Resectable | PoV, PV | Intraoperative | CellSearch | Not shown | Not shown | Patients with undetectable PoV CTCs showed a higher 18-mo survival rate (100%). |
| Zhang et al[31], 2021 | 31 | All | PoV, PV | EUS-guided | Cyttel detection kit | Not shown | Not shown | The number of PoV CTCs, especially mesenchymal CTCs, was positively correlated with the advanced stage. |
| Choi et al[32], 2022 | 33 | All | PoV, PV | Intraoperative | SMART | CTCs < 3/7.5 mL: NA, CTCs ≥ 3/7.5 mL: 16.5 mo | CTCs < 3/7.5 mL: 13.4 mo, CTCs ≥ 3/7.5 mL: 7.5 mo | Higher number of PoV CTCs was associated with higher stage, lymph node metastasis, and poorer PFS and OS. |
| Song et al[37], 2020 | 32 | Resectable | PoV, PV | Intraoperative | Microfabricated Filter | CTCs < 1/10 mL: 40.0 mo, CTCs ≥ 1/10 mL: 17.6 mo | Not shown | CTC count in the PoV was not significantly associated with OS. |
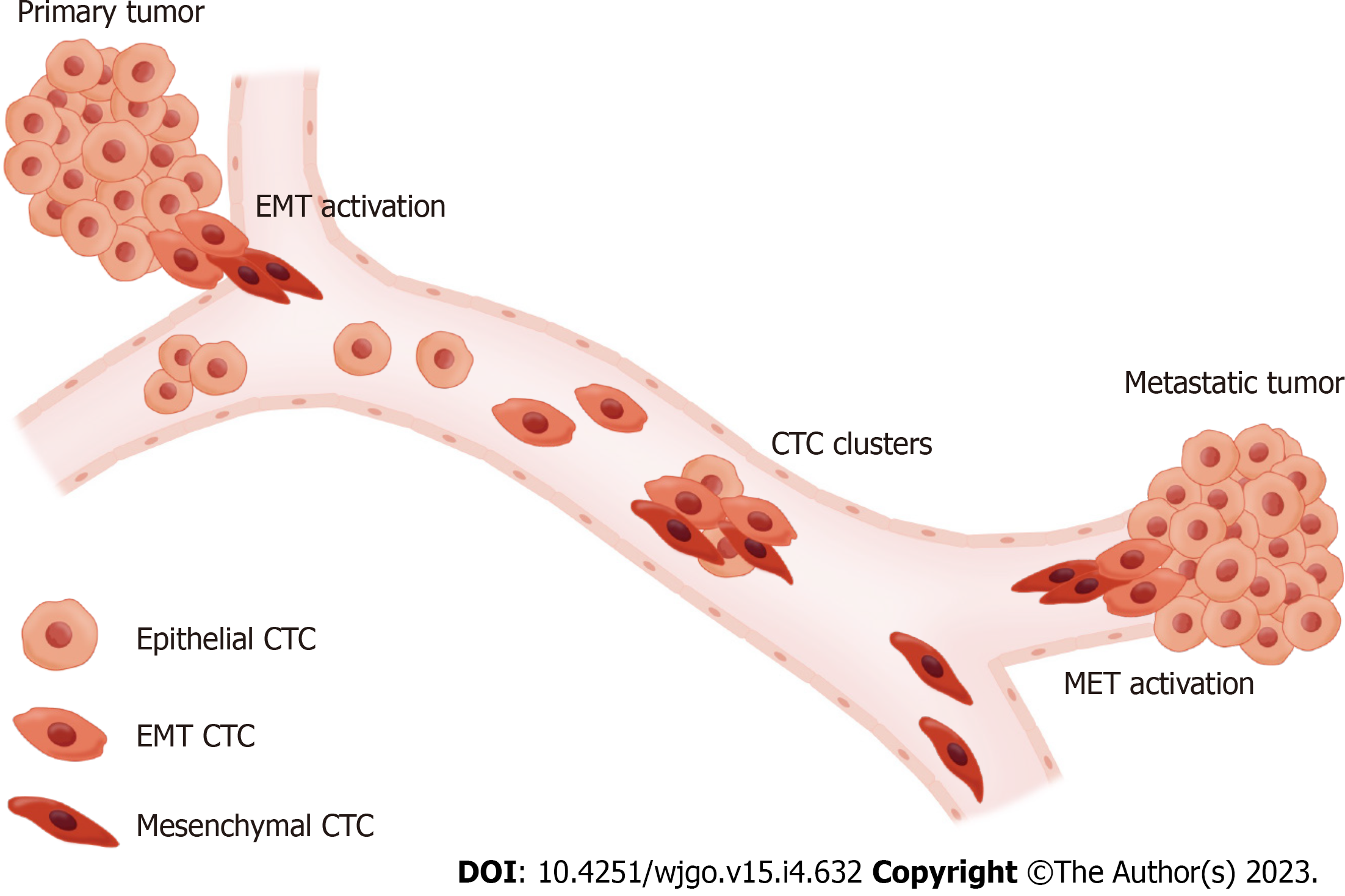
There are various circulating biomarkers for liquid biopsy, including CTCs, circulating tumor deoxyribonucleic acid (ctDNA), ribonucleic acid, and extracellular vesicles[41]. The major advantage of CTCs over other circulating markers is the ability to detect whole tumor cells; this enables the identification of markers associated with prognosis beyond the enumeration of CTCs. The concept of epithelial-mesenchymal transition (EMT) is one of the most important elements in CTC phenotyping. This refers to the process by which tumor cells attached to the basement membrane gain mesenchymal properties, which finally leads to vessel invasion and induces metastasis[42]. Based on this concept, CTCs can be classified into three subpopulations with distinct properties: epithelial CTCs, mesenchymal CTCs (M-CTCs), and epithelial-mesenchymal transition CTCs (EMT-CTCs)[43] (Figure 2). Correlations between disease progression and M-CTCs in solid tumors have been reported previously[44,45]. Zhao et al[46] analyzed the phenotype of CTCs in the peripheral blood of 107 patients with PC. Advanced stage and the presence of distant metastases were significantly associated with M-CTCs. Another study by Semaan et al[47] showed that prognostic variables, such as PFS and OS, were correlated with EMT-CTCs in peripheral blood but not with total CTC counts. Studies on the phenotype of CTCs have also been conducted using portal venous blood samples. According to a study by Pan et al[48] with 32 patients with resectable PC, M-CTCs in the portal vein were found to be a significant risk factor for metastasis-free survival and OS. Similar results were reported by Zhang et al[31]; in 31 patients with PC, a higher count of M-CTCs from portal venous blood was associated with advanced stage, lymph node, and distant metastases. However, this pattern was not observed in a study by Choi et al[32], in which no associations were observed between the phenotype of CTCs from the portal vein and prognosis. Since the abundance of CTCs in portal venous blood has been validated in previous studies, it is expected to be advantageous for phenotyping CTCs from portal venous blood. Further prospective studies with larger numbers of patients are warranted to evaluate the clinical efficacy of CTC phenotyping using portal venous samples.
Genotyping tumors for the identification of genetic mutations has become a routine practice for evaluating patients with certain solid-type cancers[49,50]. However, genotyping from primary tumor tissue has inherent limitations in that single tissue collection has a risk of selection bias from tumor heterogeneity, and acquiring tissue is not always feasible[51]. Therefore, genotypic analysis of CTCs has been conducted to reflect the genomics of primary tumors. In patients with PC, concordance of KRAS mutations in CTCs, which presents in over 90% of PC cases, has been reported repeatedly compared to primary pancreatic tumors[22,37,52]. However, a discordant rate of 42% for KRAS mutations between CTCs and primary tumors was also reported in 59 patients with PC[53]. These conflicting results may represent the natural evolution of metastatic tumors triggered by genomic instability and heterogeneity within the primary tumor[54]. Therefore, testing for other genetic mutations, such as TP53, SMAD4, and P16, which are commonly observed in PC, might be needed to confirm tumor identity[55]. Although the utility of ctDNA for tailored therapies or predicting response has been explored in many studies[56], only limited data regarding genotyping with CTCs for personalized medicine in PC are available to date. Yu et al[57] reported that an increase in SMAD4 expression levels in CTCs was associated with longer PFS and favorable treatment response in 37 patients with PC treated with gemcitabine/nab-paclitaxel. Recently, the FDA approved high-throughput next-generation sequencing-based multigene biopsy that can detect genomic mutations and polymerase chain reaction-based single-gene or multigene assays[58]. Further studies are needed to validate the clinical efficacy of the newly developed genotyping techniques for tailored therapy in patients with PC.
Several unmet needs, including early detection, preoperative risk stratification, and the development of effective and personalized chemotherapeutic agents, should be addressed to improve the prognosis of PC. Recently, the theory of a “three-step procedure” for pancreatic carcinogenesis has been widely adapted to the clinical field, giving rise to the opportunity for early diagnosis and intervention over a long period of time[14]. PC screening relies only on cross-sectional imaging or is accompanied by EUS-guided tissue acquisition in high-risk individuals[59]. To date, there have been no reports regarding the usefulness of CTCs for the early detection or screening of PC. CtDNA, another family of liquid biopsy, has been found to be useful for early PC detection with 64% sensitivity and 99% specificity when combined with well-selected plasma proteins[60]. A relevant study by Cohen et al[60]. to explore the role of CTCs in the early diagnosis of PC is registered and ongoing (ClinicalTrials.gov, NCT0207616). Further studies with larger numbers of patients are warranted to evaluate the clinical efficacy of CTCs in this field. The high number of CTCs in the peripheral blood of patients with lung and breast cancer makes CTC-guided tailored therapy more feasible to study[61-64]. By contrast, the small number of CTCs in peripheral blood is a concern for further studies on CTCs in PC. Therefore, the abundance of CTCs in portal venous blood may play a crucial role in resolving various clinical issues in the future.
Another noteworthy point is the method used to sample CTCs from the portal vein. As described in Tables 1 and 2, intraoperative sampling of portal venous blood rather than the EUS-guided approach has been more dominant. This may mean that patients who undergo portal vein sampling intraoperatively lose the opportunity to be assessed for risk of recurrence before surgery. In the future, EUS-guided portal vein CTC sampling, which preserves the patient’s normal anatomy, should be performed more widely. Standardization of CTC isolation techniques and the development of new assays that can provide clinicians with comprehensive insight into CTC heterogeneity should be investigated further.
CTCs have emerged as a new biomarker for various solid cancers over the past decade. However, their role, especially in the peripheral blood, has been limited in PC. Previous midsize studies demonstrated promising results of CTCs from the portal venous blood with a higher detection rate and better prognosis prediction than those by conventional CTC research from the peripheral blood. In the future, studies with larger numbers of patients are needed to establish the role of CTCs from the portal venous blood in early detection, risk stratification of postoperative recurrence, prediction of treatment resistance, and identification of tumor-specific biomarkers for developing targeted chemotherapeutic agents.
| 1. | Henley SJ, Ward EM, Scott S, Ma J, Anderson RN, Firth AU, Thomas CC, Islami F, Weir HK, Lewis DR, Sherman RL, Wu M, Benard VB, Richardson LC, Jemal A, Cronin K, Kohler BA. Annual report to the nation on the status of cancer, part I: National cancer statistics. Cancer. 2020;126:2225-2249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 557] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 2. | Yeo D, Bastian A, Strauss H, Saxena P, Grimison P, Rasko JEJ. Exploring the Clinical Utility of Pancreatic Cancer Circulating Tumor Cells. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 3. | Neesse A, Bauer CA, Öhlund D, Lauth M, Buchholz M, Michl P, Tuveson DA, Gress TM. Stromal biology and therapy in pancreatic cancer: ready for clinical translation? Gut. 2019;68:159-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 259] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 4. | Zhang HQ, Li J, Tan CL, Chen YH, Zheng ZJ, Liu XB. Neoadjuvant therapy in resectable pancreatic cancer: A promising curative method to improve prognosis. World J Gastrointest Oncol. 2022;14:1903-1917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Groot VP, Gemenetzis G, Blair AB, Rivero-Soto RJ, Yu J, Javed AA, Burkhart RA, Rinkes IHMB, Molenaar IQ, Cameron JL, Weiss MJ, Wolfgang CL, He J. Defining and Predicting Early Recurrence in 957 Patients With Resected Pancreatic Ductal Adenocarcinoma. Ann Surg. 2019;269:1154-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 293] [Article Influence: 48.8] [Reference Citation Analysis (1)] |
| 6. | Yang J, Xu R, Wang C, Qiu J, Ren B, You L. Early screening and diagnosis strategies of pancreatic cancer: a comprehensive review. Cancer Commun (Lond). 2021;41:1257-1274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 223] [Article Influence: 44.6] [Reference Citation Analysis (1)] |
| 7. | Massagué J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature. 2016;529:298-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1056] [Cited by in RCA: 1527] [Article Influence: 152.7] [Reference Citation Analysis (0)] |
| 8. | Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3360] [Cited by in RCA: 3450] [Article Influence: 156.8] [Reference Citation Analysis (0)] |
| 9. | Groot Koerkamp B, Rahbari NN, Büchler MW, Koch M, Weitz J. Circulating tumor cells and prognosis of patients with resectable colorectal liver metastases or widespread metastatic colorectal cancer: a meta-analysis. Ann Surg Oncol. 2013;20:2156-2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Doyen J, Alix-Panabières C, Hofman P, Parks SK, Chamorey E, Naman H, Hannoun-Lévi JM. Circulating tumor cells in prostate cancer: a potential surrogate marker of survival. Crit Rev Oncol Hematol. 2012;81:241-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Krebs MG, Sloane R, Priest L, Lancashire L, Hou JM, Greystoke A, Ward TH, Ferraldeschi R, Hughes A, Clack G, Ranson M, Dive C, Blackhall FH. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol. 2011;29:1556-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 700] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 12. | Ting DT, Wittner BS, Ligorio M, Vincent Jordan N, Shah AM, Miyamoto DT, Aceto N, Bersani F, Brannigan BW, Xega K, Ciciliano JC, Zhu H, MacKenzie OC, Trautwein J, Arora KS, Shahid M, Ellis HL, Qu N, Bardeesy N, Rivera MN, Deshpande V, Ferrone CR, Kapur R, Ramaswamy S, Shioda T, Toner M, Maheswaran S, Haber DA. Single-cell RNA sequencing identifies extracellular matrix gene expression by pancreatic circulating tumor cells. Cell Rep. 2014;8:1905-1918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 341] [Cited by in RCA: 403] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 13. | Sun YF, Guo W, Xu Y, Shi YH, Gong ZJ, Ji Y, Du M, Zhang X, Hu B, Huang A, Chen GG, Lai PBS, Cao Y, Qiu SJ, Zhou J, Yang XR, Fan J. Circulating Tumor Cells from Different Vascular Sites Exhibit Spatial Heterogeneity in Epithelial and Mesenchymal Composition and Distinct Clinical Significance in Hepatocellular Carcinoma. Clin Cancer Res. 2018;24:547-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 14. | Martini V, Timme-Bronsert S, Fichtner-Feigl S, Hoeppner J, Kulemann B. Circulating Tumor Cells in Pancreatic Cancer: Current Perspectives. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 15. | Luo K, Wang X, Zhang X, Liu Z, Huang S, Li R. The Value of Circulating Tumor Cells in the Prognosis and Treatment of Pancreatic Cancer. Front Oncol. 2022;12:933645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Dong Y, Skelley AM, Merdek KD, Sprott KM, Jiang C, Pierceall WE, Lin J, Stocum M, Carney WP, Smirnov DA. Microfluidics and circulating tumor cells. J Mol Diagn. 2013;15:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 17. | Su K, Guo L, He K, Rao M, Zhang J, Yang X, Huang W, Gu T, Xu K, Liu Y, Wang J, Chen J, Wu Z, Hu L, Zeng H, Li H, Tong J, Li X, Yang Y, Liu H, Xu Y, Tan Z, Tang X, Feng X, Chen S, Yang B, Jin H, Zhu L, Li B, Han Y. PD-L1 expression on circulating tumor cells can be a predictive biomarker to PD-1 inhibitors combined with radiotherapy and antiangiogenic therapy in advanced hepatocellular carcinoma. Front Oncol. 2022;12:873830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 18. | Yu E, Allan AL, Sanatani M, Lewis D, Warner A, Dar AR, Yaremko BP, Lowes LE, Palma DA, Raphael J, Vincent MD, Rodrigues GB, Fortin D, Inculet RI, Frechette E, Bierer J, Law J, Younus J, Malthaner RA. Circulating tumor cells detected in follow-up predict survival outcomes in tri-modality management of advanced non-metastatic esophageal cancer: a secondary analysis of the QUINTETT randomized trial. BMC Cancer. 2022;22:746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 19. | Wang Y, Yu X, Hartmann D, Zhou J. Circulating tumor cells in peripheral blood of pancreatic cancer patients and their prognostic role: a systematic review and meta-analysis. HPB (Oxford). 2020;22:660-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897-6904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1810] [Cited by in RCA: 1980] [Article Influence: 94.3] [Reference Citation Analysis (0)] |
| 21. | Poruk KE, Blackford AL, Weiss MJ, Cameron JL, He J, Goggins M, Rasheed ZA, Wolfgang CL, Wood LD. Circulating Tumor Cells Expressing Markers of Tumor-Initiating Cells Predict Poor Survival and Cancer Recurrence in Patients with Pancreatic Ductal Adenocarcinoma. Clin Cancer Res. 2017;23:2681-2690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 22. | Catenacci DV, Chapman CG, Xu P, Koons A, Konda VJ, Siddiqui UD, Waxman I. Acquisition of Portal Venous Circulating Tumor Cells From Patients With Pancreaticobiliary Cancers by Endoscopic Ultrasound. Gastroenterology. 2015;149:1794-1803.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 23. | Liu X, Li C, Li J, Yu T, Zhou G, Cheng J, Li G, Zhou Y, Lou W, Wang X, Gong G, Liu L, Chen Y. Detection of CTCs in portal vein was associated with intrahepatic metastases and prognosis in patients with advanced pancreatic cancer. J Cancer. 2018;9:2038-2045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | Gress FG. The Early History of Interventional Endoscopic Ultrasound. Gastrointest Endosc Clin N Am. 2017;27:547-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Dietrich CF. Controversies in EUS. Endosc Ultrasound. 2021;10:1-2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 26. | Weilert F, Binmoeller KF. EUS-guided vascular access and therapy. Gastrointest Endosc Clin N Am. 2012;22:303-314, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | ASGE Technology Committee; Trikudanathan G, Pannala R, Bhutani MS, Melson J, Navaneethan U, Parsi MA, Thosani N, Trindade AJ, Watson RR, Maple JT. EUS-guided portal vein interventions. Gastrointest Endosc. 2017;85:883-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Chapman CG, Waxman I. EUS-Guided Portal Venous Sampling of Circulating Tumor Cells. Curr Gastroenterol Rep. 2019;21:68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Tien YW, Kuo HC, Ho BI, Chang MC, Chang YT, Cheng MF, Chen HL, Liang TY, Wang CF, Huang CY, Shew JY, Chang YC, Lee EY, Lee WH. A High Circulating Tumor Cell Count in Portal Vein Predicts Liver Metastasis From Periampullary or Pancreatic Cancer: A High Portal Venous CTC Count Predicts Liver Metastases. Medicine (Baltimore). 2016;95:e3407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 30. | Chapman CG, Ayoub F, Swei E, Llano EM, Li B, Siddiqui UD, Waxman I. Endoscopic ultrasound acquired portal venous circulating tumor cells predict progression free survival and overall survival in patients with pancreaticobiliary cancers. Pancreatology. 2020;20:1747-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Zhang Y, Su H, Wang H, Xu C, Zhou S, Zhao J, Shen S, Xu G, Wang L, Zou X, Zhang S, Lv Y. Endoscopic Ultrasound-Guided Acquisition of Portal Venous Circulating Tumor Cells as a Potential Diagnostic and Prognostic Tool for Pancreatic Cancer. Cancer Manag Res. 2021;13:7649-7661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Choi YH, Hong TH, Yoon SB, Lee IS, Lee MA, Choi HJ, Choi MH, Jung ES. Prognostic Implications of Portal Venous Circulating Tumor Cells in Resectable Pancreatic Cancer. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 33. | Pang TCY, Po JW, Becker TM, Goldstein D, Pirola RC, Wilson JS, Apte MV. Circulating tumour cells in pancreatic cancer: A systematic review and meta-analysis of clinicopathological implications. Pancreatology. 2021;21:103-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Vilhav C, Engström C, Naredi P, Novotny A, Bourghardt-Fagman J, Iresjö BM, Asting AG, Lundholm K. Fractional uptake of circulating tumor cells into liver-lung compartments during curative resection of periampullary cancer. Oncol Lett. 2018;16:6331-6338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Poruk KE, Valero V 3rd, Saunders T, Blackford AL, Griffin JF, Poling J, Hruban RH, Anders RA, Herman J, Zheng L, Rasheed ZA, Laheru DA, Ahuja N, Weiss MJ, Cameron JL, Goggins M, Iacobuzio-Donahue CA, Wood LD, Wolfgang CL. Circulating Tumor Cell Phenotype Predicts Recurrence and Survival in Pancreatic Adenocarcinoma. Ann Surg. 2016;264:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 36. | Bissolati M, Sandri MT, Burtulo G, Zorzino L, Balzano G, Braga M. Portal vein-circulating tumor cells predict liver metastases in patients with resectable pancreatic cancer. Tumour Biol. 2015;36:991-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 37. | Song BG, Kwon W, Kim H, Lee EM, Han YM, Byun Y, Lee KB, Lee KH, Lee KT, Lee JK, Jang JY, Park JK. Detection of Circulating Tumor Cells in Resectable Pancreatic Ductal Adenocarcinoma: A Prospective Evaluation as a Prognostic Marker. Front Oncol. 2020;10:616440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 38. | Padillo-Ruiz J, Suarez G, Pereira S, Calero-Castro FJ, Tinoco J, Marin L, Bernal C, Cepeda-Franco C, Alamo JM, Almoguera F, Macher HC, Villanueva P, García-Fernandez FJ, Gallego I, Romero M, Gomez-Bravo MA, Denninghoff V, Serrano MJ. Circulating Tumor Cells Enumeration from the Portal Vein for Risk Stratification in Early Pancreatic Cancer Patients. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 39. | Parkinson DR, Dracopoli N, Petty BG, Compton C, Cristofanilli M, Deisseroth A, Hayes DF, Kapke G, Kumar P, Lee JSh, Liu MC, McCormack R, Mikulski S, Nagahara L, Pantel K, Pearson-White S, Punnoose EA, Roadcap LT, Schade AE, Scher HI, Sigman CC, Kelloff GJ. Considerations in the development of circulating tumor cell technology for clinical use. J Transl Med. 2012;10:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 264] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 40. | White MG, Lee A, Vicente D, Hall C, Kim MP, Katz MHG, Lee JE, Ikoma N, Lucci A, Tzeng CD. Measurement of Portal Vein Blood Circulating Tumor Cells is Safe and May Correlate With Outcomes in Resected Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol. 2021;28:4615-4622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Alix-Panabières C, Pantel K. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 2021;11:858-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 665] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 42. | Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 1057] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 43. | Pastushenko I, Brisebarre A, Sifrim A, Fioramonti M, Revenco T, Boumahdi S, Van Keymeulen A, Brown D, Moers V, Lemaire S, De Clercq S, Minguijón E, Balsat C, Sokolow Y, Dubois C, De Cock F, Scozzaro S, Sopena F, Lanas A, D'Haene N, Salmon I, Marine JC, Voet T, Sotiropoulou PA, Blanpain C. Identification of the tumour transition states occurring during EMT. Nature. 2018;556:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 1119] [Article Influence: 139.9] [Reference Citation Analysis (0)] |
| 44. | Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, Schwabe RF, Vahdat LT, Altorki NK, Mittal V, Gao D. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472-476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1196] [Cited by in RCA: 1471] [Article Influence: 133.7] [Reference Citation Analysis (0)] |
| 45. | Guan X, Ma F, Li C, Wu S, Hu S, Huang J, Sun X, Wang J, Luo Y, Cai R, Fan Y, Li Q, Chen S, Zhang P, Xu B. The prognostic and therapeutic implications of circulating tumor cell phenotype detection based on epithelial-mesenchymal transition markers in the first-line chemotherapy of HER2-negative metastatic breast cancer. Cancer Commun (Lond). 2019;39:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 46. | Zhao XH, Wang ZR, Chen CL, Di L, Bi ZF, Li ZH, Liu YM. Molecular detection of epithelial-mesenchymal transition markers in circulating tumor cells from pancreatic cancer patients: Potential role in clinical practice. World J Gastroenterol. 2019;25:138-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 47. | Semaan A, Bernard V, Kim DU, Lee JJ, Huang J, Kamyabi N, Stephens BM, Qiao W, Varadhachary GR, Katz MH, Shen Y, San Lucas FA, Gascoyne P, Alvarez HA, Maitra A, Guerrero PA. Characterisation of circulating tumour cell phenotypes identifies a partial-EMT sub-population for clinical stratification of pancreatic cancer. Br J Cancer. 2021;124:1970-1977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 48. | Pan Y, Li D, Yang J, Wang N, Xiao E, Tao L, Ding X, Sun P. Portal Venous Circulating Tumor Cells Undergoing Epithelial-Mesenchymal Transition Exhibit Distinct Clinical Significance in Pancreatic Ductal Adenocarcinoma. Front Oncol. 2021;11:757307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | Lamy A, Blanchard F, Le Pessot F, Sesboüé R, Di Fiore F, Bossut J, Fiant E, Frébourg T, Sabourin JC. Metastatic colorectal cancer KRAS genotyping in routine practice: results and pitfalls. Mod Pathol. 2011;24:1090-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 50. | Vanderlaan PA, Yamaguchi N, Folch E, Boucher DH, Kent MS, Gangadharan SP, Majid A, Goldstein MA, Huberman MS, Kocher ON, Costa DB. Success and failure rates of tumor genotyping techniques in routine pathological samples with non-small-cell lung cancer. Lung Cancer. 2014;84:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 51. | Diaz LA Jr, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32:579-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1413] [Cited by in RCA: 1734] [Article Influence: 144.5] [Reference Citation Analysis (0)] |
| 52. | Ankeny JS, Court CM, Hou S, Li Q, Song M, Wu D, Chen JF, Lee T, Lin M, Sho S, Rochefort MM, Girgis MD, Yao J, Wainberg ZA, Muthusamy VR, Watson RR, Donahue TR, Hines OJ, Reber HA, Graeber TG, Tseng HR, Tomlinson JS. Circulating tumour cells as a biomarker for diagnosis and staging in pancreatic cancer. Br J Cancer. 2016;114:1367-1375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 53. | Kulemann B, Rösch S, Seifert S, Timme S, Bronsert P, Seifert G, Martini V, Kuvendjiska J, Glatz T, Hussung S, Fritsch R, Becker H, Pitman MB, Hoeppner J. Pancreatic cancer: Circulating Tumor Cells and Primary Tumors show Heterogeneous KRAS Mutations. Sci Rep. 2017;7:4510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 54. | Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, Stebbings LA, Morsberger LA, Latimer C, McLaren S, Lin ML, McBride DJ, Varela I, Nik-Zainal SA, Leroy C, Jia M, Menzies A, Butler AP, Teague JW, Griffin CA, Burton J, Swerdlow H, Quail MA, Stratton MR, Iacobuzio-Donahue C, Futreal PA. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109-1113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1122] [Cited by in RCA: 1037] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 55. | Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC, Nourse C, Murtaugh LC, Harliwong I, Idrisoglu S, Manning S, Nourbakhsh E, Wani S, Fink L, Holmes O, Chin V, Anderson MJ, Kazakoff S, Leonard C, Newell F, Waddell N, Wood S, Xu Q, Wilson PJ, Cloonan N, Kassahn KS, Taylor D, Quek K, Robertson A, Pantano L, Mincarelli L, Sanchez LN, Evers L, Wu J, Pinese M, Cowley MJ, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chantrill LA, Mawson A, Humphris J, Chou A, Pajic M, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Lovell JA, Merrett ND, Toon CW, Epari K, Nguyen NQ, Barbour A, Zeps N, Moran-Jones K, Jamieson NB, Graham JS, Duthie F, Oien K, Hair J, Grützmann R, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Rusev B, Capelli P, Salvia R, Tortora G, Mukhopadhyay D, Petersen GM; Australian Pancreatic Cancer Genome Initiative, Munzy DM, Fisher WE, Karim SA, Eshleman JR, Hruban RH, Pilarsky C, Morton JP, Sansom OJ, Scarpa A, Musgrove EA, Bailey UM, Hofmann O, Sutherland RL, Wheeler DA, Gill AJ, Gibbs RA, Pearson JV, Waddell N, Biankin AV, Grimmond SM. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2848] [Cited by in RCA: 2729] [Article Influence: 272.9] [Reference Citation Analysis (8)] |
| 56. | Gorgannezhad L, Umer M, Islam MN, Nguyen NT, Shiddiky MJA. Circulating tumor DNA and liquid biopsy: opportunities, challenges, and recent advances in detection technologies. Lab Chip. 2018;18:1174-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 209] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 57. | Yu KH, Ricigliano M, McCarthy B, Chou JF, Capanu M, Cooper B, Bartlett A, Covington C, Lowery MA, O'Reilly EM. Circulating Tumor and Invasive Cell Gene Expression Profile Predicts Treatment Response and Survival in Pancreatic Adenocarcinoma. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Ignatiadis M, Sledge GW, Jeffrey SS. Liquid biopsy enters the clinic - implementation issues and future challenges. Nat Rev Clin Oncol. 2021;18:297-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 822] [Article Influence: 164.4] [Reference Citation Analysis (0)] |
| 59. | Aslanian HR, Lee JH, Canto MI. AGA Clinical Practice Update on Pancreas Cancer Screening in High-Risk Individuals: Expert Review. Gastroenterology. 2020;159:358-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 217] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 60. | Cohen JD, Javed AA, Thoburn C, Wong F, Tie J, Gibbs P, Schmidt CM, Yip-Schneider MT, Allen PJ, Schattner M, Brand RE, Singhi AD, Petersen GM, Hong SM, Kim SC, Falconi M, Doglioni C, Weiss MJ, Ahuja N, He J, Makary MA, Maitra A, Hanash SM, Dal Molin M, Wang Y, Li L, Ptak J, Dobbyn L, Schaefer J, Silliman N, Popoli M, Goggins MG, Hruban RH, Wolfgang CL, Klein AP, Tomasetti C, Papadopoulos N, Kinzler KW, Vogelstein B, Lennon AM. Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers. Proc Natl Acad Sci U S A. 2017;114:10202-10207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 452] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 61. | Königsberg R, Obermayr E, Bises G, Pfeiler G, Gneist M, Wrba F, de Santis M, Zeillinger R, Hudec M, Dittrich C. Detection of EpCAM positive and negative circulating tumor cells in metastatic breast cancer patients. Acta Oncol. 2011;50:700-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 186] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 62. | Miller MC, Doyle GV, Terstappen LW. Significance of Circulating Tumor Cells Detected by the CellSearch System in Patients with Metastatic Breast Colorectal and Prostate Cancer. J Oncol. 2010;2010:617421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 465] [Cited by in RCA: 536] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 63. | Deutsch TM, Riethdorf S, Fremd C, Feisst M, Nees J, Fischer C, Hartkopf AD, Pantel K, Trumpp A, Schütz F, Schneeweiss A, Wallwiener M. HER2-targeted therapy influences CTC status in metastatic breast cancer. Breast Cancer Res Treat. 2020;182:127-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 64. | Tamminga M, de Wit S, Schuuring E, Timens W, Terstappen LWMM, Hiltermann TJN, Groen HJM. Circulating tumor cells in lung cancer are prognostic and predictive for worse tumor response in both targeted- and chemotherapy. Transl Lung Cancer Res. 2019;8:854-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: The Korean Society of Gastroenterology, No. 1-16-2623.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arigami T, Japan; Charalampopoulou A, Italy; Kitamura K, Japan S-Editor: Zhang H L-Editor: A P-Editor: Zhang H