Published online Dec 15, 2023. doi: 10.4251/wjgo.v15.i12.2237
Peer-review started: October 26, 2023
First decision: October 30, 2023
Revised: October 31, 2023
Accepted: November 5, 2023
Article in press: November 5, 2023
Published online: December 15, 2023
Processing time: 48 Days and 20.3 Hours
Owing to the special features of biologics, deficient mismatch repair (dMMR) in patients with colon cancer has achieved little treatment efficacy from chemoradiotherapy. Immunotherapy has shown promising results for the treatment of colon cancer. The high response rate observed suggests a great option for patients presenting with unresectable tumors, as it allows for better oncological resection. Here, we aimed to highlight the significant effects of immunotherapy on dMMR in colon cancer.
A 54-year-old man diagnosed with locally unresectable dMMR colon cancer received preoperative immunotherapy (three cycles of pembrolizumab) and achieved a pathological complete response after surgery.
Immunotherapy can be used as a conversion treatment for locally unresectable colon cancer with dMMR.
Core Tip: Surgery remains the primary radical therapy for colon cancer and resection radicality is one of the most important predictors for survival. Preoperative chemotherapy has been proven to ameliorate resection radicality and survival. Due to the special features of biologics, deficient mismatch repair (dMMR) colon cancer patients achieved little treatment efficacy from chemoradiotherapy. Up to now, immunotherapy has shown promising responses in colon cancer. We aim to draw attention to the significant effect of immunotherapy on dMMR colon cancer.
- Citation: Sun Z, Liu H, Zhang GN, Xiao Y. Conversion immunotherapy for deficient mismatch repair locally unresectable colon cancer: A case report. World J Gastrointest Oncol 2023; 15(12): 2237-2241
- URL: https://www.wjgnet.com/1948-5204/full/v15/i12/2237.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i12.2237
Surgery remains the primary radical therapy for colon cancer[1,2], and resection radicality is an important predictor of local recurrence and overall survival[3]. Owing to the delayed administration of adjuvant chemotherapy, postoperative complications can cause poor oncological outcomes in patients with colorectal cancer (CRC)[4]. Hence, resection radicality should be improved, and the incidence of surgical complications should be reduced. Preoperative chemotherapy improves resection radicality and survival[5-8]. However, owing to the special features of biologics, patients with deficient mismatch repair (dMMR) colon cancer have achieved little treatment efficacy with chemoradiotherapy[9]. Immunotherapy has previously shown promising results for colon cancer[10-13]. Hence, we aimed to draw attention to its significant effect on dMMR in colon cancer. This study was written in compliance with the SCARE Guideline[14].
A 54-year-old man presented to the hospital with difficulty in defecating.
Symptoms started 2 mo before the patient was diagnosed with colon cancer in May 2023.
The patient had undergone colectomy twice owing to a poorly-differentiated adenocarcinoma of the splenic flexure colon (unknown pTNM stage) in 2007 and a moderate- to poorly-differentiated adenocarcinoma of the cecum (pT4aN2a) in 2008. Postoperative adjuvant chemotherapy was administered after both surgeries.
The patient denied any family history of malignant tumors.
Physical examination revealed normal vital signs. The digital anal examination result was also normal.
Laboratory results showed normal carcinoembryonic antigen and carbohydrate antigen 19-9. No abnormality was found in routine blood analyses.
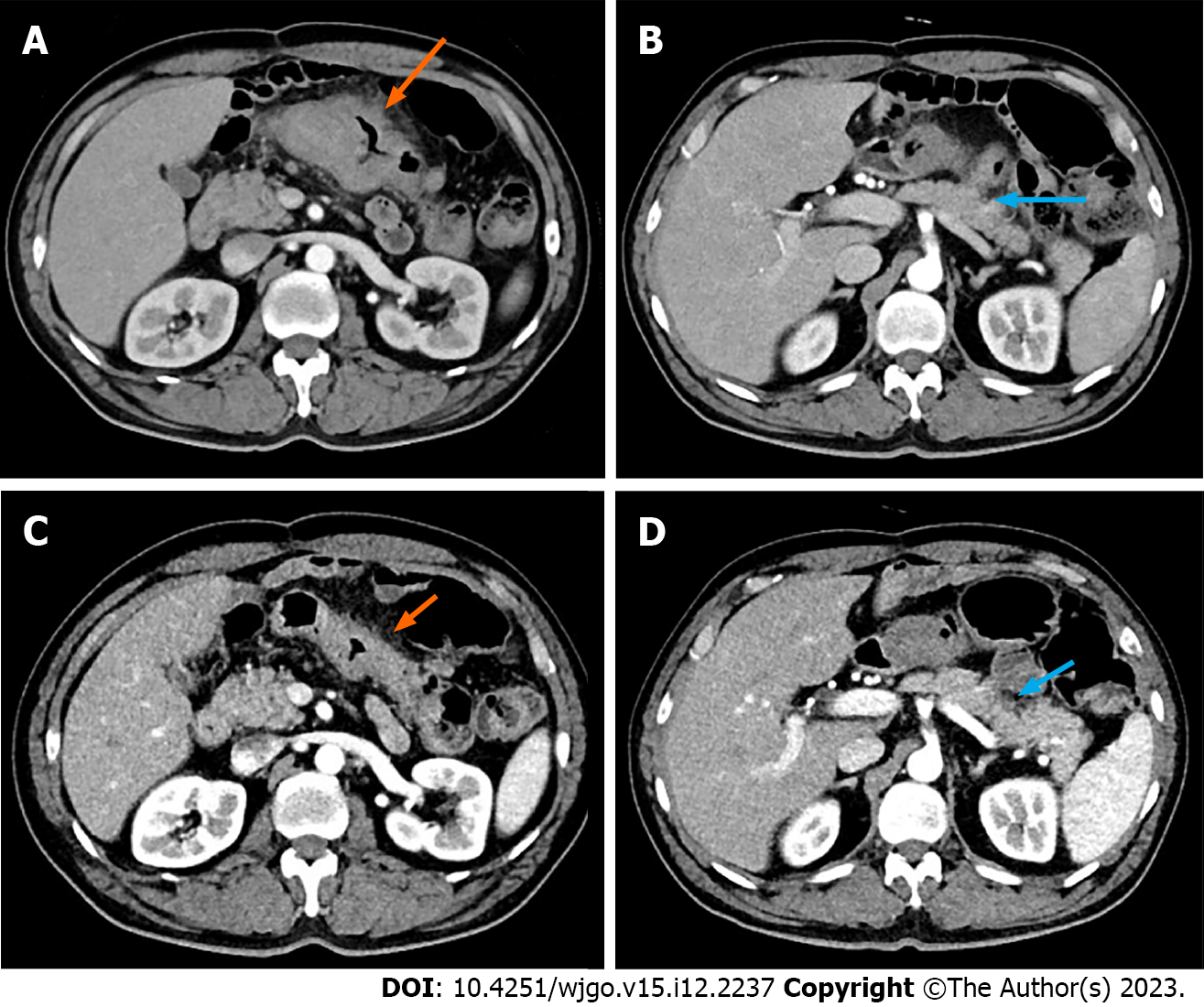
Colonoscopy revealed a large, cauliflower-like mass in the transverse colon. Histopathological examination of the biopsy specimen revealed a poorly-differentiated adenocarcinoma with dMMR. Contrast-enhanced abdominal computed tomography (CT) demonstrated a bulky tumor measuring > 10 cm in the transverse colon (Figure 1A), and several enlarged lymph nodes in the mesentery. CT did not reveal metastases to distant sites.
Considering the invasion of the pancreatic capsule (Figure 1B), negative margins were not ascertained intraoperatively.
Preoperative immunotherapy is recommended for conversion to resection. The patient received three cycles of pembrolizumab immunotherapy without complaints. Post-treatment CT showed significant regression of the tumor (Figure 1C and D), as assessed by our team.
Thus, the patient underwent open transverse colectomy with D2 lymph node dissection two weeks after the end of immunotherapy. The previous ileocolonic anastomosis was resected during surgery.
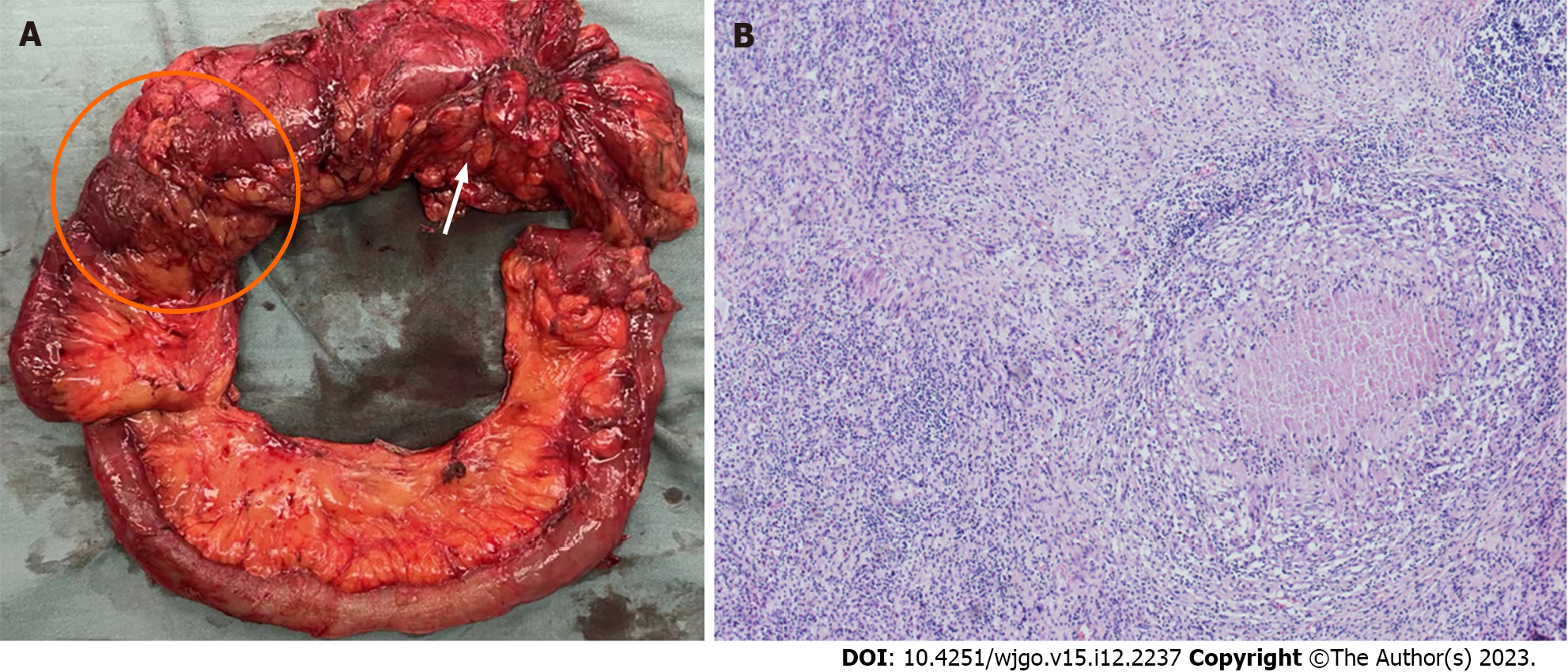
As shown in Figure 2A, gross examination revealed invasion of the serosal surface by the tumor. Interestingly, pathological examination showed that the tumor regression grade was 0, indicating complete regression with no residual tumor cells, including the lymph nodes (Figure 2B). The final stage in this patient was a pathologic complete response (pCR).
Delayed gastric emptying (DGE) occurred, and a transnasal feeding tube was placed for nutritional support. The patient was discharged from the hospital on the 39th day after surgery, when the DGE was alleviated.
To the best of our knowledge, this case is the first to provide evidence of the efficacy of immunotherapy as a conversion regimen for locally unresectable colon cancer with dMMR.
The hallmark of dMMR is the inability to repair spontaneous mutations during DNA replication, leading to hypermutation and increased tumor mutation burden[15]. Currently, dMMR is one of the major predictive biomarkers of the benefits of immune checkpoint inhibitor (ICI) benefit[16]. Thus, MMR status should be regularly tested for colon cancer to guide personalized treatment. ICI therapy aims to overcome tumor immune escape by targeting immune inhibitory molecules expressed on the surfaces of the tumor and immune cells.
Since numerous questions remain regarding dMMR and its impact on the efficacy of immunotherapy, several clinical trials have been launched to determine the optimal treatment for patients with colon cancer. NICHE-1 was the first neoadjuvant immunotherapy study to show pathological responses in 100% of dMMR tumors[10]. The PICC trial[12] reported a pCR rate of 65% in patients with locally advanced dMMR CRC who received immunotherapy. Pei et al[17] reported that 90.9% of patients with locally advanced dMMR CRC achieved pCR after neoadjuvant immunotherapy. The NICHE-2 study subsequently reported that the rates of major pathological response and pCR for locally advanced dMMR colon cancer were 95% and 67%, respectively[11]. It also reported the first survival data in which none of the patients had recurrence at a median follow-up of 13 mo, suggesting the advantage of neoadjuvant immunotherapy. More than the potential survival benefit of immunotherapy, Han et al[18] reported that neoadjuvant immunotherapy significantly reduced open surgery (83.3% vs 72.2%, P < 0.001) and multi-visceral resection rate (P = 0.025) for CRC patients. Hence, this therapy could minimize the extent of surgery and improve postoperative recovery.
The high response rate observed in patients suggests that surgery combined with neoadjuvant immunotherapy is a promising option for CRC surgeons to use in dMMR colon cancers which appear unresectable. This method is also likely to achieve better oncologic resection and organ-sparing strategies. However, the optimal duration of immunotherapy and the timing of surgery remain to be determined. Moreover, a long-term follow-up is required to assess the effects of immunotherapy on the survival of a selected subset of patients with dMMR colon cancer.
Immunotherapy can be used as a conversion treatment for locally unresectable dMMR colon cancer. However, further evidence from clinical trials is required to confirm these findings.
| 1. | Vogel JD, Felder SI, Bhama AR, Hawkins AT, Langenfeld SJ, Shaffer VO, Thorsen AJ, Weiser MR, Chang GJ, Lightner AL, Feingold DL, Paquette IM. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Colon Cancer. Dis Colon Rectum. 2022;65:148-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 235] [Article Influence: 58.8] [Reference Citation Analysis (1)] |
| 2. | Argilés G, Tabernero J, Labianca R, Hochhauser D, Salazar R, Iveson T, Laurent-Puig P, Quirke P, Yoshino T, Taieb J, Martinelli E, Arnold D; ESMO Guidelines Committee. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:1291-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 935] [Article Influence: 155.8] [Reference Citation Analysis (0)] |
| 3. | Chang H, Yu X, Xiao WW, Wang QX, Zhou WH, Zeng ZF, Ding PR, Li LR, Gao YH. Neoadjuvant chemoradiotherapy followed by surgery in patients with unresectable locally advanced colon cancer: a prospective observational study. Onco Targets Ther. 2018;11:409-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Miyamoto Y, Hiyoshi Y, Tokunaga R, Akiyama T, Daitoku N, Sakamoto Y, Yoshida N, Baba H. Postoperative complications are associated with poor survival outcome after curative resection for colorectal cancer: A propensity-score analysis. J Surg Oncol. 2020;122:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | de Gooyer JM, Verstegen MG, 't Lam-Boer J, Radema SA, Verhoeven RHA, Verhoef C, Schreinemakers JMJ, de Wilt JHW. Neoadjuvant Chemotherapy for Locally Advanced T4 Colon Cancer: A Nationwide Propensity-Score Matched Cohort Analysis. Dig Surg. 2020;37:292-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Yuan Y, Xiao WW, Xie WH, Cai PQ, Wang QX, Chang H, Chen BQ, Zhou WH, Zeng ZF, Wu XJ, Liu Q, Li LR, Zhang R, Gao YH. Neoadjuvant chemoradiotherapy for patients with unresectable radically locally advanced colon cancer: a potential improvement to overall survival and decrease to multivisceral resection. BMC Cancer. 2021;21:179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Niu SQ, Li RZ, Yuan Y, Xie WH, Wang QX, Chang H, Lu ZH, Ding PR, Li LR, Wu XJ, Zeng ZF, Xiao WW, Gao YH. Neoadjuvant chemoradiotherapy in patients with unresectable locally advanced sigmoid colon cancer: clinical feasibility and outcome. Radiat Oncol. 2021;16:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 8. | Laursen M, Dohrn N, Gögenur I, Klein MF. Neoadjuvant chemotherapy in patients undergoing colonic resection for locally advanced nonmetastatic colon cancer: A nationwide propensity score matched cohort study. Colorectal Dis. 2022;24:954-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Morton D, Seymour M, Magill L, Handley K, Glasbey J, Glimelius B, Palmer A, Seligmann J, Laurberg S, Murakami K, West N, Quirke P, Gray R; FOxTROT Collaborative Group. Preoperative Chemotherapy for Operable Colon Cancer: Mature Results of an International Randomized Controlled Trial. J Clin Oncol. 2023;41:1541-1552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 279] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 10. | Chalabi M, Fanchi LF, Dijkstra KK, Van den Berg JG, Aalbers AG, Sikorska K, Lopez-Yurda M, Grootscholten C, Beets GL, Snaebjornsson P, Maas M, Mertz M, Veninga V, Bounova G, Broeks A, Beets-Tan RG, de Wijkerslooth TR, van Lent AU, Marsman HA, Nuijten E, Kok NF, Kuiper M, Verbeek WH, Kok M, Van Leerdam ME, Schumacher TN, Voest EE, Haanen JB. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. 2020;26:566-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 1018] [Article Influence: 169.7] [Reference Citation Analysis (0)] |
| 11. | Chalabi M, Verschoor YL, van den Berg JG, Sikorska K, Beets G, Lent AV, Grootscholten MC, Aalbers A, Buller N, Marsman H, Hendriks E, Burger PWA, Aukema T, Oosterling S, Beets-Tan R, Schumacher TN, van Leerdam M, Voest EE, Haanen JBAG. LBA7 Neoadjuvant immune checkpoint inhibition in locally advanced MMR-deficient colon cancer: The NICHE-2 study. Ann Oncol. 2022;33 Suppl 7:S808-S869. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 148] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 12. | Hu H, Kang L, Zhang J, Wu Z, Wang H, Huang M, Lan P, Wu X, Wang C, Cao W, Hu J, Huang Y, Huang L, Shi L, Cai Y, Shen C, Ling J, Xie X, He X, Dou R, Zhou J, Ma T, Zhang X, Luo S, Deng W, Ling L, Liu H, Deng Y. Neoadjuvant PD-1 blockade with toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high, locally advanced, colorectal cancer (PICC): a single-centre, parallel-group, non-comparative, randomised, phase 2 trial. Lancet Gastroenterol Hepatol. 2022;7:38-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 213] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 13. | Verschoor YL, Van den Berg JE, Beets G, Sikorska K, Aalbers A, van Lent A, Grootscholten C, Huibregtse I, Marsman H, Oosterling S, van de Belt M, Kok M, Schumacher T, van Leerdam ME, Haanen JBAG, Voest EE, Chalabi M. Neoadjuvant nivolumab, ipilimumab, and celecoxib in MMR-proficient and MMR-deficient colon cancers: Final clinical analysis of the NICHE study. J Clin Oncol. 2022;40:3511-3511. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Agha RA, Franchi T, Sohrabi C, Mathew G, Kerwan A; SCARE Group. The SCARE 2020 Guideline: Updating Consensus Surgical CAse REport (SCARE) Guidelines. Int J Surg. 2020;84:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4265] [Cited by in RCA: 4757] [Article Influence: 792.8] [Reference Citation Analysis (2)] |
| 15. | Kavun A, Veselovsky E, Lebedeva A, Belova E, Kuznetsova O, Yakushina V, Grigoreva T, Mileyko V, Fedyanin M, Ivanov M. Microsatellite Instability: A Review of Molecular Epidemiology and Implications for Immune Checkpoint Inhibitor Therapy. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 16. | Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS, Zhang M, Papadopoulos N, Kinzler KW, Vogelstein B, Sears CL, Anders RA, Pardoll DM, Housseau F. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 1206] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 17. | Pei F, Wu J, Zhao Y, He W, Yao Q, Huang M, Huang J. Single-Agent Neoadjuvant Immunotherapy With a PD-1 Antibody in Locally Advanced Mismatch Repair-Deficient or Microsatellite Instability-High Colorectal Cancer. Clin Colorectal Cancer. 2023;22:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 18. | Han K, Tang JH, Liao LE, Jiang W, Sui QQ, Xiao BY, Li WR, Hong ZG, Li Y, Kong LH, Li DD, Zhang XS, Pan ZZ, Steele SR, Ding PR. Neoadjuvant Immune Checkpoint Inhibition Improves Organ Preservation in T4bM0 Colorectal Cancer With Mismatch Repair Deficiency: A Retrospective Observational Study. Dis Colon Rectum. 2023;66:e996-e1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Rizzo A, Italy; Shalaby MN, Egypt S-Editor: Yan JP L-Editor: A P-Editor: Yu HG