Published online Dec 15, 2023. doi: 10.4251/wjgo.v15.i12.2093
Peer-review started: September 12, 2023
First decision: September 26, 2023
Revised: October 7, 2023
Accepted: November 25, 2023
Article in press: November 25, 2023
Published online: December 15, 2023
Processing time: 92 Days and 19.9 Hours
Radical surgery is a common treatment for patients with gastric cancer; however, it can lead to postoperative complications and intestinal barrier dysfunction. Ultrasound-guided quadratus lumborum block is often used for postoperative analgesia, but its effects on stress response and intestinal barrier function are not well understood.
To investigate the effects of an ultrasound-guided quadratus lumborum block on stress response and intestinal barrier function in patients undergoing radical surgery for gastric cancer.
A total of 100 patients undergoing radical surgery for gastric cancer were randomly categorized into observation and control groups. Plasma adrenaline and cortisol levels, intestinal mucosal barrier indexes, and complication rates were compared between the two groups before, during, and 1 day after surgery.
The observation group had significantly lower plasma adrenaline and cortisol levels during surgery and at 1 day postoperatively than that of the control group (P < 0.05). Additionally, intestinal barrier indexes (endotoxin and D-dimer) at 1 day postoperatively were significantly lower in the observation group than in the control group (P < 0.05).
Ultrasound-guided quadratus lumborum block could reduce stress response, protect intestinal barrier function, and decrease the incidence of complications in patients undergoing radical surgery for gastric cancer. This technique has the potential for clinical applications.
Core Tip: Ultrasound-guided quadratus lumborum block reduces stress response and preserves intestinal barrier function following radical surgery for gastric cancer, potentially lowering the associated complications. This technique shows promise in providing postoperative analgesia and for improving patient outcomes. It protects the intestinal barrier function and reduces the incidence of complications in patients who undergo radical gastric cancer surgery, highlighting its potential clinical use.
- Citation: Wang XR, Xu DD, Guo MJ, Wang YX, Zhang M, Zhu DX. Effect of ultrasound-guided lumbar square muscle block on stress response in patients undergoing radical gastric cancer surgery. World J Gastrointest Oncol 2023; 15(12): 2093-2100
- URL: https://www.wjgnet.com/1948-5204/full/v15/i12/2093.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i12.2093
Ultrasound-guided quadratus lumborum block exhibited promising effects in reducing stress response and improving intestinal barrier function in patients undergoing radical surgery for gastric cancer[1-5]. The decline in the plasma adrenaline and cortisol levels[5-10], improved intestinal barrier indexes, and reduced incidence of postoperative complications underscores the potential clinical application of this technique[11-15]. However, further studies are needed to optimize its clinical application[16-21], and explore its combination with other interventions to enhance postoperative recovery and minimize complications[22,23].
Ultrasound-guided quadratus lumborum block has made significant progress as a pain management and rehabilitation promotion technique for clinical applications[24-27]. This study investigated the effects of ultrasound-guided quadratus lumborum block on stress response and intestinal barrier function in patients undergoing radical surgery for gastric cancer.
Furthermore, we did not evaluate the onset and maintenance times of the quadratus lumborum block; therefore, further studies are warranted to assess these parameters.
First, we anticipated that the ultrasound-guided quadratus lumborum block group would demonstrate improved outcomes in terms of postoperative physiological indicators and pain scores, reflecting a reduced stress response[28-30]. This could be partly attributed to the analgesic effect of the ultrasound-guided quadratus lumborum block, which diminishes pain transmission at the surgical site through local anesthesia, thereby reducing postoperative pain perception and stress response. Additionally, quadratus lumborum blocks may modulate stress response by blocking sympathetic activity and reducing the release of inflammatory mediators[31-33].
Second, regarding the assessment of intestinal barrier function, we anticipated that the ultrasound-guided quadratus lumborum block group would demonstrate enhanced outcomes[34], including reduced intestinal permeability and inflammatory response[35]. Surgical trauma and stress may lead to intestinal barrier dysfunction, increased intestinal permeability, and an inflammatory response, potentially leading to postoperative complications. Ultrasound-guided quadratus lumborum block may protect intestinal barrier function by reducing the inflammatory response to surgical trauma and maintaining intestinal blood perfusion[36,37]. In addition, some studies have suggested that these blocks may maintain intestinal health by regulating the balance of the intestinal microbiota. However, intestinal barrier function is influenced by a number of factors, with ultrasound-guided quadratus lumborum block being just one of them; other interventions and factors may also have an impact on intestinal barrier function[38,39].
First, the sample size may be limited, necessitating studies with larger sample sizes to further validate the reliability of our results. Second, the clinical application of ultrasound-guided quadratus lumborum blocks may involve the collaboration of multiple medical personnel, potentially introducing differences in operating techniques that could affect the results; hence, uniform operating standards are needed. In addition, this study only focused on the effects of postoperative stress and intestinal barrier function; other clinical outcomes and the long-term prognosis of patients require further investigation.
The plasma adrenaline, cortisol, interleukin-6, and C-reactive protein levels were significantly lower in both the groups after treatment in comparison to their levels before treatment, and the reduction in these indicators was more significant in the observation group than in the control group (P < 0.05, Table 1).
| Pro-adrenaline (pg/L) | Cortisol (ng/L) | IL-6 (ng/L) | C-reactive protein (mg/L) | ||
| Control group (n = 50) | Before treatment | 1.16 ± 0.12 | 24.55 ± 2.54 | 36.47 ± 1.64 | 34.22 ± 1.64 |
| After treatment | 0.88 ± 0.18 | 21.66 ± 2.16 | 30.08 ± 1.72 | 27.84 ± 1.19 | |
| Observation group (n = 50) | Before treatment | 1.15 ± 0.19 | 24.51 ± 2.08 | 36.39 ± 1.66 | 34.35 ± 1.91 |
| After treatment | 0.51 ± 0.09 | 17.08 ± 1.52 | 22.51 ± 1.58 | 16.78 ± 1.49 | |
| t/P | 8.682/< 0.001 | 5.814/< 0.001 | 18.037/< 0.001 | 21.122/< 0.001 | |
| t/P | 20.421/< 0.001 | 19.347/< 0.001 | 40.629/< 0.001 | 48.655/< 0.001 | |
| t/P post-treatment inter-group values | 12.333/< 0.001 | 11.632/< 0.001 | 21.743/< 0.001 | 38.908/< 0.001 |
Compared with the patients in the control group, those in the observation group demonstrated significantly improved outcomes in terms of sufentanil dosage, postoperative awakening time, postoperative time in bed, and time taken for anal discharge (P < 0.05, Table 2).
| Group | n | Sufentanil dosage (μg) | Post-operative awakening time (min) | Postoperative time in bed (h) | Time taken for anal discharge (h) |
| Observation group | 50 | 25.56 ± 4.56 | 14.52 ± 1.72 | 21.26 ± 3.41 | 12.26 ± 1.65 |
| Control group | 50 | 71.12 ± 7.45 | 25.62 ± 2.51 | 30.23 ± 4.56 | 20.17 ± 2.36 |
| t value | 28.380 | 20.533 | 8.861 | 15.458 | 0.000 |
| P value | 0.000 | 0.000 | 0.000 |
The incidence of adverse reactions was significantly lower in the observation group than in the control group (P < 0.05, Table 3).
| Leukopenia | Hepatic impairment | Nausea and vomiting | Bone marrow suppression | Renal impairment | Incidence | |
| Control group (n = 50) | 5 (11.11) | 3 (6.67) | 5 (11.11) | 4 (8.89) | 7 (15.56) | 24 (53.33) |
| Observation group (n = 50) | 2 (4.44) | 1 (2.22) | 1 (2.22) | 1 (2.22) | 2 (4.44) | 7 (15.56) |
| χ2 | 14.221 | |||||
| P value | 0.000 |
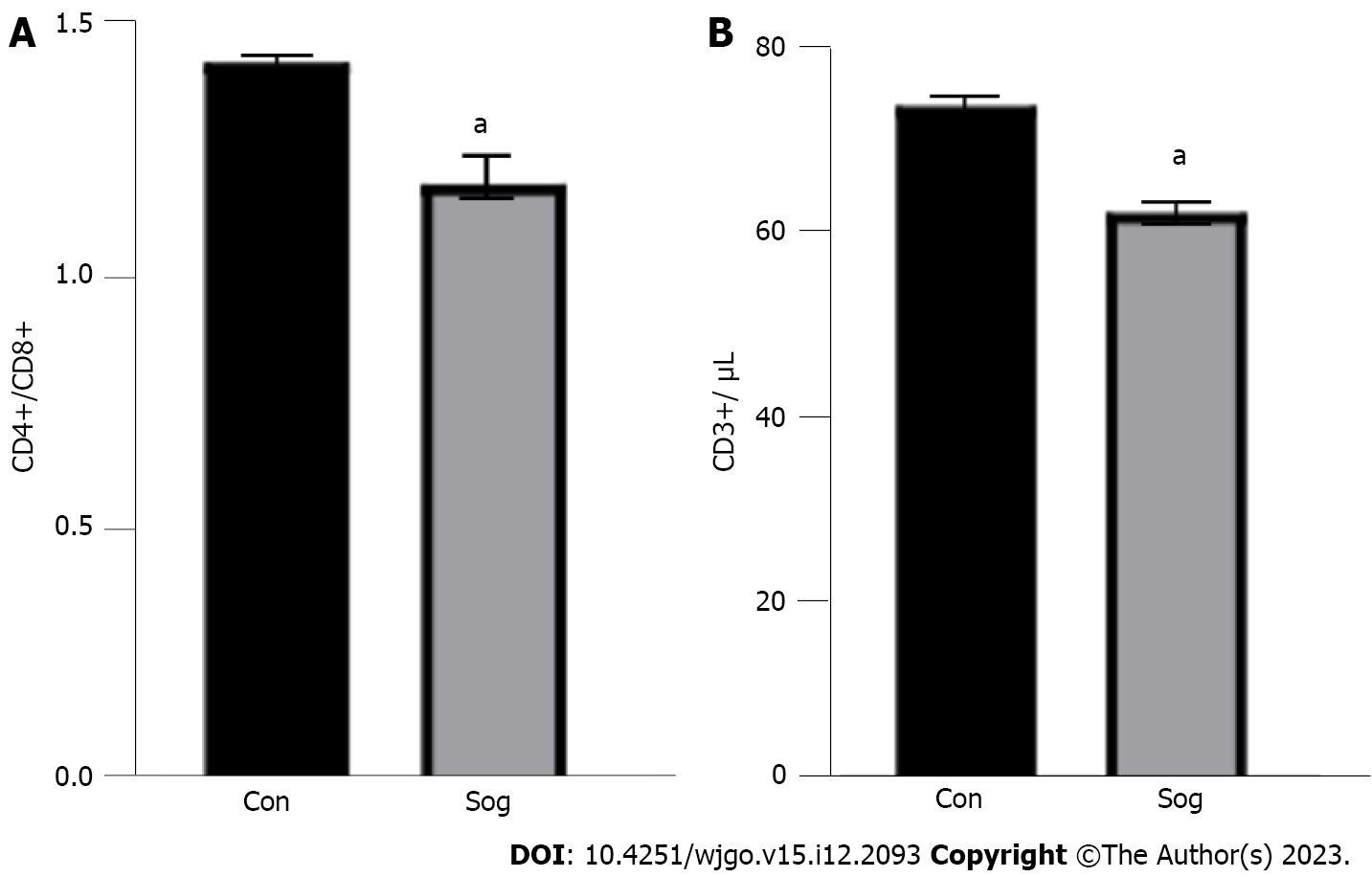
Compared with the control group, the observation group exhibited a significant decrease in the CD4+/CD8+ ratio and CD3+ count, indicating that ultrasound guidance exerts a substantial influence on the body’s immune response and a positive effect on the recovery and prognosis of the disease. In patients undergoing radical gastrectomy, ultrasound-guided quadratus lumborum block, administered at the same dose, could achieve an ideal postoperative analgesic effect and significantly reduce postoperative immune function suppression (Figure 1).
A total of 100 patients with gastric cancer who underwent radical surgery between February 2019 and July 2020 were enrolled in this study[40]. The inclusion criteria were as follows: (1) Confirmation of gastric cancer through pathological examination; (2) absence of allergies to anesthetic drugs; and (3) absence of contraindication for surgery or use of drugs affecting neuromuscular function. The patients were randomly categorized into two groups using the random number table method. The observation group included 50 patients (28 male and 22 female), aged 35–58 years (mean: 46.24 ± 1.63 years), with ASA classification grade I in 31 and grade II in 19 patients. The control group comprised 50 patients (27 male and 23 female), aged 33–58 years (mean: 45.98 ± 1.58 years), with ASA classification grade I in 32 and grade II in 18 patients.
Before surgery patients in both the groups were prepared by administering oxygen at room air; promptly establishing intravenous access; and assessing vital signs, including electrocardiography, heart rate, and blood pressure.
In the control group, the anesthetic induction protocol comprised 0.05 mg/kg imipramine + 0.3 μg/kg sufentanil + 2.0 mg/kg propofol + 0.6 mg/kg rocuronium bromide. In the observation group, an ultrasound-guided quadratus lumborum block was performed alongside the standard anesthesia protocol. Postoperatively, both the groups were connected to a self-controlled analgesic pump delivering a mixture of 2 μg/kg sufentanil + 8 mg ondansetron mixed with 100 mL saline; the pump was devoid of a background dose, and delivered a single-pressed dose of 2 mL with a lock time of 15 min.
The plasma epinephrine and cortisol levels, as well as indicators of intestinal mucosal barrier were compared between the two groups before, during, and one day postsurgery. The amount of sufentanil in both the groups was quantified, and the postoperative parameters, including awakening time, time in bed, and time taken for anal discharge were recorded in both the groups.
Additionally, the complication rate was determined at the time of discharge, and patient satisfaction was assessed using a self-designed questionnaire.
Statistical analyses were performed using the SPSS 21.0 software. The t-test was used to analyze measurement data, whereas the χ2 test was used for count data. A P value of < 0.05 indicated a statistically significant difference.
Gastric cancer is a common malignancy, and radical surgery for gastric cancer is one of the main treatment modalities. Surgical trauma and stress reactions may lead to postoperative complications and intestinal barrier dysfunction. Ultrasound-guided quadratus lumborum block is widely used for providing postoperative analgesia and promoting recovery; however, its effects on stress response and intestinal barrier function are unclear. The laparoscopic approach offers many advantages, such as less trauma, faster postoperative recovery, and fewer postoperative complications; however, surgery inevitably causes stress and unavoidable postoperative pain; therefore, a suitable surgical anesthetic is needed to ensure a successful outcomes of surgery. In the past, laparoscopic surgery for gastric cancer has often been performed under general anesthesia, yielding good results; however, general anesthesia can easily lead to central sensitization, and require a considerable amount of analgesic drugs during surgery. The use of large amounts of analgesic drugs during surgery potentially cause nausea, vomiting, intestinal paralysis, and other adverse reactions, which can easily affect the smooth implementation of surgery and may even lead to failure. With the advancements in ultrasound technology and anesthesia practices, studies have demonstrated that the combination of an ultrasound-guided anterior lumbar muscle block with conventional general anesthesia during laparoscopic surgery can achieve improved satisfactory anesthetic and analgesic effects. However, its efficacy in reducing the use of analgesics and promoting postoperative recovery remains unclear. In this study, we aimed to investigate the effects of ultrasound-guided quadratus lumborum combined with general anesthesia in laparoscopic surgery for gastric cancer to provide a valuable reference for those involved in such procedures.
Future studies should focus on optimizing the clinical application of ultrasound-guided quadratus lumborum block and exploring its efficacy in combination with other interventions to enhance postoperative recovery and minimize complications.
To investigate the effects of an ultrasound-guided quadratus lumborum block on stress response and intestinal barrier function in patients with gastric cancer.
The observation group exhibited significantly lower plasma adrenaline and cortisol levels during surgery and on the first day postoperatively compared to the control group (P < 0.05).
A total of 100 patients who underwent radical surgery for gastric cancer were randomly assigned to either the observation or the control group (50 patients each). Plasma adrenaline and cortisol levels, intestinal mucosal barrier indexes, and complication rates were compared between the two groups before, during, and on the first day after surgery.
The observation group exhibited significantly lower plasma adrenaline and cortisol levels during surgery and on the first day postoperatively compared to the control group (P < 0.05).
Ultrasound-guided quadratus lumborum block aids in preserving intestinal barrier function and reducing the incidence of postoperative complications, thereby demonstrating its potential clinical applicability.
Radical surgery for gastric cancer can lead to postoperative complications and intestinal barrier dysfunction owing to surgical trauma and stress.
| 1. | Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4091] [Cited by in RCA: 6112] [Article Influence: 407.5] [Reference Citation Analysis (0)] |
| 2. | Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, Tabarin A, Terzolo M, Tsagarakis S, Dekkers OM. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016;175:G1-G34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1045] [Cited by in RCA: 1063] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 3. | Zeiger MA, Thompson GB, Duh QY, Hamrahian AH, Angelos P, Elaraj D, Fishman E, Kharlip J; American Association of Clinical Endocrinologists; American Association of Endocrine Surgeons. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons Medical Guidelines for the Management of Adrenal Incidentalomas: executive summary of recommendations. Endocr Pract. 2009;15:450-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 233] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 4. | Dinnes J, Bancos I, Ferrante di Ruffano L, Chortis V, Davenport C, Bayliss S, Sahdev A, Guest P, Fassnacht M, Deeks JJ, Arlt W. MANAGEMENT OF ENDOCRINE DISEASE: Imaging for the diagnosis of malignancy in incidentally discovered adrenal masses: a systematic review and meta-analysis. Eur J Endocrinol. 2016;175:R51-R64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 5. | Ichijo T, Ueshiba H, Nawata H, Yanase T. A nationwide survey of adrenal incidentalomas in Japan: the first report of clinical and epidemiological features. Endocr J. 2020;67:141-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 6. | Ebbehoj A, Li D, Kaur RJ, Zhang C, Singh S, Li T, Atkinson E, Achenbach S, Khosla S, Arlt W, Young WF, Rocca WA, Bancos I. Epidemiology of adrenal tumours in Olmsted County, Minnesota, USA: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8:894-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 222] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 7. | Hong AR, Kim JH, Park KS, Kim KY, Lee JH, Kong SH, Lee SY, Shin CS, Kim SW, Kim SY. Optimal follow-up strategies for adrenal incidentalomas: reappraisal of the 2016 ESE-ENSAT guidelines in real clinical practice. Eur J Endocrinol. 2017;177:475-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Cyranska-Chyrek E, Szczepanek-Parulska E, Olejarz M, Ruchala M. Malignancy Risk and Hormonal Activity of Adrenal Incidentalomas in a Large Cohort of Patients from a Single Tertiary Reference Center. Int J Environ Res Public Health. 2019;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Bancos I, Taylor AE, Chortis V, Sitch AJ, Jenkinson C, Davidge-Pitts CJ, Lang K, Tsagarakis S, Macech M, Riester A, Deutschbein T, Pupovac ID, Kienitz T, Prete A, Papathomas TG, Gilligan LC, Bancos C, Reimondo G, Haissaguerre M, Marina L, Grytaas MA, Sajwani A, Langton K, Ivison HE, Shackleton CHL, Erickson D, Asia M, Palimeri S, Kondracka A, Spyroglou A, Ronchi CL, Simunov B, Delivanis DA, Sutcliffe RP, Tsirou I, Bednarczuk T, Reincke M, Burger-Stritt S, Feelders RA, Canu L, Haak HR, Eisenhofer G, Dennedy MC, Ueland GA, Ivovic M, Tabarin A, Terzolo M, Quinkler M, Kastelan D, Fassnacht M, Beuschlein F, Ambroziak U, Vassiliadi DA, O'Reilly MW, Young WF Jr, Biehl M, Deeks JJ, Arlt W; ENSAT EURINE-ACT Investigators. Urine steroid metabolomics for the differential diagnosis of adrenal incidentalomas in the EURINE-ACT study: a prospective test validation study. Lancet Diabetes Endocrinol. 2020;8:773-781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 171] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 10. | Cawood TJ, Hunt PJ, O'Shea D, Cole D, Soule S. Recommended evaluation of adrenal incidentalomas is costly, has high false-positive rates and confers a risk of fatal cancer that is similar to the risk of the adrenal lesion becoming malignant; time for a rethink? Eur J Endocrinol. 2009;161:513-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 201] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 11. | Cho YY, Suh S, Joung JY, Jeong H, Je D, Yoo H, Park TK, Min YK, Kim KW, Kim JH. Clinical characteristics and follow-up of Korean patients with adrenal incidentalomas. Korean J Intern Med. 2013;28:557-564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Elhassan YS, Alahdab F, Prete A, Delivanis DA, Khanna A, Prokop L, Murad MH, O'Reilly MW, Arlt W, Bancos I. Natural History of Adrenal Incidentalomas With and Without Mild Autonomous Cortisol Excess: A Systematic Review and Meta-analysis. Ann Intern Med. 2019;171:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 177] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 13. | Morelli V, Reimondo G, Giordano R, Della Casa S, Policola C, Palmieri S, Salcuni AS, Dolci A, Mendola M, Arosio M, Ambrosi B, Scillitani A, Ghigo E, Beck-Peccoz P, Terzolo M, Chiodini I. Long-term follow-up in adrenal incidentalomas: an Italian multicenter study. J Clin Endocrinol Metab. 2014;99:827-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 172] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 14. | Falcetta P, Orsolini F, Benelli E, Agretti P, Vitti P, Di Cosmo C, Tonacchera M. Clinical features, risk of mass enlargement, and development of endocrine hyperfunction in patients with adrenal incidentalomas: a long-term follow-up study. Endocrine. 2021;71:178-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Yilmaz N, Avsar E, Tazegul G, Sari R, Altunbas H, Balci MK. Clinical Characteristics and Follow-Up Results of Adrenal Incidentaloma. Exp Clin Endocrinol Diabetes. 2021;129:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Hannemann A, Wallaschofski H. Prevalence of primary aldosteronism in patient's cohorts and in population-based studies--a review of the current literature. Horm Metab Res. 2012;44:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 235] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 17. | Iacobone M, Citton M, Scarpa M, Viel G, Boscaro M, Nitti D. Systematic review of surgical treatment of subclinical Cushing's syndrome. Br J Surg. 2015;102:318-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Park J, De Luca A, Dutton H, Malcolm JC, Doyle MA. Cardiovascular Outcomes in Autonomous Cortisol Secretion and Nonfunctioning Adrenal Adenoma: A Systematic Review. J Endocr Soc. 2019;3:996-1008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Rossi R, Tauchmanova L, Luciano A, Di Martino M, Battista C, Del Viscovo L, Nuzzo V, Lombardi G. Subclinical Cushing's syndrome in patients with adrenal incidentaloma: clinical and biochemical features. J Clin Endocrinol Metab. 2000;85:1440-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 71] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Vassiliadi DA, Tsagarakis S. Diagnosis and management of primary bilateral macronodular adrenal hyperplasia. Endocr Relat Cancer. 2019;26:R567-R581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Meloche-Dumas L, Mercier F, Lacroix A. Role of unilateral adrenalectomy in bilateral adrenal hyperplasias with Cushing's syndrome. Best Pract Res Clin Endocrinol Metab. 2021;35:101486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Osswald A, Quinkler M, Di Dalmazi G, Deutschbein T, Rubinstein G, Ritzel K, Zopp S, Bertherat J, Beuschlein F, Reincke M. Long-Term Outcome of Primary Bilateral Macronodular Adrenocortical Hyperplasia After Unilateral Adrenalectomy. J Clin Endocrinol Metab. 2019;104:2985-2993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Xu Y, Rui W, Qi Y, Zhang C, Zhao J, Wang X, Wu Y, Zhu Q, Shen Z, Ning G, Zhu Y. The role of unilateral adrenalectomy in corticotropin-independent bilateral adrenocortical hyperplasias. World J Surg. 2013;37:1626-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Oßwald A, Plomer E, Dimopoulou C, Milian M, Blaser R, Ritzel K, Mickisch A, Knerr F, Stanojevic M, Hallfeldt K, Schopohl J, Kuhn KA, Stalla G, Beuschlein F, Reincke M. Favorable long-term outcomes of bilateral adrenalectomy in Cushing's disease. Eur J Endocrinol. 2014;171:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Szabo Yamashita T, Sada A, Bancos I, Young WF Jr, Dy BM, Farley DR, Lyden ML, Thompson GB, McKenzie TJ. Differences in outcomes of bilateral adrenalectomy in patients with ectopic ACTH producing tumor of known and unknown origin. Am J Surg. 2021;221:460-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Thompson SK, Hayman AV, Ludlam WH, Deveney CW, Loriaux DL, Sheppard BC. Improved quality of life after bilateral laparoscopic adrenalectomy for Cushing's disease: a 10-year experience. Ann Surg. 2007;245:790-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Di Dalmazi G, Berr CM, Fassnacht M, Beuschlein F, Reincke M. Adrenal function after adrenalectomy for subclinical hypercortisolism and Cushing's syndrome: a systematic review of the literature. J Clin Endocrinol Metab. 2014;99:2637-2645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Else T, Williams AR, Sabolch A, Jolly S, Miller BS, Hammer GD. Adjuvant therapies and patient and tumor characteristics associated with survival of adult patients with adrenocortical carcinoma. J Clin Endocrinol Metab. 2014;99:455-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 29. | Bilimoria KY, Shen WT, Elaraj D, Bentrem DJ, Winchester DJ, Kebebew E, Sturgeon C. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer. 2008;113:3130-3136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 370] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 30. | Kebebew E, Reiff E, Duh QY, Clark OH, McMillan A. Extent of disease at presentation and outcome for adrenocortical carcinoma: have we made progress? World J Surg. 2006;30:872-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 325] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 31. | Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, Naruse M, Pacak K, Young WF Jr; Endocrine Society. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:1915-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1592] [Cited by in RCA: 1857] [Article Influence: 154.8] [Reference Citation Analysis (0)] |
| 32. | Kunz PL, Reidy-Lagunes D, Anthony LB, Bertino EM, Brendtro K, Chan JA, Chen H, Jensen RT, Kim MK, Klimstra DS, Kulke MH, Liu EH, Metz DC, Phan AT, Sippel RS, Strosberg JR, Yao JC; North American Neuroendocrine Tumor Society. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013;42:557-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 455] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 33. | Dahia PL. Pheochromocytoma and paraganglioma pathogenesis: learning from genetic heterogeneity. Nat Rev Cancer. 2014;14:108-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 398] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 34. | Fishbein L, Leshchiner I, Walter V, Danilova L, Robertson AG, Johnson AR, Lichtenberg TM, Murray BA, Ghayee HK, Else T, Ling S, Jefferys SR, de Cubas AA, Wenz B, Korpershoek E, Amelio AL, Makowski L, Rathmell WK, Gimenez-Roqueplo AP, Giordano TJ, Asa SL, Tischler AS; Cancer Genome Atlas Research Network, Pacak K, Nathanson KL, Wilkerson MD. Comprehensive Molecular Characterization of Pheochromocytoma and Paraganglioma. Cancer Cell. 2017;31:181-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 584] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 35. | Grubbs EG, Rich TA, Ng C, Bhosale PR, Jimenez C, Evans DB, Lee JE, Perrier ND. Long-term outcomes of surgical treatment for hereditary pheochromocytoma. J Am Coll Surg. 2013;216:280-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Neumann HPH, Tsoy U, Bancos I, Amodru V, Walz MK, Tirosh A, Kaur RJ, McKenzie T, Qi X, Bandgar T, Petrov R, Yukina MY, Roslyakova A, van der Horst-Schrivers ANA, Berends AMA, Hoff AO, Castroneves LA, Ferrara AM, Rizzati S, Mian C, Dvorakova S, Hasse-Lazar K, Kvachenyuk A, Peczkowska M, Loli P, Erenler F, Krauss T, Almeida MQ, Liu L, Zhu F, Recasens M, Wohllk N, Corssmit EPM, Shafigullina Z, Calissendorff J, Grozinsky-Glasberg S, Kunavisarut T, Schalin-Jäntti C, Castinetti F, Vlcek P, Beltsevich D, Egorov VI, Schiavi F, Links TP, Lechan RM, Bausch B, Young WF Jr, Eng C; International Bilateral-Pheochromocytoma-Registry Group. Comparison of Pheochromocytoma-Specific Morbidity and Mortality Among Adults With Bilateral Pheochromocytomas Undergoing Total Adrenalectomy vs Cortical-Sparing Adrenalectomy. JAMA Netw Open. 2019;2:e198898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 37. | Maestroni U, Ferretti S, Ziglioli F, Campobasso D, Cerasi D, Cortellini P. [Laparoscopic adrenalectomy in giant masses]. Urologia. 2011;78 Suppl 18:S54-S58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Kebebew E, Siperstein AE, Duh QY. Laparoscopic adrenalectomy: the optimal surgical approach. J Laparoendosc Adv Surg Tech A. 2001;11:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Anderson KL Jr, Thomas SM, Adam MA, Pontius LN, Stang MT, Scheri RP, Roman SA, Sosa JA. Each procedure matters: threshold for surgeon volume to minimize complications and decrease cost associated with adrenalectomy. Surgery. 2018;163:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 40. | Fischer A, Schöffski O, Nießen A, Hamm A, Langan EA, Büchler MW, Billmann F. Retroperitoneoscopic adrenalectomy may be superior to laparoscopic transperitoneal adrenalectomy in terms of costs and profit: a retrospective pair-matched cohort analysis. Surg Endosc. 2023;37:8104-8115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house Author and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ramos AC, Brazil; Varon C, France S-Editor: Lin C L-Editor: A P-Editor: Lin C