Published online Jul 15, 2022. doi: 10.4251/wjgo.v14.i7.1363
Peer-review started: March 20, 2022
First decision: April 17, 2022
Revised: April 30, 2022
Accepted: June 26, 2022
Article in press: June 26, 2022
Published online: July 15, 2022
Processing time: 114 Days and 18.9 Hours
Situs inversus totalis (SIT) is an extremely rare congenital malformation characterized by mirror displacement of the thoracoabdominal organs such as the heart, liver, spleen, and stomach. Herein, we describe a patient with SIT complicated with cholangiocarcinoma who underwent successful pancreaticoduodenectomy with the assistance of a da Vinci robot.
A 58-year-old female presented to the hospital with paroxysmal pain in her left upper abdomen, accompanied by jaundice and staining of the sclera as chief complaints. Imaging examination detected a mass at the distal end of the common bile duct, with inverted thoracic and abdominal organs. Endoscopic retrograde cholangiopancreatography forceps biopsy revealed the presence of a well-differentiated adenocarcinoma. The patient successfully underwent robotic-assisted pancreaticoduodenectomy; the operation lasted 300 min, the intraoperative blood loss was 500 mL, and there were no intraoperative and postoperative complications.
SIT is not directly related to the formation of cholangiocarcinoma. Detailed preoperative imaging examination is conducive to disease diagnosis and also convenient for determining the feasibility of tumor resection. Robot-assisted pancreaticoduodenectomy for SIT complicated with cholangiocarcinoma provides a safe, feasible, minimally invasive, and complication-free alternative with adequate preoperative planning combined with meticulous intraoperative procedures.
Core Tip: Situs inversus totalis (SIT) is an extremely rare congenital malformation characterized by a mirror image displacement of the thoracoabdominal organs such as the heart, liver, spleen and stomach. SIT combined with choledochal cancer is even rarer, and da Vinci robot-assisted pancreaticoduodenectomy in patients with SIT combined with choledochal cancer has not been reported. This case demonstrates that preoperative thorough planning, intraoperative precise anatomical knowledge, effective teamwork, meticulous treatment, and postoperative care are feasible with the aid of the da Vinci robot for pancreaticoduodenectomy in patients with SIT.
- Citation: Li BB, Lu SL, He X, Lei B, Yao JN, Feng SC, Yu SP. Da Vinci robot-assisted pancreato-duodenectomy in a patient with situs inversus totalis: A case report and review of literature. World J Gastrointest Oncol 2022; 14(7): 1363-1371
- URL: https://www.wjgnet.com/1948-5204/full/v14/i7/1363.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i7.1363
Situs inversus totalis (SIT) is characterized by complete mirror displacement of the anatomical positions of organs in the thoracoabdominal cavity. It has been reported that its incidence is 1/8000-1/25000 with a male to female ratio of 3:2[1-3]. Although the exact cause of SIT remains unknown, it is generally accepted that it is caused by autosomal abnormalities[4]. In addition, some mutations, such as Dynein Axonemal Heavy Chain 11 and NME7, have been shown to be closely related to SIT[5,6]. Patients with SIT typically exhibit no obvious symptoms, and their health and life expectancy appear to be unaffected[7]. Cholangiocarcinoma is a rare malignant tumor originating from bile duct epithelial cells, accounting for merely 3% of digestive tract malignant tumors[8]. It has an insidious onset, a high degree of malignancy, and a poor prognosis[9]. The da Vinci surgical robot system appeared at the end of the 20th century, and the fourth generation of the robot was launched in 2014, with qualitative improvements in flexibility, accuracy, and imaging clarity. Notably, a remote observation and guidance system was developed in the second half of 2014[10,11]. According to reports, the da Vinci robot has evolved into its fifth generation[12]. To date, several cases of complete visceral choledochal carcinoma have been reported[7,13-18], but da Vinci robot-assisted pancreatoduodenectomy in a patient with SIT has not been reported so far. This is the case report of a patient with SIT complicated with cholangiocarcinoma who underwent pancreaticoduodenectomy assisted by a da Vinci surgical robot. We detailed the diagnosis and management of this case, as well as a thorough evaluation of the associated literature.
A 58-year-old female patient presented to our hospital in November 2021 with jaundice, yellow sclera, paroxysmal pain in the left upper abdomen, itching, fatigue, tea-like urine, and weight loss of 1.5 kg.
The patient developed symptoms 2 mo ago with recurrent upper left abdominal pain.
The patient underwent surgery for cholecystolithiasis 10 years ago.
The patient had no similar family history or genetic history of the disease.
Physical examination on admission revealed yellow discoloration of the skin and sclera, no chest deformity, and cardiac sound in the right chest. The abdomen was soft, while the upper abdomen was tender. There was no rebound tenderness or muscle tension. The liver and spleen were not palpable under the rib edge, and the activities of both lower limbs were unremarkable.
Preoperative auxiliary examinations were as follows: Total bilirubin 126.2 μmol/L (normal range: 3.4-20.5 μmol/L); Direct bilirubin 97.2 μmol/L (normal range: 0-6.8 μmol/L); Indirect bilirubin 29.2 μmol/L (normal range: 3.1-14.3 μmol/L); Aspartate aminotransferase 19.0 U/L (normal range: 13-40 U/L); Alanine aminotransferase (ALT) 28.0 U/L (normal range: 7-45 U/L); Alkaline phosphatase 255 U/L (normal range: 50-135 U/L); Abnormal prothrombin 76.60 ng/mL (normal range: 0-40 ng/mL); Carbohydrate antigen 19-9 197.96 U/mL (normal range: 0-37.00 ng/mL). Other blood biochemical indexes were within the normal range.
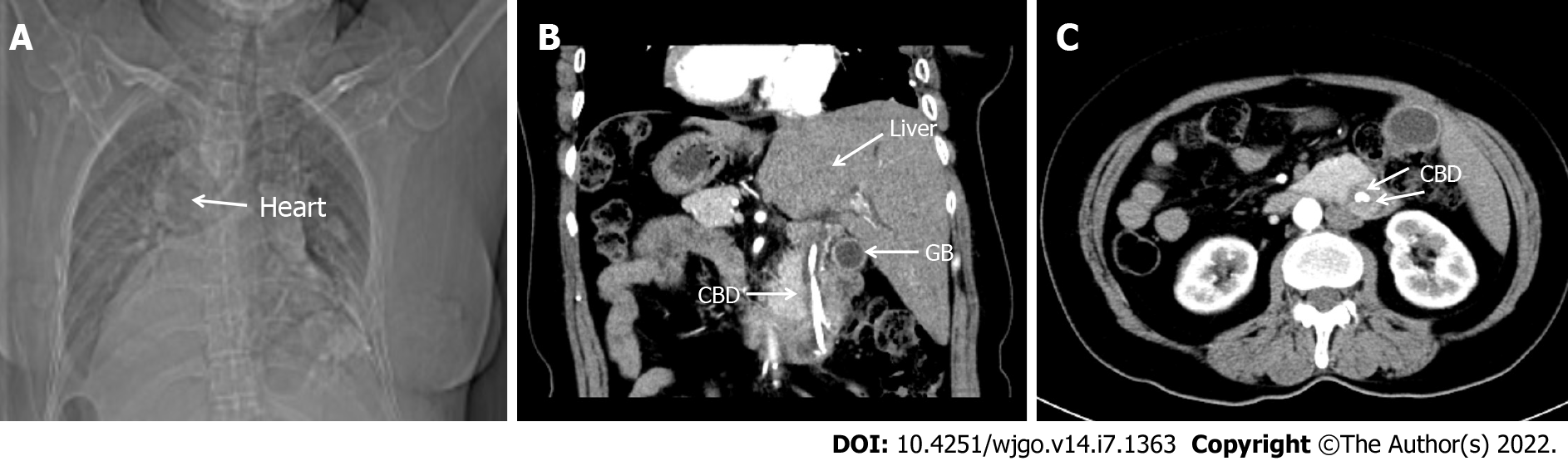
The chest X-ray exposed that the right heart was mirrored (Figure 1A). Multi-slice spiral computed tomography CT (MSCT) revealed complete transposition of the thoracic and abdominal viscera, as well as a tumor in the lower segment of the common bile duct, with minimal intestinal obstruction. The intrahepatic bile duct, extrahepatic bile duct, and pancreatic duct were dilated, and cholecystolithiasis with chronic cholecystitis was diagnosed (Figure 1B and C). Endoscopic retrograde cholangiopancreatography (ERCP) was performed, and the tissue was taken for pathological biopsy; the results showed a well-differentiated adenocarcinoma. Magnetic resonance imaging (MRI) showed intraluminal growth of cholangiocarcinoma in the lower part of the common bile duct with dilatation of the bile duct and pancreatic duct.
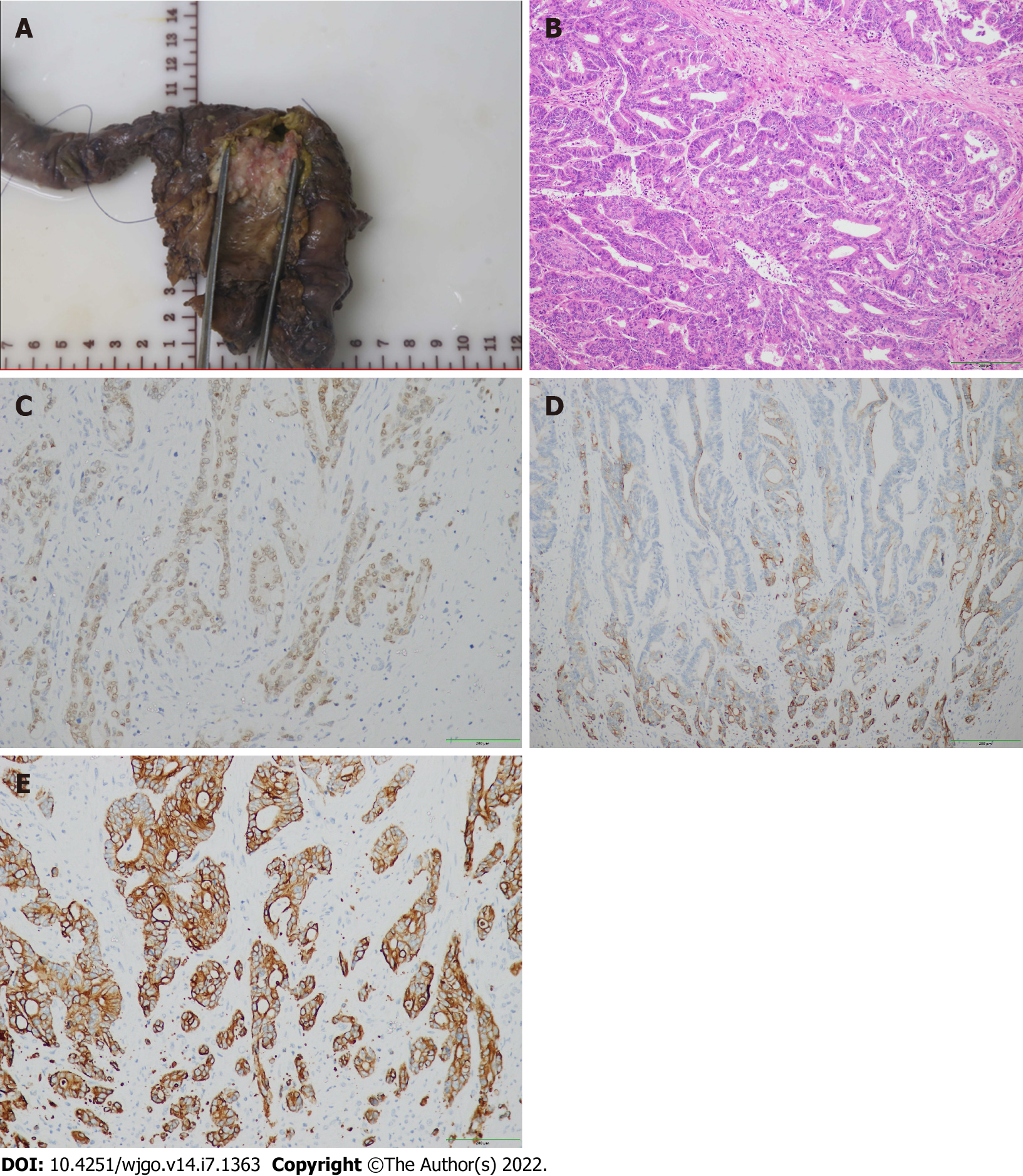
Postoperative histological results were as follows: (1) Well-differentiated adenocarcinoma of the common bile duct with invasion of the duodenal wall. No vascular tumor thrombus and nerve invasion were identified; (2) There were no signs of malignancy in the gastric, duodenal, and pancreatic margins; (3) Each of the 6 Lymph nodes manifested reactive hyperplasia; and (4) Cholecystolithiasis with chronic cholecystitis.5. Immunohistochemical staining was positive for CDX-2, cytokeratin (CK) 7 and CK19 (Figure 2).
According to the American Joint Committee on Cancer/International Alliance for Cancer Control TNM staging system, the patient's tumor stage was IIB stage[19]. The pathological stage of the patient was pT3N0M0.
Prior to surgery, the anesthesiologist assessed the patient's perioperative risk score. According to the American Society of Anesthesiologists Physical Status classification system (ASA PS)[20], the ASA score of the patient was P2.
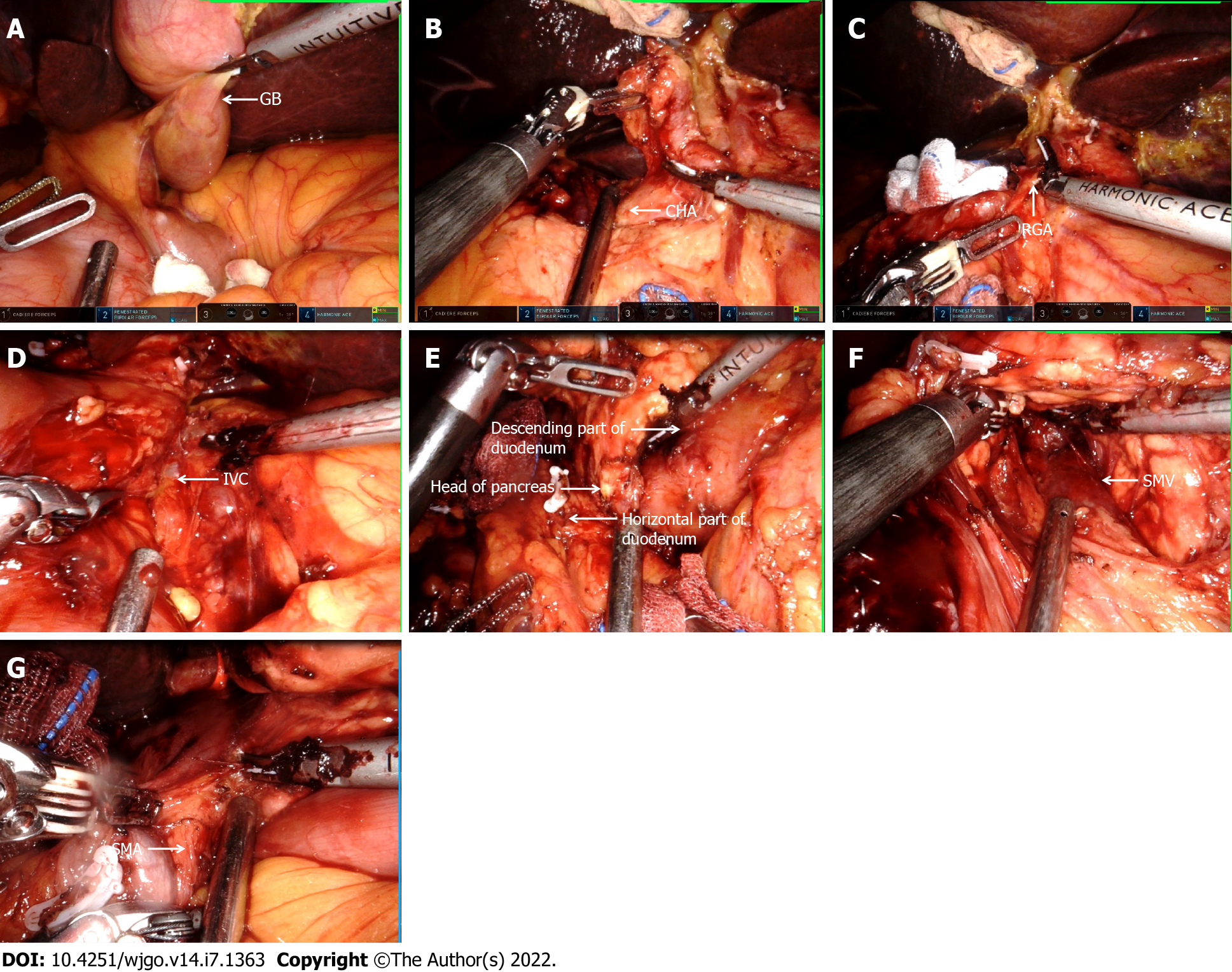
The detailed procedure of the operation is as follows: (1) The surgery was prepared in accordance with the specifications of the da Vinci System. The layout of the operation window and the instrument arm are illustrated in Figure 3; (2) Upon entering the abdominal cavity, it was observed that the abdominal organs were fully inverted (Figure 4A); (3) The gallbladder was anterogradely dissected while the cystic duct was retained. Then, the common hepatic artery was exposed (Figure 4B). Following this, the right gastric artery, the left hepatic artery, and the right hepatic artery were exposed. Next, the suprapyloric lymph node (NO. 5), anterosuperior lymph node of common hepatic artery (NO. 8a), and hepatoduodenal ligament lymph nodes (along the hepatic artery) (NO. 12a) were dissected, and the portal vein and its left and right branches were exposed. Afterward, hepatoduodenal ligament lymph nodes (along the portal vein) (NO. 12p) were dissected, the common hepatic duct and common bile duct were exposed, and hepatoduodenal ligament lymph nodes (along the bile duct) (NO. 12b) were dissected (Figure 4C). Finally, the hepatic portal vein was skeletonized, and the common bile duct was excised; (4) The gastrocolic and hepatogastric ligaments were dissected. Thereafter, the right blood vessels of the gastric reticulum were clamped and cut off, and the subpyloric lymph nodes (NO. 6) were dissected. A Kocher incision was made to expose the inferior vena cava (Figure 4D). Most of the pancreatic head covered the descending and horizontal part of the duodenum, making it difficult to expose the superior mesenteric artery (Figure 4E). The superior mesenteric vein was subsequently exposed along the lower margin of the pancreas, following which the posterior space of the pancreas was separated. Next, the pancreas was cut off, and the location of the pancreatic duct was determined (Figure 4F). The superior mesenteric artery was protected under direct vision. The uncinate process was subsequently cut off along the right side of the superior mesenteric vein. Lastly, the pancreaticoduodenum was resected (Figure 4G); (5) Pancreaticojejunostomy and choledochojejunostomy were performed. The specimens were removed from the abdominal cavity via a median 8 cm incision in the upper abdomen, and gastrojejunostomy was executed through this incision; and (6) A total of 3 drainage tubes were placed in Wen's foramen, behind the pancreaticojejunostomy, and in the pelvic cavity. The operation lasted 300 min, and the intraoperative bleeding was only 500 mL.
There were no severe complications during or after the operation. The patient stayed in the hospital for a total of 30 d and was discharged 22 d following surgery. At the last follow-up (March 15, 2022), there was no evidence of recurrence, and tumor markers had fallen within the normal range. After treatment, he lived completely independently and is still alive.
At present, it is generally acknowledged that SIT with malignancies is a rare coincidence, although SIT with gastric cancer, rectal cancer, pancreatic cancer, liver cancer, and other tumors have been reported[21-24]. Indeed, only 8 cases of SIT complicated with choledochal carcinoma have been reported so far[7,13-18]. The clinicopathological features of the 8 previously reported cases and our case are summarized in Table 1. The cohort consisted of 6 men and 3 women, ranging from 33-years-old to 74-years-old, including 3 from Japan, 2 from China, 2 from the United States, 1 from Italy, and 1 from Morocco. Eight out of the 9 patients underwent the Whipple procedure, and 1 patient underwent choledochectomy and Roux-en-Y hepaticojejunostomy. Histopathological results determined that there were 4 cases of well-differentiated adenocarcinoma, 1 case of medium-differentiated adenocarcinoma, 1 case of poorly-differentiated adenocarcinoma, and 1 case of high-grade intraepithelial neoplasia.
| Case | Ref. | Country | Age/sex | Size | Jaundice | Alkaline phosphatase | Treatment | Histological type | Stage | Postoperative complication |
| 1 | Organ et al[15], 1991 | United States | 68/F | 2 cm | Yes | 905 U/L | Whipple | m | IIIB | NA |
| 2 | Tsunoda et al[17], 2006 | Japan | 65/M | NA | NA | NA | Whipple | NA | NA | None |
| 3 | Benhammane et al[14], 2012 | Morocco | 33/M | 15 mm | Yes | NA | Whipple | w | IIB | None |
| 4 | Kyuno et al[7], 2013 | Japan | 74/M | NA | NA | NA | Whipple | w | IIB | Pancreatic fistula |
| 5 | Kyuno et al[7], 2013 | Japan | 67/M | NA | NA | NA | Whipple | w | IA | Pancreatic fistula |
| 6 | Togliani et al[13], 2013 | Italy | 67/M | 2 cm | Yes | NA | Whipple | NA | NA | NA |
| 7 | Zhang et al[16], 2019 | China | 61/M | 1.8 cm × 1.5 cm × 1.5 cm | Yes | 1011 U/L | Choledochectomy + Roux-en-Y hepaticojejunostomy | l | IIB | None |
| 8 | Coronel et al[18], 2020 | United States | 67/F | 11 mm | Yes | 570 U/L | Whiple | h | 0 | NA |
| 9 | The present case | China | 58/F | 3 cm × 1.9 cm × 0.7 cm | Yes | 255 U/L | Whiple | w | IIB | None |
Most of the early clinical manifestations of cholangiocarcinoma are atypical, and patients seek medical treatment owing to epigastric discomfort, abdominal pain, abdominal distension, loss of appetite, and other symptoms[25]. A minority of patients with cholangiocarcinoma caused by the tumor may develop symptoms of typical obstructive jaundice with severe colic[26]. However, SIT generally lacks specific clinical manifestations, considering it is frequently accompanied by other diseases or discovered during physical examination[27]. By reviewing the literature, it was noted that the reported clinical manifestations of SIT complicated with cholangiocarcinoma include jaundice, abdominal pain, weight loss, and other symptoms. Among the cohort, 5 cases developed jaundice, as was the case in our patient. Thus, jaundice can be considered the primary clinical manifestation of SIT complicated with cholangiocarcinoma. Earlier studies have reported that MSCT, MRI, endoscopic ultrasound, and ERCP are of tremendous value in the diagnosis and preoperative evaluation of SIT complicated with cholangiocarcinoma[7,18].
The following points need to be considered when performing robot-assisted pancreaticoduodenectomy in SIT patients. Compared to healthy individuals, the gall bladder, bile duct, and gastrointestinal tract form a symmetric structure in SIT patients. Therefore, surgeons must be accustomed to preoperative images of SIT patients to avoid prolonged operation time due to unfamiliarity with the anatomy of SIT patients. Additionally, we uncovered for the first time that the thoracic and abdominal organs of SIT patients with cholangiocarcinoma have not only left and right reverse positions but also anterior and posterior reverse positions. During the operation, the pancreatic head of the patient covered the descending and horizontal parts of the duodenum, which was inverted from its normal anatomical structure. Considering the atypical anatomy of this patient, there was no reference for the preoperative procedure. In addition to the conventional operation with three mechanical arms, two auxiliary operation holes were used to improve operability and safety. Due to the atypical anatomy of SIT, routine thinking should be avoided during the operation, and the operation should be initiated with familiarity and a clear anatomical position to avoid accidental injury. During the operation, with the help of a da Vinci robot 3D and a highly clear magnified view, the method of small steps was used to dissect and separate; for the bleeding or blurred parts of the visual field, different from normal anatomy, one should try to avoid blind anatomical separation. For example, when the Kocher technique is used to dissociate the descending part of the duodenum and other parts, the anatomy is not forced to follow the conventional technique, but in the da Vinci robot high-definition field of vision, from shallow to deep, rapid, initial resection of tiny tissue, abnormal bleeding, and other cases in a timely and effective manner. The humanoid wrist function of the da Vinci robot also highlights its superiority to laparoscopic surgeries in narrow spaces and unconventional suture operation, as well as the effect of open surgery. The patient recovered well following surgery, without developing complications such as biliary fistula, pancreatic leakage, gastric emptying dysfunction, pseudocolinestherasis deficits, and airway obstruction[28-31]. Furthermore, incision healing was also significantly better than open surgery.
SIT is a rare genetic disease, and da Vinci robotic surgery is the current poster child for minimally invasive surgery. We believe that with thorough preoperative planning, precise intraoperative anatomical knowledge, effective teamwork, meticulous treatment, and postoperative care, da Vinci robotic-assisted pancreaticoduodenectomy in patients with SIT is feasible and developmental.
I would like to express my gratitude to all those who helped me during the writing of this thesis and their efforts in the management of this patient.
| 1. | Gentile BA, Tighe DA. Situs Inversus Totalis. N Engl J Med. 2019;380:e45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Chen W, Guo Z, Qian L, Wang L. Comorbidities in situs inversus totalis: A hospital-based study. Birth Defects Res. 2020;112:418-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Chen LJ, Qiu X, Sun H, Xu PF, Yin FM, Xu LJ. Two types of lung cancer with situs inversus totalis: a case report and review of the literature. J Int Med Res. 2020;48:300060520944107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Burvill A, Blackham R, Hamdorf J. Laparoscopic sleeve gastrectomy in a patient with situs inversus totalis and Kartagener syndrome: an unusual surgical conundrum. BMJ Case Rep. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Bartoloni L, Blouin JL, Pan Y, Gehrig C, Maiti AK, Scamuffa N, Rossier C, Jorissen M, Armengot M, Meeks M, Mitchison HM, Chung EM, Delozier-Blanchet CD, Craigen WJ, Antonarakis SE. Mutations in the DNAH11 (axonemal heavy chain dynein type 11) gene cause one form of situs inversus totalis and most likely primary ciliary dyskinesia. Proc Natl Acad Sci U S A. 2002;99:10282-10286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 237] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 6. | Reish O, Aspit L, Zouella A, Roth Y, Polak-Charcon S, Baboushkin T, Benyamini L, Scheetz TE, Mussaffi H, Sheffield VC, Parvari R. A Homozygous Nme7 Mutation Is Associated with Situs Inversus Totalis. Hum Mutat. 2016;37:727-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Kyuno D, Kimura Y, Imamura M, Uchiyama M, Ishii M, Meguro M, Kawamoto M, Mizuguchi T, Hirata K. Pancreaticoduodenectomy for biliary tract carcinoma with situs inversus totalis: difficulties and technical notes based on two cases. World J Surg Oncol. 2013;11:312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Krasinskas AM. Cholangiocarcinoma. Surg Pathol Clin. 2018;11:403-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 9. | Labib PL, Goodchild G, Pereira SP. Molecular Pathogenesis of Cholangiocarcinoma. BMC Cancer. 2019;19:185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 221] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 10. | Aldousari SA, Buabbas AJ, Yaiesh SM, Alyousef RJ, Alenezi AN. Multiple perceptions of robotic-assisted surgery among surgeons and patients: a cross-sectional study. J Robot Surg. 2021;15:529-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Ferguson JM, Pitt B, Kuntz A, Granna J, Kavoussi NL, Nimmagadda N, Barth EJ, Herrell SD 3rd, Webster RJ 3rd. Comparing the accuracy of the da Vinci Xi and da Vinci Si for image guidance and automation. Int J Med Robot. 2020;16:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Shin HR, Lee K, Yu HW, Kim SJ, Chai YJ, Choi JY, Lee KE. Comparison of Perioperative Outcomes Using the da Vinci S, Si, X, and Xi Robotic Platforms for BABA Robotic Thyroidectomy. Medicina (Kaunas). 2021;57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Togliani T, Pilati S, Mantovani N, Savioli A, Gaetti L, Negri S, Fusaroli P. Extrahepatic cholangiocarcinoma in a patient with situs inversus totalis diagnosed by endoscopic ultrasound. Endoscopy. 2013;45 Suppl 2 UCTN:E229-E230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Benhammane H, Kharmoum S, Terraz S, Berney T, Nguyen-Tang T, Genevay M, El Mesbahi O, Roth A. Common bile duct adenocarcinoma in a patient with situs inversus totalis: report of a rare case. BMC Res Notes. 2012;5:681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Organ BC, Skandalakis LJ, Gray SW, Skandalakis JE. Cancer of bile duct with situs inversus. Arch Surg. 1991;126:1150-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Zhang SM, Chen ZG, Ma LL, Wu XA, Hua SH. Common bile duct adenocarcinoma in a patient with situs inversus totalis:a case report. Anhui Med Pharm J. 2019;23. [DOI] [Full Text] |
| 17. | Tsunoda S, Miyashita T, Murata M. Pancreaticoduodenectomy for common bile duct cancer in a patient with situs inversus totalis: a case report. Int Surg. 2006;91:24-27. [PubMed] |
| 18. | Coronel M, Lanke G, Cambell D, Coronel E, Tzeng CD, Foo W, Lee JH. Performing Endoscopic Retrograde Cholagiopancreatpgraphy and Endoscopic Ultrasound for Management of Malignant Bile Duct Obstruction in a Patient With a Situs Inversus Totalis. ACG Case Rep J. 2020;7:e00483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Adsay NV, Bagci P, Tajiri T, Oliva I, Ohike N, Balci S, Gonzalez RS, Basturk O, Jang KT, Roa JC. Pathologic staging of pancreatic, ampullary, biliary, and gallbladder cancers: pitfalls and practical limitations of the current AJCC/UICC TNM staging system and opportunities for improvement. Semin Diagn Pathol. 2012;29:127-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Horvath B, Kloesel B, Todd MM, Cole DJ, Prielipp RC. The Evolution, Current Value, and Future of the American Society of Anesthesiologists Physical Status Classification System. Anesthesiology. 2021;135:904-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 233] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 21. | Suh BJ. A Case of Gastric Cancer with Situs Inversus Totalis. Case Rep Oncol. 2017;10:130-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Kasai S, Hino H, Shiomi A, Kagawa H, Manabe S, Yamaoka Y, Kato S, Hanaoka M, Kinugasa Y. Robotic-assisted surgery for rectal cancer with situs inversus totalis: A case report. Asian J Endosc Surg. 2021;14:803-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Hussan MA, Yang Z, Dong X, Yang H, Li N, Qiao S. A laparoscopic pancreaticoduodenectomy for pancreatic adenocarcinoma in a patient with situs inversus totalis. J Surg Case Rep. 2021;2021:rjab316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Liao KX, Li JW. ASO Author Reflections: A New Minimally Invasive Procedure for Hepatocellular Carcinoma with Situs Inversus Totalis. Ann Surg Oncol. 2021;28:6832-6833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Fong ZV, Brownlee SA, Qadan M, Tanabe KK. The Clinical Management of Cholangiocarcinoma in the United States and Europe: A Comprehensive and Evidence-Based Comparison of Guidelines. Ann Surg Oncol. 2021;28:2660-2674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 26. | Turgeon MK, Maithel SK. Cholangiocarcinoma: a site-specific update on the current state of surgical management and multi-modality therapy. Chin Clin Oncol. 2020;9:4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Téllez-Beltrán D, González-Muñoz A, Barón-Cifuentes V, Pradilla-Gómez JM. Abdominal aortic aneurysm in a patient with situs inversus totalis. Cir Cir. 2020;88:79-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | El Nakeeb A, El Sorogy M, Hamed H, Said R, Elrefai M, Ezzat H, Askar W, Elsabbagh AM. Biliary leakage following pancreaticoduodenectomy: Prevalence, risk factors and management. Hepatobiliary Pancreat Dis Int. 2019;18:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, Nimura Y, Nagino M. Hepatopancreatoduodenectomy for cholangiocarcinoma: a single-center review of 85 consecutive patients. Ann Surg. 2012;256:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 30. | Hogg C. Primary ciliary dyskinesia: when to suspect the diagnosis and how to confirm it. Paediatr Respir Rev. 2009;10:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Ferkol T, Leigh M. Primary ciliary dyskinesia and newborn respiratory distress. Semin Perinatol. 2006;30:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chang A, Thailand; Isac S, Romania; Telkes G, Hungary S-Editor: Yan JP L-Editor: Filipodia P-Editor: Yan JP