Published online Jul 15, 2022. doi: 10.4251/wjgo.v14.i7.1348
Peer-review started: December 15, 2021
First decision: April 17, 2022
Revised: April 28, 2022
Accepted: June 27, 2022
Article in press: June 27, 2022
Published online: July 15, 2022
Processing time: 209 Days and 15.7 Hours
Perivascular epithelioid cell tumor (PEComa) represents a group of rare mesenchymal tumors. PEComa can occur in many organs but is rare in the colorectum, especially in children. Furthermore, PEComa is a rare cause of intussusception, the telescoping of a segment of the gastrointestinal tract into an adjacent one. We describe a rare case of pediatric PEComa complicated with intussusception and anal incarceration, and conduct a review of the current literature.
A 12-year-old girl presented with abdominal pain and abdominal ultrasound suggested intussusception. Endoscopic direct-vision intussusception treatment and colonoscopy was performed. A spherical tumor was discovered in the transverse colon and removed by surgery. Postoperative pathologic analyses revealed that the tumor volume was 5.0 cm × 4.5 cm × 3.0 cm and the tumor tissue was located in the submucosa of the colon, arranged in an alveolar pattern. The cell morphology was regular, no neoplastic necrosis was observed, and nuclear fission was rare. The immunohistochemical staining results were as follows: Human melanoma black 45 (HMB 45) (+), cluster of differentiation 31 (CD31) (+), cytokeratin (-), melanoma-associated antigen recognized by T cells (-), smooth muscle actin (-), molleya (-), desmin (-), S-100 (-), CD117 (-), and Ki67 (positive rate in hot spot < 5%). Combined with the results of pathology and immunohistochemistry, we diagnosed the tumor as PEComa. Postoperative recovery was good at the 4 mo follow-up.
The diagnosis of PEComa mainly depends on pathology and immunohistochemistry. Radical resection is the preferred treatment method.
Core Tip: Perivascular epithelioid cell tumor (PEComa) of the colon is rarely encountered in the clinic, especially in pediatric patients. We describe a rare case of PEComa complicated with intussusception and anal incarceration in a 12-year-old female. We performed endoscopic direct-vision intussusception treatment and surgical removal. The diagnosis of PEComa mainly depends on pathology and immunohistochemistry. Radical resection is the preferred treatment method.
- Citation: Kou L, Zheng WW, Jia L, Wang XL, Zhou JH, Hao JR, Liu Z, Gao FY. Pediatric case of colonic perivascular epithelioid cell tumor complicated with intussusception and anal incarceration: A case report. World J Gastrointest Oncol 2022; 14(7): 1348-1355
- URL: https://www.wjgnet.com/1948-5204/full/v14/i7/1348.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i7.1348
Colonic perivascular epithelioid cell tumor (PEComa) is rare in clinical practice, especially in children. Intussusception caused by PEComa is even rarer. This report describes a pediatric case of colonic PEComa with intussusception and anal incarceration treated with endoscopic intussusception reduction. This is the first report of such a case. Furthermore, we review the studies on colorectal PEComa indexed in the PubMed database and accessed with the keywords “Colonic PEComa” and “Rectal PEComa”. A total of 30 cases were retrieved, and we provide a detailed analysis and summarization of these cases here.
A 12-year-old girl presented with abdominal pain as the first manifestation.
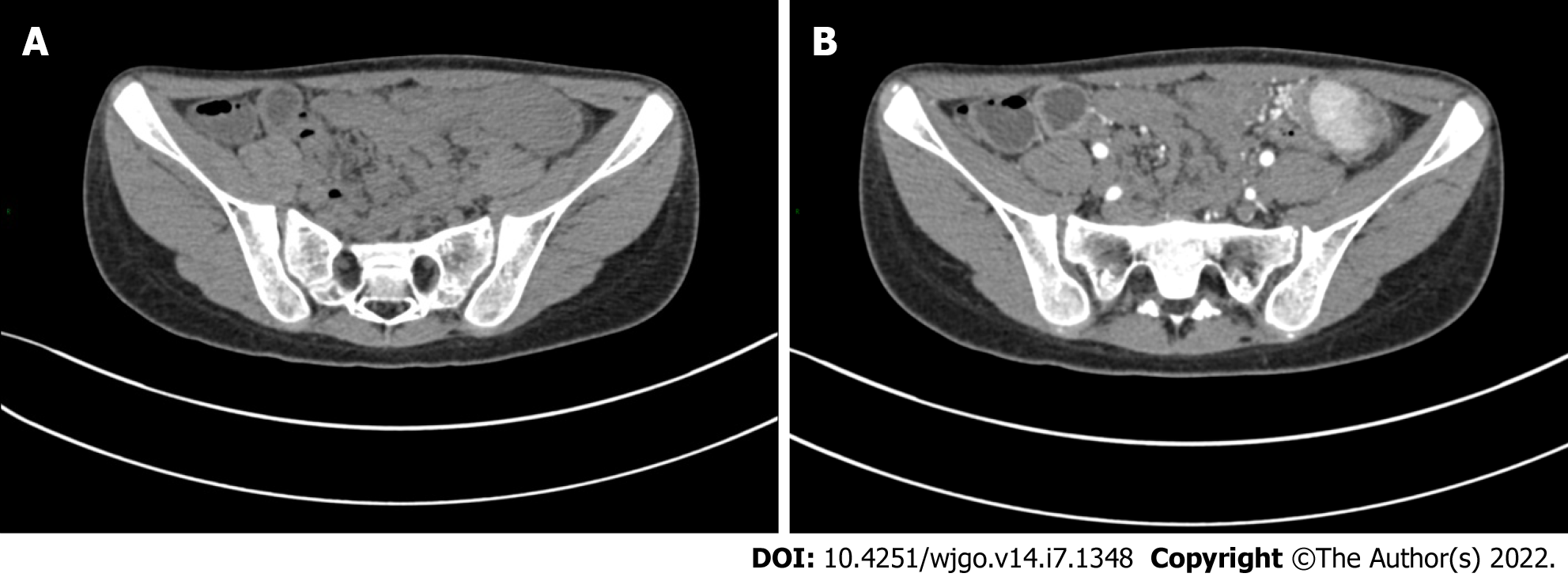
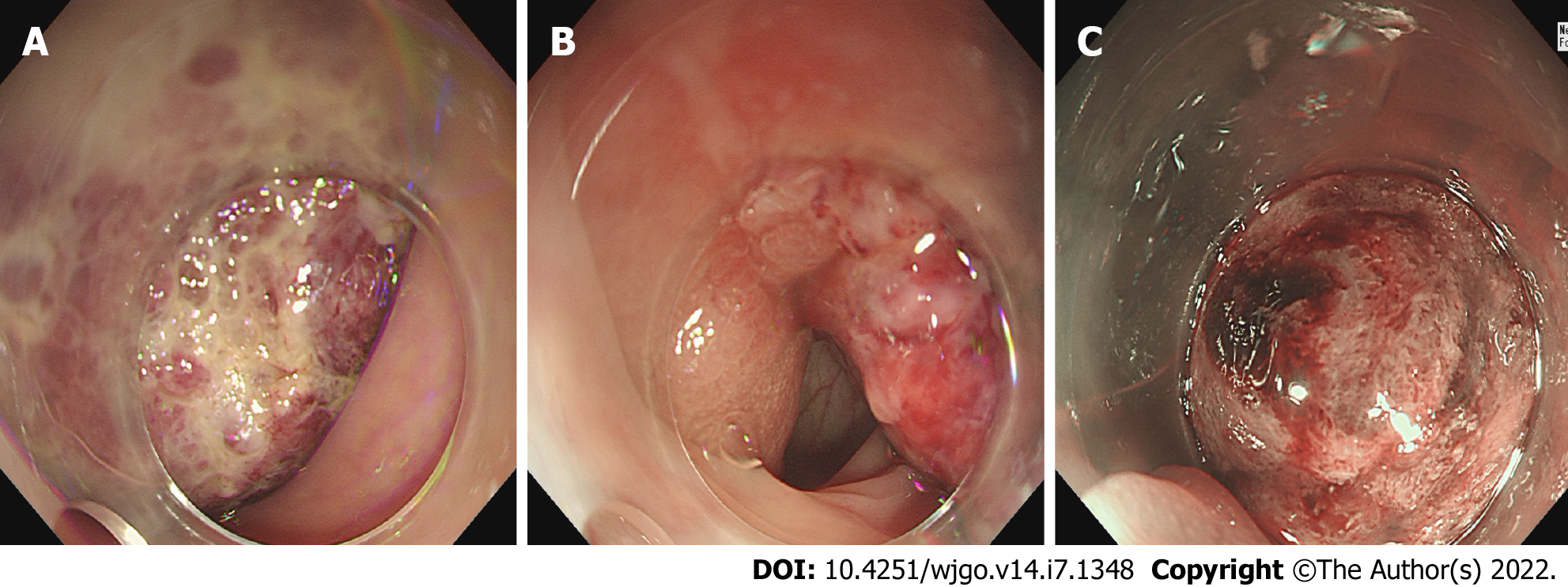
The patient had developed paroxysmal angina pectoris around the umbilicus and lower abdomen 17 d prior, accompanied by nausea and vomiting. Intussusception was diagnosed in a local hospital by ultrasound, and was reduced by air enema. Contrast-enhanced computed tomography (CT) scan showed abnormal enhancement on the left side of the transverse colon with intussusception, which was considered as polyps (Figure 1). Supplementary colonoscopy showed a spherical protuberance of 5 cm in diameter in the transverse colon (Figure 2). The patient was transferred to our hospital for further diagnosis and treatment.
The patient had no previous medical history.
There was no relevant personal or family history of colon tumor.
The patient’s vital signs were stable, the abdomen was flat and soft, the left lower abdomen was tender, and there was mild rebound pain.
Results of routine blood, liver function, and coagulation and tumor marker tests were within the normal ranges.
Combined with the microscopy findings and considering the high risk associated with endoscopy, after discussion with the pediatric surgeons, pediatricians, pathologists, and ultrasonographers, we decided to remove the tumor via general surgery.
PEComa was diagnosed by immunohistochemistry.
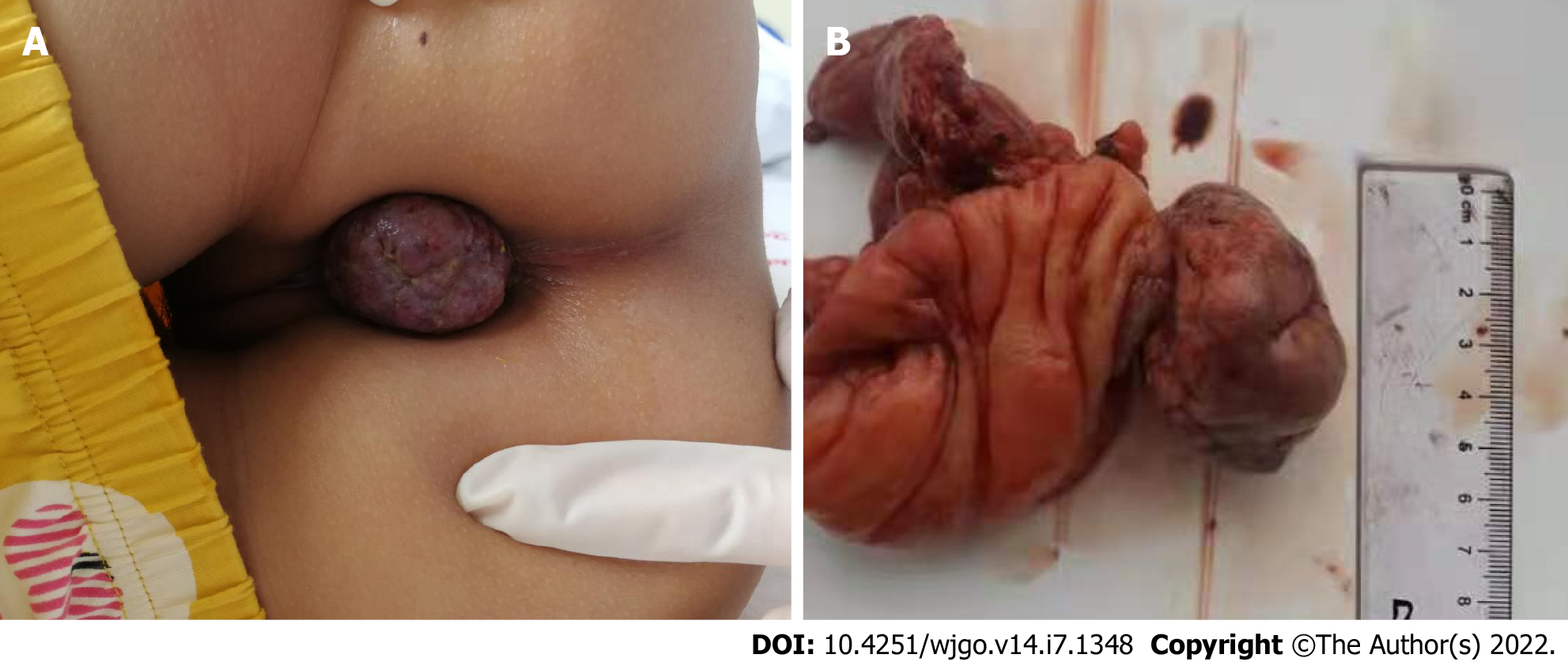
We performed surgery on the patient, a tumor was found in the transverse colon near the spleen, of about 6 cm × 4 cm × 3 cm in size, with a wide pedicle connected to the bowel. It had good mobility, a hard texture, and a rich blood supply. Edema of the surrounding bowel wall and mesentery was found, separating the mesentery in turn and being ligated to the affected mesenteric vessels. We completely removed the tumor, in addition to about 3 cm of the affected bowel (Figure 3).
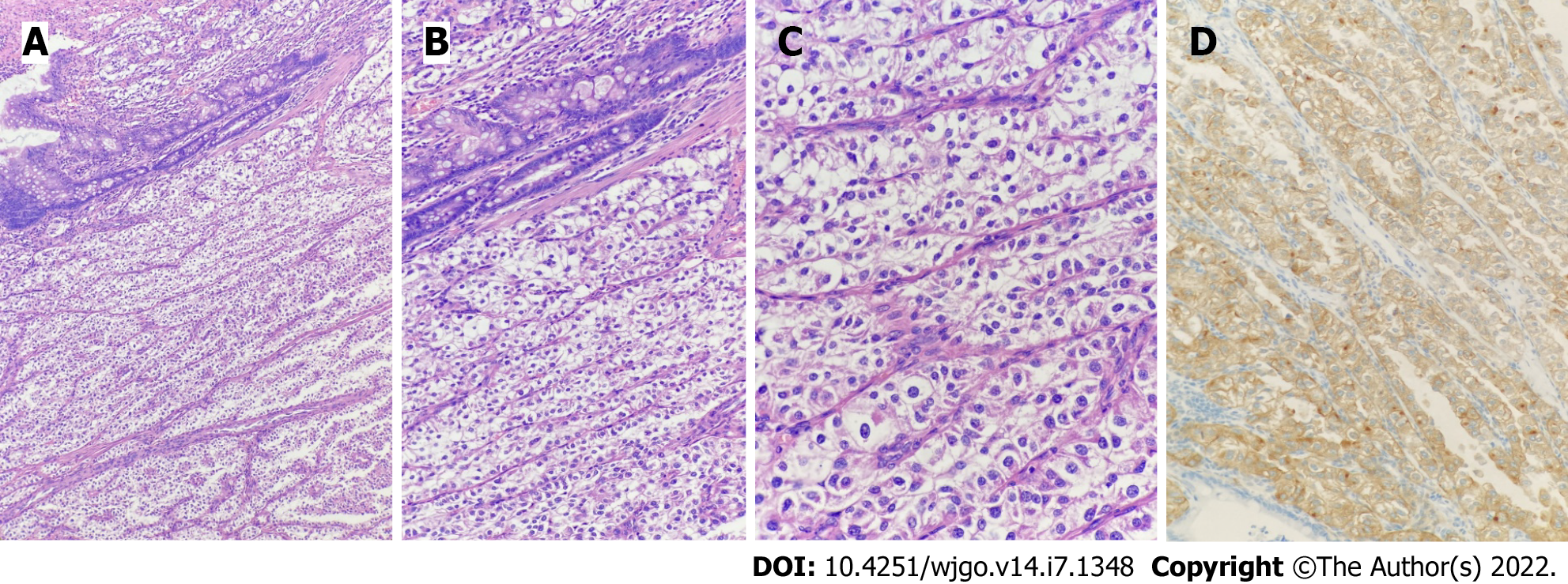
Postoperative pathology showed that the tumor volume was 5.0 cm × 4.5 cm × 3.0 cm and the tumor tissue was located in the submucosa of colon, arranged in an acinar shape with mild cell morphology, no tumor necrosis, and rare instances of mitosis. The immunohistochemical staining results were as follows: Human melanoma black 45 (HMB-45) (+), cluster of differentiation 31 (CD31) (+), cytokeratin (-), melanoma-associated antigen recognized by T cells (-), smooth muscle actin (-), molleya (-), desmin (-), S-100 (-), CD117 (-), and Ki67 (hot spot positive rate < 5%) (Figure 4).
The patient recovered well after the operation, and no abnormalities were found at the 6 mo follow-up.
PEComa represents a group of mesenchymal tumors characterized by perivascular epithelioid cells[1]. The etiology is still unclear, and some scholars consider it to be related to the gene mutation of the tuberous sclerosis complex[2]. Histologically, it is mainly composed of blood vessels, spindle cells or epithelioid cells, and fat. The proportion of the three components varies, which leads to large differences in imaging manifestations; it can manifest as poorly differentiated soft tissue tumors or as sclerosing tumors. Its density or signal performance is also closely related to the tumor cell components, but most of these tumors are characterized by a soft tissue mass with a regular shape, clear boundary, high density, and low signal intensity[3].
PEComa diagnosis depends on the pathology and immunohistochemistry findings. According to the World Health Organization classification of digestive system tumors published in 2019, the basic and ideal diagnostic criteria of PEComa are: epithelioid cells and (or) spindle cells in tissues, eosinophilic granular or transparent cytoplasm; nestlike, trabecular or lamellar structure; and co-expression of melanocytes and smooth muscle markers[4]. At present, there is no definitive standard for the diagnosis of benign and malignant PEComa. Folpe et al[5] divided the tumors into benign, malignant, and undetermined malignant potential. The malignant features included: tumor size > 5 cm, marginal infiltration, atypical nuclear, mitotic image ≥ 1/50 high-power field, tumor necrosis, and vascular invasion. Benign tumors are considered malignant when they have more than two of the aforementioned features; cases where the diagnosis of malignant potential is uncertain and there is tumor necrosis, including obvious nuclear atypia and high proliferation index, need close follow-up[4]. Considering the pathological results of this case, we considered the tumor to be benign; however, due to the patient’s young age and large tumor volume, close follow-up is still needed.
PEComa is rarely reported. Cecal PEComa was first reported by Birkhaeuser et al[6] in 2004. Since then, a total of 30 cases (Table 1)[6-32] of colorectal PEComa have been reported (as determined upon performance of a detailed PubMed search), including 18 females and 12 males, of ages ranging from 5.5-years-old to 69-years-old; most of these patients were adults, and only 7 (23%) were younger than 15-years-old. There was a significant sex difference among the adults but no significant sex difference among the children, consistent with the findings reported by Fadare[33], who proposed that PEComa may be a hormone-dependent tumor. PEComa can occur in all parts of the colon, although they occur more often in the left colon (9 cases in the sigmoid colon[11,12,15,16,18,21,22,26,31], 5 in the rectum[6,7,13,28,29], 3 in the descending colon[9,16,27], 4 in the ascending colon[16,19,30], 7 in the cecum[8,14,20,23-25,32], 1 in the transverse colon[10], and 1 in the right colon[17]).
| Ref. | Age (yr) | Sex | Symptom | Location | Size (mm) | Metastasis | Treatment | Follow-up |
| 1 Birkhaeuser et al[6] | 35 | F | Bleeding | Cecum | 35 | No | SR | NER at 5 yr |
| 2 Genevay et al[7] | 36 | F | Anemia and rectorrhagia | Cecum | 35 | No | SR | NA |
| 3 Evert et al[8] | 56 | F | Rectal obstruction loss | Rectum | 80 × 50 | Lung metastasis | NA | NA |
| 4 Yamamoto et al[9] | 43 | F | Abdominal pain | Descending | 80 | No | SR | DOD at 38 mo |
| 5 Baek et al[10] | 16 | F | NA | Transverse | 25 | No | ER | NER at 24 mo |
| 6 Pisharody et al[11] | 11 | M | Bleeding | Sigmoid | 30 | Lymph node metastasis | SR | NER at 5 mo |
| 7 Righi et al[12] | 11 | M | NA | Sigmoid | 35 | NA | SR | NA |
| 8 Qu et al[13] | 43 | F | NA | Cecum | 20 | No | SR | NER at 25 mo |
| 9 Ryan et al[14] | 15 | F | Bleeding | Rectum | 37 | Lymph node metastasis | SR and AC | NER at 5 mo |
| 10 Tanaka et al[15] | 14 | F | Physical examination | Sigmoid | 40 | No | SR | NA |
| 11 Shi et al[16] | 38 | F | Abdominal pain | Ascending | 60 | No | SR | NER at 8 mo |
| 12 Shi et al[16] | 42 | M | Abdominal pain | Sigmoid | 45 | No | SR | NER at 15 mo |
| 13 Shi et al[16] | 36 | M | Abdominal pain | Descending | 48 | No | SR | NER at 32 mo |
| 14 Shi et al[16] | 45 | F | Abdominal pain | Ascending | 35 | No | SR | NER at 36 mo |
| 15 Gross et al[17] | 5.5 | M | Abdominal pain and fever | Right | 50 | No | SR | NER at 15 yr |
| 16 Freeman et al[18] | 17 | F | Bleeding | Sigmoid | NA | No | SR | NA |
| 17 Park et al[19] | 7 | M | Abdominal pain and bleeding | Ascending | 37 | No | SR and IFN therapy | NER at 26 mo |
| 18 Mar et al[20] | 11 | F | Prolapsed mass | Rectum | 20 | No | ER | NA |
| 19 Lee et al[21] | 62 | F | Abdominal pain and melena | Sigmoid | 50 | NA | NA | NA |
| 20 Cho et al[22] | 62 | F | Bleeding | Sigmoid | 50 | No | SR | NER at 16 mo |
| 21 Scheppach et al[23] | 23 | M | Abdominal pain and bleeding | Rectum | NA | Lymph node and liver metastasis | SR and AC | DOD at 23 mo |
| 22 Im et al[24] | 17 | M | Bleeding | Rectum | 30 | No | ER | NER at 10 mo |
| 23 Kanazawa et al[25] | 55 | F | Physical examination | Rectum | 25 | No | ER | NER at 12 mo |
| 24 Cheng et al[26] | 40 | M | Dyschezia | Sigmoid | 70 × 60 | No | SR | Pancreatic metastasis at 27 mo |
| 25 Iwamoto et al[27] | 42 | F | Physical examination | Descending | NA | No | SR | NA |
| 26 Lin et al[28] | 28 | M | Abdominal pain and bleeding | Cecum | 88 | No | SR | Liver metastasis at 49 mo |
| 27 Iwa et al[29] | 69 | M | Physical examination | Cecum | 41 × 32 | No | SR | NA |
| 28 Bennett et al[30] | 67 | F | Physical examination | Ascending | 80 | No | ER | NA |
| 29 Cheng et al[31] | 17 | M | Bleeding | Sigmoid | NA | Lymph node metastasis | SR and AC | NER at 24 mo |
| 30 Yeon et al[32] | 45 | F | Physical examination | Rectum | 20 | No | SR | NA |
The diameter of the reported tumors have ranged from 0.8 cm to 8.0 cm. There are no specific symptoms. The tumor can manifest abdominal pain, diarrhea, abdominal distension, hematochezia, or other symptoms of gastrointestinal tumors[19]. The most common clinical manifestation is abdominal pain (44%). Two intussusception cases have been reported. Among the 30 cases, 23 patients underwent surgery and 4 of them given postoperative adjuvant chemotherapy[14,19,26,31]. In total, 5 underwent endoscopic mucosal resection[10,20,24,25,30], and 1 patient underwent endoscopic mucosal dissection after pathological diagnosis. No recurrence was found during follow-up. There have been 10 malignant PEComa cases reported[8,9,11,14,15,19,23,26,28,31]; among them, 2 patients died[9,23] and 2 were lost to follow-up but involving the pancreas and liver metastasis respectively[28,31]. Combined with limited case analysis, the prognosis of malignant PEComa is poor.
Ileocolic intussusception is one of the most common abdominal emergencies involving children who are less than 3-years-old[34]. The pathophysiology underlying the majority of pediatric intussusception cases is thought to be secondary to a transient viral illness[35]. In adults, 70%-90% of intussusception can be found to have a clear cause, and about 40% are caused by a primary or secondary malignant tumor[36]. Here, we have reported the first pediatric case of benign PEComa in the transverse colon with intussusception, tumor prolapse, and incarceration outside the anus.
At present, benign PEComa has no adjuvant drug treatment. The main treatment for colon PEComa is radical resection, with a good prognosis. Long-term clinical and CT follow-up is recommended. Scheppach et al[23] administered sirolimus, doxorubicin, ifosfamide, citabine and docetaxel successively after surgery, which had no obvious effect. Park et al[19] reported on a 7-year-old boy with poorly differentiated PEComa in the ascending colon, who received adjuvant interferon-alpha for 1 year after surgery. There was no recurrence after 26 mo of follow-up. That was the first report of interferon-alpha for the treatment of PEComa in the colon. In recent years, an increasing number of targeted drugs have been used in PEComa. Studies have shown that mechanistic target of rapamycin inhibitors are the most effective drugs for the treatment of advanced/metastatic PEComa[37].
PEComa is a special type of mesenchymal tissue tumor, which is rarely encountered in the clinic and lacks specific clinical manifestations. The diagnosis depends on pathology and immunohistochemistry findings. Radical resection is the preferred treatment method, and there is no standardized treatment for postoperative adjuvant therapy. Targeted drug application is gradually increasing and has achieved certain results but still needs further research.
| 1. | Hornick JL, Fletcher CD. PEComa: what do we know so far? Histopathology. 2006;48:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 326] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 2. | Thway K, Fisher C. PEComa: morphology and genetics of a complex tumor family. Ann Diagn Pathol. 2015;19:359-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 160] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 3. | Tan Y, Zhang H, Xiao EH. Perivascular epithelioid cell tumour: dynamic CT, MRI and clinicopathological characteristics--analysis of 32 cases and review of the literature. Clin Radiol. 2013;68:555-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | WHO Classification of tumours Editorial Board. WHO Classification of tumours of the digestive system. 5th ed. Lyon: IARC Press, 2019: 485-488. |
| 5. | Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol. 2005;29:1558-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 666] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 6. | Birkhaeuser F, Ackermann C, Flueckiger T, Guenin MO, Kern B, Tondelli P, Peterli R. First description of a PEComa (perivascular epithelioid cell tumor) of the colon: report of a case and review of the literature. Dis Colon Rectum. 2004;47:1734-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Genevay M, Mc Kee T, Zimmer G, Cathomas G, Guillou L. Digestive PEComas: a solution when the diagnosis fails to "fit". Ann Diagn Pathol. 2004;8:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Evert M, Wardelmann E, Nestler G, Schulz HU, Roessner A, Röcken C. Abdominopelvic perivascular epithelioid cell sarcoma (malignant PEComa) mimicking gastrointestinal stromal tumour of the rectum. Histopathology. 2005;46:115-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Yamamoto H, Oda Y, Yao T, Oiwa T, Kobayashi C, Tamiya S, Kawaguchi K, Hino O, Tsuneyoshi M. Malignant perivascular epithelioid cell tumor of the colon: report of a case with molecular analysis. Pathol Int. 2006;56:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Baek JH, Chung MG, Jung DH, Oh JH. Perivascular epithelioid cell tumor (PEComa) in the transverse colon of an adolescent: a case report. Tumori. 2007;93:106-108. [PubMed] |
| 11. | Pisharody U, Craver RD, Brown RF, Gardner R, Schmidt-Sommerfeld E. Metastatic perivascular epithelioid cell tumor of the colon in a child. J Pediatr Gastroenterol Nutr. 2008;46:598-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Righi A, Dimosthenous K, Rosai J. PEComa: another member of the MiT tumor family? Int J Surg Pathol. 2008;16:16-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Qu GM, Hu JC, Cai L, Lang ZQ. Perivascular epithelioid cell tumor of the cecum: a case report and review of literatures. Chin Med J (Engl). 2009;122:1713-1715. [PubMed] |
| 14. | Ryan P, Nguyen VH, Gholoum S, Carpineta L, Abish S, Ahmed NN, Laberge JM, Riddell RH. Polypoid PEComa in the rectum of a 15-year-old girl: case report and review of PEComa in the gastrointestinal tract. Am J Surg Pathol. 2009;33:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Tanaka M, Kato K, Gomi K, Matsumoto M, Kudo H, Shinkai M, Ohama Y, Kigasawa H, Tanaka Y. Perivascular epithelioid cell tumor with SFPQ/PSF-TFE3 gene fusion in a patient with advanced neuroblastoma. Am J Surg Pathol. 2009;33:1416-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Shi HY, Wei LX, Sun L, Guo AT. Clinicopathologic analysis of 4 perivascular epithelioid cell tumors (PEComas) of the gastrointestinal tract. Int J Surg Pathol. 2010;18:243-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Gross E, Vernea F, Weintraub M, Koplewitz BZ. Perivascular epithelioid cell tumor of the ascending colon mesentery in a child: case report and review of the literature. J Pediatr Surg. 2010;45:830-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Freeman HJ, Webber DL. Perivascular epithelioid cell neoplasm of the colon. World J Gastrointest Oncol. 2010;2:205-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Park SJ, Han DK, Baek HJ, Chung SY, Nam JH, Kook H, Hwang TJ. Perivascular epithelioid cell tumor (PEComa) of the ascending colon: the implication of IFN-α2b treatment. Korean J Pediatr. 2010;53:975-978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Maran-Gonzalez A, Baldet P, Costes V. [Polypoid PEComa: case report and literature review]. Ann Pathol. 2011;31:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Lee M, Cho KJ, Yu C, Park Y, Kim JC, Kim J, Yu E, Kim MJ. Perivascular epithelioid cell tumor of the sigmoid colon with transcription factor E3 expression. Ann Diagn Pathol. 2012;16:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Cho YW, Kim KJ, Ye BD, Byeon JS, Myung SJ, Yang SK, Kim JH. [A case of a perivascular epithelioid cell tumor mimicking colon cancer]. Korean J Gastroenterol. 2012;60:377-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Scheppach W, Reissmann N, Sprinz T, Schippers E, Schoettker B, Mueller JG. PEComa of the colon resistant to sirolimus but responsive to doxorubicin/ifosfamide. World J Gastroenterol. 2013;19:1657-1660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Im S, Yoo C, Jung JH, Choi HJ, Yoo J, Kang CS. Primary perivascular epithelioid cell tumor in the rectum: a case report and review of the literature. Pathol Res Pract. 2013;209:244-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Kanazawa A, Fujii S, Godai TI, Ishibe A, Oshima T, Fukushima T, Ota M, Yukawa N, Rino Y, Imada T, Ito J, Nozawa A, Masuda M, Kunisaki C. Perivascular epithelioid cell tumor of the rectum: report of a case and review of the literature. World J Surg Oncol. 2014;12:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Cheng J, Deng M, Gao J, Tao K. A recurrent perivascular epithelioid cell tumor of sigmoid colon with pancreatic metastasis: an extremely rare case report and review of the literature. Int J Colorectal Dis. 2016;31:1237-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Iwamoto R, Kataoka TR, Furuhata A, Ono K, Hirota S, Kawada K, Sakai Y, Haga H. Perivascular epithelioid cell tumor of the descending colon mimicking a gastrointestinal stromal tumor: a case report. World J Surg Oncol. 2016;14:285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Lin KH, Chang NJ, Liou LR, Su MS, Tsao MJ, Huang ML. Successful management of perivascular epithelioid cell tumor of the rectum with recurrent liver metastases: A case report. Medicine (Baltimore). 2018;97:e11679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Iwa N, Yutani C, Kobayashi TK. Presence of eosinophilic intracytoplasmic inclusions diagnosed by fine needle aspiration cytology in perivascular epithelioid cell tumor (PEComa) arising from the cecum. Diagn Cytopathol. 2019;47:359-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Bennett J, Laury R, Dai H, Walde C, Kasi A. A Curious Case of Colonic Perivascular Epithelioid Cell Tumor: A Unique Diagnosis With Variable Presentations. Cureus. 2020;12:e11164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Cheng HC, Kuo CY, Huang CW, Shih HH, Lin CH, Wang JY. Unusual paediatric sigmoid perivascular epithelioid cell tumour with regional lymph node metastasis treated using gemcitabine and docetaxel: a case report and literature review. J Int Med Res. 2021;49:3000605211041509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Yeon HJ, Sung NS, Roh SJ, Choi WJ, Park YW. PEComa in the rectum: A case report and review of the literature on epithelioid angiomyolipoma. Int J Surg Case Rep. 2021;86:106301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Fadare O. Perivascular epithelioid cell tumor (PEComa) of the uterus: an outcome-based clinicopathologic analysis of 41 reported cases. Adv Anat Pathol. 2008;15:63-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Applegate KE. Intussusception in children: evidence-based diagnosis and treatment. Pediatr Radiol. 2009;39 Suppl 2:S140-S143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 35. | Burnett E, Kabir F, Van Trang N, Rayamajhi A, Satter SM, Liu J, Yousafzai MT, Anh DD, Basnet AT, Flora MS, Houpt E, Qazi SH, Canh TM, Rayamajhi AK, Saha BK, Saddal NS, Muneer S, Hung PH, Islam T, Ali SA, Tate JE, Yen C, Parashar UD. Infectious Etiologies of Intussusception Among Children <2 Years Old in 4 Asian Countries. J Infect Dis. 2020;221:1499-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Akbulut S. Unusual cause of adult intussusception: diffuse large B-cell non-Hodgkin's lymphoma: a case report and review. Eur Rev Med Pharmacol Sci. 2012;16:1938-1946. [PubMed] |
| 37. | Unluoglu S, Bayol U, Korkmaz N, Ozenen B, Ipekci F, Pala EE. Perivascular epithelioid cell tumor of the ileum presenting as diverticulitis. Case Rep Pathol. 2012;2012:476941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Akbulut S, Turkey; Kim YW, South Korea S-Editor: Chen YL L-Editor: A P-Editor: Chen YL