Published online Jun 15, 2022. doi: 10.4251/wjgo.v14.i6.1141
Peer-review started: November 14, 2021
First decision: December 9, 2021
Revised: December 19, 2021
Accepted: May 13, 2022
Article in press: May 13, 2022
Published online: June 15, 2022
Processing time: 207 Days and 20.4 Hours
Operation is the primary therapeutic option for patients with distal gastrectomy. Braun anastomosis is usually performed after Billroth II reconstruction, which is wildly applied on distal gastrectomy because it is believed to benefit patients. However, studies are needed to confirm that.
To identify whether the addition of Braun anastomosis to Billroth II recon
A total of 143 patients with gastric cancer underwent laparoscopy-assisted distal gastrectomy at Centre 1 of PLA general hospital between January 2015 and December 2019. Clinical data of the patients were collected, and 93 of the 143 patients were followed up. These 93 patients were divided into two groups: Group 1 (Billroth II reconstruction, 33 patients); and Group 2 (Billroth II reconstruction combined with Braun anastomosis, 60 patients). Postoperative complication follow-up data and relevant clinical data were compared between the two groups.
There were no significant differences between Group 1 and Group 2 in postoperative complications (6.1% vs 6.7%, P = 0.679), anal exhaust time or blood loss. The follow-up prevalence of reflux gastritis indicated no significant difference between Group 1 and Group 2 (68.2% vs 51.7%, P = 0.109). The follow-up European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core-30 scores revealed no evident difference between Group 1 and Group 2 as well. Group 1 had a shorter operating time than Group 2 on average (234.6 min vs 262.0 min, P = 0.017).
Combined with Billroth II reconstruction, Braun anastomosis has been applied due to its ability to reduce the prevalence of reflux gastritis. Whereas in this study, the prevalence of reflux gastritis showed no significant difference, leading to a conclusion that under the circumstance of Braun anastomosis costing more time and more money, simple Billroth II reconstruction should be widely applied.
Core Tip: Braun anastomosis is usually performed after Billroth II reconstruction, which is wildly applied on distal gastrectomy because it is believed to benefit patients. This study indicated that the addition of Braun anastomosis to Billroth II reconstruction makes no significant difference in reducing the incidence of reflux gastritis.
- Citation: Li XG, Song QY, Wu D, Li S, Zhang BL, Zhang LY, Guan D, Wang XX, Liu L. Does the addition of Braun anastomosis to Billroth II reconstruction on laparoscopic-assisted distal gastrectomy benefit patients? World J Gastrointest Oncol 2022; 14(6): 1141-1147
- URL: https://www.wjgnet.com/1948-5204/full/v14/i6/1141.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i6.1141
Billroth I, Billroth II and Roux-en-Y reconstruction are the three most wildly applied reconstructions for distal gastrectomy[1]. Among these reconstructions, Billroth II reconstruction is recognized to reduce a high proportion of patients with reflux gastritis, which decreases patient quality of life[2] and potentially leads to malignancy, gastritis and reflux esophagitis[3,4]. In accordance with recent studies, the incidence of reflux gastritis after Billroth II reconstruction varies from 40% to 90%[5-7]. The addition of Braun anastomosis has been performed after Billroth II reconstruction since 1885, aiming to reduce complications after Billroth II reconstruction.
However, based on the working experiences in the hospital, it was observed that patients who underwent Braun anastomosis could get serious reflux gastritis as well. Moreover, one recent study[8] found that the addition of a Braun anastomosis is not effective in preventing enterogastric bile reflux. Other studies suggested that Braun anastomosis has a minor impact on the incidence of reflux gastritis to pancreatoduodenectomy[9,10] and one anastomosis gastric bypass[11]. Thus, whether Braun anastomosis can truly decrease the incidence of bile reflux to distal gastrectomy remains unknown.
The current study aimed to identify whether Braun anastomosis can truly decrease the incidence of bile reflux and improve the quality of life of the patients after Billroth II reconstruction on laparoscopic distal gastrectomy.
This retrospective cohort study was approved by our ethics committee at our institution. Between January 2015 and December 2019, a total of 143 patients with distal gastric cancer converted Billroth II reconstruction were collected in the 1st center of People’s Liberation Army General Hospital (PLA general hospital), Beijing, China. Of these patients, follow-up data was available for 93. These 93 patients were divided into two groups: Group 1 (Billroth II reconstruction, 33 patients); and Group 2 (Billroth II reconstruction combined with Braun anastomosis, 60 patients).
Laparoscopic-assisted distal gastrectomy with D2 lymphadenectomy was performed on all of the patients under the conduct of the Japanese classification of gastric carcinoma and the guidelines for the treatment of gastric carcinoma[12,13]. The arteries and veins were cut in the laparoscopic vision and then a small incision (less than 10 cm) was made in the center of the abdominal wall.
In Group 1, a small opening was made in the jejunum 20 cm away from the Treitz ligament on the anti-mesenteric margin and the residual gastric wall. The Billroth II anastomosis was performed with a 60 mm linear stapler in the end. In Group 2, jejunum-jejunum anastomosis was made 40 cm from the afferent limb.
Clinical data of the patients was collected, and 93 of the 143 patients were followed up. The follow-up data included: (1) The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core-30 scores[14] of patients; and (2) The number of patients with reflux gastritis. All of the follow-up was completed between January 2021 and June 2021. Postoperative complications, relevant clinical data and follow-up data were compared as well.
The inclusion criteria included: (1) Age from 18 to 75; (2) Pathologically diagnosed as distal gastric cancer; (3) Cancer pathological stage I-III (the 8th edition of the American Joint Committee on Cancer[15]); and (4) Complete clinical details. Exclusion criteria included: patients with serious heart disease or brain disease that influenced quality of life. A total of 143 patients were selected. Clinical data of the patients were collected and 93 of the 143 patients are followed up. Among the other 50 patients, 40 patients were out of contact and 10 patients were dead.
The main outcomes of this study were the incidence of reflux gastritis after the operation and The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core-30 scores, which is wildly applied in a variety of clinical studies[16-18]. Patients were called and required to answer 30 questions from The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core-30. Scores were calculated based on five multi-item functional scales (emotional, physical, role, social and cognitive function), of which higher scores indicate better quality of life; three multi-item and six single-item symptom scores, of which higher scores indicate poorer quality of life. Reflux gastritis was diagnosed according to the gastroscope reports.
All statistical analyses were performed with the support of SPSS v23.0 for Windows software. Continuous variables were expressed as mean ± SD and compared by Student’s t-test. Categorical variables were analyzed by Pearson χ2 test. A two-tailed P value < 0.05 was considered statistically significant.
There were 33 patients in Group 1 and 60 patients in Group 2. The age, pathological tumor stage, sex, mean blood loss and mean exhaust time between the two groups was similar, while group 2 had a significantly longer mean operation time (Table 1).
| Characteristic | Billroth 2 | Billroth 2 + Braun | P value |
| Age (yr) | 56.8 ± 9.9 | 57.2 ± 11.4 | 0.834 |
| Sex | 0.784 | ||
| Male | 41 | 56 | |
| Female | 18 | 28 | |
| Pathological tumor stage | 0.89 | ||
| 1 | 5 | 7 | |
| 2 | 16 | 30 | |
| 3 | 12 | 23 | |
| Operation time (min) | 234.6 ± 47.7 | 262.0 ± 64.9 | 0.017 |
| Blood loss (mL) | 160.6 ± 130.9 | 136.2 ± 107.9 | 0.224 |
| Anal exhaust time (d) | 5.0 ± 2.0 | 3.8 ± 1.1 | 0.348 |
In comparison of postoperative complications, 1 of the 33 patients in Group 1 suffered from bile reflux and 2 patients had anastomotic fistula. In Group 2, 1 patient had anastomosis bleeding and 3 patients had anastomotic fistula. The total incidence of postoperative complications indicated no significant difference between the two groups (Table 2).
| Complication | Billroth 2 | Billroth 2 + Braun | P value |
| Bile reflux | 1 | 0 | 1 |
| Anastomosis bleeding | 0 | 1 | 1 |
| Anastomotic fistula | 2 | 3 | 1 |
| Total | 3 | 4 | 0.696 |
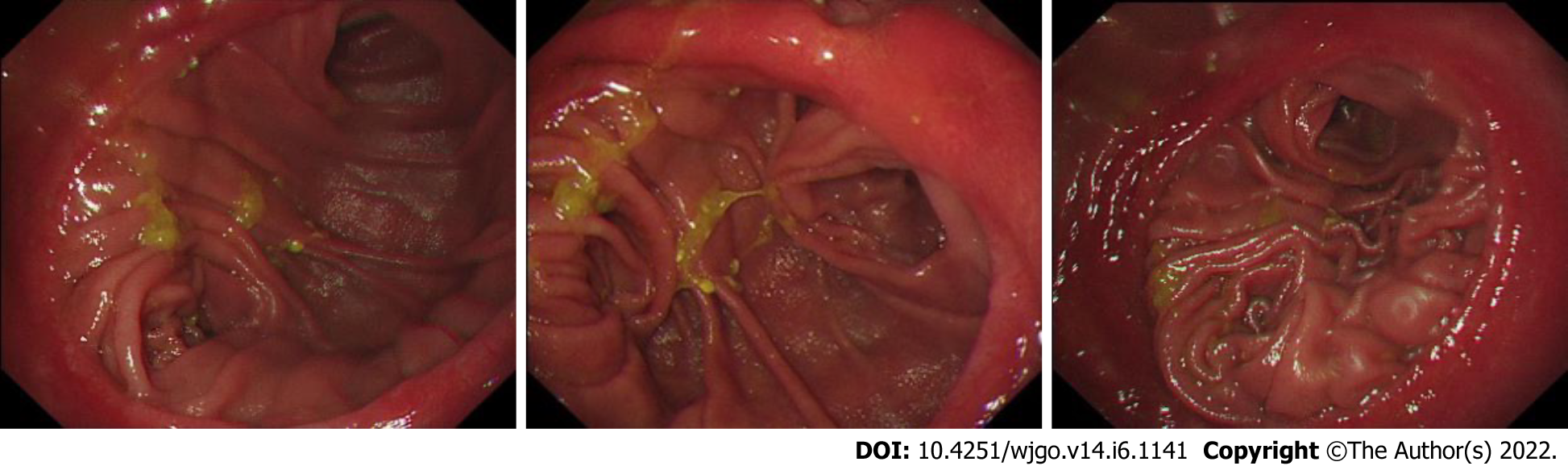
During follow-up, 11 patients in Group 1 and 29 patients in Group 2 had reflux gastritis on their gastroscope report during the postoperative review. The total incidence of reflux gastritis showed no significant difference (66.7% vs 51.7%, P = 0.109). For five multi-item functional scales (physical, emotional, role, cognitive and social function), three multi-item and six single-item symptom scores, it showed no significant difference between these two groups (Figure 1 and Table 3).
| Group 1 | Group 2 | P value | |
| Reflux gastritis | 0.109 | ||
| No | 11 | 29 | |
| Yes | 22 | 31 | |
| Incidence | 66.70% | 51.70% | |
| Multi-item functional scales | |||
| Physical function | 97.6 ± 0.95 | 92.7 ± 1.40 | 0.107 |
| Cognitive function | 98.9 ± 1.01 | 95.8 ± 1.40 | 0.126 |
| Emotional function | 94.4 ± 1.98 | 93.6 ± 1.54 | 0.744 |
| Role function | 97.5 ± 1.64 | 96.4 ± 1.19 | 0.592 |
| Social function | 96.5 ± 1.58 | 94.2 ± 1.67 | 0.369 |
| Total function | 84.3 ± 3.81 | 83.1 ± 2.25 | 0.757 |
| Symptom | |||
| Fatigue | 1.85 ± 5.49 | 6.48 ± 9.21 | 0.1 |
| Nausea/vomiting | 2.78 ± 6.80 | 1.94 ± 4.70 | 0.695 |
| Pain | 0.01 ± 3.46 | 2.08 ± 4.76 | 0.258 |
| Dyspnea | 0.51 ± 2.90 | 1.39 ± 5.57 | 0.398 |
| Appetite loss | 1.52 ± 6.41 | 4.17 ± 10.00 | 0.173 |
| Insomnia | 3.03 ± 7.74 | 3.61 ± 9.75 | 0.769 |
| Constipation | 1.01 ± 5.80 | 3.06 ± 7.19 | 0.165 |
| Diarrhea | 4.55 ± 10.44 | 4.72 ± 10.22 | 0.937 |
| Financial difficulty | 2.02 ± 5.52 | 5.28 ± 11.68 | 0.135 |
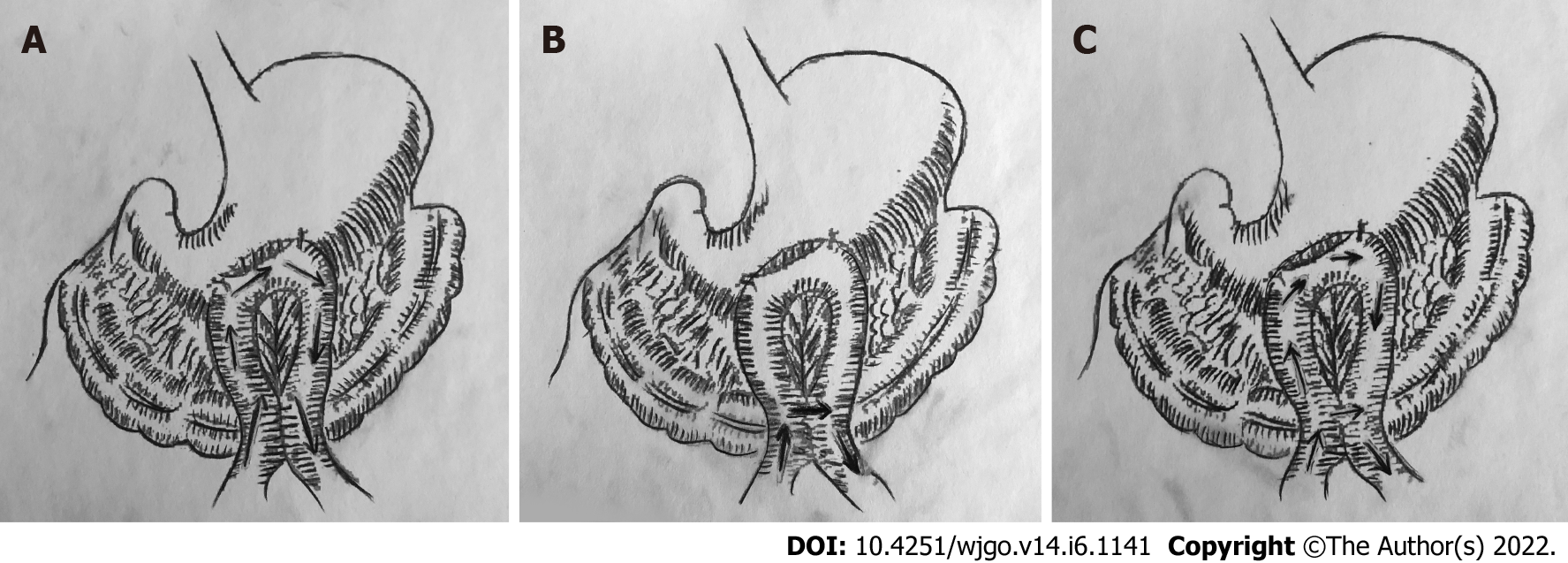
Billroth II reconstruction was invented in 1885 by Billroth as a modification of Billroth I. Due to the unique structure of Billroth II reconstruction, bile will flow through the residual stomach to the afferent loop, causing reflux gastritis (Figure 2A). Billroth II reconstruction is recognized with complications including anorexia, loss of appetite, dumping syndrome, nutritional anemia and alkaline reflux esophagitis[19]. In a previous study, the addition of Braun anastomosis was regarded as a method to reduce the incidence of reflux gastritis. That is because Braun anastomosis could relieve the afferent loop pressure[20,21], making bile flow through the jejunum- jejunum anastomosis, rather than the residual stomach (Figure 2B).
In this study, the incidence of reflux gastritis in Group 1 was lower than that in Group 2, but the difference was insignificant. It is indicated that bile may flow through both the residual stomach and the jejunum- jejunum anastomosis (Figure 2C). More experiments are needed to ensure this judgement.
In terms of postoperation complications, Group 1 and Group 2 were similar. The five multi-item functional scales (physical, emotional, role, cognitive and social function), three multi-item and six single-item symptom scores showed no significant difference as well.
In conclusion, this study indicated that the addition of Braun anastomosis to Billroth II reconstruction made no significant difference in reducing the incidence of reflux gastritis. The addition can hardly improve the quality of life of the patients but extends the operation time. Thus, the addition of Braun anastomosis is not necessary, and simple Billroth II reconstruction should be wildly applied.
Braun anastomosis is usually performed after Billroth II reconstruction on laparoscopy-assisted distal gastrectomy because it is believed to benefit patients. But we found that patients who underwent Braun anastomosis still had serious complications after operation. Thus, studies are needed to confirm that.
To determine whether the addition of Braun anastomosis to Billroth II reconstruction on laparoscopy-assisted distal gastrectomy benefits patients.
To study the role of Braun anastomosis in laparoscopy-assisted distal gastrectomy.
The clinical data of the addition of Braun anastomosis to Billroth II reconstruction on laparoscopy-assisted distal gastrectomy for patients with distal gastric cancer were compared. Patient follow-up data were analyzed. Operation time, blood loss, anal exhaust time and prevalence rate of reflux gastritis between the groups were examined.
Postoperative complications were reported in 3 of the 33 patients in the Billroth II reconstruction group and 4 out of 60 patients in the Billroth II reconstruction combined with Braun anastomosis group. The total incidence of postoperative complications indicated no significant difference between the two groups. During follow-up, 11 patients in the Billroth II reconstruction group and 29 patients in the Billroth II reconstruction combined with Braun anastomosis group had reflux gastritis. The total incidence of reflux gastritis showed no significant difference (66.7% vs 51.7%, P = 0.109). Five multi-item functional scales (physical, emotional, role, cognitive and social function), three multi-item and six single-item symptom scores showed no significant difference between these two groups.
The addition of Braun anastomosis to Billroth II reconstruction on laparoscopy-assisted distal gastrectomy did not show any benefit to patients with distal gastrectomy.
A prospective study with more patients is required to verify the conclusions of this study.
| 1. | Hirao M, Takiguchi S, Imamura H, Yamamoto K, Kurokawa Y, Fujita J, Kobayashi K, Kimura Y, Mori M, Doki Y; Osaka University Clinical Research Group for Gastroenterological Study. Comparison of Billroth I and Roux-en-Y reconstruction after distal gastrectomy for gastric cancer: one-year postoperative effects assessed by a multi-institutional RCT. Ann Surg Oncol. 2013;20:1591-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 2. | Svensson JO. Duodenogastric reflux after gastric surgery. Scand J Gastroenterol. 1983;18:729-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Osugi H, Fukuhara K, Takada N, Takemura M, Kinoshita H. Reconstructive procedure after distal gastrectomy to prevent remnant gastritis. Hepatogastroenterology. 2004;51:1215-1218. [PubMed] |
| 4. | Sato T, Miwa K, Sahara H, Segawa M, Hattori T. The sequential model of Barrett's esophagus and adenocarcinoma induced by duodeno-esophageal reflux without exogenous carcinogens. Anticancer Res. 2002;22:39-44. [PubMed] |
| 5. | Yang D, He L, Tong WH, Jia ZF, Su TR, Wang Q. Randomized controlled trial of uncut Roux-en-Y vs Billroth II reconstruction after distal gastrectomy for gastric cancer: Which technique is better for avoiding biliary reflux and gastritis? World J Gastroenterol. 2017;23:6350-6356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 6. | Ren Z, Wang WX. Comparison of Billroth I, Billroth II, and Roux-en-Y Reconstruction After Totally Laparoscopic Distal Gastrectomy: A Randomized Controlled Study. Adv Ther. 2019;36:2997-3006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Pribadi RR, Rani AA, Abdullah M. Challenges of endoscopic retrograde cholangiopancreatography in patients with Billroth II gastrointestinal anatomy: A review article. J Dig Dis. 2019;20:631-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Lee MS, Ahn SH, Lee JH, Park DJ, Lee HJ, Kim HH, Yang HK, Kim N, Lee WW. What is the best reconstruction method after distal gastrectomy for gastric cancer? Surg Endosc. 2012;26:1539-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 9. | Fujieda H, Yokoyama Y, Hirata A, Usui H, Sakatoku Y, Fukaya M, Nagino M. Does Braun Anastomosis Have an Impact on the Incidence of Delayed Gastric Emptying and the Extent of Intragastric Bile Reflux Following Pancreatoduodenectomy? Dig Surg. 2017;34:462-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Wang L, Su Ap, Zhang Y, Yang M, Yue Pj, Tian Bl. Reduction of alkaline reflux gastritis and marginal ulcer by modified Braun enteroenterostomy in gastroenterologic reconstruction after pancreaticoduodenectomy. J Surg Res. 2014;189:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Olmi S, Oldani A, Cesana G, Ciccarese F, Uccelli M, De Carli SM, Villa R, David G, Giorgi R, Zanoni AAG. Laparoscopic One Anastomosis Gastric Bypass Versus Laparoscopic One Anastomosis Gastric Bypass with Braun Anastomosis: What's Better? J Laparoendosc Adv Surg Tech A. 2019;29:1469-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Nakajima T. Gastric cancer treatment guidelines in Japan. Gastric Cancer. 2002;5:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 421] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 13. | Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition -. Gastric Cancer. 1998;1:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 964] [Reference Citation Analysis (0)] |
| 14. | Fayers PM, Aaronson NK, Bjordal K. EORTC Scoring Manual. 3rd ed. EORTC Quality of Life Study Group. Brussels: European Organisation for Research and Treatment of Cancer, 2001. |
| 15. | Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washinghton MK. AJCC Cancer Staging Manual. Springer, 2017. |
| 16. | Nolte S, Liegl G, Petersen MA, Aaronson NK, Costantini A, Fayers PM, Groenvold M, Holzner B, Johnson CD, Kemmler G, Tomaszewski KA, Waldmann A, Young TE, Rose M; EORTC Quality of Life Group. General population normative data for the EORTC QLQ-C30 health-related quality of life questionnaire based on 15,386 persons across 13 European countries, Canada and the Unites States. Eur J Cancer. 2019;107:153-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 332] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 17. | Husson O, de Rooij BH, Kieffer J, Oerlemans S, Mols F, Aaronson NK, van der Graaf WTA, van de Poll-Franse LV. The EORTC QLQ-C30 Summary Score as Prognostic Factor for Survival of Patients with Cancer in the "Real-World": Results from the Population-Based PROFILES Registry. Oncologist. 2020;25:e722-e732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 173] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 18. | Schmidt ME, Wiskemann J, Armbrust P, Schneeweiss A, Ulrich CM, Steindorf K. Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: A randomized controlled trial. Int J Cancer. 2015;137:471-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 209] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 19. | Shirbeigi L, Halavati N, Abdi L, Aliasl J. Dietary and Medicinal Herbal Recommendation for Management of Primary Bile Reflux Gastritis in Traditional Persian Medicine. Iran J Public Health. 2015;44:1166-1168. [PubMed] |
| 20. | Wang F, Zu HL, Jiang H, Kang Y, Dong PD, Xue YW. Clinical investigation of combined Billroth II with Braun anastomosis for patients with gastric cancer. Hepatogastroenterology. 2014;61:1812-1816. [PubMed] |
| 21. | Vogel SB, Drane WE, Woodward ER. Clinical and radionuclide evaluation of bile diversion by Braun enteroenterostomy: prevention and treatment of alkaline reflux gastritis. An alternative to Roux-en-Y diversion. Ann Surg. 1994;219:458-65; discussion 465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Katagiri R, Japan; Lee EW, South Korea; Yashiro M, Japan S-Editor: Yan JP L-Editor: Filipodia P-Editor: Yan JP