Published online Apr 15, 2022. doi: 10.4251/wjgo.v14.i4.897
Peer-review started: August 7, 2021
First decision: October 3, 2021
Revised: October 8, 2021
Accepted: February 22, 2022
Article in press: February 22, 2022
Published online: April 15, 2022
Processing time: 251 Days and 5.7 Hours
Inflammatory indices are considered to be potential prognostic biomarkers for patients with gastric cancer (GC). However, there is no evidence defining the prognostic significance of inflammatory indices for GC with different tumor infiltrative pattern (INF) types.
To evaluate the significance of inflammatory indices and INF types in predicting the prognosis of patients with GC.
A total of 962 patients who underwent radical gastrectomy were retrospectively selected for this study. Patients were categorized into the expansive growth type (INFa), the intermediate type (INFb), and the infiltrative growth type (INFc) groups. The cutoff values of inflammatory indices were analyzed by receiver operating characteristic curves. The Kaplan–Meier method and log-rank test were used to analyze overall survival (OS). The chi-square test was used to analyze the association between inflammatory indices and clinical characteristics. The independent risk factors for prognosis in each group were analyzed by univariate and multivariate analyses based on logistic regression. Nomogram models were constructed by R studio.
The INFc group had the worst OS (P < 0.001). The systemic immune-inflammation index (P = 0.039) and metastatic lymph node ratio (mLNR) (P = 0.003) were independent risk factors for prognosis in the INFa group. The platelet-lymphocyte ratio (PLR) (P = 0.018), age (P = 0.026), body mass index (P = 0.003), and postsurgical tumor node metastasis (pTNM) stage (P < 0.001) were independent risk factors for prognosis in the INFb group. The PLR (P = 0.021), pTNM stage (P = 0.028), age (P = 0.021), and mLNR (P = 0.002) were independent risk factors for prognosis in the INFc group. The area under the curve of the nomogram model for predicting 5-year survival in the INFa group, INFb group, and INFc group was 0.787, 0.823, and 0.781, respectively.
The outcome of different INF types GC patients could be assessed by nomograms based on different inflammatory indices and clinicopathologic features.
Core Tip: This is a retrospective study to analyze the relationship between peripheral circulating immune cells, inflammatory indices and the tumor infiltrative pattern (INF) types and to verify their ability to evaluate the outcome of gastric cancer (GC) patients. Our results showed that the systemic immune-inflammation index and platelet–lymphocyte ratio were independent prognostic factors for the expansive growth type, the intermediate type, and the infiltrative growth type groups. Based on different inflammatory indicators and clinicopathologic features, the nomogram models can predict the prognosis of different INF types GC patients, which deserve further testing and extension in clinical practice.
- Citation: Wang YF, Yin X, Fang TY, Wang YM, Zhang L, Zhang XH, Zhang DX, Zhang Y, Wang XB, Wang H, Xue YW. Prognostic significance of serum inflammation indices for different tumor infiltrative pattern types of gastric cancer. World J Gastrointest Oncol 2022; 14(4): 897-919
- URL: https://www.wjgnet.com/1948-5204/full/v14/i4/897.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i4.897
Gastric cancer (GC) is the sixth most common cancer and the third leading cause of cancer-related death, with more than 865000 deaths every year[1]. To better predict the individual prognosis of GC patients according to tumor biological characteristics, physicians had proposed a variety of classifications[2,3], including the tumor infiltrative pattern (INF) proposed in 1977[4]. The INF types are defined in the Japanese Classification of Gastric Carcinoma[5], which categorizes GC as the expansive growth type (INFa), the intermediate type (INFb), and the infiltrative growth type (INFc). Previous studies have shown that different INF types differ in clinicopathological features and prognosis and can be used as predictors of postoperative recurrence and prognosis in GC patients[6-9]. However, GC is a highly heterogeneous malignant tumor, and the prognosis of patients with the same INF may also show significant differences. Therefore, it is worth further exploring the prognosis of GC patients with different INF types to guide clinical treatment.
With the popularization and development of immunotherapy, the significant role of tumor immunity in malignant tumors has gradually attracted the attention of clinical experts[10]. From 2014 to 2018, the postsurgical tumor node metastasis-immunology (pTNM-I) stage proposed by Galon et al[11] and Pagès et al[12] was well applied in colon cancer patients. It suggests that the traditional pTNM staging combined with tumor immunity can give more serviceable prognostic and treatment information. Nevertheless, tumor heterogeneity will lead to the restriction of immunohistochemical detection of GC by site selection. As a part of tumor immunity, the peripheral blood immune system also has a significant effect on tumor progression. Our previous studies have confirmed that the inflammatory index plays an important role in the early diagnosis of GC and the evaluation of the outcome of GC patients with different Lauren classification[13,14]. In addition, in liver metastases, different tumor growth pattern subtypes have different levels of lymphocyte infiltration, indicating that they have different immune states in the tumor microenvironment[15]. However, no study has analyzed the relationship between INF and the peripheral blood immune inflammatory response in GC. Therefore, whether the immune difference and prognosis of GC patients with different INF types can be evaluated by peripheral blood inflammatory indices deserves further exploration.
From September 2012 and July 2015, 962 patients at Harbin Medical University Cancer Hospital were included in the study. The clinical applicability of different inflammatory indices, including the neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), and systemic immune-inflammation index (SII), was evaluated. At the same time, the difference of peripheral blood immune cells in different INF types was further analyzed. Finally, we constructed predictive models by combining inflammatory indices with the clinicopathological features of patients based on INF types.
All GC patients underwent radical gastrectomy according to their respective conditions[16]. GC was diagnosed by tissue specimens obtained during gastroscopy and confirmed by pathologists examining tissue samples after surgery. The patients underwent routine pre-operative auxiliary examinations, including abdominal ultrasound, gastric computed tomography (CT)/magnetic resonance imaging, chest X-ray, tumor markers examination, hematology examination, and electrocardiogram during hospitalization. If necessary, patients underwent positron emission tomography (PET)/CT. Patients were followed up until the date of death or for 5 years, whichever came first.
The exclusion criteria were as follows: (1) preoperative neoadjuvant therapy; (2) platelet therapy was performed within 3 mo before surgery; (3) severe heart disease; (4) active hemorrhage; (5) intravascular coagulation; (6) severe infection; (7) hematological malignancies; and (8) steroid drug treatment.
Formulation of postoperative chemotherapy regimens were according to the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology[17]. For GC patients with stage II or III, oxaliplatin + capecitabine and oxaliplatin + S-1 were the main treatment options. A total of 315 patients who underwent complete postoperative chemotherapy were included in our study.
Clinicopathological data of patients were saved in the Gastric Cancer Information Management System v1.2 of Harbin Medical University Cancer Hospital (Copyright No. 2013SR087424, http://www.sgihmu.com), including age, gender, body mass index (BMI), Borrmann type, tumor location, tumor diameter, histological type, metastatic lymph node ratio (mLNR), nerve infiltration, vascular infiltration, resection, pTNM stage, postoperative chemotherapy and laboratory examination. pTNM stage is consistent with the eighth edition of the American Joint Commission on Cancer. Other auxiliary examinations (CT, ultrasound, and gastroscopy) or tumor markers were performed on all patients every 3-6 mo postoperatively. In addition, PET/CT examinations are performed as needed.
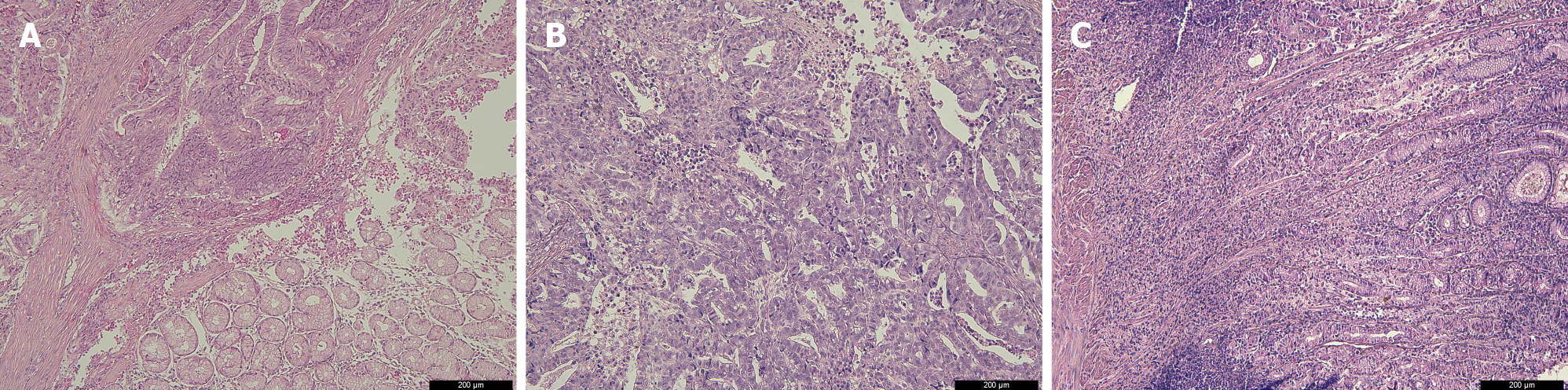
The pathological INF types were diagnosed by two institutional pathologists, which using paraffin sections stained with hematoxylin and eosin. GC tissue specimens were classified as INFa (tumor grows expansively and has an obvious boundary with the surrounding tissue), INFb (tumor shows an intermediate type between the expansive growth type and the infiltrative growth type), and INFc (tumor grows infiltratively and has no obvious boundary with the surrounding tissue), according to the Japanese Classification of Gastric Carcinoma[5] (Figure 1).
Blood samples were taken on an empty stomach the day after admission. Venous blood was collected from the cubital vein. Then, it was sent to the blood laboratory to separate the serum and calculate the corresponding inflammatory index. NLR = neutrophil count (N)/lymphocyte count (L), PLR = platelet count (P)/L, SII = N × P/L.
Overall survival (OS) was defined as the time from surgery to death or the last surviving follow-up visit. OS is shown as the mean and 95%CI. Receiver operating characteristic curve (ROC) analysis and the area under the curve (AUC) were used to compare the diagnostic significance of each inflammatory index. The Youden index was used to analyzed the optimal cutoff value of each inflammatory index, which was calculated by the equation: sensitivity-(1-specificity). The optimal cutoff value of each inflammatory index was determined by the maximum value of the Youden index. The chi-square test was used to analyze the association between the inflammatory indices and clinicopathological factors. Survival curves for different INF types were analyzed by the log-rank test and Kaplan–Meier method. Based on logistic regression, univariate and multivariate analyses were used to analyze the independent prognostic factors for different INF types. Hazard ratios (HRs) and 95%CIs of each factor were shown. Boxplots and scatterplots were drawn by GraphPad Prism 8. The nomogram models were drawn through R studio by the “SvyNom” and “rms” packages. SPSS version 25.0 (SPSS Inc., Chicago, IL, United States) was used for analysis, and P < 0.05 was considered statistically significant.
In the INFa group, the age range was 33-87 years (median 55 years), and the ratio of male to female was 133:50. In the INFb group, the age range was 30-79 years (median 60 years), and the ratio of male to female was 248:83. In the INFc group, the age range was 24-85 years (median 57 years), and the ratio of male to female was 308:140. Table 1 shows the clinicopathological features of the three groups.
| Characteristics | INFa (n = 183) | INFb (n = 331) | INFc (n = 448) |
| Sex | |||
| Male | 133 (72.7) | 248 (74.9) | 308 (68.8) |
| Female | 50 (27.3) | 83 (25.1) | 140 (31.2) |
| Age (yr) | 58.81 ± 9.59 | 59.30 ± 9.73 | 57.06 ± 10.55 |
| Borrmann type | |||
| 0-1 | 31 (16.9) | 51 (15.4) | 21 (4.7) |
| 2 | 62 (33.9) | 105 (31.7) | 104 (23.2) |
| 3 | 74 (40.4) | 138 (41.7) | 242 (54.0) |
| 4-5 | 16 (8.8) | 37 (11.2) | 81 (18.1) |
| Tumor location | |||
| Lower third | 156 (85.2) | 227 (68.6) | 329 (73.4) |
| Middle third | 21 (11.5) | 64 (19.3) | 75 (16.8) |
| Upper third | 4 (2.2) | 33 (10.0) | 31 (6.9) |
| Entire stomach | 2 (1.1) | 7 (2.1) | 13 (2.9) |
| Tumor size (mm) | 44.40 ± 24.85 | 53.06 ± 25.78 | 56.31 ± 27.38 |
| pTNM stage | |||
| I | 72 (39.3) | 70 (21.1) | 36 (8.0) |
| II | 60 (32.8) | 127 (38.4) | 137 (30.6) |
| III | 51 (27.9) | 134 (40.5) | 275 (61.4) |
| Histological type | |||
| Well and medium differentiation | 112 (61.2) | 149 (45.0) | 126 (28.1) |
| Poor differentiation | 46 (25.1) | 88 (26.6) | 207 (46.2) |
| Others | 25 (13.7) | 94 (28.4) | 115 (25.7) |
| mLNR | 0.09 ± 0.16 | 0.12 ± 0.17 | 0.19 ± 0.22 |
| Vascular infiltration | |||
| No | 137 (74.9) | 231 (69.8) | 317 (70.8) |
| Yes | 46 (25.1) | 100 (30.2) | 131 (29.2) |
| Nerve infiltration | |||
| No | 123 (67.2) | 198 (59.8) | 251 (56.0) |
| Yes | 60 (32.8) | 133 (40.2) | 197 (44.0) |
| Postoperative chemotherapy | |||
| Yes | 58 (31.7) | 90 (27.2) | 167 (37.3) |
| No | 125 (68.3) | 241 (72.8) | 281 (62.7) |
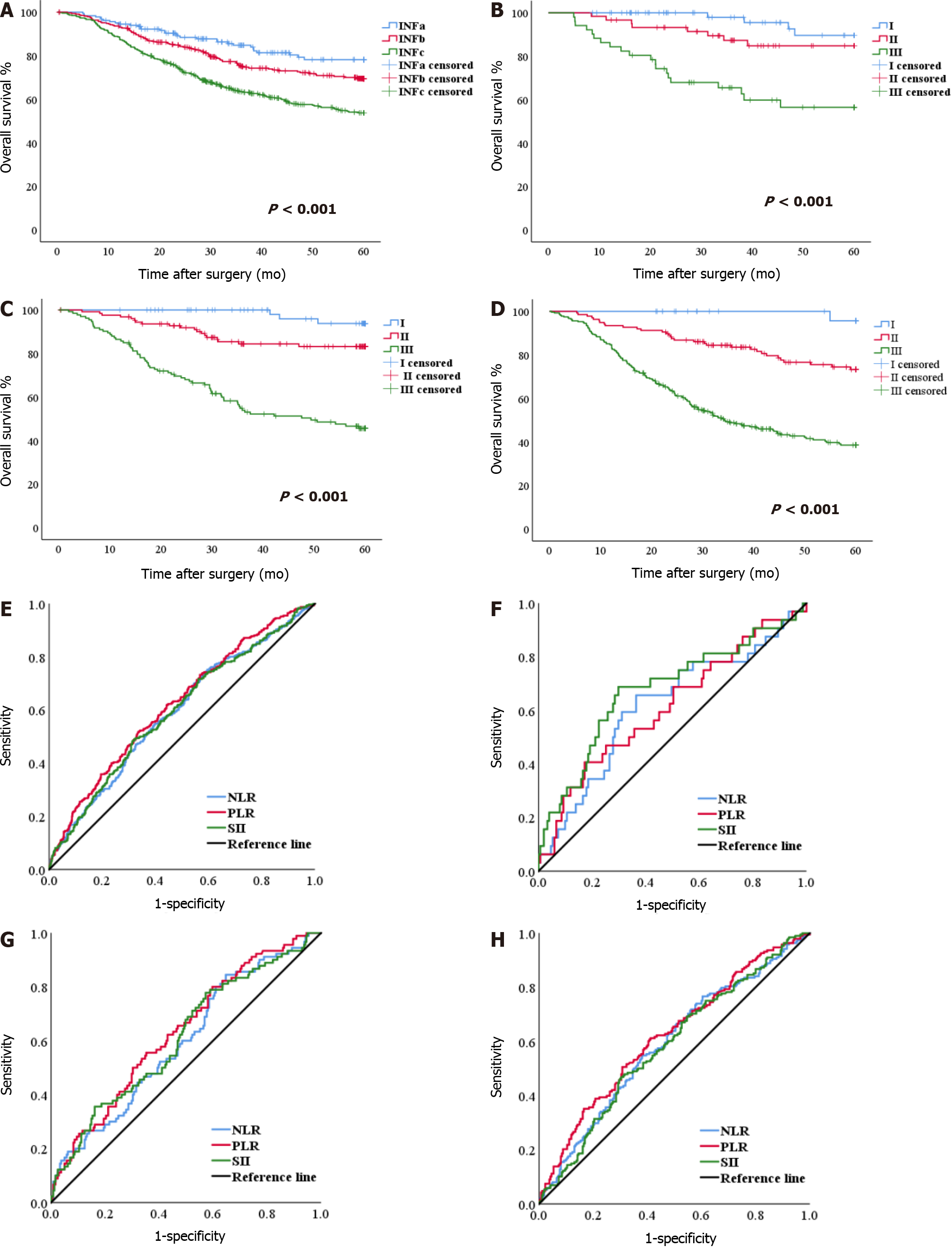
There were statistically significant differences in OS among the INFa, INFb and INFc groups (P < 0.001). The OS of patients with INFc was worse than the OS of patients with INFa and INFb (Figure 2A). In the three groups, the OS of patients with stage I, II, and III GC was significantly different (all P < 0.001) (Figure 2B-D). These results are shown in the supplementary materials (Supplementary Table 1).
The NLR, PLR, and SII scores of 1.99, 126.90, and 529.24, respectively, were calculated as the most appropriate cutoff thresholds by the Youden index of the ROC for all patients based on preoperative hematology. The AUCs were 0.591 (95%CI: 0.553-0.630), 0.620 (95%CI: 0.583-0.658), and 0.594 (95%CI: 0.555-0.632), respectively (Figure 2E). The AUCs of NLR, PLR, and SII for the INFa, INFb and INFc groups are shown in the Supplementary Table 2 (Figure 2F-H).
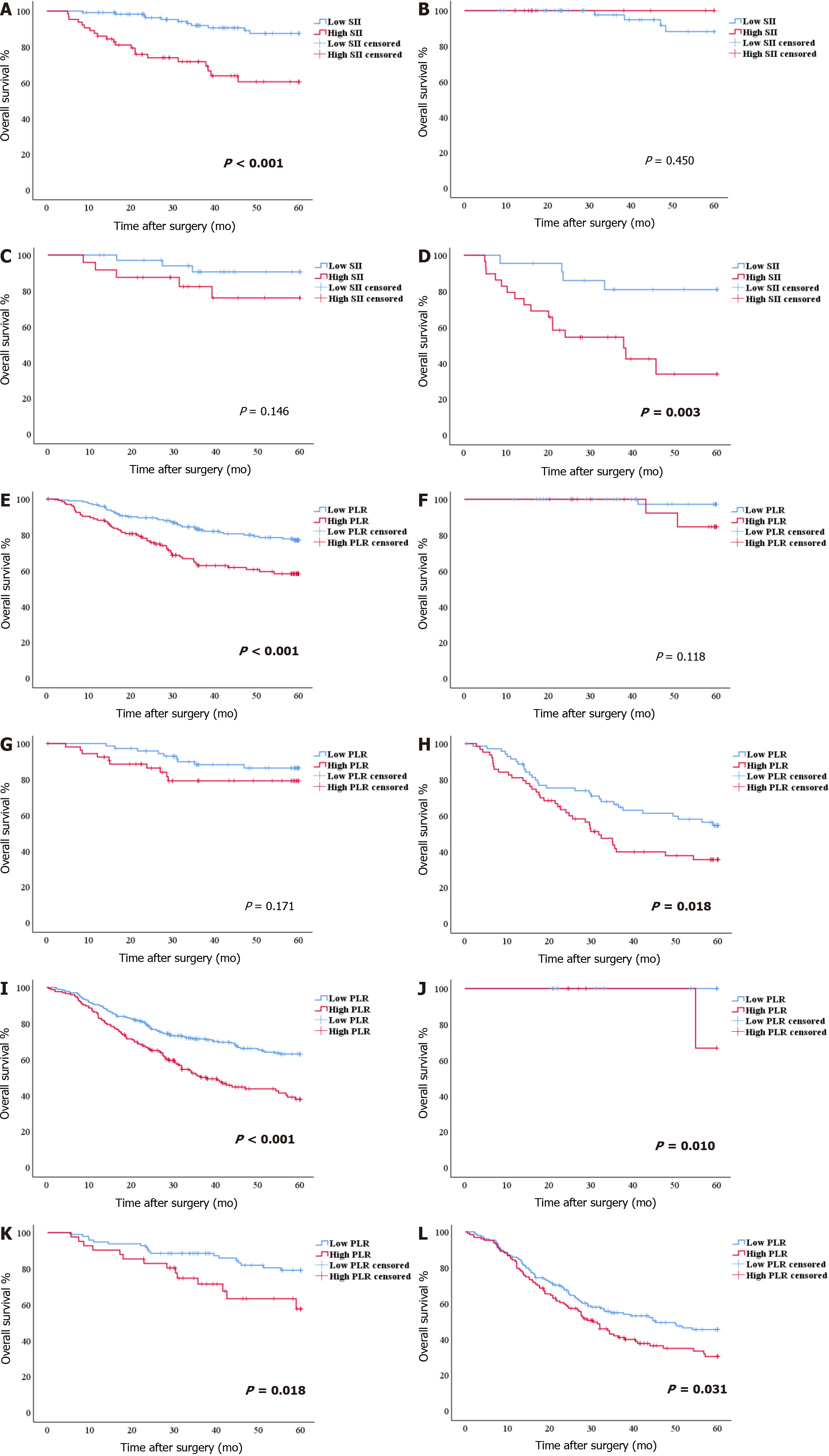
In patients with INFa, a significant difference in OS was found between patients with SII > 523.01 and those with SII ≤ 523.01 (OS: 56.55 mo vs 44.98 mo, P < 0.001; HR: 4.502, 95%CI: 2.166-9.355). According to the pTNM stage, for stages I and II, the difference in OS between patients with SII > 523.01 and those with SII ≤ 523.01 was not significant (P = 0.450 and P = 0.146). For stage III, patients with SII ≤ 523.01 had better survival (P = 0.003) (Figure 3A-D). The SII score was significantly associated with carbohydrate antigen 19-9 (CA19-9), Borrmann type, tumor diameter, histological type, pTNM stage, and mLNR, as determined by the chi-square test of clinicopathologic features (all P < 0.05) (Table 2).
| Characteristics | INFa | INFb | INFc | ||||||
| SII ≤ 523.01 (119) | SII > 523.01 (64) | P value | PLR ≤ 134.02 (195) | PLR > 134.02 (136) | P value | PLR ≤ 134.02 (273) | PLR > 134.02 (175) | P value | |
| Sex | 0.443 | 0.979 | 0.489 | ||||||
| Male | 84 (70.6) | 49 (76.6) | 146 (74.9) | 102 (75.0) | 191 (70.0) | 117 (66.9) | |||
| Female | 35 (29.4) | 15 (23.4) | 49 (25.1) | 34 (25.0) | 82 (30.0) | 58 (33.1) | |||
| Age (yr) | 0.126 | 0.878 | < 0.001 | ||||||
| ≤ 60 | 68 (57.1) | 29 (45.3) | 103 (52.8) | 73 (53.7) | 191 (70.0) | 86 (49.1) | |||
| > 60 | 51 (42.9) | 35 (54.7) | 92 (47.2) | 63 (46.3) | 82 (30.0) | 89 (50.9) | |||
| BMI | 0.105 | 0.038 | 0.592 | ||||||
| ≤ 22.49 | 52 (43.7) | 36 (56.3) | 85 (43.6) | 75 (55.1) | 138 (50.5) | 93 (53.1) | |||
| > 22.49 | 67 (56.3) | 28 (43.7) | 110 (56.4) | 61 (44.9) | 135 (49.5) | 82 (46.9) | |||
| CEA | 0.064 | 0.608 | 0.782 | ||||||
| ≤ 5 ng/mL | 108 (90.8) | 52 (81.2) | 162 (83.1) | 110 (80.9) | 235 (86.1) | 149 (85.1) | |||
| > 5 ng/mL | 11 (9.2) | 12 (18.8) | 33 (16.9) | 26 (19.1) | 38 (13.9) | 26 (14.9) | |||
| CA19-9 | 0.014 | 0.198 | 0.156 | ||||||
| ≤ 37 U/mL | 112 (94.1) | 53 (82.8) | 168 (86.2) | 110 (83.7) | 246 (90.1) | 150 (85.7) | |||
| > 37 U/mL | 7 (5.9) | 11 (17.2) | 27 (13.8) | 26 (16.3) | 27 (9.9) | 25 (14.3) | |||
| Borrmann type | 0.005 | 0.051 | 0.454 | ||||||
| 0-2 | 71 (59.7) | 22 (34.4) | 84 (43.1) | 72 (53.0) | 80 (29.3) | 45 (25.7) | |||
| 3 | 40 (33.6) | 34 (53.1) | 92 (47.2) | 46 (33.8) | 141 (51.6) | 101 (57.7) | |||
| 4 | 8 (6.7) | 8 (12.5) | 19 (9.7) | 18 (13.2) | 52 (19.1) | 29 (16.6) | |||
| Tumor diameter (mm) | < 0.001 | < 0.001 | < 0.001 | ||||||
| ≤ 50 | 95 (79.8) | 31 (48.4) | 131 (67.2) | 63 (46.3) | 172 (63.0) | 75 (42.9) | |||
| > 50 | 24 (20.2) | 33 (51.6) | 64 (32.8) | 73 (53.7) | 101 (37.0) | 100 (57.1) | |||
| Tumor location | 0.992 | 0.051 | 0.423 | ||||||
| Middle and upper third | 16 (13.4) | 9 (14.0) | 57 (29.2) | 40 (29.4) | 62 (22.7) | 44 (25.1) | |||
| Lower third | 102 (85.7) | 54 (84.4) | 137 (70.3) | 90 (66.2) | 205 (75.1) | 124 (70.9) | |||
| Entire stomach | 1 (0.9) | 1 (1.6) | 1 (0.5) | 6 (4.4) | 6 (2.2) | 7 (4.0) | |||
| Histological type | < 0.001 | 0.041 | < 0.001 | ||||||
| Well and medium differentiation | 74 (62.2) | 38 (59.4) | 99 (50.8) | 50 (36.8) | 78 (28.6) | 48 (27.4) | |||
| Poor differentiation | 2 (1.7) | 19 (30.0) | 47 (24.1) | 41 (30.1) | 120 (43.9) | 87 (49.7) | |||
| Others | 18 (15.1) | 7 (10.9) | 49 (25.1) | 45 (33.1) | 75 (27.5) | 40 (22.9) | |||
| pTNM stage | < 0.001 | 0.022 | < 0.001 | ||||||
| I | 61 (51.3) | 11 (17.2) | 51 (26.2) | 19 (14.0) | 29 (10.6) | 7 (4.0) | |||
| II | 36 (30.2) | 24 (37.5) | 73 (37.4) | 54 (39.7) | 96 (35.2) | 41 (23.4) | |||
| III | 22 (18.5) | 29 (45.3) | 71 (36.4) | 63 (46.3) | 148 (54.2) | 127 (72.6) | |||
| mLNR | 0.019 | 0.290 | 0.001 | ||||||
| ≤ 0.07 | 84 (70.6) | 34 (53.1) | 109 (55.9) | 68 (50.0) | 131 (48.0) | 56 (32.0) | |||
| > 0.07 | 35 (29.4) | 30 (46.9) | 86 (44.1) | 68 (50.0) | 142 (52.0) | 119 (68.0) | |||
| Vascular infiltration | 0.494 | 0.030 | 0.146 | ||||||
| No | 91 (76.5) | 46 (71.9) | 145 (74.4) | 86 (63.2) | 200 (73.3) | 117 (66.9) | |||
| Yes | 28 (23.5) | 18 (28.1) | 50 (25.6) | 50 (36.8) | 73 (26.7) | 58 (33.1) | |||
| Nerve infiltration | 0.098 | 0.592 | 0.238 | ||||||
| No | 85 (71.4) | 38 (59.4) | 119 (61.0) | 79 (58.1) | 159 (58.2) | 92 (52.6) | |||
| Yes | 34 (28.6) | 26 (40.6) | 76 (39.0) | 57 (41.9) | 114 (41.8) | 83 (47.4) | |||
In patients with INFb, a significant difference in OS was found between patients with PLR > 134.02 and those with PLR ≤ 134.02 (OS: 52.47 mo vs 44.37 mo, P < 0.001; HR: 2.191, 95%CI: 1.444-3.323). According to the pTNM stage, for stages I and II, the difference in OS between patients with PLR > 134.02 and those with PLR ≤ 134.02 was not significant (P = 0.118 and P = 0.171). For stage III, the OS of patients with PLR ≤ 134.02 was better (P = 0.018) (Figure 3E-H). The PLR score was significantly associated with BMI, tumor diameter, histological type, pTNM stage, and mLNR, as determined by the chi-square test of clinicopathologic features (all P < 0.05) (Table 2).
In patients with INFc, a significant difference in OS was found between patients with PLR > 134.02 and those with PLR > 134.02 (OS: 46.53 mo vs 37.97 mo, P < 0.001; HR: 1.956, 95%CI: 1.467-2.307). According to the pTNM stage, for stages I, II, and III, patients with PLR ≤ 134.02 had better survival (P = 0.010, P = 0.018 and P = 0.031) (Figure 3I-L). The PLR score was significantly associated with age, tumor diameter, histological type, pTNM stage, and mLNR, as determined by the chi-square test of clinicopathologic features (all P < 0.05) (Table 2).
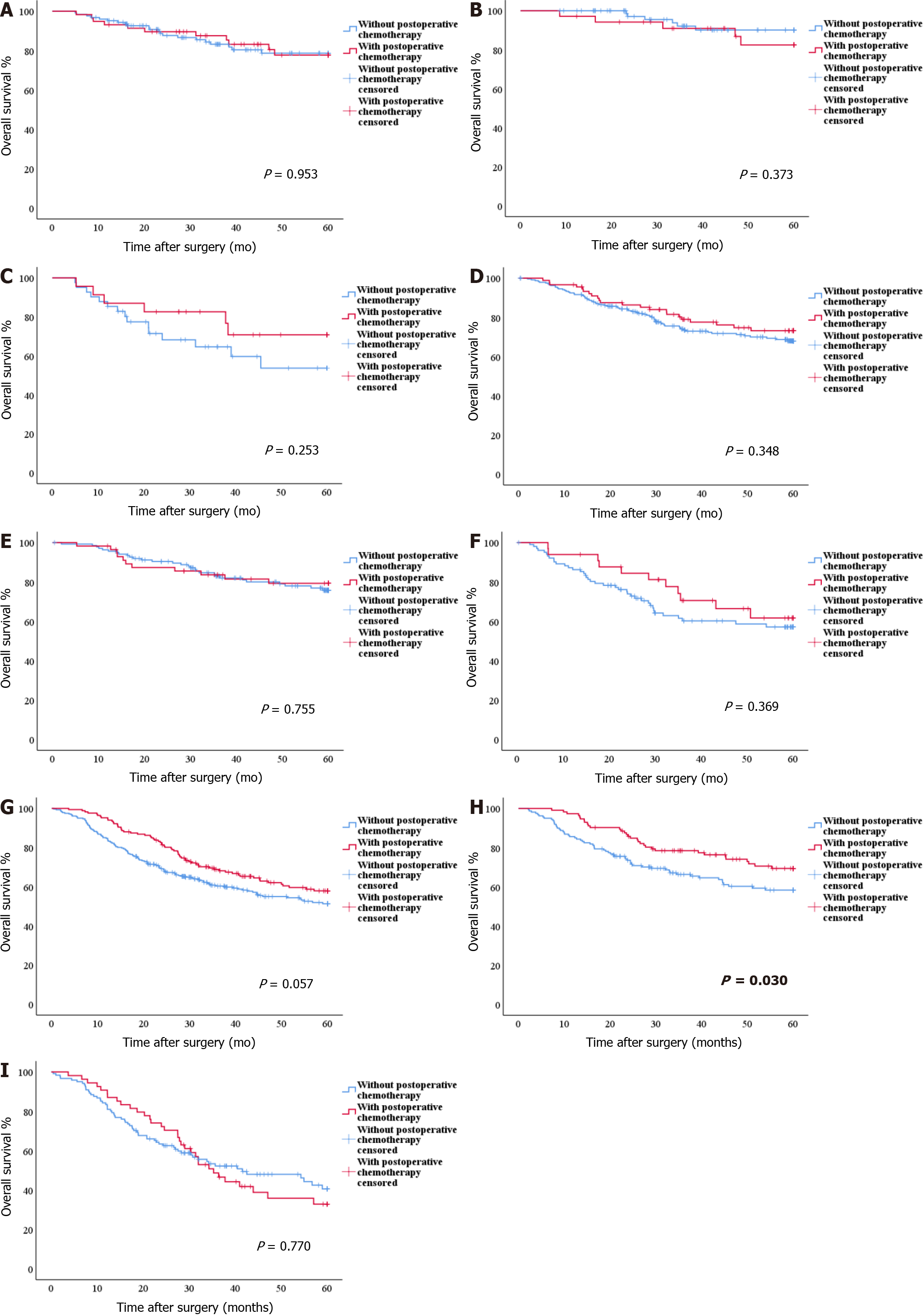
For INFa and INFb GC, there were no statistically significant differences in OS between patients with and without postoperative chemotherapy (all P > 0.05) (Figure 4A-F). For INFc GC, in the PLR ≤ 134.02 group, the OS of patients with postoperative chemotherapy was significantly better than that of patients without postoperative chemotherapy (OS: 50.22 mo vs 43.90 mo, P = 0.030) (Figure 4H). However, in the overall group and the PLR > 134.02 group, there were no statistically significant differences in OS between patients with and without postoperative chemotherapy (P = 0.057 and P = 0.770) (Figure 4G and I).
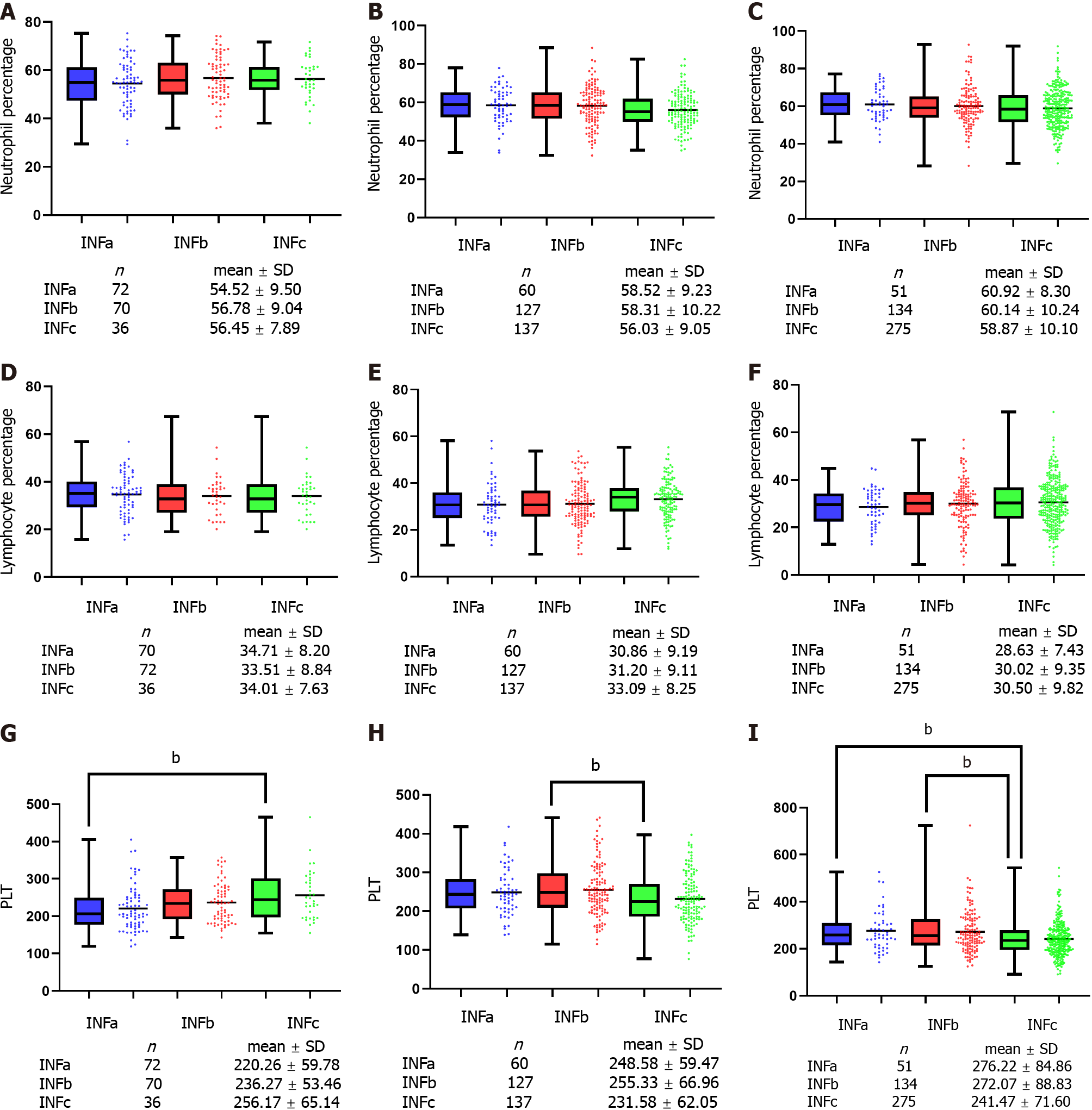
According to pTNM stage, peripheral circulating immune cell parameters, including neutrophil percentage, lymphocyte percentage and platelet count were analyzed. For I-III stage GC patients, the percentages of neutrophils and lymphocytes were not significantly different among the three groups (P > 0.05) (Figure 5A-F). For patients with stage I GC, the platelet count of INFc group was significantly higher than that of INFa group (P < 0.05) (Figure 5G). For patients with stage II GC, the platelet count of INFc group was significantly lower than that of INFb group (P < 0.05) (Figure 5H). For patients with stage III GC, the platelet count of INFc group was significantly lower than that of INFa and INFb group (P < 0.05) (Figure 5I).
To identify the independent risk factors for prognosis in the three groups, univariate and multivariate analyses based on the logistic risk regression model were implemented. In the INFa group, univariate analysis and multivariate analysis showed that SII (P = 0.039) and mLNR (P = 0.003) were independent prognostic factors for INFa GC (Table 3). In the INFb group, univariate analysis and multivariate analysis showed that age (P = 0.026), BMI (P = 0.003), PLR (P = 0.018) and pTNM stage (P < 0.001) were independent risk factors for the prognosis of INFb GC (Table 4). In the INFc group, univariate analysis and multivariate analysis showed that age (P = 0.021), PLR (P = 0.021), pTNM stage (P = 0.028) and mLNR (P = 0.002) were independent prognostic factors for INFc GC (Table 5).
| Characteristics | INFa | |||
| Univariate analysis | Multivariate analysis | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Sex | 0.584 | |||
| Male | 1 | |||
| Female | 1.261 (0.550-2.894) | |||
| Age (yr) | 1.019 (0.979-1.061) | 0.349 | ||
| BMI | 0.880 (0.772-1.003) | 0.055 | ||
| SII | 1.002 (1.001-1.003) | 0.001 | 1.001 (1.000-1.003) | 0.039 |
| ALT | 0.988 (0.954-1.023) | 0.491 | ||
| Total protein | 0.968 (0.916-1.023) | 0.251 | ||
| CEA | 1.030 (0.996-1.065) | 0.087 | ||
| CA19-9 | 1.002 (0.998-1.006) | 0.278 | ||
| Borrmann type | 0.007 | 0.272 | ||
| 0-1 | 1 | 1 | ||
| 2 | 3.818 (0.448-32.517) | 0.220 | 5.312 (0.450-62.711) | 0.185 |
| 3 | 8.947 (1.135-70.530) | 0.038 | 5.450 (0.496-59.870) | 0.166 |
| 4-5 | 23.333 (2.525-215.647) | 0.005 | 14.134 (0.944-211.701) | 0.055 |
| Tumor location | 0.691 | |||
| Lower third | 1 | |||
| Middle third | 1.176 (0.366-3.782) | 0.785 | ||
| Upper third | 1.667 (0.167-16.657) | 0.664 | ||
| Entire stomach | 5.000 (0.303-82.520) | 0.261 | ||
| Tumor size (mm) | 1.032 (1.016-1.049) | < 0.001 | 1.012 (0.989-1.034) | 0.310 |
| pTNM stage | < 0.001 | 0.796 | ||
| I | 1 | 1 | ||
| II | 2.615 (0.747-9.159) | 0.133 | 0.660 (0.154-2.830) | 0.576 |
| III | 10.968 (3.457-34.794) | < 0.001 | 0.532 (0.080-3.525) | 0.513 |
| Histological type | 0.160 | |||
| Well and medium differentiation | 1 | |||
| Poor differentiation | 2.282 (0.972-5.360) | 0.058 | ||
| Others | 1.617 (0.527-4.959) | 0.401 | ||
| mLNR | 438.799 (38.101-5053.535) | < 0.001 | 471.355 (8.218-27035.275) | 0.003 |
| Vascular infiltration | 0.080 | |||
| No | 1 | |||
| Yes | 2.065 (0.917-4.647) | |||
| Nerve infiltration | 0.025 | 0.621 | ||
| No | 1 | 1 | ||
| Yes | 2.432 (1.118-5.288) | 1.286 (0.474-3.490) | ||
| Postoperative chemotherapy | 0.720 | |||
| Yes | 1 | |||
| No | 1.159 (0.517-2.597) | |||
| Characteristics | INFb | |||
| Univariate analysis | Multivariate analysis | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Sex | 0.115 | |||
| Male | 1 | |||
| Female | 0.617 (0.339-1.124) | |||
| Age (yr) | 1.033 (1.007-1.061) | 0.014 | 1.036 (1.004-1.069) | 0.026 |
| BMI | 0.814 (0.746-0.888) | < 0.001 | 0.861 (0.781-0.950) | 0.003 |
| PLR | 1.008 (1.003-1.012) | < 0.001 | 1.006 (1.001-1.012) | 0.018 |
| ALT | 0.976 (0.949-1.004) | 0.094 | ||
| Total protein | 0.975 (0.946-1.004) | 0.092 | ||
| CEA | 1.004 (0.995-1.014) | 0.338 | ||
| CA19-9 | 1.003 (1.001-1.006) | 0.012 | 1.000 (0.998-1.003) | 0.650 |
| Borrmann type | 0.005 | 0.226 | ||
| 0-1 | 1 | 1 | ||
| 2 | 1.031 (0.430-2.473) | 0.945 | 0.715 (0.246-2.079) | 0.538 |
| 3 | 2.410 (1.081-5.372) | 0.031 | 1.530 (0.554-4.223) | 0.412 |
| 4-5 | 3.182 (1.201-8.429) | 0.020 | 1.339 (0.374-4.797) | 0.654 |
| Tumor location | 0.088 | - | - | |
| Lower third | 1 | |||
| Middle third | 1.195 (0.641-2.228) | 0.575 | ||
| Upper third | 1.527 (0.697-3.345) | 0.290 | ||
| Entire stomach | 7.634 (1.441-40.446) | 0.017 | ||
| Tumor size (mm) | 1.021 (1.012-1.032) | < 0.001 | 1.007 (0.994-1.021) | 0.302 |
| pTNM stage | < 0.001 | < 0.001 | ||
| I | 1 | 1 | ||
| II | 3.929 (1.120-13.785) | 0.033 | 2.036 (0.528-7.856) | 0.302 |
| III | 23.010 (6.895-76.795) | < 0.001 | 8.306 (2.053-33.597) | 0.003 |
| Histological types | 0.739 | |||
| Well and medium differentiation | 1 | |||
| Poor differentiation | 1.105 (0.617-1.977) | 0.738 | ||
| Others | 0.853 (0.472-1.542) | 0.599 | ||
| mLNR | 87.343 (18.612-409.881) | < 0.001 | 2.267 (0.291-17.641) | 0.434 |
| Vascular infiltration | < 0.001 | 0.149 | ||
| No | 1 | 1 | ||
| Yes | 3.160 (1.897-5.263) | 1.585 (0.848-2.963) | ||
| Nerve infiltration | 0.003 | 0.859 | ||
| No | 1 | 1 | ||
| Yes | 2.097 (1.283-3.428) | 1.057 (0.573-1.951) | ||
| Postoperative chemotherapy | 0.493 | |||
| Yes | 1 | |||
| No | 0.823 (0.472-1.436) | |||
| Characteristics | INFc | |||
| Univariate analysis | Multivariate analysis | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Sex | 0.570 | |||
| Male | 1 | |||
| Female | 0.889 (0.592-1.335) | |||
| Age (yr) | 1.028 (1.009-1.047) | 0.003 | 1.026 (1.004-1.049) | 0.021 |
| BMI | 0.965 (0.915-1.017) | 0.184 | ||
| PLR | 1.007 (1.004-1.010) | < 0.001 | 1.004 (1.001-1.008) | 0.021 |
| ALT | 0.984 (0.966-1.003) | 0.096 | ||
| Total protein | 0.995 (0.973-1.018) | 0.683 | ||
| CEA | 1.006 (0.998-1.014) | 0.161 | ||
| CA19-9 | 1.000 (1.000-1.006) | 0.020 | 1.001 (0.999-1.004) | 0.387 |
| Borrmann type | < 0.001 | 0.055 | ||
| 0-1 | 1 | 1 | ||
| 2 | 2.104 (0.574-7.708) | 0.262 | 1.193 (0.290-4.903) | 0.807 |
| 3 | 4.298 (1.233-14.981) | 0.022 | 1.781 (0.460-6.891) | 0.403 |
| 4-5 | 14.250 (3.837-52.919) | < 0.001 | 3.735 (0.835-16.696) | 0.085 |
| Tumor location | < 0.001 | 0.059 | ||
| Lower third | 1 | 1 | ||
| Middle third | 2.791 (1.669-4.670) | < 0.001 | 1.952 (1.052-3.622) | 0.034 |
| Upper third | 1.985 (0.947-4.160) | 0.069 | 1.955 (0.820-4.658) | 0.130 |
| Entire stomach | 22.330 (2.867-173.902) | 0.003 | 4.687 (0.523-41.965) | 0.167 |
| Tumor size (mm) | 1.028 (1.020-1.037) | < 0.001 | 1.004 (0.993-1.016) | 0.433 |
| pTNM stage | < 0.001 | 0.028 | ||
| I | 1 | 1 | ||
| II | 10.667 (1.405-80.956) | 0.022 | 8.549 (1.073-68.125) | 0.043 |
| III | 45.208 (6.106-334.716) | < 0.001 | 13.795 (1.693-112.382) | 0.014 |
| Histological types | 0.643 | |||
| Well and medium differentiation | 1 | |||
| Poor differentiation | 1.026 (0.656-1.604) | 0.912 | ||
| Others | 0.826 (0.493-1.384) | 0.468 | ||
| mLNR | 93.645 (30.921-283.603) | < 0.001 | 11.042 (2.407-50.664) | 0.002 |
| Vascular infiltration | < 0.001 | 0.783 | ||
| No | 1 | 1 | ||
| Yes | 2.899 (1.905-4.411) | 1.080 (0.623-1.874) | ||
| Nerve infiltration | 0.029 | 0.471 | ||
| No | 1 | 1 | ||
| Yes | 1.524 (1.044-2.226) | 1.187 (0.745-1.892) | ||
| Postoperative chemotherapy | 0.315 | |||
| Yes | 1 | |||
| No | 0.891 (0.554-1.209) | |||
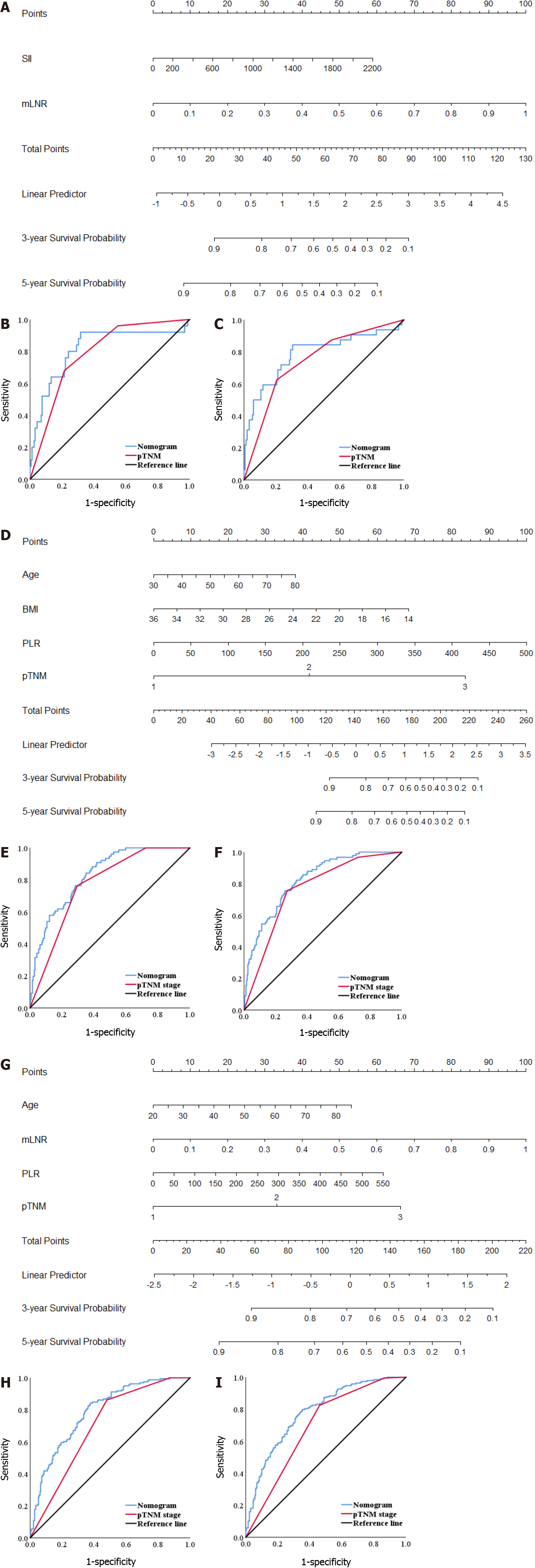
We combined independent prognostic risk factors to construct nomogram models to evaluate the prognosis of patients in different INF groups (Figure 6A, D and G). For predicting the survival of patients with INFa, INFb and INFc GC within 3 and 5 years after radical resection, ROC analysis showed that the AUCs of the nomogram models were both greater than those of pTNM stage alone (Figure 6B, C, E, F, H and I). The results of the nomogram models are shown in the Supplemen
In East Asia, INF has gradually become a clinicopathological feature for routine evaluation in surgical specimens[7]. Hematoxylin eosin staining is easy to determine it, which is convenient for clinical application. Recent retrospective studies have further demonstrated that INF can be an ideal predictor of recurrence and metastasis patterns after radical gastrectomy. INFc is an independent risk factor for peritoneal metastasis and is more prone to lymph node metastasis, while the liver metastasis rate of INFa/b is significantly higher than that of INFc[6,8,9]. Our study also confirmed that the prognosis of patients with INFa and INFb GC was better than that of patients with INFc GC. This evidence shows that INF can effectively help clinical experts predict the recurrence pattern and prognosis of GC patients. In addition, with the great achievements of tumor immunotherapy, it has been found that the tumor microenvironment and immune inflammatory response in peripheral blood can effectively reduce the impact of GC heterogeneity, providing more comprehensive information for the personalized treatment of patients[14,18]. This study was aimed at investigating the peripheral blood immune response is great value to different INF types GC patients in evaluating prognosis and individualized chemotherapy.
As the significant role of the systemic immune inflammatory response in gastrointestinal tumors has gradually become widely recognized by clinical experts, inflammatory indices, such as NLR, PLR and LMR, have been indicated to be prognostic markers of GC, esophageal cancer or colorectal cancer[19,20]. These indices can also evaluate the possible benefits and prospects of immunotherapy according to their baseline levels to support personalized immunotherapy[21]. As a part of systemic immunity, peripheral blood immunity also plays a significant role in the process of tumor metastasis. The proliferation of tumor cells causes them to break through mechanical pressure, escape immune monitoring, enter peripheral veins and form circulating tumor cells (CTCs)[22]. In this process, the close relationship between neutrophils and CTCs promotes the cell cycle process of CTCs and expands the metastatic potential of CTCs[23]. In addition, cytokines, such as tumor necrosis factor α released by CTC-associated neutrophils, play a key role in promoting tumor cell proliferation[23]. Lymphocytes can restrain the migration and proliferation of CTCs by secreting cytokines such as perforin/granzyme[24]. In addition, platelets can form aggregates with CTCs to escape host immune monitoring and release transforming growth factor β (TGF-β), inducing epithelial-mesenchymal transformation to promote distant metastasis[25]. The current research results showed that the higher the levels of the inflammatory indices SII and PLR are, the greater the possibility of early distant metastasis and the worse the prognosis. The AUC of the SII was higher in patients with INFa GC in ROC analysis, and the SII was an independent prognostic factor. This finding also showed that the pivotal role of neutrophils, lymphocytes and platelets for patients with INFa GC in the external circulation immunity. Besides, PLR also was a key predictor of the prognosis of patients with INFb and INFc GC. This finding further suggests that in peripheral circulating immunity of different INF types, the subsets of immune cells that play a major part are different.
In this study, we found that there was no significant difference in the percentages of neutrophils and lymphocytes among the three groups. In patients with stage II and III GC, the platelet count of INFc group was significantly lower than that of INFa and INFb group, which seems to contradict the conclusion that the prognosis of patients with INFc GC was poor. The reason for this may be that different INF groups differ greatly in their sensitivity to peripheral immune cells. Due to the influence of GC heterogeneity, the immune microenvironment of different subtypes of GC patients is greatly different[26]. Because of the unique biological characteristics of infiltrative growth with no distinct border with the surrounding tissue, tumor cells in patients with INFc GC may be more likely to enter the peripheral circulation to form CTCs. This results in that even if the platelets of patients with INFc GC being lower than those of patients with INFa and INFb GC in the same period, their peripheral circulating platelets are more likely to form aggregates with CTCs in patients with INFc GC, thus avoiding host immune monitoring and causing distant metastasis. In addition, we found that the platelet count of patients with INFc GC did not increase with increasing pTNM stage. The platelet count of patients with stage I disease was higher than that of patients with stage II and III disease. This may be related to the small number of INFc patients with stage I disease included in our study. Therefore, our next research direction is to explore the distribution and function of immune cell subsets according to INF.
We found that for the INFc group, patients with a low PLR were more sensitive to postoperative chemotherapy. The therapeutic effect of chemotherapy on different individuals depends not only on the sensitivity of cancer cells to chemotherapeutic drugs, but also on the immune status of tumor microenvironment. Ohe et al[27] also found that GC patients with a low PLR were more sensitive to chemotherapy. Platelets in peripheral blood are released from damaged endothelial cells by cisplatin-based chemotherapy, adhere to and accumulate on the vascular wall through von Willebrand factor[28]. Coalescented and activated platelets secrete multifarious cytokines, such as TGF-β and vascular endothelial growth factor A (VEGF-A). They induce the up regulation of cancer cell metastasis and drug resistance by acting on epithelial-mesenchymal transformation[29,30]. In addition, peripheral blood lymphocytes continually enter and exit lymph nodes, which leads to the initiation and activation of antigen-presenting dendritic cells (DCs). DCs can recognize the neoantigens of tumor cells induced by chemotherapy immunogenicity and make tumor cells sensitive to T cell-mediated killing[31]. This evidence suggests that patients with a low PLR GC are more sensitive to chemotherapy than other patients due to fewer platelets and more lymphocytes in their peripheral circulation. Our study also found that chemotherapy had better efficacy in the INFc group than in the other two groups. Wang et al[32] found that the survival rate of patients with early neutropenia after postoperative chemotherapy was higher. In addition, neutrophils and platelets in peripheral blood can inhibit natural killer cells by releasing chemical mediators, such as interleukin-1 and VEGF-A, and advance the immune resistance and escape of CTCs to chemotherapy drugs[33-36]. Our results showed that the percentages of neutrophils and platelets in INFc were lower than those in INFa and INFb in stage II and III GC patients, which may be why INFc is more sensitive to chemotherapy. Therefore, it is necessary to further investigate whether targeted therapy can improve postoperative survival in patients with INFa and INFb GC.
Clinically, some clinicians have realized that pTNM stage depends on postoperative pathology can provide efficacious but imperfect information for individual treatment. The same stage patients show significant individual differences in prognosis. Many studies have suggested that tumor immunity can bring into play an efficacious supplementary role[37,38]. Analyzing the proportion of immune cells in body fluids can effectively evaluate the prognosis of metastatic GC patients and provide them with more personalized treatment[39]. Li et al[20] constructed a nomogram based on inflammation and nutritional markers to predict the prognosis of GC patients receiving neoadjuvant chemotherapy and D2 Lymph node dissection. As a result, this type of prediction model constructed by combining inflammatory markers with clinicopathological features has the advantages of more accurate and individualized evaluation of patient prognosis and reducing the differences caused by heterogeneity. Based on the logistic risk regression model, our study found that the SII and mLNR were independent risk factors related to the prognosis of INFa GC patients. PLR, age, BMI and pTNM stage are independent risk factors related to the prognosis of INFb GC patients. PLR, age, pTNM stage and mLNR are independent risk factors related to the prognosis of INFc GC patients. Then, the nomogram models were constructed to predict the prognosis of patients with different INF types. We found that the nomogram models were better than the conventional pTNM stage alone in predicting the prognosis of patients with different INF types of GC within 3 years and 5 years after radical resection. The prediction models for evaluating the prognosis of different INF types patients combining inflammatory biomarkers and clinicopathological features were effective, which deserve further testing and extension in clinical practice.
There were some limitations in this study. First, the existence of internal bias and heterogeneity were inevitable as it was a retrospective study. Second, this was a single-center study, focusing only on Asian populations. Whether these results are widely applicable to other populations needs to be further studied by enlarging the sample size and source.
The SII and PLR were independent risk factors for the prognosis of patients with GC in the INFa, INFb and INFc groups. The nomogram based on these two inflammatory biomarkers combined with clinicopathologic features can evaluate the prognosis of GC patients with different INF types, and its predictive ability is better than that of the traditional pTNM stage alone.
Gastric cancer (GC) is an important public health burden worldwide. In East Asia, the tumor infiltrative pattern (INF) has gradually become a clinicopathologic feature routinely evaluated in surgically resected specimens. The INF type categorizes GC as the expansive growth type (INFa), the intermediate type (INFb), and the infiltrative growth type (INFc). Different INF types differ in clinicopathological features and prognosis and can be used as predictors of postoperative recurrence and prognosis in GC patients. Many studies have shown that inflammatory indices are potential prognostic indices for GC patients. However, there is no evidence defining the prognostic significance of immune inflammatory indices for GC with different INF types.
Evaluating whether inflammatory indices have prognostic significance for GC with different INF types will provide a basis for clinicians to treat and predict the prognosis of these patients in the future.
To analyze the relationships among peripheral circulating immune cells, inflammatory indices and INF types and to evaluate their ability to evaluate the outcome of patients with GC.
This retrospective study analyzed the clinicopathological characteristics and long-term survival data of 962 patients who underwent radical gastrectomy. Patients were categorized into the INFa, INFb, and INFc groups. The differences of clinicopathological features between the three groups were analyzed by chi-square test. The cutoff values of inflammatory indices were analyzed by receiver operating characteristic curves. The Kaplan–Meier and log-rank tests were used to analyze overall survival (OS). The independent risk factors for patients prognosis were analyzed by univariate and multivariate analyses based on the logistic regression. The nomogram models were constructed by R studio.
Based on the postoperative pathology report, there were 183, 331 and 448 patients in the INFa, INFb, and INFc groups, respectively. The OS of the INFc group was significantly lower than that of the other two groups (P < 0.001). The systemic immune-inflammation index (P = 0.039) and metastatic lymph node ratio (mLNR) (P = 0.003) were independent risk factors for prognosis in the INFa group. The platelet–lymphocyte ratio (PLR) (P = 0.018), age (P = 0.026), body mass index (P = 0.003), and postsurgical tumor node metastasis (pTNM) stage (P < 0.001) were independent risk factors for prognosis in the INFb group. The PLR (P = 0.021), age (P = 0.021), pTNM stage (P = 0.028), and mLNR (P = 0.002) were independent risk factors for prognosis in the INFc group. The area under the curve of the nomogram model for predicting 5-year survival in the INFa group, INFb group, and INFc group was 0.787, 0.823, and 0.781, respectively.
The nomogram model based on different inflammatory indices and clinicopathological features can be used to evaluate the prognosis of different INF types GC patients.
Further multicentric studies are needed to expansion of the sample size and external validation of nomogram model was performed to determine its predictive ability.
| 1. | Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, Abdelalim A, Abdoli A, Abdollahpour I, Abdulle ASM, Abebe ND, Abraha HN, Abu-Raddad LJ, Abualhasan A, Adedeji IA, Advani SM, Afarideh M, Afshari M, Aghaali M, Agius D, Agrawal S, Ahmadi A, Ahmadian E, Ahmadpour E, Ahmed MB, Akbari ME, Akinyemiju T, Al-Aly Z, AlAbdulKader AM, Alahdab F, Alam T, Alamene GM, Alemnew BTT, Alene KA, Alinia C, Alipour V, Aljunid SM, Bakeshei FA, Almadi MAH, Almasi-Hashiani A, Alsharif U, Alsowaidi S, Alvis-Guzman N, Amini E, Amini S, Amoako YA, Anbari Z, Anber NH, Andrei CL, Anjomshoa M, Ansari F, Ansariadi A, Appiah SCY, Arab-Zozani M, Arabloo J, Arefi Z, Aremu O, Areri HA, Artaman A, Asayesh H, Asfaw ET, Ashagre AF, Assadi R, Ataeinia B, Atalay HT, Ataro Z, Atique S, Ausloos M, Avila-Burgos L, Avokpaho EFGA, Awasthi A, Awoke N, Ayala Quintanilla BP, Ayanore MA, Ayele HT, Babaee E, Bacha U, Badawi A, Bagherzadeh M, Bagli E, Balakrishnan S, Balouchi A, Bärnighausen TW, Battista RJ, Behzadifar M, Bekele BB, Belay YB, Belayneh YM, Berfield KKS, Berhane A, Bernabe E, Beuran M, Bhakta N, Bhattacharyya K, Biadgo B, Bijani A, Bin Sayeed MS, Birungi C, Bisignano C, Bitew H, Bjørge T, Bleyer A, Bogale KA, Bojia HA, Borzì AM, Bosetti C, Bou-Orm IR, Brenner H, Brewer JD, Briko AN, Briko NI, Bustamante-Teixeira MT, Butt ZA, Carreras G, Carrero JJ, Carvalho F, Castro C, Castro F, Catalá-López F, Cerin E, Chaiah Y, Chanie WF, Chattu VK, Chaturvedi P, Chauhan NS, Chehrazi M, Chiang PP, Chichiabellu TY, Chido-Amajuoyi OG, Chimed-Ochir O, Choi JJ, Christopher DJ, Chu DT, Constantin MM, Costa VM, Crocetti E, Crowe CS, Curado MP, Dahlawi SMA, Damiani G, Darwish AH, Daryani A, das Neves J, Demeke FM, Demis AB, Demissie BW, Demoz GT, Denova-Gutiérrez E, Derakhshani A, Deribe KS, Desai R, Desalegn BB, Desta M, Dey S, Dharmaratne SD, Dhimal M, Diaz D, Dinberu MTT, Djalalinia S, Doku DT, Drake TM, Dubey M, Dubljanin E, Duken EE, Ebrahimi H, Effiong A, Eftekhari A, El Sayed I, Zaki MES, El-Jaafary SI, El-Khatib Z, Elemineh DA, Elkout H, Ellenbogen RG, Elsharkawy A, Emamian MH, Endalew DA, Endries AY, Eshrati B, Fadhil I, Fallah Omrani V, Faramarzi M, Farhangi MA, Farioli A, Farzadfar F, Fentahun N, Fernandes E, Feyissa GT, Filip I, Fischer F, Fisher JL, Force LM, Foroutan M, Freitas M, Fukumoto T, Futran ND, Gallus S, Gankpe FG, Gayesa RT, Gebrehiwot TT, Gebremeskel GG, Gedefaw GA, Gelaw BK, Geta B, Getachew S, Gezae KE, Ghafourifard M, Ghajar A, Ghashghaee A, Gholamian A, Gill PS, Ginindza TTG, Girmay A, Gizaw M, Gomez RS, Gopalani SV, Gorini G, Goulart BNG, Grada A, Ribeiro Guerra M, Guimaraes ALS, Gupta PC, Gupta R, Hadkhale K, Haj-Mirzaian A, Hamadeh RR, Hamidi S, Hanfore LK, Haro JM, Hasankhani M, Hasanzadeh A, Hassen HY, Hay RJ, Hay SI, Henok A, Henry NJ, Herteliu C, Hidru HD, Hoang CL, Hole MK, Hoogar P, Horita N, Hosgood HD, Hosseini M, Hosseinzadeh M, Hostiuc M, Hostiuc S, Househ M, Hussen MM, Ileanu B, Ilic MD, Innos K, Irvani SSN, Iseh KR, Islam SMS, Islami F, Jafari Balalami N, Jafarinia M, Jahangiry L, Jahani MA, Jahanmehr N, Jakovljevic M, James SL, Javanbakht M, Jayaraman S, Jee SH, Jenabi E, Jha RP, Jonas JB, Jonnagaddala J, Joo T, Jungari SB, Jürisson M, Kabir A, Kamangar F, Karch A, Karimi N, Karimian A, Kasaeian A, Kasahun GG, Kassa B, Kassa TD, Kassaw MW, Kaul A, Keiyoro PN, Kelbore AG, Kerbo AA, Khader YS, Khalilarjmandi M, Khan EA, Khan G, Khang YH, Khatab K, Khater A, Khayamzadeh M, Khazaee-Pool M, Khazaei S, Khoja AT, Khosravi MH, Khubchandani J, Kianipour N, Kim D, Kim YJ, Kisa A, Kisa S, Kissimova-Skarbek K, Komaki H, Koyanagi A, Krohn KJ, Bicer BK, Kugbey N, Kumar V, Kuupiel D, La Vecchia C, Lad DP, Lake EA, Lakew AM, Lal DK, Lami FH, Lan Q, Lasrado S, Lauriola P, Lazarus JV, Leigh J, Leshargie CT, Liao Y, Limenih MA, Listl S, Lopez AD, Lopukhov PD, Lunevicius R, Madadin M, Magdeldin S, El Razek HMA, Majeed A, Maleki A, Malekzadeh R, Manafi A, Manafi N, Manamo WA, Mansourian M, Mansournia MA, Mantovani LG, Maroufizadeh S, Martini SMS, Mashamba-Thompson TP, Massenburg BB, Maswabi MT, Mathur MR, McAlinden C, McKee M, Meheretu HAA, Mehrotra R, Mehta V, Meier T, Melaku YA, Meles GG, Meles HG, Melese A, Melku M, Memiah PTN, Mendoza W, Menezes RG, Merat S, Meretoja TJ, Mestrovic T, Miazgowski B, Miazgowski T, Mihretie KMM, Miller TR, Mills EJ, Mir SM, Mirzaei H, Mirzaei HR, Mishra R, Moazen B, Mohammad DK, Mohammad KA, Mohammad Y, Darwesh AM, Mohammadbeigi A, Mohammadi H, Mohammadi M, Mohammadian M, Mohammadian-Hafshejani A, Mohammadoo-Khorasani M, Mohammadpourhodki R, Mohammed AS, Mohammed JA, Mohammed S, Mohebi F, Mokdad AH, Monasta L, Moodley Y, Moosazadeh M, Moossavi M, Moradi G, Moradi-Joo M, Moradi-Lakeh M, Moradpour F, Morawska L, Morgado-da-Costa J, Morisaki N, Morrison SD, Mosapour A, Mousavi SM, Muche AA, Muhammed OSS, Musa J, Nabhan AF, Naderi M, Nagarajan AJ, Nagel G, Nahvijou A, Naik G, Najafi F, Naldi L, Nam HS, Nasiri N, Nazari J, Negoi I, Neupane S, Newcomb PA, Nggada HA, Ngunjiri JW, Nguyen CT, Nikniaz L, Ningrum DNA, Nirayo YL, Nixon MR, Nnaji CA, Nojomi M, Nosratnejad S, Shiadeh MN, Obsa MS, Ofori-Asenso R, Ogbo FA, Oh IH, Olagunju AT, Olagunju TO, Oluwasanu MM, Omonisi AE, Onwujekwe OE, Oommen AM, Oren E, Ortega-Altamirano DDV, Ota E, Otstavnov SS, Owolabi MO, P A M, Padubidri JR, Pakhale S, Pakpour AH, Pana A, Park EK, Parsian H, Pashaei T, Patel S, Patil ST, Pennini A, Pereira DM, Piccinelli C, Pillay JD, Pirestani M, Pishgar F, Postma MJ, Pourjafar H, Pourmalek F, Pourshams A, Prakash S, Prasad N, Qorbani M, Rabiee M, Rabiee N, Radfar A, Rafiei A, Rahim F, Rahimi M, Rahman MA, Rajati F, Rana SM, Raoofi S, Rath GK, Rawaf DL, Rawaf S, Reiner RC, Renzaho AMN, Rezaei N, Rezapour A, Ribeiro AI, Ribeiro D, Ronfani L, Roro EM, Roshandel G, Rostami A, Saad RS, Sabbagh P, Sabour S, Saddik B, Safiri S, Sahebkar A, Salahshoor MR, Salehi F, Salem H, Salem MR, Salimzadeh H, Salomon JA, Samy AM, Sanabria J, Santric Milicevic MM, Sartorius B, Sarveazad A, Sathian B, Satpathy M, Savic M, Sawhney M, Sayyah M, Schneider IJC, Schöttker B, Sekerija M, Sepanlou SG, Sepehrimanesh M, Seyedmousavi S, Shaahmadi F, Shabaninejad H, Shahbaz M, Shaikh MA, Shamshirian A, Shamsizadeh M, Sharafi H, Sharafi Z, Sharif M, Sharifi A, Sharifi H, Sharma R, Sheikh A, Shirkoohi R, Shukla SR, Si S, Siabani S, Silva DAS, Silveira DGA, Singh A, Singh JA, Sisay S, Sitas F, Sobngwi E, Soofi M, Soriano JB, Stathopoulou V, Sufiyan MB, Tabarés-Seisdedos R, Tabuchi T, Takahashi K, Tamtaji OR, Tarawneh MR, Tassew SG, Taymoori P, Tehrani-Banihashemi A, Temsah MH, Temsah O, Tesfay BE, Tesfay FH, Teshale MY, Tessema GA, Thapa S, Tlaye KG, Topor-Madry R, Tovani-Palone MR, Traini E, Tran BX, Tran KB, Tsadik AG, Ullah I, Uthman OA, Vacante M, Vaezi M, Varona Pérez P, Veisani Y, Vidale S, Violante FS, Vlassov V, Vollset SE, Vos T, Vosoughi K, Vu GT, Vujcic IS, Wabinga H, Wachamo TM, Wagnew FS, Waheed Y, Weldegebreal F, Weldesamuel GT, Wijeratne T, Wondafrash DZ, Wonde TE, Wondmieneh AB, Workie HM, Yadav R, Yadegar A, Yadollahpour A, Yaseri M, Yazdi-Feyzabadi V, Yeshaneh A, Yimam MA, Yimer EM, Yisma E, Yonemoto N, Younis MZ, Yousefi B, Yousefifard M, Yu C, Zabeh E, Zadnik V, Moghadam TZ, Zaidi Z, Zamani M, Zandian H, Zangeneh A, Zaki L, Zendehdel K, Zenebe ZM, Zewale TA, Ziapour A, Zodpey S, Murray CJL. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5:1749-1768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1723] [Cited by in RCA: 1839] [Article Influence: 262.7] [Reference Citation Analysis (0)] |
| 2. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4011] [Cited by in RCA: 4390] [Article Influence: 146.3] [Reference Citation Analysis (1)] |
| 3. | Song XH, Zhang WH, Kai-Liu, Chen XL, Zhao LY, Chen XZ, Kun-Yang, Zhou ZG, Hu JK. Prognostic impact of Borrmann classification on advanced gastric cancer: a retrospective cohort from a single institution in western China. World J Surg Oncol. 2020;18:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 4. | Ming SC. Gastric carcinoma. A pathobiological classification. Cancer. 1977;39:2475-2485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 5. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2950] [Article Influence: 196.7] [Reference Citation Analysis (1)] |
| 6. | Saito H, Miyatani K, Takaya S, Kuroda H, Matsunaga T, Fukumoto Y, Osaki T, Ikeguchi M. Tumor infiltration pattern into the surrounding tissue has prognostic significance in advanced gastric cancer. Virchows Arch. 2015;467:519-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Maehara Y, Oshiro T, Adachi Y, Ohno S, Akazawa K, Sugimachi K. Growth pattern and prognosis of gastric cancer invading the subserosa. J Surg Oncol. 1994;55:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Kanda M, Mizuno A, Fujii T, Shimoyama Y, Yamada S, Tanaka C, Kobayashi D, Koike M, Iwata N, Niwa Y, Hayashi M, Takami H, Nakayama G, Sugimoto H, Fujiwara M, Kodera Y. Tumor Infiltrative Pattern Predicts Sites of Recurrence After Curative Gastrectomy for Stages 2 and 3 Gastric Cancer. Ann Surg Oncol. 2016;23:1934-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Nakagawa N, Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, Murai T, Asada T, Ishiyama A, Matsushita H, Tanaka C, Kobayashi D, Fujiwara M, Murotani K, Kodera Y. Pathological tumor infiltrative pattern and sites of initial recurrence in stage II/III gastric cancer: Propensity score matching analysis of a multi-institutional dataset. Cancer Med. 2018;7:6020-6029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1659] [Cited by in RCA: 2177] [Article Influence: 217.7] [Reference Citation Analysis (0)] |
| 11. | Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4318] [Cited by in RCA: 5018] [Article Influence: 250.9] [Reference Citation Analysis (19)] |
| 12. | Pagès F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, Lugli A, Zlobec I, Rau TT, Berger MD, Nagtegaal ID, Vink-Börger E, Hartmann A, Geppert C, Kolwelter J, Merkel S, Grützmann R, Van den Eynde M, Jouret-Mourin A, Kartheuser A, Léonard D, Remue C, Wang JY, Bavi P, Roehrl MHA, Ohashi PS, Nguyen LT, Han S, MacGregor HL, Hafezi-Bakhtiari S, Wouters BG, Masucci GV, Andersson EK, Zavadova E, Vocka M, Spacek J, Petruzelka L, Konopasek B, Dundr P, Skalova H, Nemejcova K, Botti G, Tatangelo F, Delrio P, Ciliberto G, Maio M, Laghi L, Grizzi F, Fredriksen T, Buttard B, Angelova M, Vasaturo A, Maby P, Church SE, Angell HK, Lafontaine L, Bruni D, El Sissy C, Haicheur N, Kirilovsky A, Berger A, Lagorce C, Meyers JP, Paustian C, Feng Z, Ballesteros-Merino C, Dijkstra J, van de Water C, van Lent-van Vliet S, Knijn N, Mușină AM, Scripcariu DV, Popivanova B, Xu M, Fujita T, Hazama S, Suzuki N, Nagano H, Okuno K, Torigoe T, Sato N, Furuhata T, Takemasa I, Itoh K, Patel PS, Vora HH, Shah B, Patel JB, Rajvik KN, Pandya SJ, Shukla SN, Wang Y, Zhang G, Kawakami Y, Marincola FM, Ascierto PA, Sargent DJ, Fox BA, Galon J. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391:2128-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 1573] [Article Influence: 196.6] [Reference Citation Analysis (0)] |
| 13. | Fang T, Wang Y, Yin X, Zhai Z, Zhang Y, Yang Y, You Q, Li Z, Ma Y, Li C, Song H, Shi H, Yu X, Gao H, Sun Y, Xie R, Xue Y. Diagnostic Sensitivity of NLR and PLR in Early Diagnosis of Gastric Cancer. J Immunol Res. 2020;2020:9146042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 14. | Yin X, Fang T, Wang Y, Zhang D, Li C, Xue Y. Prognostic significance of serum inflammation indexes in different Lauren classification of gastric cancer. Cancer Med. 2021;10:1103-1119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | van Dam PJ, Daelemans S, Ross E, Waumans Y, Van Laere S, Latacz E, Van Steen R, De Pooter C, Kockx M, Dirix L, Vermeulen PB. Histopathological growth patterns as a candidate biomarker for immunomodulatory therapy. Semin Cancer Biol. 2018;52:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1415] [Article Influence: 283.0] [Reference Citation Analysis (2)] |
| 17. | Ajani JA, D'Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, Denlinger CS, Fanta P, Farjah F, Fuchs CS, Gerdes H, Gibson M, Glasgow RE, Hayman JA, Hochwald S, Hofstetter WL, Ilson DH, Jaroszewski D, Johung KL, Keswani RN, Kleinberg LR, Korn WM, Leong S, Linn C, Lockhart AC, Ly QP, Mulcahy MF, Orringer MB, Perry KA, Poultsides GA, Scott WJ, Strong VE, Washington MK, Weksler B, Willett CG, Wright CD, Zelman D, McMillian N, Sundar H. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:1286-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 689] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 18. | Jiang Y, Xie J, Huang W, Chen H, Xi S, Han Z, Huang L, Lin T, Zhao LY, Hu YF, Yu J, Cai SR, Li T, Li G. Tumor Immune Microenvironment and Chemosensitivity Signature for Predicting Response to Chemotherapy in Gastric Cancer. Cancer Immunol Res. 2019;7:2065-2073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 19. | Zhang X, Hu D, Lin X, Zhang H, Xia Y, Lin J, Zheng X, Peng F, Jie J, Niu W. Prognostic Value of an Inflammation-Related Index in 6,865 Chinese Patients With Postoperative Digestive Tract Cancers: The FIESTA Study. Front Oncol. 2019;9:427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Li Z, Li S, Ying X, Zhang L, Shan F, Jia Y, Ji J. The clinical value and usage of inflammatory and nutritional markers in survival prediction for gastric cancer patients with neoadjuvant chemotherapy and D2 Lymphadenectomy. Gastric Cancer. 2020;23:540-549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 21. | Bilen MA, Martini DJ, Liu Y, Lewis C, Collins HH, Shabto JM, Akce M, Kissick HT, Carthon BC, Shaib WL, Alese OB, Pillai RN, Steuer CE, Wu CS, Lawson DH, Kudchadkar RR, El-Rayes BF, Master VA, Ramalingam SS, Owonikoko TK, Harvey RD. The prognostic and predictive impact of inflammatory biomarkers in patients who have advanced-stage cancer treated with immunotherapy. Cancer. 2019;125:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 22. | Mohme M, Riethdorf S, Pantel K. Circulating and disseminated tumour cells - mechanisms of immune surveillance and escape. Nat Rev Clin Oncol. 2017;14:155-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 463] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 23. | Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, Scheidmann MC, Donato C, Scherrer R, Singer J, Beisel C, Kurzeder C, Heinzelmann-Schwarz V, Rochlitz C, Weber WP, Beerenwinkel N, Aceto N. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566:553-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 939] [Article Influence: 134.1] [Reference Citation Analysis (0)] |
| 24. | Ferrone C, Dranoff G. Dual roles for immunity in gastrointestinal cancers. J Clin Oncol. 2010;28:4045-4051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 196] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 25. | Kanikarla-Marie P, Lam M, Menter DG, Kopetz S. Platelets, circulating tumor cells, and the circulome. Cancer Metastasis Rev. 2017;36:235-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Derks S, de Klerk LK, Xu X, Fleitas T, Liu KX, Liu Y, Dietlein F, Margolis C, Chiaravalli AM, Da Silva AC, Ogino S, Akarca FG, Freeman GJ, Rodig SJ, Hornick JL, van Allen E, Li B, Liu SX, Thorsson V, Bass AJ. Characterizing diversity in the tumor-immune microenvironment of distinct subclasses of gastroesophageal adenocarcinomas. Ann Oncol. 2020;31:1011-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 27. | Ohe Y, Fushida S, Yamaguchi T, Kinoshita J, Saito H, Okamoto K, Nakamura K, Tajima H, Ninomiya I, Ohta T. Peripheral Blood Platelet-Lymphocyte Ratio Is Good Predictor of Chemosensitivity and Prognosis in Gastric Cancer Patients. Cancer Manag Res. 2020;12:1303-1311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Dieckmann KP, Struss WJ, Budde U. Evidence for acute vascular toxicity of cisplatin-based chemotherapy in patients with germ cell tumour. Anticancer Res. 2011;31:4501-4505. [PubMed] |
| 29. | Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1461] [Article Influence: 97.4] [Reference Citation Analysis (0)] |
| 30. | Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1766] [Cited by in RCA: 2277] [Article Influence: 133.9] [Reference Citation Analysis (0)] |
| 31. | Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2818] [Cited by in RCA: 2535] [Article Influence: 181.1] [Reference Citation Analysis (0)] |
| 32. | Wang Y, Chen Y, Yin H, Gu X, Shi Y, Dai G. Timing of chemotherapy-induced neutropenia is a prognostic factor in patients with advanced gastric cancer undergoing first-line chemotherapy with oxaliplatin and capecitabine: a retrospective study. Cancer Med. 2018;7:997-1005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Proctor MJ, Morrison DS, Talwar D, Balmer SM, O'Reilly DS, Foulis AK, Horgan PG, McMillan DC. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow Inflammation Outcome Study. Br J Cancer. 2011;104:726-734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 306] [Cited by in RCA: 446] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 34. | Wang TT, Zhao YL, Peng LS, Chen N, Chen W, Lv YP, Mao FY, Zhang JY, Cheng P, Teng YS, Fu XL, Yu PW, Guo G, Luo P, Zhuang Y, Zou QM. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway. Gut. 2017;66:1900-1911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 403] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 35. | Zhang D, Zhou J, Tang D, Zhou L, Chou L, Chou KY, Tao L, Lu LM. Neutrophil infiltration mediated by CXCL5 accumulation in the laryngeal squamous cell carcinoma microenvironment: A mechanism by which tumour cells escape immune surveillance. Clin Immunol. 2017;175:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Nagtegaal ID, Marijnen CA, Kranenbarg EK, Mulder-Stapel A, Hermans J, van de Velde CJ, van Krieken JH. Local and distant recurrences in rectal cancer patients are predicted by the nonspecific immune response; specific immune response has only a systemic effect--a histopathological and immunohistochemical study. BMC Cancer. 2001;1:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 111] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 37. | Kirkwood JM, Butterfield LH, Tarhini AA, Zarour H, Kalinski P, Ferrone S. Immunotherapy of cancer in 2012. CA Cancer J Clin. 2012;62:309-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 332] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 38. | Cheung KS, Leung WK, Seto WK. Application of Big Data analysis in gastrointestinal research. World J Gastroenterol. 2019;25:2990-3008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 39. | Park HS, Kwon WS, Park S, Jo E, Lim SJ, Lee CK, Lee JB, Jung M, Kim HS, Beom SH, Park JY, Kim TS, Chung HC, Rha SY. Comprehensive immune profiling and immune-monitoring using body fluid of patients with metastatic gastric cancer. J Immunother Cancer. 2019;7:268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nakai Y S-Editor: Zhang H L-Editor: A P-Editor: Yuan YY