Published online Apr 15, 2022. doi: 10.4251/wjgo.v14.i4.808
Peer-review started: March 17, 2021
First decision: May 3, 2021
Revised: May 15, 2021
Accepted: March 25, 2022
Article in press: March 25, 2022
Published online: April 15, 2022
Processing time: 393 Days and 10.4 Hours
Vasoactive intestinal peptide (VIP) secreting tumour (VIPoma) is a rare functional neuroendocrine tumour that typically arises from pancreatic islet cells. These present as sporadic, solitary pancreatic neoplasias with an estimated incidence of one in ten million individuals per year. Only around 5% of VIPomas are associated with multiple endocrine neoplasia type I syndrome. Excessive VIP secretion produces a clinical syndrome characterized by refractory watery diarrhoea, hypokalemia and metabolic acidosis. These coupled with elevated plasma levels of VIP are diagnostic. The majority of VIPomas are malignant and have already metastasized at the time of diagnosis (60%). Metastases occur most frequently in the liver, or regional lymph nodes, lungs, kidneys and bones. Some reports of skin metastases have been documented. Complete surgical resection continues to be the only potentially curative treatment. However, when the neoplasia cannot be excised completely, surgical debulking may provide palliative benefit. Other palliative options have included recently the peptide receptor radionuclide therapy which has shown to be effective and well-tolerated. This article will review all aspects of pancreatic VIPomas highlighting aspects such as clinical presentation, diagnosis and management.
Core Tip: Vasoactive intestinal peptide (VIP) secreting tumour (VIPoma) is a rare functional neuroendocrine tumour that typically arises from pancreatic islet cells. It is usually sporadic but may present as part of the endocrine neoplasia type I syndrome in 5% of cases. Excessive VIP secretion produces a refractory secretory diarrhoea which left untreated will cause patient's death. The majority of VIPomas are malignant and have already metastasized at the time of diagnosis (60%), being the liver, or regional lymph nodes the most frequent site for metastases. Complete surgical resection is the only potentially curative treatment, however, surgical debulking may provide palliative benefit. Other palliative options include somatostatin analogues and recently the peptide receptor radionuclide therapy which has shown to be effective and well-tolerated.
- Citation: Una Cidon E. Vasoactive intestinal peptide secreting tumour: An overview. World J Gastrointest Oncol 2022; 14(4): 808-819
- URL: https://www.wjgnet.com/1948-5204/full/v14/i4/808.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i4.808
Vasoactive intestinal peptide (VIP) secreting tumour (VIPoma) is a rare functional neuroendocrine tumour (NET) secreting VIP in an uncontrolled manner. It is a non-beta pancreatic islet cell tumour that comprises < 10% of all pancreatic NETs[1].
VIPomas were initially reported by Priest and Alexander in 1957. Verner and Morrison in 1958, described a syndrome called WDHA (watery diarrhoea, hypokalemia, achlorhydria) as a consequence of a pancreatic malignancy that caused death due to dehydration and shock[2]. These tumours are also called Verner-Morrison syndrome, pancreatic cholera and WDHA syndrome, in view of the most frequent symptoms[2]. Its estimated incidence is of 1/10000000 individuals per year[3], affecting more women (65%) than men (35%)[4] and it usually appears in the 4th decade[3].
These originate in amine precursor uptake and decarboxylation cells of the gastro-enteropancreatic endocrine system and in adrenal or extra-adrenal neurogenic locations[5]. VIPomas are sporadic in 90% of cases, generally presenting as solitary lesions greater than 3 cm[5], with only in 5% of the cases being multicentric[6]. Pancreatic VIPomas might also be part of multiple endocrine neoplasia (MEN) 1 syndrome[6], which is an autosomal dominant syndrome that combines malignant lesions in the parathyroid glands, pancreatic islet cells, and the pituitary[7]. As well as its participation in familial MEN 1[5] and related disorders, the MEN1 locus has been involved in the development of MEN 1-type sporadic endocrine malignancies.
Pancreatic VIPomas are extremely rare in children, where it has been shown that VIP originates mainly from ganglioneuromas, ganglioneuroblastomas, neurofibromas, or most commonly from other neoplasias in the adrenal area[1,8]. Only a small number of neuroblastomas and ganglioneuroblastomas produce VIP, but this characteristic indicates a more favourable prognosis[8]. Patients with neurogenic neoplasias, usually show normal levels of gastrin, insulin, pancreatic polypeptide and somatostatin, as opposed to those with pancreatic VIPomas[8]. Most VIPomas are intrapancreatic, the majority of them in the body and tail while 25% are located in the head of the pancreas[9]. However, there are cases of extrapancreatic origin, mostly in adrenal glands (35%), followed by paraspinal retroperitoneal ganglia (30-35%), posterior mediastinum (20%), head and neck in 1%-5% and pelvis in 2%-3%; rare locations include the thymus, lung, kidney or anterior mediastinum[10,11].
VIPomas most relevant symptoms are caused by the exaggerated, uncontrolled secretion of VIP. However, other products such as prostaglandin E2, may be secreted as well by this neoplasia[12].
VIP is generated as a precursor substance with a signal peptide of 22 amino acids, then cleaved to the active form of 28 amino acids[12,13]. This product encoding gene is in chromosome 6[14]. It works by attachment to receptors on intestinal epithelial cells and inducing activation of adenylate cyclase and adenosine 3’, 5’- cyclic phosphate (cAMP) production[11]. As such, it will control smooth muscle activity, blood flow in the gastrointestinal tract[15-17] and epithelial cell secretion. This will result in profuse refractory watery diarrhoea leading to water and electrolyte depletion, mainly hypokalemia[18-20]. Some studies have shown that there is a local control of VIP gene expression, suggesting that there is a post transcriptional regulation which may be crucial for normal VIP secretion[21].
VIP also shows a vasodilator effect (with flushing), glucogenolytic effect on the liver (with subsequent hyperglycaemia) and diminish gastric acid secretion[22] leading to hypochlorhydria/achlorhydria[23]. Hyperglycemia and impaired glucose tolerance affect ≤ 50% of patients[24]. The reasons behind these issues are in relation to direct glycogenolytic activity of the VIP on the liver and the inhibitory effect of hypokalemia on pancreatic islets cells insulin release. Hypercalcemia can also be seen without MEN1 syndrome or raised parathyroid hormone levels. It is believed that elevated bone resorption can be involved, however, it is not clear. In addition, the dehydration, as a consequence of severe diarrhoea, might also aggravate the hypercalcemia. This is seen in 50% of patients[25]. Hypochlorhydria or achlorhydria is described in 75% of patients with VIPoma as a result of the inhibition of histamine and pentagastrin-stimulated gastric acid secretion[26].
Diagnosis requires demonstration of secretory diarrhoea. Then the laboratory assessment (elevated VIP serum levels are required) and imaging studies will complete the diagnostic tools. Typically, the diagnosis is often delayed and diarrhoea may persist for years before a VIPoma is confirmed[27].
VIPomas are diagnosed when watery diarrhoea, hypokalemia, and achlorhydria are observed in the context of raised serum VIP[1]. Stool volumes of less than 700 mL/d virtually rule out a VIPoma. Generally in this context, stool volumes exceed 3 L/d. Stool osmolality must be compatible with a secretory diarrhoea, that is stool osmotic gap of < 50[8]. VIP levels are determined by radioimmunoassay and these are generally 2-10 times the normal range (20-30 pmol/L) in patients with VIPoma[9]. Figures > 75 pg/mL are consistent with a VIPoma[10], although levels usually reach 160-250 pmol/L or higher.
These levels should be drawn after fasting and a protease inhibitor (such as aprotinin) must be added to the blood sample, otherwise, VIP is degraded rapidly. Moreover, the sample must be kept frozen at -70 °C until it is processed. These levels should be determined when the patient is symptomatic (as VIP release from the tumour fluctuates and it is normal in between the episodes of diarrhoea) and should be repeated to verify the diagnosis. Hence, a normal figure may be a false negative[28].
Hypokalemia and non-anion gap acidosis are also frequently seen in VIPomas[1], same as hypochlorhydria or achlorhydria that may be assessed by checking gastric pH or basal gastric acid output l[1]. Measuring the stool weight and levels of potassium, it could be confirmed the high gastrointestinal potassium losses. Renal function must be assessed by blood urea nitrogen and serum creatinine levels. Magnesium should be determined as well[11]. Stool weight with potassium measurements will confirm the high gastrointestinal potassium losses.
VIPomas may release also other hormones, such as neurotensin, calcitonin or pancreatic polypeptide. Moreover, 66% of the cases with VIPoma will also show raised levels of gastrin and insulin[29]. It has been published one case in the literature, where a patient with VIPoma had increased dopamine levels suggesting that neuroendocrine cells are able to secrete both catecholamines, as well as pancreatic peptides[30].
Imaging studies will initially check the pancreas, as it is known that 90% of VIPomas will be found there. These techniques are crucial not only to localize the neoplasia, but also to determine its size and the stage at diagnosis to help establish the treatment pathway[31]. In most cases, finding the VIPoma is easy as most will be larger than 3cm at diagnosis. Computed tomography (CT), magnetic resonance imaging (MRI) and somatostatin receptor scintigraphy (SRS) are three imaging techniques that may be utilised to find the tumour. Some published articles have used 99mTc sestamibi[32] to locate the neoplasia. Unfortunately there are no staging criteria for VIPomas.
CT: Multiphasic CT is crucial in determining the size, location, as well as involvement of adjacent structures, vessels, lymph nodes and presence of calcification[9,15]. CT will search for neck, mediastinal, or retroperitoneal masses and will identify the primary neoplasia in the majority of the cases. It will also help detecting or excluding liver metastases[10]. Peng et al[33] examined 31 patients and reported that CT was able to identify correctly all VIPomas in the body and tail of the pancreas. However, only 71% of the tumours in pancreatic head were identified. This neoplasia shows as a hyperattenuating lesion on arterial phase followed by an obscure mass on venous depiction. Calcifications may be detected as well. These are hypervascularized tumours rich in cells and fibrous tissue which is poorly supplied and therefore the contrast agent is held within the lesion[11].
MRI: MRI is useful for assessment of spinal tumours[34] or if CT is contraindicated (e.g., patient is allergic to iodine contrast or in renal failure). VIPomas are best seen on T1-weighted, fat-suppressed images as these are low signal-intensity tumours. MRI has better sensitivity for liver metastases detection. These may be observed as intensive peripheral ring enhancement on immediate post-gadolinium spoiled gradient-echo images[35]. This technique can detect tumours as small as 1 cm[36] and MRI should be performed in those cases with indeterminate lesions.
SRS: 90% of pancreatic NETs have a high number of somatostatin receptors[8]. Therefore, using radionuclide-labelled octreotide or lanreotide may be useful for studying abnormalities found on a CT or for identification of hidden or distant metastases[6]. It might help after surgery as well, if postoperative changes diminish the accuracy of a CT. This technique’s sensitivity for all pancreatic NETs is 80-90%; 92% for neoplasias > 1 cm[6].
Endoscopic ultrasound: This will help determine the accurate extent of the disease and it allows a biopsy of the pancreatic lesion.
Single-photon emission CT: Research has suggested that the use of single-photon emission CT (SPECT) might improve the value of somatostatin receptor scintigraphy for the localization of NETs, including VIPomas[37]. Several different radiotracers can be bound to octreotide, and applied together with SPECT or positron emission tomography (PET) imaging to localize areas of enhanced uptake.
PET: 18F-deoxyglucose-PET imaging has also been used to diagnose NETs. However, it may not be as sensitive as somatostatin receptor scintigraphy[38]. The FDA approved the newer functional PET technique with 68-Ga DOTATATE injection as the radioactive diagnostic product for the identification of somatostatin receptor-positive NETs in adult and paediatric patients. PET-CT Gallium-68 dotatate is 97% sensitive for the identification of VIPomas. Contrast enhanced CT and MRI sensitivities are 80 and 85%, respectively[8,11]. Moreover, a recent publication has suggested a role of the high sensitivity Ga-PET/CT not only in the identification of NETs but also in VIPomas prognostication and risk stratification[30].
Immunohistochemically: VIPomas stain positively for VIP, somatostatin, neuronspecific enolase, chromogranin A, synaptophysin and cytokeratin[39,40].
Other techniques: Chest radiography may help with identification/Location of paravertebral masses[41]. Endoscopic retrograde cholangiopancreatography might demonstrate blockage of the major pancreatic duct and perhaps some calcifications in the pancreatic body. Transabdominal ultrasonography help exclude liver metastases, which might show as hepatic calcifications. Electrocardiography can demonstrate QRS widening and T-wave flattening in cases with a very significant hypokalemia. Colonoscopy is another technique that might help with diferential diagnosis, to rule out a villous adenoma as another cause of potassium-losing diarrhoea.
Other causes of secretory diarrhoea should be checked, mainly the laxative abuse. Patients with VIPomas should show high levels of VIP specially during a bout of diarrhoea. These will confirm the diagnosis. However, mild elevations may occur with short bowel syndrome and inflammatory diseases as well[42].
The onset of VIPomas is subtle and its symptoms can bevague. In most case, these neoplasias have already metastasized at diagnosis. A Chinese study with 41 patients showed that the average time from the appearance of symptoms to the final diagnosis was > 15 mo, although patients experience a range of distinguishing signs[43].
The major symptom of VIPomas is long-standing profuse watery diarrhoea of approximately 10 watery stools per day. This diarrhoea persists even after 72 h of fasting[44] as opposed as osmotic diarrhoea[44]. In actual fact, the majority of cases develop diarrhea (89%), weight loss (72%), and hypokalemia (67%)[29], as a consequence of VIP binding to intestinal epithelial cells, upregulating cAMP and leading to secretion of electrolytes into the bowel, causing profuse watery diarrhea[29]. These issues occur as a result of VIP binding to intestinal epithelial cells, thereby upregulating cAMP and leading to secretion of electrolytes into the bowel lumen, causing profuse watery diarrhea[2]. Stool volumes are during fasting at least 20 mL/kg/d but exceed 50 mL/kg/d in most cases. Non-fasting volumes exceed > 3000 mL/d. Faecal osmolality is accounted for by twice the sum of the sodium and potassium levels, evidencing the electrolyte loss. The stools are generally tea coloured and odourless without blood or mucus[1,2]. Initially diarrhoea may evolve in episodes, whereas it becomes constant as the neoplasm progresses. Unfortunately, the diarrhoea may be present for years before the diagnosis is made. As such, in around 33% of patients, the diarrhoea has been present for less than one year before the diagnosis, but 1/4 of patients have diarrhoea for a minimum of 5 years before the diagnosis is established. Faecal excretion of large amounts of potassium and bicarbonate will produce hypokalemia and non–anion gap acidosis. VIPomas will then lead to significant dehydration (45%-95%) and electrolytes imbalance, most frequently hypokalemia (70%-100%), achlorhydria (35%-76%), hypomagnesemia, hypophosphatemia and metabolic acidosis, all with linked symptoms[45].
Other frequent symptoms include muscular weakness, sickness (nausea, vomiting), lethargy and abdominal painful cramps or bloating. In some cases, skin rash has been shown as well[10] . Flushing similar to that seen with carcinoid syndrome appears in around 33% of patients during the episode of diarrhoea[33]. This flushing is secondary to prostaglandin production by the neoplasm. Patients will be exhausted and suffer from noticeable weight losses and renal failure, unless able to replace the lost fluids and electrolytes and flushing (15%-30%)[24,27]. Finally, ischemic stroke attributed to high haematocrit due to diarrhoea has been mentioned in an extremely rare case report[46].
This is very a very frequent electrolyte imbalance in patients with VIPoma-induced diarrhoea[27]. Moderate hypokalemia (2.5–3mmol) may produce confusion, disorientation, weakness, constipation, and muscles discomfort or cramps during exercise. Severe hypokalemia (levels under 2.5mmol) may result in extreme weakness and paralysis (flaccid paralysis). Sometimes it may produce respiratory distress and respiratory failure. Changes in ECG (flattened in T waves) can also be found and some cardiac arrythmias may be leading to cardiac arrest.
VIPomas can cause a large amount of gasrointestinal loss and bicarbonate wasting through stool. This fact, will lead to metabolic acidosis, contributing as well in hypokalemia[47].
This appear as secondary to the direct gastric acid inhibitory effect of VIP, which consequently result in a diminished gastric acid production. Hypochlorhydria appears in 20%-50% of patients with VIPomas[25]. These changes will end up in malabsorption of several electrolytes and vitamins[26].
Hyperglycemia (20%-50%) due to a profound glycogenolytic effects of VIP on the liver that leads to reduced glucose intake by tissues[25]. Hypercalcemia occurs in 25%-50% of cases[25]. It is not clear why hypercalcemia might appear but it may be linked to dehydration, electrolyte disturbances, paraneoplastic syndrome or coincidental MEN-1 syndrome with hyperparathyroidism[15]. Hypomagnesemia may happen as well as a consequence of diarrhoea and lead to tetany in some patients. Other signs of VIPoma may comprise bloating, nausea, vomiting, cutaneous rash, lethargy and weight loss[48].
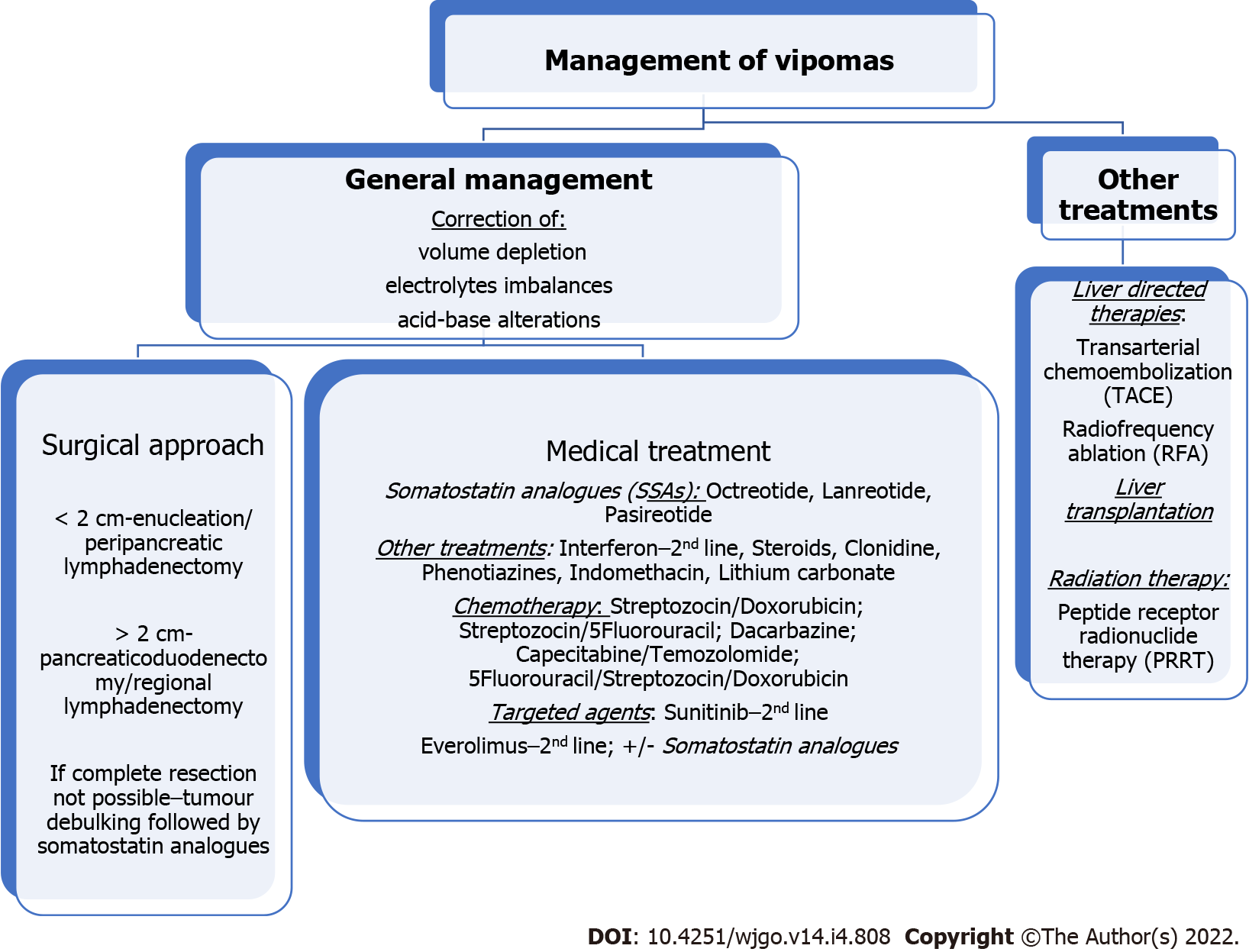
The management of VIPomas includes medical and surgical treatment. Its mortality rate is closely linked to uncontrolled WDHA syndrome. This will lead to a sustained dehydration with significant electrolyte and acid-base disturbances, and consequently renal failure, cardiac arrhythmias, neuromuscular deficits, shock, cardiac arrest and eventually death[49]. Therefore, the initial therapy for a VIPoma aims at controlling the symptoms and correcting any volume depletion, electrolytes and acid-base alterations. This entails a rapid replacement of fluids and electrolytes. The ideal fluid replacement should be with an isotonic electrolyte solution with adequate sodium, potassium and base if needed. In many cases, patients will need intravenous replacements and hospital admission[48]. In the absence of finding a neoplasia, symptomatic therapy is advised. This treatment in conjunction with octreotide, will improve preoperative electrolyte balance[30] if surgery is considered (Figure 1).
Somatostatin analogues (SSAs) inhibit VIP secretion and are used to control symptoms[15]. Somatostatin is a peptide that reduces the secretion of an ample set of hormones[50]. Various studies on functional NETs have shown that controlling hormone levels is crucial to reduce patients’ morbidity and mortality[51]. SSAs (octreotide, lanreotide and pasireotide) replicate the effect of somatostatin on G-coupled receptors of cell membrane and will reduce VIP secretion. This will impact positively on diarrhoea control. SSAs will also inhibitit tumour growth[31] in more than 90% of patients[33]. The CLARINET trial (Controlled study of Lanreotide Antiproliferative Response in NETs) reported antiproliferative effects of lanreotide in NETs[52]. SSAs showed rates of tumour stabilization in 50%-60% of the pNETs[22]. Some authors have even suggested that that SSAs may produce a reduction in tumour size. Although this is still under debate, neoadjuvant therapy with octreotide has been applied in 69.2% of cases[45,53]. Overall, several studies have reported rates of diarrhoea control in more than 50% of the patients, while significant improvements are seen in 25%.
Unfortunately, there are some issues in relation to SSAs. One is the clearly documented resistance with long-term octreotide use, leading to the requirement of significantly high doses to achieve the wanted effect[54]. Another problem is the fact that the diarrhoea reappears when the SSAs is stopped. Thus, octreotide should carry on unless the tumour can be surgically completely removed. Octreotide is a synthetic longacting SSA that stops VIP secretion and is approved for treatment of VIPomas[55]. SSAs are generally well tolerated, although adverse events might occur. Indigestion, bloating, vomiting, bloating, diarrhoea with steatorrhea due to fat malabsorption, and mild glucose intolerance; However, these side effects tend to fade over time[52] (Figure 1).
Interferon alfa is added to the treatment management when the highest tolerable dose of octreotide do not control symptoms. It may also cause a modest reduction in tumour size[56]. Interferon alpha (IFN-α) is approved for symptom control (3-5 million IU sc three times weekly) with similar results to SSAs. Due to its less favourable toxicity profile (fatigue, weight loss and, more rarely, depression), this is used in second-line as a supplemental therapy joined to SSA in cases with refractory syndrome[57] (Figure 1).
Steroids may diminish symptoms in 50% of patients. This treatment may be used for those patients who did not show a good response to SSAs[58] (Figure 1).
Before the availability of octreotide, some patients had shown responses with high dose of prednisone, clonidine, phenotiazine, indomethacin, lithium carbonate, etc[58]. Additional loperamide and opiates may be used as well[59] (Figure 1).
The activity of cytotoxic chemotherapy in metastatic VIPomas is difficult to assess as many series have published the results for all histologic subtypes of pNETs together. Overall response rate (ORR) is disappointing and as such, chemotherapy is not curative. Moertel et al[60] use a combination of streptozotocin and doxorubicin as the standard therapy for progressive or symptomatic unresectable NETs, including VIPomas. This combination reduced diarrhoea and diminished tumour size in 69% (in 14% it showed a complete regression). Further studies have reported that patients with advanced disease may respond to streptozocin-based chemotherapy, being doxorubicin/streptozotocin combination the gold standard with 5-fluorouracil replacing doxorubicin when the latter is contraindicated[10]. When the standard chemotherapy and SSAs lose effectiveness, 5-FU may be used in combination with interferon alfa.
Other chemotherapy drugs are dacarbazine and more recently capecitabine and temozolomide (CAPTEM). This last combination has shown efficacy from retrospective studies in pancreatic NETs where the number of VIPomas was very small. The study by Strosberg et al[61] showed that CAPTEM was able to normalise serum VIP level in one patient with VIPoma. They have only included two patients with thisdisease, the others had different NETs. Kouvaraki et al[62] reported that patients with locally advanced or metastatic pancreatic NETs that received 5-fluorouracil, doxorubicin, streptozozin showed a 40% of ORR, and showed longer progression free survival (PFS) and overall survival (OS). The extension of liver metastases seems to be the most important predictor of result. The median time to response was 4 mo. This study included only 2 cases of VIPoma and the authors’ data suggest that chromogranin A level after two to four cycles of this combination is a useful surrogate marker for the prediction of response. Temozolomide alone can also be recommended as an alternative chemotherapy in pancreatic NETs. There are no established second-line treatment, but regimens that can be used are 5-FU/Leucovorin/irinotecan (FOLFIRI), 5-FU/Leucovorin/oxaliplatin (FOLFOX), CAPTEM bevacizu
Chemotherapy may be considered as an alternative to hepatic-directed therapies such as resection, ablation or hepatic artery embolization, but it fails to control the hormonal syndrome[8]. It should be maintained at least for one or two cycles and it may show significant benefits at the end of the first mo. There is little experience with adjuvant chemotherapy after surgical resection in NET G1/G2. However, in aggressive NENs (NEC G3), adjuvant platinum-based chemotherapy can be used, although prospective clinical trials are advised[63]. Schizas et al[45] in their systematic review had reported that 6.8% of patients received systemic adjuvant therapy. The number of cases is very small as to be able to draw general conclusions. A multicenter trial evaluated 80 cases with metastatic NETs who were randomised to receive lanreotide, interferon alpha, or both. The authors did not find any significant differences between the arms of the study. Partial response was reported in 4% to 7.1%, stable disease in 17.9%-28% and progressive disease in 50%-56%[56]. Another study of 14 patients with metastatic NETs who received indium in-111 octreotide showed stable disease in 50 % of cases, partial response in 14%, and disease progression in 36%[64]. A quality-of-life study in 13 of these patients found a significant benefit in this[65,66].
European Neuroendocrine Tumour Society (ENETS) have published a position statement on peptide receptor radionuclide therapy (PRRT) for pancreatic neuroendocrine tumours (pNETS)[67]. The early findings with radioembolization with resin 90 Y-microspheres in liver metastases from a variety of NETs have been encouraging. Findings reported complete response in 2.7%, partial response in 60.5%, stabilisation in 22.7% and progressive disease in 4.9%. The median OS documented was 70 mo and no cases of radiation liver failure were reported[68]. Second-line therapy for VIPomas includes IFN-α as mentioned earlier in this article, everolimus and sunitinib[69-72]. ENETS 2016 guidelines approved everolimus and sunitinib as antiproliferative therapies in cases of progressive pNETs, after failure of SSA or chemotherapy.
A randomized multicentric trial evaluating sunitinib (a tyrosine kinase inhibitor), included 171 advanced well-differentiated NETs, including patients with VIPomas. The results showed an ORR of 9.3% vs 0%, PFS 11.4 mo vs 5.5 mo in the sunitinib and placebo groups respectively. Nine deaths were reported with sunitinib (10%) vs 21 in the placebo group (25%). These results seem to be similar to those obtained with chemotherapy, but with a more favourable toxicity[69,70]. Sunitinib inhibits several receptor tyrosine kinase key to tumour growth, neoangiogenesis and dissemination[73].
mTOR is a serine-threonine kinase involved in cell growth control and cell apoptosis. Its effects are mediated through phosphoinositide 3-kinase/Akt pathway and stimulates downstream protein kinases crucial to cell cycle progression. mTOR inhibitors, alone or combined with octreotide have been studied in patients in pancreatic NETs. Everolimus is a selective mTOR inhibitor with antiangiogenic activity as well.
The RADIANT 1 is a multicentric single arm phase II trial that evaluates everolimus alone or in combination with octreotide in 160 cases of metastatic pNETs after chemotherapy failure[71]. The ORR was 9.6% in those patients not receiving octreotide, with a median PFS of 9.7 mo and OS 24.9 mo. A smaller group of 45 cases received everolimus and continued to receive octreotide. In these cases, the ORR was 4.4% with PFS of 16.7 mo and OS was not reached at the time of data cutoff.
The RADIANT 2 was a phase III trial randomising patients with NETs to everolimus[72] and SSA or to placebo and SSA. 429 patients were included, 6% pNETs. A PFS of 16.4 mo vs 11.3 favoured the combination.
RADIANT -3 trial is a phase III study that has been recently reported[74]. It included 410 cases with radiologic disease progression. Patients were randomized to receive everolimus or usual treatment which could include SSA. Findings showed a median PFS of 11 vs 4.6 mo and 34% vs 9% were reported alive and free of progression at 18 mo with everolimus or usual treatment respectively. 24% of patients in this trial had somatostatinomas, gastrinomas, insulinomas, glucagonomas or VIPomas. This means that everolimus may be used across the spectrum of pNET subtypes. In addition, authors reported that the benefit of everolimus was found in different subgroups of sex, age, geographic regions, race, performance status and previous therapy applied (chemotherapy, radiation or octreotide). As there is a risk of pneumonitis with this therapy, perhaps sunitinib would be a better option in those patients with underlying severe lung disease (Figure 1).
Well-designed randomized clinical trials have significantly improved our treatment options for patients with these tumours. However, we are still far away from an ideal situation and as such, further research is crucial, although difficult, specially taking into account that VIPomas comprise only < 10% of pancreatic NETs.
Several trials have been carried out testing different agents. Some of these trials have finished recruitment and are still awaiting results such as NCT01466036, a phase II study of cabozantinib in advanced pancreatic NETs and carcinoids[73]. It recruited 62 cases and the primary end point is ORR. The final data collection date was expected in March 2021. Another one is NCT02893930, a phase II with sapanisertib[75] in patients with metastatic or refractory pancreatic NETs that cannot be surgically removed. It has been last updated in May 2021 but not results posted yet. NCT00075439 is a phase II study evaluating gefitinib[76] in patients with progressive metastatic NETs which has finished recruitment as well but awaiting results. EPO906 has been assessed in phase II trial in metastatic carcinoids and other NETs, including VIPomas. It has completed recruitment in 2007 but not available results. Other trials have been withdrawn such as the phase 1 trial with Veliparib (ABT-888) in combination with capecitabine and temozolomide in advanced well-differentiated NETs (NCT02831179)[77]. Another study with cabozantinib (a phase III) is still recruiting patients (NCT03375320). It will assess cabozantinib vs placebo in cases with advanced NETs or carcinoids[78].
Bevacizumab has also been investigated in a phase II study of everolimus and octreotide with or without bevacizumab in cases with advanced or metastatic pancreatic NETs that are not amenable for surgery. This has finished recruitment. PFS is the primary end point, being secondary end points ORR and OS. One hundred and fifty patients were recruited. At the most recent update (May 2021), the PFS results showed 14 m vs 16.7 m without and with bevacizumab respectively. OS is favouring as well the arm with bevacizumab with 34 m vs 37.6 m respectively. Although the study is not finished yet, results seem to favour the arm with bevacizumab. What we do not know yet is how many patients with VIPoma were included (NCT01229943)[79].
Data from other two clinical trials, SANET-p (NCT02589821) (NCT02588170)[80,81] have shown PFS benefit with surufatinib, with a tolerable safety[80] and SANET-ep pattern. Surufatinib is a new oral angio-immuno kinase inhibitor. It inhibits selectively the tyrosine kinase activity related to the vascular endothelial and fibroblast growth factor receptors, both inhibiting angiogenesis, and colony stimulating factor-1 receptor, which controls tumour-associated macrophages, stimulating an immune response against tumour cells. The FDA has conceded to surufatinib, an Orphan drug designation for pancreatic NET in 2019 and two Fast Track Designations for development in pancreatic and extra-pancreatic NETs in 2020.
In the SANET-p trial , 172 patients with pNETs were randomised to surufatinib or placebo. At a median follow-up of 19.3 mo in the experimental arm and 11.1 mo in the placebo arm, the investigator-assessed PFS was 10.9 mo (95%CI, 7.5-13.8) vs 3.7 mo (95%CI, 2.8-5.6) for surufatinib and placebo respectively (HR, 0.49; 95%CI, 0.32-0.76; P = 0.0011), being the most frequent adverse event of grade 3 or higher with surufatinib vs placebo, hypertension (38% vs 7%), proteinuria (10% vs 2%), and hypertriglyceridemia (7% vs 0%). Serious AE occurred in 22% of surufatinib arm vs 7% with placebo. Three patients died surufatinib, two of them due to AE and one due to disease progression. One died in the placebo arm due to disease progression.
We should continue to research further to identify actionable mutations or predictive factors for targeted therapy response to better select patients’ treatment. Also further efforts are needed to increase knowledge about the optimal sequential therapy that could impact positively in survival and also in quality of life.
VIPoma is a rare functional NET that typically presents as sporadic, solitary pancreatic neoplasia with only 5% of cases associated with MEN type I syndrome. It is characterised by a special clinical syndrome of refractory watery diarrhoea, electrolyte and acid-base imbalances related to the excessive VIP secretion.
The only curative option of treatment would be a complete surgical removal. Unfortunately, the majority of VIPomas have have already metastasized at the time of diagnosis leaving only palliative options for these patients. However, surgical debulking for these patients could be considered as it will help control symptoms and prolong survival. Other options include SSA and the newer chemotherapy regimens such as temozolomide, or drugs such as sunitinib or everolimus. Moreover, recent incorporation of treatment with PRRT has shown significant benefits and it is a safe addition to surgery or as a palliative treatment for those cases of widespread metastatic disease or unresectable primary tumour. As a priority, and regardless of the treatment to follow, all patients should have the water depletion, electrolyte imbalance and acid-base profile corrected.
With all these facts in mind, the prognosis may improve but hopefully further multinational clinical trials enrolling more patients with VIPoma can be carried out to get further insight in this rare but challenging disease.
To my colleagues, current and past, that have always been there.
| 1. | VERNER JV, MORRISON AB. Islet cell tumor and a syndrome of refractory watery diarrhea and hypokalemia. Am J Med. 1958;25:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 329] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Jensen RT, Cadiot G, Brandi ML, de Herder WW, Kaltsas G, Komminoth P, Scoazec JY, Salazar R, Sauvanet A, Kianmanesh R; Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: functional pancreatic endocrine tumor syndromes. Neuroendocrinology. 2012;95:98-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 465] [Cited by in RCA: 381] [Article Influence: 27.2] [Reference Citation Analysis (1)] |
| 3. | Yao JC, Eisner MP, Leary C, Dagohoy C, Phan A, Rashid A, Hassan M, Evans DB. Population based study of islet cell carcinoma. Ann Surg Oncol. 2007;14:3492-3500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 246] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 4. | Long RG, Bryant MG, Mitchell SJ, Adrian TE, Polak JM, Bloom SR. Clinicopathological study of pancreatic and ganglioneuroblastoma tumours secreting vasoactive intestinal polypeptide (vipomas). Br Med J (Clin Res Ed). 1981;282:1767-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 92] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Batcher E, Madaj P, Gianoukakis AG. Pancreatic neuroendocrine tumors. Endocr Res. 2011;36:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Parbhu SK, Adler DG. Pancreatic neuroendocrine tumors: contemporary diagnosis and management. Hosp Pract (1995). 2016;44:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Fujiya A, Kato M, Shibata T, Sobajima H. VIPoma with multiple endocrine neoplasia type 1 identified as an atypical gene mutation. BMJ Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Ghaferi AA, Chojnacki KA, Long WD, Cameron JL, Yeo CJ. Pancreatic VIPomas: subject review and one institutional experience. J Gastrointest Surg. 2008;12:382-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Chen Y, Shi D, Dong F, Han SG, Qian ZH, Yang LI, Wang Y, Yu RS, Li QH, Fu YB. Multiple-phase spiral CT findings of pancreatic vasoactive intestinal peptide-secreting tumor: A case report. Oncol Lett. 2015;10:2351-2354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Krejs GJ. VIPoma syndrome. Am J Med. 1987;82:37-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 67] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Belei OA, Heredea ER, Boeriu E, Marcovici TM, Cerbu S, Mărginean O, Iacob ER, Iacob D, Motoc AGM, Boia ES. Verner-Morrison syndrome. Literature review. Rom J Morphol Embryol. 2017;58:371-376. [PubMed] |
| 12. | Maggi CA, Giachetti A, Dey RD, Said SI. Neuropeptides as regulators of airway function: vasoactive intestinal peptide and the tachykinins. Physiol Rev. 1995;75:277-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 106] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Linder S, Barkhem T, Norberg A, Persson H, Schalling M, Hökfelt T, Magnusson G. Structure and expression of the gene encoding the vasoactive intestinal peptide precursor. Proc Natl Acad Sci U S A. 1987;84:605-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Gozes I, Nakai H, Byers M, Avidor R, Weinstein Y, Shani Y, Shows TB. Sequential expression in the nervous system of c-myb and VIP genes, located in human chromosomal region 6q24. Somat Cell Mol Genet. 1987;13:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Said SI, Mutt V. Polypeptide with broad biological activity: isolation from small intestine. Science. 1970;169:1217-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1205] [Cited by in RCA: 1146] [Article Influence: 20.5] [Reference Citation Analysis (13)] |
| 16. | Said SI, Mutt V. Potent peripheral and splanchnic vasodilator peptide from normal gut. Nature. 1970;225:863-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 242] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Dockray GJ. Vasoactive intestinal polypeptide and related peptides. In: Walsh JH, Dockray GJ. Gut Hormones: Biochemistry and Physiology, 1st ed. New York: Raven Press, 1994: 447. |
| 18. | Meriney DK. Pathophysiology and management of VIPoma: a case study. Oncol Nurs Forum. 1996;23:941-8; quiz 949. [PubMed] |
| 19. | Bloom SR, Yiangou Y, Polak JM. Vasoactive intestinal peptide secreting tumors. Pathophysiological and clinical correlations. Ann N Y Acad Sci. 1988;527:518-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | CHARLES B, COCHRANE WA. Islet cell tumour of the pancreas with chronic diarrhoea and hypokalaemia-a recently recognized syndrome. Can Med Assoc J. 1960;82:579-586. [PubMed] |
| 21. | Gozes I, Shani Y, Rostène WH. Developmental expression of the VIP-gene in brain and intestine. Brain Res. 1987;388:137-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Friesen SR. Update on the diagnosis and treatment of rare neuroendocrine tumors. Surg Clin North Am. 1987;67:379-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 43] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Mansour JC, Chen H. Pancreatic endocrine tumors. J Surg Res. 2004;120:139-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology. 2008;135:1469-1492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 637] [Cited by in RCA: 552] [Article Influence: 30.7] [Reference Citation Analysis (2)] |
| 25. | Piet R, Dunckley H, Lee K, Herbison AE. Vasoactive Intestinal Peptide Excites GnRH Neurons in Male and Female Mice. Endocrinology. 2016;157:3621-3630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Remme CA, de Groot GH, Schrijver G. Diagnosis and treatment of VIPoma in a female patient. Eur J Gastroenterol Hepatol. 2006;18:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Brentjens R, Saltz L. Islet cell tumors of the pancreas: the medical oncologist's perspective. Surg Clin North Am. 2001;81:527-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | de Herder WW. Biochemistry of neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab. 2007;21:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Smith SL, Branton SA, Avino AJ, Martin JK, Klingler PJ, Thompson GB, Grant CS, van Heerden JA. Vasoactive intestinal polypeptide secreting islet cell tumors: a 15-year experience and review of the literature. Surgery. 1998;124:1050-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Nilubol N, Freedman EM, Quezado MM, Patel D, Kebebew E. Pancreatic Neuroendocrine Tumor Secreting Vasoactive Intestinal Peptide and Dopamine With Pulmonary Emboli: A Case Report. J Clin Endocrinol Metab. 2016;101:3564-3567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Grozinsky-Glasberg S, Mazeh H, Gross DJ. Clinical features of pancreatic neuroendocrine tumors. J Hepatobiliary Pancreat Sci. 2015;22:578-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Cesani F, Ernst R, Walser E, Villanueva-Meyer J. Tc-99m sestamibi imaging of a pancreatic VIPoma and parathyroid adenoma in a patient with multiple type I endocrine neoplasia. Clin Nucl Med. 1994;19:532-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Peng SY, Li JT, Liu YB, Fang HQ, Wu YL, Peng CH, Wang XB, Qian HR. Diagnosis and treatment of VIPoma in China: (case report and 31 cases review) diagnosis and treatment of VIPoma. Pancreas. 2004;28:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Semelka RC, Custodio CM, Cem Balci N, Woosley JT. Neuroendocrine tumors of the pancreas: spectrum of appearances on MRI. J Magn Reson Imaging. 2000;11:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 35. | Sofka CM, Semelka RC, Marcos HB, Woosley JT. MR imaging of metastatic pancreatic VIPoma. Magn Reson Imaging. 1997;15:1205-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Eldor R, Glaser B, Fraenkel M, Doviner V, Salmon A, Gross DJ. Glucagonoma and the glucagonoma syndrome - cumulative experience with an elusive endocrine tumour. Clin Endocrinol (Oxf). 2011;74:593-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 37. | Schillaci O, Corleto VD, Annibale B, Scopinaro F, Delle Fave G. Single photon emission computed tomography procedure improves accuracy of somatostatin receptor scintigraphy in gastro-entero pancreatic tumours. Ital J Gastroenterol Hepatol. 1999;31 Suppl 2:S186-S189. [PubMed] |
| 38. | Virgolini I, Traub T, Novotny C, Leimer M, Füger B, Li SR, Patri P, Pangerl T, Angelberger P, Raderer M, Andreae F, Kurtaran A, Dudczak R. New trends in peptide receptor radioligands. Q J Nucl Med. 2001;45:153-159. [PubMed] |
| 39. | Anderson MA, Carpenter S, Thompson NW, Nostrant TT, Elta GH, Scheiman JM. Endoscopic ultrasound is highly accurate and directs management in patients with neuroendocrine tumors of the pancreas. Am J Gastroenterol. 2000;95:2271-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 235] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 40. | Ram R, Natanzi N, Saadat P, Eliav D, Vadmal MS. Skin metastasis of pancreatic vasoactive intestinal polypeptide tumor: case report and review of the literature. Arch Dermatol. 2006;142:946-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 41. | Rastogi V, Singh D, Mazza JJ, Parajuli D, Yale SH. Flushing Disorders Associated with Gastrointestinal Symptoms: Part 1, Neuroendocrine Tumors, Mast Cell Disorders and Hyperbasophila. Clin Med Res. 2018;16:16-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Zandee WT, Hofland J, de Herder WW. Vasoactive Intestinal Peptide Tumor (VIPoma). 2021 Aug 28. In: Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–. [PubMed] |
| 43. | Chen C, Zheng Z, Li B, Zhou L, Pang J, Wu W, Zheng C, Zhao Y. Pancreatic VIPomas from China: Case reports and literature review. Pancreatology. 2019;19:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Godard-Sebillotte C, Dramé M, Fagour C, Basileu T, Godaert L. When Symptomatic Treatment Becomes Antitumor Treatment for Vipoma: Opportunity for Frail Elderly Adults. J Am Geriatr Soc. 2016;64:449-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Schizas D, Mastoraki A, Bagias G, Patras R, Moris D, Lazaridis II, Arkadopoulos N, Felekouras E. Clinicopathological data and treatment modalities for pancreatic vipomas: a systematic review. J BUON. 2019;24:415-423. [PubMed] |
| 46. | Anderson CW, Bennett JJ. Clinical Presentation and Diagnosis of Pancreatic Neuroendocrine Tumors. Surg Oncol Clin N Am. 2016;25:363-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 47. | Mekhjian HS, O'Dorisio TM. VIPoma syndrome. Semin Oncol. 1987;14:282-291. [PubMed] |
| 48. | Abu-Zaid A, Azzam A, Abudan Z, Algouhi A, Almana H, Amin T. Sporadic pancreatic vasoactive intestinal peptide-producing tumor (VIPoma) in a 47-year-old male. Hematol Oncol Stem Cell Ther. 2014;7:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Soga J, Yakuwa Y. Vipoma/diarrheogenic syndrome: a statistical evaluation of 241 reported cases. J Exp Clin Cancer Res. 1998;17:389-400. [PubMed] |
| 50. | Zhang X, Zhou L, Liu Y, Li W, Gao H, Wang Y, Yao B, Jiang D, Hu P. Surgical resection of vasoactive intestinal peptideoma with hepatic metastasis aids symptom palliation: A case report. Exp Ther Med. 2016;11:783-787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 51. | Ito T, Igarashi H, Uehara H, Jensen RT. Pharmacotherapy of Zollinger-Ellison syndrome. Expert Opin Pharmacother. 2013;14:307-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Caplin ME, Pavel M, Phan AT, Ćwikła JB, Sedláčková E, Thanh XT, Wolin EM, Ruszniewski P; CLARINET Investigators. Lanreotide autogel/depot in advanced enteropancreatic neuroendocrine tumours: final results of the CLARINET open-label extension study. Endocrine. 2021;71:502-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 53. | Harris AG, O'Dorisio TM, Woltering EA, Anthony LB, Burton FR, Geller RB, Grendell JH, Levin B, Redfern JS. Consensus statement: octreotide dose titration in secretory diarrhea. Diarrhea Management Consensus Development Panel. Dig Dis Sci. 1995;40:1464-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 54. | Nguyen HN, Backes B, Lammert F, Wildberger J, Winograd R, Busch N, Rieband H, Matern S. Long-term survival after diagnosis of hepatic metastatic VIPoma: report of two cases with disparate courses and review of therapeutic options. Dig Dis Sci. 1999;44:1148-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 55. | Plouin PF, Bertherat J, Chatellier G, Billaud E, Azizi M, Grouzmann E, Epelbaum J. Short-term effects of octreotide on blood pressure and plasma catecholamines and neuropeptide Y levels in patients with phaeochromocytoma: a placebo-controlled trial. Clin Endocrinol (Oxf). 1995;42:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 56. | Faiss S, Pape UF, Böhmig M, Dörffel Y, Mansmann U, Golder W, Riecken EO, Wiedenmann B; International Lanreotide and Interferon Alfa Study Group. Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors--the International Lanreotide and Interferon Alfa Study Group. J Clin Oncol. 2003;21:2689-2696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 335] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 57. | Oberg K. Interferon in the management of neuroendocrine GEP-tumors: a review. Digestion. 2000;62 Suppl 1:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 114] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 58. | O'Dorisio TM, Mekhjian HS, Gaginella TS. Medical therapy of VIPomas. Endocrinol Metab Clin North Am. 1989;18:545-556. [PubMed] |
| 59. | Pasricha G, Padhi P, Daboul N, Monga DK. Management of Well-differentiated Gastroenteropancreatic Neuroendocrine Tumors (GEPNETs): A Review. Clin Ther. 2017;39:2146-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Moertel CG, Lefkopoulo M, Lipsitz S, Hahn RG, Klaassen D. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992;326:519-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 673] [Cited by in RCA: 588] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 61. | Strosberg JR, Fine RL, Choi J, Nasir A, Coppola D, Chen DT, Helm J, Kvols L. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer. 2011;117:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 556] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 62. | Kouvaraki MA, Ajani JA, Hoff P, Wolff R, Evans DB, Lozano R, Yao JC. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol. 2004;22:4762-4771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 411] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 63. | Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, Berruti A; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:844-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 793] [Article Influence: 132.2] [Reference Citation Analysis (0)] |
| 64. | Ozkan E, Tokmak E, Kucuk NO. Efficacy of adding high-dose In-111 octreotide therapy during Sandostatin treatment in patients with disseminated neuroendocrine tumors: clinical results of 14 patients. Ann Nucl Med. 2011;25:425-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 65. | Traub-Weidinger T, Raderer M, Uffmann M, Angelberger P, Kurtaran A, Leimer M, Preitfellner J, Dudczak R, Virgolini I. Improved quality of life in patients treated with Peptide radionuclides. World J Nucl Med. 2011;10:115-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 66. | Kwekkeboom DJ, de Herder WW, Krenning EP. Somatostatin receptor-targeted radionuclide therapy in patients with gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:173-185, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 67. | Kwekkeboom DJ, Krenning EP, Lebtahi R, Komminoth P, Kos-Kudła B, de Herder WW, Plöckinger U; Mallorca Consensus Conference participants; European Neuroendocrine Tumor Society. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: peptide receptor radionuclide therapy with radiolabeled somatostatin analogs. Neuroendocrinology. 2009;90:220-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 68. | Kennedy AS, Dezarn WA, McNeillie P, Coldwell D, Nutting C, Carter D, Murthy R, Rose S, Warner RR, Liu D, Palmedo H, Overton C, Jones B, Salem R. Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: early results in 148 patients. Am J Clin Oncol. 2008;31:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 276] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 69. | Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, Chen JS, Hörsch D, Hammel P, Wiedenmann B, Van Cutsem E, Patyna S, Lu DR, Blanckmeister C, Chao R, Ruszniewski P. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2032] [Cited by in RCA: 1878] [Article Influence: 125.2] [Reference Citation Analysis (0)] |
| 70. | Vinik AI, Raymond E. Pancreatic neuroendocrine tumors: approach to treatment with focus on sunitinib. Therap Adv Gastroenterol. 2013;6:396-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 71. | Yao JC, Lombard-Bohas C, Baudin E, Kvols LK, Rougier P, Ruszniewski P, Hoosen S, St Peter J, Haas T, Lebwohl D, Van Cutsem E, Kulke MH, Hobday TJ, O'Dorisio TM, Shah MH, Cadiot G, Luppi G, Posey JA, Wiedenmann B. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. J Clin Oncol. 2010;28:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 476] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 72. | Strosberg JR, Yao JC, Bajetta E, Aout M, Bakker B, Hainsworth JD, Ruszniewski PB, Van Cutsem E, Öberg K, Pavel ME. Efficacy of octreotide long-acting repeatable in neuroendocrine tumors: RADIANT-2 placebo arm post hoc analysis. Endocr Relat Cancer. 2015;22:933-940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 73. | Chan J. Cabozantinib in Advanced Pancreatic Neuroendocrine and Carcinoid Tumors. [accessed 2021 July 15]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT01466036. |
| 74. | Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG, Tomassetti P, Pavel ME, Hoosen S, Haas T, Lincy J, Lebwohl D, Öberg K; RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3) Study Group. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2039] [Cited by in RCA: 2163] [Article Influence: 144.2] [Reference Citation Analysis (0)] |
| 75. | Rajdev L. Sapanisertib in Treating Patients With Metastatic or Refractory Pancreatic Neuroendocrine Tumor That Cannot Be Removed by Surgery. [accessed 2021 November 23]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02893930. |
| 76. | Hobday T. Gefitinib in Treating Patients With Progressive Metastatic Neuroendocrine Tumors. [accessed 2022 January 23]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT00075439. |
| 77. | Berlin J. Veliparib, Capecitabine, and Temozolomide in Patients With Advanced, Metastatic, and Recurrent Neuroendocrine Tumor. [accessed 2017 September 28]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02831179. |
| 78. | Chan JA. Testing Cabozantinib in Patients With Advanced Pancreatic Neuroendocrine and Carcinoid Tumors. [accessed 2022 February 3]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03375320. |
| 79. | Kulke MH. Everolimus and Octreotide Acetate With or Without Bevacizumab in Treating Patients With Locally Advanced or Metastatic Pancreatic Neuroendocrine Tumors That Cannot Be Removed by Surgery. [accessed 2021 September 9]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT01229943. |
| 80. | Fan S. Phase III Study of Surufatinib in Treating Advanced Pancreatic Neuroendocrine Tumors. [accessed 2021 August 31]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02589821. |
| 81. | Fan S. Phase III Study of Surufatinib in Treating Advanced Extrapancreatic Neuroendocrine Tumors. [accessed 2021 April 15]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02588170. |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Xiao Y, China S-Editor: Chang KL L-Editor: A P-Editor: Chang KL