Published online Mar 15, 2022. doi: 10.4251/wjgo.v14.i3.568
Peer-review started: May 22, 2021
First decision: October 3, 2021
Revised: November 12, 2021
Accepted: February 15, 2022
Article in press: February 15, 2022
Published online: March 15, 2022
Processing time: 292 Days and 2.7 Hours
Barrett's esophagus (BE) is the precursor to esophageal adenocarcinoma (EAC). Progression to cancer typically occurs in a stepwise fashion through worsening dysplasia and ultimately, invasive neoplasia. Established EAC with deep involvement of the esophageal wall and/or metastatic disease is invariably associated with poor long-term survival rates. This guides the rationale of surveillance of Barrett’s in an attempt to treat lesions at an earlier, and potentially curative stage. The last two decades have seen a paradigm shift in management of Barrett’s with rapid expansion in the role of endoscopic eradication therapy (EET) for management of dysplastic and early neoplastic BE, and there have been substantial changes to international consensus guidelines for management of early BE based on evolving evidence. This review aims to assist the physician in the therapeutic decision-making process with patients by comprehensive review and summary of literature surrounding natural history of Barrett’s by histological stage, and the effectiveness of interventions in attenuating the risk posed by its natural history. Key findings were as follows. Non-dysplastic Barrett’s is associated with extremely low risk of progression, and interventions cannot be justified. The annual risk of cancer progression in low grade dysplasia is between 1%-3%; EET can be offered though evidence for its benefit remains confined to highly select settings. High-grade dysplasia progresses to cancer in 5%-10% per year; EET is similarly effective to and less morbid than surgery and should be routinely performed for this indication. Risk of nodal metastases in intramucosal cancer is 2%-4%, which is comparable to operative mortality rate, so EET is usually preferred. Submucosal cancer is associated with nodal metastases in 14%-41% hence surgery remains standard of care, except for select situations.
Core Tip: Barrett’s esophagus (BE) is an important premalignant condition. The last two decades have seen treatment paradigms increasingly shift towards endoscopic eradication therapy for dysplastic and early neoplastic cases, where it appears safe and effective. We herein provide a comprehensive review of the literature relating to Barrett’s natural history and comparative efficacy of surveillance, endoscopic and surgical therapies for BE by histological stage.
- Citation: Choi KKH, Sanagapalli S. Barrett’s esophagus: Review of natural history and comparative efficacy of endoscopic and surgical therapies. World J Gastrointest Oncol 2022; 14(3): 568-586
- URL: https://www.wjgnet.com/1948-5204/full/v14/i3/568.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i3.568
Barrett’s esophagus (BE) is an acquired condition characterised by metaplastic change of esophageal mucosal cells in response to chronic gastro-esophageal reflux. While the very definition of BE is variable (and controversial), it is most commonly diagnosed in the presence of salmon-colored mucosa extending at least 1 cm proximal to the gastroesophageal junction, where there is histopathological confirmation of replacement of normal squamous epithelium by metaplastic intestinal-type columnar epithelium. Its clinical importance primarily relates to its established status as a precursor lesion to esophageal adenocarcinoma (EAC)[1]. Worldwide, esophageal cancer ranks seventh in incidence and sixth in overall mortality[2], and is subdivided into squamous cell carcinoma and adenocarcinoma. The incidence of EAC is rising in both Western and Eastern parts of the world[3,4], with EAC now becoming the dominant type of esophageal cancer in high-income, Western countries[3-7]. EAC is associated with high morbidity and mortality, and is commonly diagnosed late with metastatic disease[5,8].
On the other hand, it is clear that in the majority of cases, EAC arises within a segment of pre-existing BE[9]. BE is thought to progress to EAC in a stepwise fashion via the development of dysplasia and finally, neoplasia. Hence it is logical that surveillance of patients with established BE may prevent the poor outcomes associated with EAC by the detection of treatable premalignant or earlier stage localized malignant lesions, and this seems to have been borne out in some data[10]. Recent data from the United States show metastatic EAC at diagnosis has a 5-year survival rate of 4.3%, whereas local disease has a 40.3% 5-year survival[11]. This forms the rationale for Barrett’s surveillance programs that are recommended by international societies[12-14]. Concomitant with increased surveillance of BE, recent decades have also seen significant advances in therapeutic options for premalignant Barrett’s, with endoscopic therapies now having entered widespread use for premalignant BE and for some cases of early EAC. This has led to significant changes in international consensus recommendations for management of BE, though these are not always entirely in agreement with each other. Controversy in management of BE persists, primarily arising from persistent uncertainties regarding natural history and identification of dysplasia.
The purpose of this review therefore is to assist the treating physician in efficient decision making in patients with BE or early EAC by reviewing the current literature regarding natural history of BE, and comparing this to our current understanding of the risks and expected efficacy of current management options including surveillance, endoscopic therapy and surgery.
A comprehensive Medline search was performed using the following keywords and phrases: “Barrett’s esophagus, non-dysplastic Barrett’s esophagus/oesophagus, low grade dysplasia, high grade dysplasia, surveillance, esophageal cancer, Barrett’s endoscopic therapy, endoscopic eradication therapy, radiofrequency ablation, endoscopic resection, esophagectomy, lymph node metastasis, adenocarcinoma, intramucosal adenocarcinoma, T1a esophageal/oesophageal adenocarcinoma, submucosa adenocarcinoma T1b esophageal/oesophageal adenocarcinoma, meta-analysis, systematic review”. There was a focus on original and high-quality research. In addition, we manually reviewed reference lists of all citing references to ensure no relevant articles were excluded.
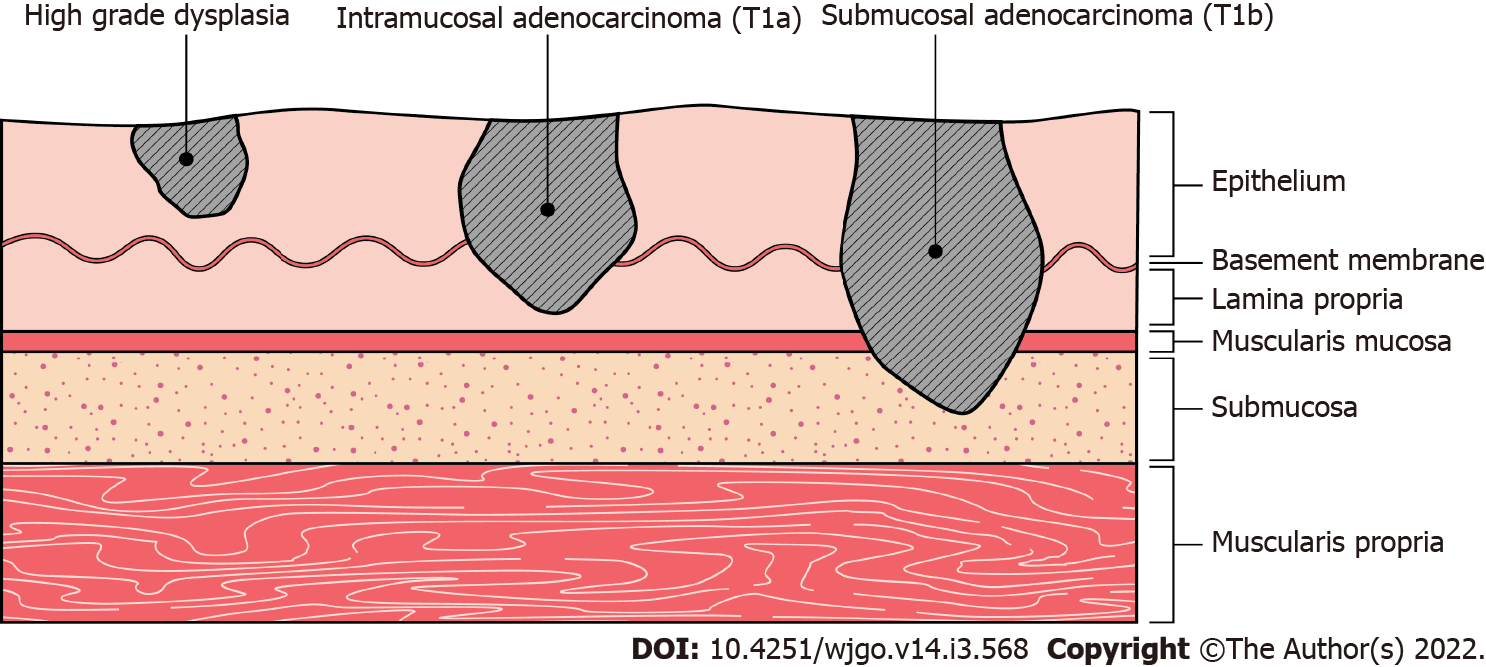
Since EAC is thought to arise in a stepwise histopathological progression from BE (Figure 1)[15], the optimal management strategy is primarily dependent on the degree of dysplastic and neoplastic stage. There is considerable variability in nomenclature, but for the purposes of this review the following classification will be used[16].
Non-dysplastic BE: Intestinal metaplasia without histological features of dysplasia.
BE with low grade dysplasia (LGD): Intestinal metaplasia with histological features of low-grade dysplasia or intra-epithelial neoplasia.
BE with high grade dysplasia (HGD): Intestinal metaplasia with histological features of high-grade dysplasia or intra-epithelial neoplasia. HGD is synonymous with carcinoma in situ, Tis[17], non-invasive carcinoma, suspicion of invasive carcinoma, or defined by malignant cells confined by the basement membrane.
Intramucosal EAC: Invasion of neoplastic cells beyond the basement membrane into the mucosa but not into the submucosa (T1a[17]).
Submucosal EAC: Invasion of neoplastic cells beyond the basement membrane into the submucosa (T1b[17]).
T2 EAC: Invasion beyond submucosa but confined to the muscularis propia[17].
Consideration of natural history is essential when evaluating the utility of interventions for any condition. In BE, the most important and clinically relevant endpoint is development of adenocarcinoma, and will be the focus of review for natural history studies of premalignant BE. Where early stage adenocarcinoma has already developed within BE, the endpoint of interest is nodal metastases, since this is the major factor determining appropriateness of endoscopic or surgical therapy.
Non-dysplastic Barrett’s: There is a large body of data examining the risk of cancer progression for non-dysplastic BE. Several meta analyses incorporating multiple retrospective case series have reported annual progression rates of non-dysplastic BE to cancer of between 0.33%–0.70%[18-41]. Within this range, Shaheen et al[18] showed an inverse relationship between study size and cancer risk whereby small studies tended report higher progression rates[18]. Meta-analyses reporting higher progression rates also tended to incorporate a significant minority of LGD cases that were not separated in analyses[20,21,23] (Supplementary Table 1).
Population-based studies have reported rates of progression at the lower end of the abovementioned range. De Jonge et al[29] showed from a registry in the Netherlands including more than 38000 subjects an annual progression rates of 0.39% after careful exclusion of prevalent HGD and EAC cases[29] and even lower rates have been found in other national databases[30,31]. The only prospective natural history study in patients with non-dysplastic BE followed 150 subjects over 5.5 years that led to 3 cases of EAC (annual progression rate 0.36%)[25]. Taken in sum, the annual risk of cancer in those with non-dysplastic BE is felt to be below 0.5%.
Barrett’s with low grade dysplasia: There is marked heterogeneity in the reported rate of progression of LGD-BE to EAC. This is now thought to primarily relate to the significant variability in the classification of LGD by pathologists. Traditionally, the risk of progression of LGD was deemed low. Several large, multicenter series suggested that the annualized risk of progression to EAC was less than 1%[25-27,34,42]. However, due to concerns including non-centralized histopathology reading, marked interobserver variability in dysplasia diagnosis, short follow up duration and significant rates of regression of dysplasia in follow up, the possibility of overstaging of dysplasia in these studies was raised. Several population-based studies based on national registry data from the United States and Europe also reported similar rates of between 0.24% and 0.92%[29,31,33,43]. Such data is subject to the same limitations as cohort studies as they include patients from smaller centers where overdiagnosis of LGD is even more likely to occur.
Several studies have attempted to address the issue of overstaging of dysplasia and suggested that the true rate of progression to EAC may be higher. Curvers et al[24] had pathology specimens from 147 subjects with LGD re-examined by an expert panel who downstaged the diagnosis in 85%. Of the minority who were confirmed to have LGD by the expert panel, the annualized risk of progression to EAC was 3.3%. This was significantly higher than in those who were downstaged to non-dysplastic BE where progression rate to EAC or HGD was only 0.49%, thus providing a convincing argument that inconsistency in pathological diagnosis was the major factor in variability in reported progression rates. Duits et al[37,44] similarly demonstrated that the majority of cases of LGD-BE diagnosed in community centers are downstaged by a centralized expert panel; but those who are confirmed dysplastic have a higher rate of progression than previously thought[37,44]. The control arm of the SURF trial, examining outcomes in LGD-BE, also found that when LGD was confirmed by an expert panel of experienced pathologists, the rate of progression to EAC was 2.9% per annum[45]. In contrast, a recent well-designed RCT with expert GI pathologists and central pathology review, showed that even after downstaging 26% of patients initially thought to have LGD, progression of ‘true’ LGD to EAC in those under surveillance was a low 2.4% at three years[46]. The authors identified nearly 1/3 of their initial diagnosis of LGD spontaneously regressed raising the issue of potential consensual misclassification of the diagnosis of LGD. Even in the presence of agreement between multiple expert pathologists k-values may still be suboptimal[37,47,48], thus not completely eliminating the issue of overdiagnosis in LGD.
Therefore, the risk of progression of LGD-BE depends upon the rigor by which it is diagnosed. There can be significant variability in diagnosis depending on local expertise and experience. It is clear that diagnosis in community centers can be unreliable, and when the diagnosis of LGD is made, biopsies should be repeated and examined by at least two expert gastrointestinal pathologists. If a conclusive diagnosis of LGD remains, then the annualized risk of progression to cancer may approximate 1%–3% (Supplementary Table 2).
Barrett’s with high grade dysplasia: There is a paucity of high-quality literature describing true progression rates of HGD-BE to EAC. Only three small single center observational studies exist reporting annual incidence rates of cancer between 5% and 8.7%[49-51]. Two well-conducted meta-analyses primarily comprising the abovementioned studies reported identical weighted risks of cancer of 6.6% per annum. A single randomized controlled trial included in the meta-analyses followed 70 subjects with HGD-BE over the course of 3.3 years. 19 of these patients developed EAC giving an annual progression rate of 8.14%[52].
While not as significant of an issue as for LGD, pathological overstaging of dysplasia may also be a problem in HGD-BE[53]. Two additional studies suggested that when HGD-BE was characterized following consensus amongst more than one pathologist, the annual risk of progression was higher and between 19%-31.25%[32,42]. Regardless, the annual risk of cancer in HGD-BE is certainly high and is at least in the range of 5%-10% (Supplementary Table 3).
Conceptually, lymph node metastasis is the major factor that precludes the curative potential of endoscopic therapy for early adenocarcinoma in BE. Lymph node metastasis is also an important outcome as it leads to higher mortality[54-59], tumor recurrence[57,60,61] and is an indication for systemic therapy[62,63]. Most data that assesses risk of lymph node metastases comes from retrospective studies with histopathological lymph node dissection samples from esophagectomy specimens, therefore is significantly limited by selection bias.
Barrett’s with high grade dysplasia: HGD, that is, neoplasia confined to the mucosa that does not extend through the basement membrane confirmed by expert gastrointestinal pathologists, has a negligible lymph node metastasis rate[54,60,64-67]. This was confirmed by a systematic review in 2012 which compiled 524 subjects with HGD-BE showing a lymph node metastasis rate of 0%[68].
Intramucosal adenocarcinoma: There is a wide range in the reported rate of lymph node metastasis in intramucosal cancer, ranging between 0% and 9.5%[55-61,64,67-78]. However most of these studies arise from retrospective surgical series suffering from small sample sizes and selection bias[55-61,64,67,69-78]. Larger population database studies tend to suggest much lower rates of lymph node metastasis. A recent retrospective cohort study comprising 782 patients and using the National Cancer Database capturing 70% of all cancers in the United States, showed a relatively low lymph node metastasis rate of 3.6% for intramucosal adenocarcinoma[54]. The reliability of this study stems from including patients with clear staging and adequate lymph node sampling[54]. Another large United States database identified 3595 individuals with intramucosal adenocarcinoma who had undetected lymph node metastatic rate of 8.7%[74], though 16% of the cohort had squamous cell carcinoma which may tend to metastasize to lymph nodes earlier[79]. Further, a systematic review including 1350 patients with intramucosal adenocarcinoma identified 26 individuals with metastasis to surrounding lymph nodes. After prevalence rates were weighted for study sample size, a lymph node metastasis rate of 1.93% was reported.
It appears intramucosal adenocarcinoma has an approximate lymph node metastasis risk between 2%–4% (Table 1). Those with high risk features (invasion into the muscularis mucosae, poor differentiation, and lymphovascular invasion) may have greater risk of metastasis[61].
| Ref. | Type | n1 | LNMrate | 5-yr DFS or DSS | 5-yr OS |
| Rice et al[97] | Retrospective | 53 | 2% | - | 77% |
| Liu et al[61] | Retrospective | 53 | - | 100% | 91% |
| Prasad et al[75] | Retrospective | 46 | 8.6% | 97% | 95% |
| Pennathur et al[59] | Retrospective | 29 | 7% | 82% | 73% |
| Wang et al[109] | Retrospective | 60; T1a 32%; HGD 68% | - | - | 88% |
| Sepesi et al[72] | Retrospective | 25 | 0% | - | 85% |
| Zehetner et al[96] | Retrospective | 48 | - | 88% | 94% (3 yr) |
| Hölscher et al[56] | Retrospective | 70; SCC 29% | 0% | - | 87% |
| Leers et al[55] | Retrospective | 75 | 1.3% | 98% | 82% |
| Pech et al[95] | Retrospective | 38 | - | 100% (3.7 yr) | 93% |
| Ngamruengphong et al[120] | Retrospective | 671 | - | - | 76% |
| Lorenz et al[57] | Retrospective | 42 | 8.7% | 93.4% | 91% |
| Newton et al[54] | Retrospective | 303 | 3.6% | - | 80% |
| Marino et al[121] | Retrospective | 1317 | - | - | 79% |
| Semenkovich et al[74] | Retrospective | 428; SCC 16% | 8.7% | - | 80% |
Submucosal adenocarcinoma: An even wider discrepancy exists in lymph node metastasis for submucosal adenocarcinoma ranging from 14% to 41% (Table 2)[55-61,64,67,69-78]. Such variation is explained by a number of factors. The number of lymph nodes resected during esophagectomy vary widely, and are often not reported; those with greater numbers of nodes excised tend to show higher metastasis rates[73].
| Ref. | Type | n | LNM rate | 5-yr DFS | 5-yr OS |
| Rice et al[97] | Retrospective | 31 | 5% | - | 60% |
| Liu et al[61] | Retrospective | 37 | - | 60% | 58% |
| Pennathur et al[59] | Retrospective | 71 | 27% | 62% | 60% |
| Sepesi et al[72] | Retrospective | 29 | 31% | - | 60% |
| Hölscher et al[56] | Retrospective | 101; SCC 35% | 34% | - | 66% |
| Leers et al[55] | Retrospective | 51 | 22% | 79%DSS | 71% |
| Ngamruengphong et al[120] | Retrospective | 523 | - | - | 64% |
| Lorenz et al[57] | Retrospective | 168 | 20.6% | 85% | 74% |
| Schölvinck et al[78] | Retrospective | 26 | 17% (n = 69 including EET group) | - | Median survival: 51 mo |
| Schwameis et al[76] | Retrospective | 32 | 22% | - | 84% |
| Newton et al[54] | Retrospective (NCDB) | 512 | 23.4% | - | 64.4% |
| Semenkovich et al[74] | Retrospective (NCDB) | 1146; SCC 16% | 14% | - | 60% |
| Otaki et al[77] | Retrospective | 68 | 14.7% | 92% | 89% |
Further, other factors may significantly impact rates of lymph node metastases in submucosal disease. Lymphovascular invasion, poor differentiation and size (2 cm) are prognostic factors known to increase the risk of lymph node metastasis[54,55,57,58,60,64,65,67,69,70,72,73,80]. A study by Sepesi et al[72] contained a cohort of submucosal adenocarcinoma patients with almost a third exhibiting poor differentiation and found a lymph node metastasis rate of 31%[72]. In contrast, a large retrospective study containing 14000 subjects identified a lymph node metastasis rate of 8.6% when tumors were smaller than 2 cm and well to moderately differentiated[64]. Even lower rates of 1.9% have been reported where invasion depth into the submucosa was shallow and no other poor prognostic features were present[81]. Several other studies identify depth of submucosal invasion as another independent risk factor for nodal metastasis in submucosal disease, but this is not a universal finding[56-58].
Surveillance of BE is recommended by all international societies for all patients who have a history of non-dysplastic BE, and is one of the strategies available for LGD-BE[12-14]. Surveillance involves dye-based[82] or virtual chromoendoscopy[83] in combination with white light endoscopy[13] using a systematic 4-quadrant biopsy protocol (Seattle protocol)[84]. The surveillance interval is determined by a risk appraisal based on the prior endoscopic and histological findings.
Barrett’s endoscopic eradication therapy (EET) has become an established therapeutic modality for dysplastic and early neoplastic BE. EET is an umbrella term given to a multimodal therapeutic strategy whereby nodular components of the BE segment are endoscopically resected, with subsequent treatment of residual flat components of the segment with ablative therapies.
Resection: Resection is the first component to successful EET. It relies on a careful high-quality endoscopic examination with white light as well as an enhanced imaging modality (dye-based or virtual) for detection of nodular or irregular lesions. Resection is vital from a therapeutic standpoint but also assists in staging by providing depth of tumor invasion that cannot be ascertained from mucosal biopsies alone[85]. The most widely used resection technique in BE is endoscopic mucosal resection (EMR). EMR can be performed using the cap and snare technique or by multi-band mucosectomy[86,87].
Endoscopic submucosal dissection (ESD) is an advanced resection technique that has theoretical advantages of allowing en bloc resection and thorough assessment of lateral and deep margins of the specimen. However, ESD is technically challenging, time consuming, has a steep learning curve, and is not as widely available. Further, it has not been clearly shown to be superior to EMR for neoplasia remission, recurrence or need for surgery in BE[88]. At present, it is usually reserved for large lesions with endoscopic evidence of submucosal invasion[89].
Ablative therapy: Ablation always follows resection other than in the scenario where all visible intestinal metaplasia has been endoscopically resected. It is typically applied to LGD-BE or flat HGD-BE. There are numerous modalities of ablative therapy, however the technique with the best efficacy, ease of use and favorable safety profile is radiofrequency ablation (RFA)[90]. RFA is applied using a catheter with distal balloon or other attachment bringing electrodes in contact with the esophageal mucosa[42].
A recent meta-analysis assessing adverse events of EET with most included studies using a combination of RFA and EMR showed an overall adverse event rate of 8.8%. The most noteworthy is stricture formation, which represented 5.6% of all patients, although strictures can almost always be treated safely with endoscopic dilatation with durable response[42,45,85,91,92]. Other serious adverse events included bleeding in 1% and 0.6% rate of perforation. Post-procedural chest pain in the absence of other serious complication occurs in 1.5%–5.4%[42,45,46]. No deaths attributable to endoscopic therapy were recorded[93].
En-bloc esophagectomy and lymphadenectomy of the mediastinal and abdominal nodes via an abdominal or right transthoracic approach is the standard surgical approach to adenocarcinoma arising within a Barrett’s segment[57,73]. For tumors in the distal two thirds of the esophagus, esophagectomy is typically performed with the Ivor-Lewis technique, via laparotomy and right thoracotomy. Tumors located in the upper third of the esophagus are typically managed via the McKeown technique[58].
Esophagectomy has traditionally been considered a relatively high-risk surgery with significant morbidity and mortality rates. Adenocarcinoma specific 90-d mortality has been reported in up to 8.7%[94]. However, early stage carcinoma limited up to submucosa tends to be associated with much more favorable operative risks. When esophagectomy is performed for such early disease, operative mortality ranges between 0% and 5% (Supplementary Table 4)[54,57,59,75-78,95-97]. Serious adverse events, however, remain relatively common and include anastomotic leaks and tracheal injury[98]. Even when the immediate postoperative course is benign, foregut function is permanently altered, and there can be long-term (and in some cases, permanent) impairment of quality of life due to dysphagia, vomiting, reflux symptoms, abdominal pain, and dumping syndrome[99].
Endoscopic eradication therapy: Surveillance with repeat endoscopy every 3–5 years is recommended for non-dysplastic BE[12-14], however there is little data examining ablative therapy. Wani et al[22] suggested in a meta analyses that ablative therapies reduced the annual incidence of EAC from 0.60% to 0.16%[22], though the included studies were of varying quality. A single prospective multi-center trial including 50 patients reported complete eradication of intestinal metaplasia rate of 92% at 5 years of follow up. Of the 8% who recurred, all were retreated and eradication of intestinal metaplasia re-achieved. There was no progression to EAC for the duration of the study with no recorded mortalities, serious adverse effects or strictures[90].
Due to the low progression rates of non-dysplastic BE to cancer it is unlikely that any study will ever demonstrate a benefit of ablative therapy in preventing progression to cancer, let alone a mortality benefit. Due to the very low risk profile of non-dysplastic BE, EET is not indicated given that it is not entirely devoid of risk.
Endoscopic eradication therapy: The management of LGD-BE is the most controversial aspect of the management of BE. Retrospective data suggested that EET was highly effective in eradicating intestinal metaplasia in LGD-BE[92]. In terms of the efficacy of RFA for preventing progression of LGD-BE to cancer, a systematic review and meta-analysis including 19 studies and a total of 2700 patients found that compared to surveillance, RFA was associated with relative risk of disease progression to HGD or EAC of 0.14[100]. Three randomized controlled trials examining this question have been published to date. The SURF trial compared RFA against surveillance in patients with LGD-BE without visible lesions. Progression to EAC was reduced by 7.4% (1.5% in RFA arm vs 8.8% in control arm) over a median 3 year follow up period[45]. Long term, no further EAC occurred in the ablation arm compared with 10.3% rate of cancer observed in the control arm over 73 mo. On intention to treat analysis, the number needed to treat was 11.4 to prevent cancer. Notably, all 23 progressors to HGD or EAC subsequently achieved complete eradication of cancer and dysplasia by the end of the extended study[101]. Subsequently the AIM DYSPLASIA study showed 5% of LGD-BE patients receiving RFA progressed to HGD compared with 14% in the sham arm over the 12-mo study period. No cancers developed in either arm[42]. The study was extended for 3 more years and only 1 subject from the sham arm developed intramucosal adenocarcinoma, which was cured with EMR[91]. However, these studies are limited by roughly half of subjects not reaching their third year of follow up. A recent multi-center RCT by Barret et al[46] retained a near entirety of their cohort of 82 patients for up to 3 years and did not show statistical significance in neoplastic progression rates (12.5% RFA vs 26.2% surveillance, P = 0.15). The most notable finding, and likely explanation for the negative result, was that RFA was much less effective with significantly lower rates of eradication of dysplasia and intestinal metaplasia (55% and 35% respectively) compared to the earlier studies (Table 3). The lower efficacy of RFA in this study may be attributed to several factors, most importantly a less aggressive protocol (maximum number of ablation sessions was capped at 4). There was a suggestion of a learning curve and operator effect, with significant difference in success rates between low and high-volume centers[46]. Further, this seemed to be a less ‘aggressive’ cohort of LGD-BE with much lower rate of neoplastic progression, and higher rate of spontaneous remission of LGD-BE, compared to the former studies. There is no data on the surgical efficacy of LGD-BE.
| Ref. | Type | n | CE-IM | CE-D | NNT to prevent disease progression | Annual disease progression, treatment vs placebo (P value) |
| Wani et al[22] | Meta-analysis | 1512 | - | - | 65.5 (EAC) | 0.16% vs 1.7% (P = 0.99) (EAC) |
| Shaheen et al[42] | RCT | 64 | 81% | 90.5% | 11.3 (HGD) | 5% vs 14% (HGD) (P = 0.33) |
| Shaheen et al[91] | Retrospective | 52 | 98% | 98% | NA | NA |
| Bulsiewicz et al[92] | Retrospective | 41 | 93% | 100% | NA | NA |
| Phoa et al[45] | RCT | 136 | 88.2% | 92.6% | 13.6 (EAC) | 1.5% vs 8.8% at 3 yr (EAC) (P = 0.03) |
| Qumseya et al[100] | Meta-analysis | 2746 | - | - | 16 (EAC) | NA |
| Pouw et al[101] | Retrospective | 83 | 90% | 90% | 11.4 (EAC) | NA |
| Barret et al[46] | RCT | 82 | 37.5% | 52.5% | - | 5% vs 2.4% at 3 yr: (EAC) (P = 0.52) |
With conflicting findings from high-quality randomized controlled trials, the decision to offer EET for LGD-BE remains nuanced and several factors need to be considered in the decision-making process. Firstly, RFA only provides a benefit when LGD cases are carefully confirmed by expert pathologists to avoid overdiagnosis and identifying a highly select LGD-BE cohort with rates of progression comparable to that typically associated with HGD-BE[42,45]. This is not representative of most patients diagnosed with LGD-BE. Second, a commitment to an aggressive RFA protocol with potential for several sessions (often 4 or more) needs to be made in order for RFA to be successful in reducing risk of cancer progression. Third, it appears that RFA is more likely to be successful in high-volume centers. Fourth, one must bear in mind that when under surveillance by experts, cancers that evolve from LGD-BE tend to be early and appear to be amenable to curable therapy. Therefore, based on available data, one could argue that the long-term outcome for those under surveillance is no worse, even if HGD or cancer developed.
Endoscopic eradication therapy: Studies of varying quality demonstrate that RFA reduces annual progression of HGD to EAC to a range between 0.6%–2.4%[22,42,91,102-105] compared to no treatment, which has an estimated rate of 5%–10% as described above. To date, there is one RCT that has randomized RFA of HGD against a control arm. RFA reduced progression to cancer from 19% to 2.4%, and the number needed to treat was six[42]. This trial was extended by 3 more years with cross-over from the sham arm to RFA, leading durable remission and an annual progression rate to EAC of 0.60%[91]. Only one other prospective study recruited 75 consecutive subjects with HGD-BE, finding that all patients who achieved complete eradication of BE with EET had no progression to EAC over a follow up period of 31 mo[85].
Once the threshold of HGD has been reached, subjects are also at risk of developing other areas of HGD within their Barrett’s mucosa[50]. Further, those who achieve complete endoscopic eradication of Barrett’s mucosa are far less likely to progress to cancer compared to those where this is only partially achieved[85,103-105]. Thus, a logical step is to eradicate all surrounding Barrett’s tissue once a diagnosis of HGD has been made. In patients with HGD, EET is effective in eradicating all dysplasia in 79%–97%, and intestinal metaplasia in 51.2%–94%[85,92,96,102-105] (Table 4). Low eradication rates are explained by non-standardized and incomplete RFA treatment sessions[102] and inclusion of treatment-experienced subjects representing resistant disease due to fibrosis[103]. Furthermore, experienced centers and contemporary data report higher rates of complete eradication of dysplasia and intestinal metaplasia above 90%[92]. Haidry et al[104] compared early and late cohorts, finding rise in eradication of intestinal metaplasia from 57% to 83%[104].
| Ref. | Type1 | n | CE-IM | CE-D | NNT to prevent disease progression | Annual disease progression, treatment vs placebo (P value) |
| Overholt et al[52] | RCT (PDT) | 208 | 52% | 77% (including HGD) | 22 | 3.6% vs 8.14% (P = 0.006) |
| Ganz et al[102] | Retrospective | 92 | 54% | 80% | NA | 1.4% |
| Wani et al[22] | Meta-analysis | 236 | - | - | 20.4 | 1.7% vs 6.6% (P = 0.02) |
| Shaheen et al[42] | RCT | 63 | 73.8% | 81% | 6 | 2.4% vs 19% (P = 0.04) |
| Shaheen et al[91] | Retrospective | 54 | 89% | 93% | NA | 0.6% |
| Moss et al[85] | Prospective (SRER) | 35 | 94% | 94% | NA | Nil |
| Zehetner et al[96] | Retrospective | 22 | 89% | 89.5% | NA | Nil |
| Okoro et al[103] | Retrospective | 35 | 51.2% | 79% | NA | 2.3% (2 yr) |
| Bulsiewicz et al[92] | Retrospective | 118 | 90% | 97% | NA | NA |
| Haidry et al[104] | Retrospective | 122 | 85% | 92% | NA | 2.5% (3 yr) |
| Li et al[105] | Retrospective | 832 | 83.4% | 92.1% | NA | 3% (2.8 yr) |
Therefore, EET is effective in reducing annual cancer progression risk by 5-fold, to approximately 2% by eradicating areas of HGD as well as surrounding Barrett’s mucosa. The risk of cancer appears to be reliably attenuated when all residual Barrett’s mucosa is completely treated. Overall 5-year survival rate appears to be very high at 90%, even in those who do not achieve complete eradication of Barrett’s mucosa[85].
Surgery: We can presume individuals have complete eradication of dysplasia and intestinal metaplasia on the day of esophagectomy. Nevertheless, there is paucity of high quality evidence of overall survival and recurrence following esophagectomy for HGD-BE, as most studies are retrospective with small numbers. Five retrospective studies that had referred patients for surgery after biopsy or esophagectomy confirmation of HGD-BE showed promising 5-year survival rates ranging from 83%-97%[97,106-109] with disease free survival at 5 years surpassing 94% in 2 of these studies[106,107]. Another study by Edwards et al[110] reported an 82% survival rate after a median of 2.7 years in a small cohort of eleven[110]. These disease free survival rates are not markedly different to those following EET for HGD. However, rates in surgical series incorporate a mixed population, with up to 40% of HGD-BE subjects referred for esophagectomy having evidence of infiltration past the basement membrane corresponding to intramucosal adenocarcinoma[97,106,108,110], reflecting a period where endoscopic assessment was not as accurate as the modern era with subtle lesions likely missed.
Therefore, esophagectomy for HGD shows 5-year overall survival rates above 83% and there is some data to suggest disease-free survival at 5 years exceeds 94%.
Endoscopic eradication therapy: Successful endoscopic eradication rates of intramucosal adenocarcinoma is reported to occur in between 82%–100%[75,92,95,96,104,105,111-119]. Pech’s large cohort involved 1000 prospective patients over a 15-year period with successful endoscopic resection of cancer and HGD in 96.3%. These patients were closely followed up giving rise to a long term remission rate of 93.8% at 5 years[117]. Another prospective study by Phoa et al[119] followed 132 subjects with a significant proportion having intramucosal adenocarcinoma. 92% achieved cure of cancer and dysplasia with a quarter of patients reaching 3 year follow up having durable response rate of 95%[119]. A number of other prospective trials have shown successful endoscopic eradication rates of intramucosal adenocarcinoma exceeding 97%[111-115].
Although initial remission rates are promising, long term outcomes may be more relevant. Three prospective studies exceeding 100 subjects show durability rates of 93.8%–100% over a follow up period ranging between 3–5 years[95,111,117]. Remaining data showing endoscopic eradication rates are displayed in Table 5. Despite these limitations it is clear that residual or recurrent EAC is easily managed by further EET[75,95,96,111,112,115,117,119]. Pech et al[117] showed retreatment with EET was successful in 115 out of 140 subjects[117]. Further, esophagectomy also appears to remain a valid treatment option for treatment failures with minimal risk of lymph node metastasis[75,115-117,119].
| Ref. | Type1 | n2 | Eradication of T1a | 5-yr OS |
| Ell et al[111] | Prospective | 100 | 99% | 98% |
| Pech et al[112] | Prospective (EMR +/- PDT) | 349; HGD 17.5% | 97.4% (including HGD) | NA |
| Pouw et al[113] | Prospective (RFA +/- EMR) | 44; HGD up to 27% | 100% | NA |
| Prasad et al[75] | Retrospective (PDT) | 132 | 94% | 83% |
| Pouw et al[114] | Prospective (EMR + RFA) | 24; HGD 25%; T1b 8% | 100% | NA |
| Pech et al[95] | Retrospective (EMR +/- APC) | 79 | 98.7% | 96% |
| Van Vilsteren et al[115] | RCT | 47; HGD up to 40% | 97.9% | NA |
| Zehetner et al[96] | Retrospective | 18 | 82% (14/17); 3/17 subsequently successfully treated under surveillance | NA |
| Bulsiewicz et al[92] | Retrospective | 29 | 93% | NA |
| Ngamruengphong et al[120] | Retrospective | 229; HGD 24% | - | 60% |
| Saligram et al[116] | Retrospective | 54 | 96% | 89% (over 2 yr) |
| Pech et al[117] | Prospective | 1000 | 96.3% (including HGD) | 91.5% |
| Haidry et al[104] | Retrospective | 63 | 97.5% (combined with HGD cohort) | NA |
| Agoston et al[118] | Retrospective | 79 | 86% | NA |
| Li et al[105] | Retrospective | 162 | 97.5% | NA |
| Phoa et al[119] | Prospective | 132; ND/LGD 8.4%; HGD 30%; T1b 1.7% | 92% | NA |
| Marino et al[121] | Retrospective | 856 | - | 71.8% |
| Semenkovich et al[74] | Retrospective | 1123 | - | 70% |
Reported survival rates of subjects with intramucosal adenocarcinoma who have undergone EET are between 60%–100%[74,75,95,111,120,121]. Lower survival rates are felt to be secondary to selection bias in these observational studies whereby those with frailty, age and comorbidities are more likely to receive less invasive EET than surgery[74,120]. Further, deaths are predominantly due to causes unrelated to EAC[75,95,112,115-117], for example, Pech et al[117] reported only 2 in 1000 subjects with tumor-associated deaths[117].
Intramucosal adenocarcinoma can be successfully treated with EET in greater than 90% of cases with durable remission in the vast majority. 5-year overall survival is an estimated 80%, with deaths predominantly attributable to other causes.
Surgery: There are several large surgical series reporting overall survival rates whose findings are severely limited by lack of data on follow up protocols, imaging modalities for surveillance and comorbidities[54,74]. However, at least eight good quality retrospective studies with follow up of 4 years or more reported estimated 5-year overall survival between 73%-93% (Table 1)[55-57,59,72,75,95,97]. The largest of these retrospective studies contained 75 subjects with intramucosal adenocarcinoma from a single center with detailed follow up protocol over a median duration of 50 mo. The 5-year overall survival rate was 92% with 5 year disease specific survival an estimated 98%[55].
Surgery provides definitive therapy of cancer as well as Barrett’s mucosa leading to high 5-year overall survival rates of approximately 80%. Most deaths are attributable to non-EAC related causes and correlate to even greater rates of disease-free survival approaching 100%.
Endoscopic eradication therapy: There are no prospective or randomized controlled studies that assess the survival benefit of endoscopic therapy for submucosal adenocarcinoma. Endoscopic eradication of submucosally invasive adenocarcinoma is reportedly achieved in 63%-100% following EET[77,81,122] (Table 6). Manner et al[81] retrospectively studied efficacy of EET in 61 subjects with low risk submucosal disease, defined as macroscopically polypoid or flat, minor invasion depth into the submucosa, good to moderate differentiation and with no lymphovascular invasion. Cancer eradication was achieved in 87% and durable response was sustained in 83.6% over a mean reaching 4 years. 5-year overall survival was 84%. Only 1 patient required esophagectomy for lymph node metastasis found during surveillance after complete endoscopic remission was achieved[81]. However, this study and others did not uniformly apply ablative therapy following endoscopic resection[77,81,122], thus possibly underreporting true eradication rates. When disease recurrence occurs after initial EET, successful retreatment appears to be achievable with minimal risk of lymph node metastasis[81,122].
| Ref. | Type | n | Eradication of cancer | Survival |
| Manner et al[81] | Retrospective | 61 | 87% (including HGD) | 5-yr OS 84% |
| Ngamruengphong et al[120] | Retrospective | 39 | - | 5-yr OS 66% |
| Schölvinck et al[78] | Retrospective | 43 | - | Median survival: 46 mo |
| Künzli et al[122] | Retrospective (RFA or APC) | 35 | 100% | - |
| Semenkovich et al[74] | Retrospective | 588 | - | 5-yr OS 50% |
| Otaki et al[77] | Retrospective (RFA/APC/Cryo) | 73 | 63% (including HGD) | 5-yr OS 59% |
Of 5-year overall survival rates of submucosal adenocarcinoma undergoing EET range from 50%–87%[74,77,81]. Low survival rates were associated with several factors including high risk histological features and extensive comorbidities, with EET often performed in patients deemed unfit for surgery with the majority of subsequent deaths attributable to other causes[77,78].
Complete eradication of cancer may be achievable in up to 87% in low risk submucosal adenocarcinoma. Reported overall survival is very low, though this primarily relates to the frail and comorbid demographic that typically is selected for EET. There remains a role for endoscopic therapy with curative intent in low-risk submucosal disease. Especially in those with comorbidities, EET is a reasonable option in the setting of low-risk histological features.
Surgery: There are numerous retrospective studies of varying size and quality that report overall survival and recurrence rates of submucosal EAC. 5-year overall survival rates for submucosal adenocarcinoma range between 58%–89%[55-57,59,61,72,76,77,97]. Four studies report 5-year disease free survival rates between 60%–92%[57,59,61,77], with contemporary series typically reporting higher overall and cancer-specific survival rates[56,59,61,72,97] . Disease-free survival is typically significantly higher than overall survival given the high rates of non-cancer related deaths in this cohort[61,72,78]. Esophagectomy appears effective in treating submucosal tumors regardless of the presence of high risk features. Otaki et al[77] showed a 5-year overall survival rate of 89% despite the majority of patients having at least 1 high risk feature[77].
Surgery appears to be a very effective and curative option in submucosal EAC. Survival rates may reach up to 80% in appropriate surgical candidates, with a significant portion of deaths being unrelated to EAC.
We recommend surveillance endoscopy for patients with non-dysplastic BE. EET is not justified in non-dysplastic BE due to the extremely low rates of cancer progression (Table 7).
| Stage | Annualized risk of cancer | Recommended management | Risks of intervention | Post-intervention cancer risk |
| NDBE | 0.5% | Surveillance | Negligible | NA |
| LGD1 | 1%–3% | Surveillance or EET | Stricture 6%; Chest pain 5%; Bleeding 1%; Perforation 1% | 1% per year |
| HGD | 5%–10% | EET | Stricture 6%; Chest pain 5%; Bleeding 1%; Perforation 1% | 2% per year |
We recommend that LGD be always confirmed by expert gastrointestinal pathologists. If confirmed, such patients should all enter a close surveillance program at a high-volume specialized Barrett’s center. EET can be offered, as long as the following caveats are understood: (1) Only a small minority will progress; (2) Benefit of RFA seems confined to aggressive RFA protocols performed in expert centers; (3) It appears that in patients under surveillance by expert hands any progression to HGD or cancer can be detected early and completely treated without any adverse consequences; and (4) Adverse events occur following RFA in an estimated 10%, however rarely severe.
After the confirmation of HGD-BE by expert gastrointestinal pathologists, we recommend referral to an expert Barrett’s center with repeat endoscopy within 4 wk of diagnosis. We recommend all visible lesions be treated with EMR initially which provides additional staging information, followed by sequential RFA until eradication of all visible intestinal metaplasia is achieved. HGD-BE without visible lesions should commence treatment with RFA. The risk of lymph node metastasis is negligible in HGD-BE[68], and surgery should not be offered.
We recommend EET for management of intramucosal adenocarcinoma over surgery. While the literature suggests that cancer-free survival may be modestly higher for surgery, EET is far less morbid, recurrences following EET can usually be managed endoscopically, and for persistent failures salvage esophagectomy is not precluded. Where high-risk histological features are present, surgery may be a greater consideration (Table 8).
| Invasive Barrett’s esophagus by stage | Risk of nodal metastases | Recommended management | Risks of intervention | 5-yr disease free survival | 5-yr overall survival |
| Intramucosal adenocarcinoma | 2%–4% | EET | Stricture 6%; Chest pain 5%; Bleeding 1%; Perforation 1% | NA | Estimated 80% |
| Submucosal adenocarcinoma | 14%–41% | Surgery | Mortality 3%; Adverse events up to 62%; Long-term symptoms due to altered upper gut function | Estimated 70% | Estimated 75% |
We recommend surgery as standard therapy for submucosal adenocarcinoma due to high risk of lymph node metastasis. The role of EET is confined to comorbid or elderly patients at high surgical risk, especially where there are low risk histological features.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Xu H, Yalçınkaya İ S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Cameron AJ, Souto EO, Smyrk TC. Small adenocarcinomas of the esophagogastric junction: association with intestinal metaplasia and dysplasia. Am J Gastroenterol. 2002;97:1375-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56607] [Article Influence: 7075.9] [Reference Citation Analysis (134)] |
| 3. | Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64:381-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 944] [Cited by in RCA: 1053] [Article Influence: 95.7] [Reference Citation Analysis (0)] |
| 4. | Edgren G, Adami HO, Weiderpass E, Nyrén O. A global assessment of the oesophageal adenocarcinoma epidemic. Gut. 2013;62:1406-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 269] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 5. | Hur C, Miller M, Kong CY, Dowling EC, Nattinger KJ, Dunn M, Feuer EJ. Trends in esophageal adenocarcinoma incidence and mortality. Cancer. 2013;119:1149-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 400] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 6. | Rubenstein JH, Shaheen NJ. Epidemiology, Diagnosis, and Management of Esophageal Adenocarcinoma. Gastroenterology. 2015;149:302-17.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 271] [Article Influence: 24.6] [Reference Citation Analysis (1)] |
| 7. | Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371:2499-2509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 1032] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 8. | Hagen JA, DeMeester SR, Peters JH, Chandrasoma P, DeMeester TR. Curative resection for esophageal adenocarcinoma: analysis of 100 en bloc esophagectomies. Ann Surg. 2001;234:520-30; discussion 530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 278] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 9. | Sawas T, Killcoyne S, Iyer PG, Wang KK, Smyrk TC, Kisiel JB, Qin Y, Ahlquist DA, Rustgi AK, Costa RJ, Gerstung M, Fitzgerald RC, Katzka DA; OCCAMS Consortium. Identification of Prognostic Phenotypes of Esophageal Adenocarcinoma in 2 Independent Cohorts. Gastroenterology. 2018;155:1720-1728.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | El-Serag HB, Naik AD, Duan Z, Shakhatreh M, Helm A, Pathak A, Hinojosa-Lindsey M, Hou J, Nguyen T, Chen J, Kramer JR. Surveillance endoscopy is associated with improved outcomes of oesophageal adenocarcinoma detected in patients with Barrett's oesophagus. Gut. 2016;65:1252-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 11. | NIH. Cancer Stats Facts: Esophageal Cancer. [cited 1 May 2021]. Available from: https://seer.cancer.gov/statfacts/html/esoph.html. |
| 12. | Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG Clinical Guideline: Diagnosis and Management of Barrett's Esophagus. Am J Gastroenterol. 2016;111:30-50; quiz 51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 990] [Cited by in RCA: 1081] [Article Influence: 108.1] [Reference Citation Analysis (0)] |
| 13. | ASGE Standards of Practice Committee; Qumseya B, Sultan S, Bain P, Jamil L, Jacobson B, Anandasabapathy S, Agrawal D, Buxbaum JL, Fishman DS, Gurudu SR, Jue TL, Kripalani S, Lee JK, Khashab MA, Naveed M, Thosani NC, Yang J, DeWitt J, Wani S; ASGE Standards of Practice Committee Chair. ASGE guideline on screening and surveillance of Barrett's esophagus. Gastrointest Endosc. 2019;90:335-359.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 299] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 14. | Weusten B, Bisschops R, Coron E, Dinis-Ribeiro M, Dumonceau JM, Esteban JM, Hassan C, Pech O, Repici A, Bergman J, di Pietro M. Endoscopic management of Barrett's esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2017;49:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 386] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 15. | Hameeteman W, Tytgat GN, Houthoff HJ, van den Tweel JG. Barrett's esophagus: development of dysplasia and adenocarcinoma. Gastroenterology. 1989;96:1249-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 552] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 16. | Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF, Geboes K, Hattori T, Hirota T, Itabashi M, Iwafuchi M, Iwashita A, Kim YI, Kirchner T, Klimpfinger M, Koike M, Lauwers GY, Lewin KJ, Oberhuber G, Offner F, Price AB, Rubio CA, Shimizu M, Shimoda T, Sipponen P, Solcia E, Stolte M, Watanabe H, Yamabe H. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1463] [Cited by in RCA: 1575] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 17. | Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg. 2017;6:119-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 566] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 18. | Shaheen NJ, Crosby MA, Bozymski EM, Sandler RS. Is there publication bias in the reporting of cancer risk in Barrett's esophagus? Gastroenterology. 2000;119:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 516] [Article Influence: 19.8] [Reference Citation Analysis (1)] |
| 19. | Chang EY, Morris CD, Seltman AK, O'Rourke RW, Chan BK, Hunter JG, Jobe BA. The effect of antireflux surgery on esophageal carcinogenesis in patients with barrett esophagus: a systematic review. Ann Surg. 2007;246:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Thomas T, Abrams KR, De Caestecker JS, Robinson RJ. Meta analysis: Cancer risk in Barrett's oesophagus. Aliment Pharmacol Ther. 2007;26:1465-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Yousef F, Cardwell C, Cantwell MM, Galway K, Johnston BT, Murray L. The incidence of esophageal cancer and high-grade dysplasia in Barrett's esophagus: a systematic review and meta-analysis. Am J Epidemiol. 2008;168:237-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 292] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 22. | Wani S, Puli SR, Shaheen NJ, Westhoff B, Slehria S, Bansal A, Rastogi A, Sayana H, Sharma P. Esophageal adenocarcinoma in Barrett's esophagus after endoscopic ablative therapy: a meta-analysis and systematic review. Am J Gastroenterol. 2009;104:502-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 23. | Sikkema M, de Jonge PJF, Steyerberg EW, Kuipers EJ. Risk of Esophageal Adenocarcinoma and Mortality in Patients With Barrett's Esophagus: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2010;8:235-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 258] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 24. | Curvers WL, ten Kate FJ, Krishnadath KK, Visser M, Elzer B, Baak LC, Bohmer C, Mallant-Hent RC, van Oijen A, Naber AH, Scholten P, Busch OR, Blaauwgeers HG, Meijer GA, Bergman JJ. Low-grade dysplasia in Barrett's esophagus: overdiagnosed and underestimated. Am J Gastroenterol. 2010;105:1523-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 339] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 25. | Conio M, Blanchi S, Lapertosa G, Ferraris R, Sablich R, Marchi S, D'Onofrio V, Lacchin T, Iaquinto G, Missale G, Ravelli P, Cestari R, Benedetti G, Macrì G, Fiocca R, Munizzi F, Filiberti R. Long-term endoscopic surveillance of patients with Barrett's esophagus. Incidence of dysplasia and adenocarcinoma: a prospective study. Am J Gastroenterol. 2003;98:1931-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 159] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 26. | Dulai GS, Shekelle PG, Jensen DM, Spiegel BM, Chen J, Oh D, Kahn KL. Dysplasia and risk of further neoplastic progression in a regional Veterans Administration Barrett's cohort. Am J Gastroenterol. 2005;100:775-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Sharma P, Falk GW, Weston AP, Reker D, Johnston M, Sampliner RE. Dysplasia and cancer in a large multicenter cohort of patients with Barrett's esophagus. Clin Gastroenterol Hepatol. 2006;4:566-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 278] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 28. | Lim CH, Treanor D, Dixon MF, Axon AT. Low-grade dysplasia in Barrett's esophagus has a high risk of progression. Endoscopy. 2007;39:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | de Jonge PJ, van Blankenstein M, Looman CW, Casparie MK, Meijer GA, Kuipers EJ. Risk of malignant progression in patients with Barrett's oesophagus: a Dutch nationwide cohort study. Gut. 2010;59:1030-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 212] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 30. | Bhat S, Coleman HG, Yousef F, Johnston BT, McManus DT, Gavin AT, Murray LJ. Risk of malignant progression in Barrett's esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2011;103:1049-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 529] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 31. | Hvid-Jensen F, Pedersen L, Drewes AM, Sørensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med. 2011;365:1375-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 985] [Cited by in RCA: 1003] [Article Influence: 66.9] [Reference Citation Analysis (1)] |
| 32. | Montgomery E, Goldblum JR, Greenson JK, Haber MM, Lamps LW, Lauwers GY, Lazenby AJ, Lewin DN, Robert ME, Washington K, Zahurak ML, Hart J. Dysplasia as a predictive marker for invasive carcinoma in Barrett esophagus: a follow-up study based on 138 cases from a diagnostic variability study. Hum Pathol. 2001;32:379-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 213] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 33. | Schouten LJ, Steevens J, Huysentruyt CJ, Coffeng CE, Keulemans YC, van Leeuwen FE, Driessen AL, van den Brandt PA. Total cancer incidence and overall mortality are not increased among patients with Barrett's esophagus. Clin Gastroenterol Hepatol. 2011;9:754-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Wani S, Falk G, Hall M, Gaddam S, Wang A, Gupta N, Singh M, Singh V, Chuang KY, Boolchand V, Gavini H, Kuczynski J, Sud P, Reddymasu S, Bansal A, Rastogi A, Mathur SC, Young P, Cash B, Lieberman DA, Sampliner RE, Sharma P. Patients with nondysplastic Barrett's esophagus have low risks for developing dysplasia or esophageal adenocarcinoma. Clin Gastroenterol Hepatol. 2011;9:220-7; quiz e26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 35. | Desai TK, Krishnan K, Samala N, Singh J, Cluley J, Perla S, Howden CW. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett's oesophagus: a meta-analysis. Gut. 2012;61:970-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 427] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 36. | Gaddam S, Singh M, Balasubramanian G, Thota P, Gupta N, Wani S, Higbee AD, Mathur SC, Horwhat JD, Rastogi A, Young PE, Cash BD, Bansal A, Vargo JJ, Falk GW, Lieberman DA, Sampliner RE, Sharma P. Persistence of nondysplastic Barrett's esophagus identifies patients at lower risk for esophageal adenocarcinoma: results from a large multicenter cohort. Gastroenterology. 2013;145:548-53.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 37. | Duits LC, Phoa KN, Curvers WL, Ten Kate FJ, Meijer GA, Seldenrijk CA, Offerhaus GJ, Visser M, Meijer SL, Krishnadath KK, Tijssen JG, Mallant-Hent RC, Bergman JJ. Barrett's oesophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut. 2015;64:700-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 187] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 38. | Pereira AD, Chaves P. Low risk of adenocarcinoma and high-grade dysplasia in patients with non-dysplastic Barrett's esophagus: Results from a cohort from a country with low esophageal adenocarcinoma incidence. United European Gastroenterol J. 2016;4:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Visrodia K, Iyer PG, Schleck CD, Zinsmeister AR, Katzka DA. Yield of Repeat Endoscopy in Barrett's Esophagus with No Dysplasia and Low-Grade Dysplasia: A Population-Based Study. Dig Dis Sci. 2016;61:158-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Krishnamoorthi R, Lewis JT, Krishna M, Crews NJ, Johnson ML, Dierkhising RA, Ginos BF, Wang KK, Wolfsen HC, Fleischer DE, Ramirez FC, Buttar NS, Katzka DA, Iyer PG. Predictors of Progression in Barrett's Esophagus with Low-Grade Dysplasia: Results from a Multicenter Prospective BE Registry. Am J Gastroenterol. 2017;112:867-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 41. | Peters Y, Honing J, Kievit W, Kestens C, Pestman W, Nagtegaal ID, van der Post RS, Siersema PD. Incidence of Progression of Persistent Nondysplastic Barrett's Esophagus to Malignancy. Clin Gastroenterol Hepatol. 2019;17:869-877.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 42. | Shaheen NJ, Sharma P, Overholt BF, Wolfsen HC, Sampliner RE, Wang KK, Galanko JA, Bronner MP, Goldblum JR, Bennett AE, Jobe BA, Eisen GM, Fennerty MB, Hunter JG, Fleischer DE, Sharma VK, Hawes RH, Hoffman BJ, Rothstein RI, Gordon SR, Mashimo H, Chang KJ, Muthusamy VR, Edmundowicz SA, Spechler SJ, Siddiqui AA, Souza RF, Infantolino A, Falk GW, Kimmey MB, Madanick RD, Chak A, Lightdale CJ. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med. 2009;360:2277-2288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1146] [Cited by in RCA: 999] [Article Influence: 58.8] [Reference Citation Analysis (2)] |
| 43. | Jung KW, Talley NJ, Romero Y, Katzka DA, Schleck CD, Zinsmeister AR, Dunagan KT, Lutzke LS, Wu TT, Wang KK, Frederickson M, Geno DM, Locke GR, Prasad GA. Epidemiology and natural history of intestinal metaplasia of the gastroesophageal junction and Barrett's esophagus: a population-based study. Am J Gastroenterol. 2011;106:1447-55; quiz 1456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 155] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 44. | Duits LC, van der Wel MJ, Cotton CC, Phoa KN, Ten Kate FJW, Seldenrijk CA, Offerhaus GJA, Visser M, Meijer SL, Mallant-Hent RC, Krishnadath KK, Pouw RE, Tijssen JGP, Shaheen NJ, Bergman JJGHM. Patients With Barrett's Esophagus and Confirmed Persistent Low-Grade Dysplasia Are at Increased Risk for Progression to Neoplasia. Gastroenterology. 2017;152:993-1001.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 45. | Phoa KN, van Vilsteren FG, Weusten BL, Bisschops R, Schoon EJ, Ragunath K, Fullarton G, Di Pietro M, Ravi N, Visser M, Offerhaus GJ, Seldenrijk CA, Meijer SL, ten Kate FJ, Tijssen JG, Bergman JJ. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA. 2014;311:1209-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 456] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 46. | Barret M, Pioche M, Terris B, Ponchon T, Cholet F, Zerbib F, Chabrun E, Le Rhun M, Coron E, Giovannini M, Caillol F, Laugier R, Jacques J, Legros R, Boustiere C, Rahmi G, Metivier-Cesbron E, Vanbiervliet G, Bauret P, Escourrou J, Branche J, Jilet L, Abdoul H, Kaddour N, Leblanc S, Bensoussan M, Prat F, Chaussade S. Endoscopic radiofrequency ablation or surveillance in patients with Barrett's oesophagus with confirmed low-grade dysplasia: a multicentre randomised trial. Gut. 2021;70:1014-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 47. | Wani S, Falk GW, Post J, Yerian L, Hall M, Wang A, Gupta N, Gaddam S, Singh M, Singh V, Chuang KY, Boolchand V, Gavini H, Kuczynski J, Sud P, Bansal A, Rastogi A, Mathur SC, Young P, Cash B, Goldblum J, Lieberman DA, Sampliner RE, Sharma P. Risk factors for progression of low-grade dysplasia in patients with Barrett's esophagus. Gastroenterology. 2011;141:1179-1186, 1186.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 187] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 48. | Kerkhof M, van Dekken H, Steyerberg EW, Meijer GA, Mulder AH, de Bruïne A, Driessen A, ten Kate FJ, Kusters JG, Kuipers EJ, Siersema PD; CYBAR study group. Grading of dysplasia in Barrett's oesophagus: substantial interobserver variation between general and gastrointestinal pathologists. Histopathology. 2007;50:920-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 191] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 49. | Schnell TG, Sontag SJ, Chejfec G, Aranha G, Metz A, O'Connell S, Seidel UJ, Sonnenberg A. Long-term nonsurgical management of Barrett's esophagus with high-grade dysplasia. Gastroenterology. 2001;120:1607-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 389] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 50. | Weston AP, Sharma P, Topalovski M, Richards R, Cherian R, Dixon A. Long-term follow-up of Barrett's high-grade dysplasia. Am J Gastroenterol. 2000;95:1888-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 174] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 51. | Reid BJ, Levine DS, Longton G, Blount PL, Rabinovitch PS. Predictors of progression to cancer in Barrett's esophagus: baseline histology and flow cytometry identify low- and high-risk patient subsets. Am J Gastroenterol. 2000;95:1669-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 191] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 52. | Overholt BF, Lightdale CJ, Wang KK, Canto MI, Burdick S, Haggitt RC, Bronner MP, Taylor SL, Grace MG, Depot M; International Photodynamic Group for High-Grade Dysplasia in Barrett's Esophagus. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett's esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc. 2005;62:488-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 353] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 53. | Sangle NA, Taylor SL, Emond MJ, Depot M, Overholt BF, Bronner MP. Overdiagnosis of high-grade dysplasia in Barrett's esophagus: a multicenter, international study. Mod Pathol. 2015;28:758-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 54. | Newton AD, Predina JD, Xia L, Roses RE, Karakousis GC, Dempsey DT, Williams NN, Kucharczuk JC, Singhal S. Surgical Management of Early-Stage Esophageal Adenocarcinoma Based on Lymph Node Metastasis Risk. Ann Surg Oncol. 2018;25:318-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 55. | Leers JM, DeMeester SR, Oezcelik A, Klipfel N, Ayazi S, Abate E, Zehetner J, Lipham JC, Chan L, Hagen JA, DeMeester TR. The prevalence of lymph node metastases in patients with T1 esophageal adenocarcinoma a retrospective review of esophagectomy specimens. Ann Surg. 2011;253:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 190] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 56. | Hölscher AH, Bollschweiler E, Schröder W, Metzger R, Gutschow C, Drebber U. Prognostic impact of upper, middle, and lower third mucosal or submucosal infiltration in early esophageal cancer. Ann Surg. 2011;254:802-7; discussion 807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 57. | Lorenz D, Origer J, Pauthner M, Graupe F, Fisseler-Eckhoff A, Stolte M, Pech O, Ell C. Prognostic risk factors of early esophageal adenocarcinomas. Ann Surg. 2014;259:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 58. | Ancona E, Rampado S, Cassaro M, Battaglia G, Ruol A, Castoro C, Portale G, Cavallin F, Rugge M. Prediction of lymph node status in superficial esophageal carcinoma. Ann Surg Oncol. 2008;15:3278-3288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 59. | Pennathur A, Farkas A, Krasinskas AM, Ferson PF, Gooding WE, Gibson MK, Schuchert MJ, Landreneau RJ, Luketich JD. Esophagectomy for T1 esophageal cancer: outcomes in 100 patients and implications for endoscopic therapy. Ann Thorac Surg. 2009;87:1048-1054; discussion 1054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 217] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 60. | Westerterp M, Koppert LB, Buskens CJ, Tilanus HW, ten Kate FJ, Bergman JJ, Siersema PD, van Dekken H, van Lanschot JJ. Outcome of surgical treatment for early adenocarcinoma of the esophagus or gastro-esophageal junction. Virchows Arch. 2005;446:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 225] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 61. | Liu L, Hofstetter WL, Rashid A, Swisher SG, Correa AM, Ajani JA, Hamilton SR, Wu TT. Significance of the depth of tumor invasion and lymph node metastasis in superficially invasive (T1) esophageal adenocarcinoma. Am J Surg Pathol. 2005;29:1079-1085. [PubMed] |
| 62. | van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, Verheul HM, Spillenaar Bilgen EJ, van Dekken H, van der Sangen MJ, Rozema T, Biermann K, Beukema JC, Piet AH, van Rij CM, Reinders JG, Tilanus HW, van der Gaast A; CROSS Group. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3288] [Cited by in RCA: 4243] [Article Influence: 303.1] [Reference Citation Analysis (3)] |
| 63. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, Denlinger CS, Enzinger PC, Fanta P, Farjah F, Gerdes H, Gibson M, Glasgow RE, Hayman JA, Hochwald S, Hofstetter WL, Ilson DH, Jaroszewski D, Johung KL, Keswani RN, Kleinberg LR, Leong S, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Paluri RK, Park H, Perry KA, Pimiento J, Poultsides GA, Roses R, Strong VE, Wiesner G, Willett CG, Wright CD, McMillian NR, Pluchino LA. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:855-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 718] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 64. | Gamboa AM, Kim S, Force SD, Staley CA, Woods KE, Kooby DA, Maithel SK, Luke JA, Shaffer KM, Dacha S, Saba NF, Keilin SA, Cai Q, El-Rayes BF, Chen Z, Willingham FF. Treatment allocation in patients with early-stage esophageal adenocarcinoma: Prevalence and predictors of lymph node involvement. Cancer. 2016;122:2150-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 65. | Ishihara R, Oyama T, Abe S, Takahashi H, Ono H, Fujisaki J, Kaise M, Goda K, Kawada K, Koike T, Takeuchi M, Matsuda R, Hirasawa D, Yamada M, Kodaira J, Tanaka M, Omae M, Matsui A, Kanesaka T, Takahashi A, Hirooka S, Saito M, Tsuji Y, Maeda Y, Yamashita H, Oda I, Tomita Y, Matsunaga T, Terai S, Ozawa S, Kawano T, Seto Y. Risk of metastasis in adenocarcinoma of the esophagus: a multicenter retrospective study in a Japanese population. J Gastroenterol. 2017;52:800-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 66. | Williams VA, Watson TJ, Herbella FA, Gellersen O, Raymond D, Jones C, Peters JH. Esophagectomy for high grade dysplasia is safe, curative, and results in good alimentary outcome. J Gastrointest Surg. 2007;11:1589-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 67. | Buskens CJ, Westerterp M, Lagarde SM, Bergman JJ, ten Kate FJ, van Lanschot JJ. Prediction of appropriateness of local endoscopic treatment for high-grade dysplasia and early adenocarcinoma by EUS and histopathologic features. Gastrointest Endosc. 2004;60:703-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 178] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 68. | Dunbar KB, Spechler SJ. The risk of lymph-node metastases in patients with high-grade dysplasia or intramucosal carcinoma in Barrett's esophagus: a systematic review. Am J Gastroenterol. 2012;107:850-62; quiz 863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 69. | Davison JM, Landau MS, Luketich JD, McGrath KM, Foxwell TJ, Landsittel DP, Gibson MK, Nason KS. A Model Based on Pathologic Features of Superficial Esophageal Adenocarcinoma Complements Clinical Node Staging in Determining Risk of Metastasis to Lymph Nodes. Clin Gastroenterol Hepatol. 2016;14:369-377.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 70. | Lee L, Ronellenfitsch U, Hofstetter WL, Darling G, Gaiser T, Lippert C, Gilbert S, Seely AJ, Mulder DS, Ferri LE. Predicting lymph node metastases in early esophageal adenocarcinoma using a simple scoring system. J Am Coll Surg. 2013;217:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 71. | Gockel I, Domeyer M, Sgourakis GG, Schimanski CC, Moehler M, Kirkpatrick CJ, Lang H, Junginger T, Hansen T. Prediction model of lymph node metastasis in superficial esophageal adenocarcinoma and squamous cell cancer including D2-40 immunostaining. J Surg Oncol. 2009;100:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 72. | Sepesi B, Watson TJ, Zhou D, Polomsky M, Litle VR, Jones CE, Raymond DP, Hu R, Qiu X, Peters JH. Are endoscopic therapies appropriate for superficial submucosal esophageal adenocarcinoma? J Am Coll Surg. 2010;210:418-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 73. | Bollschweiler E, Baldus SE, Schröder W, Prenzel K, Gutschow C, Schneider PM, Hölscher AH. High rate of lymph-node metastasis in submucosal esophageal squamous-cell carcinomas and adenocarcinomas. Endoscopy. 2006;38:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 204] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 74. | Semenkovich TR, Hudson JL, Subramanian M, Mullady DK, Meyers BF, Puri V, Kozower BD. Trends in Treatment of T1N0 Esophageal Cancer. Ann Surg. 2019;270:434-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 75. | Prasad GA, Wu TT, Wigle DA, Buttar NS, Wongkeesong LM, Dunagan KT, Lutzke LS, Borkenhagen LS, Wang KK. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett's esophagus. Gastroenterology. 2009;137:815-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 258] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 76. | Schwameis K, Green KM, Worrell SG, Samaan J, Cooper S, Tatishchev S, Oh DS, Hagen JA, DeMeester SR. Outcome with Primary En-bloc Esophagectomy for Submucosal Esophageal Adenocarcinoma. Ann Surg Oncol. 2017;24:3921-3925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 77. | Otaki F, Ma GK, Krigel A, Dierkhising RA, Lewis JT, Blevins CH, Gopalakrishnan NP, Ravindran A, Johnson ML, Leggett CL, Wigle D, Wang KK, Falk GW, Abrams JA, Nakagawa H, Rustgi AK, Wang TC, Lightdale CJ, Ginsberg GG, Iyer PG. Outcomes of patients with submucosal (T1b) esophageal adenocarcinoma: a multicenter cohort study. Gastrointest Endosc. 2020;92:31-39.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 78. | Schölvinck D, Künzli H, Meijer S, Seldenrijk K, van Berge Henegouwen M, Bergman J, Weusten B. Management of patients with T1b esophageal adenocarcinoma: a retrospective cohort study on patient management and risk of metastatic disease. Surg Endosc. 2016;30:4102-4113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 79. | Gockel I, Sgourakis G, Lyros O, Polotzek U, Schimanski CC, Lang H, Hoppo T, Jobe BA. Risk of lymph node metastasis in submucosal esophageal cancer: a review of surgically resected patients. Expert Rev Gastroenterol Hepatol. 2011;5:371-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 80. | Shimada H, Nabeya Y, Matsubara H, Okazumi S, Shiratori T, Shimizu T, Aoki T, Shuto K, Akutsu Y, Ochiai T. Prediction of lymph node status in patients with superficial esophageal carcinoma: analysis of 160 surgically resected cancers. Am J Surg. 2006;191:250-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 81. | Manner H, Pech O, Heldmann Y, May A, Pohl J, Behrens A, Gossner L, Stolte M, Vieth M, Ell C. Efficacy, safety, and long-term results of endoscopic treatment for early stage adenocarcinoma of the esophagus with low-risk sm1 invasion. Clin Gastroenterol Hepatol. 2013;11:630-5; quiz e45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 82. | Sharma P, Savides TJ, Canto MI, Corley DA, Falk GW, Goldblum JR, Wang KK, Wallace MB, Wolfsen HC; ASGE Technology and Standards of Practice Committee. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on imaging in Barrett's Esophagus. Gastrointest Endosc. 2012;76:252-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 83. | Sharma P, Bergman JJ, Goda K, Kato M, Messmann H, Alsop BR, Gupta N, Vennalaganti P, Hall M, Konda V, Koons A, Penner O, Goldblum JR, Waxman I. Development and Validation of a Classification System to Identify High-Grade Dysplasia and Esophageal Adenocarcinoma in Barrett's Esophagus Using Narrow-Band Imaging. Gastroenterology. 2016;150:591-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 169] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 84. | Levine DS, Haggitt RC, Blount PL, Rabinovitch PS, Rusch VW, Reid BJ. An endoscopic biopsy protocol can differentiate high-grade dysplasia from early adenocarcinoma in Barrett's esophagus. Gastroenterology. 1993;105:40-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 408] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 85. | Moss A, Bourke MJ, Hourigan LF, Gupta S, Williams SJ, Tran K, Swan MP, Hopper AD, Kwan V, Bailey AA. Endoscopic resection for Barrett's high-grade dysplasia and early esophageal adenocarcinoma: an essential staging procedure with long-term therapeutic benefit. Am J Gastroenterol. 2010;105:1276-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 86. | Pouw RE, van Vilsteren FG, Peters FP, Alvarez Herrero L, Ten Kate FJ, Visser M, Schenk BE, Schoon EJ, Peters FT, Houben M, Bisschops R, Weusten BL, Bergman JJ. Randomized trial on endoscopic resection-cap vs multiband mucosectomy for piecemeal endoscopic resection of early Barrett's neoplasia. Gastrointest Endosc. 2011;74:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 87. | May A, Gossner L, Behrens A, Kohnen R, Vieth M, Stolte M, Ell C. A prospective randomized trial of two different endoscopic resection techniques for early stage cancer of the esophagus. Gastrointest Endosc. 2003;58:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 88. | Terheggen G, Horn EM, Vieth M, Gabbert H, Enderle M, Neugebauer A, Schumacher B, Neuhaus H. A randomised trial of endoscopic submucosal dissection vs endoscopic mucosal resection for early Barrett's neoplasia. Gut. 2017;66:783-793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 171] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 89. | Sun F, Yuan P, Chen T, Hu J. Efficacy and complication of endoscopic submucosal dissection for superficial esophageal carcinoma: a systematic review and meta-analysis. J Cardiothorac Surg. 2014;9:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 90. | Fleischer DE, Overholt BF, Sharma VK, Reymunde A, Kimmey MB, Chuttani R, Chang KJ, Muthasamy R, Lightdale CJ, Santiago N, Pleskow DK, Dean PJ, Wang KK. Endoscopic radiofrequency ablation for Barrett's esophagus: 5-year outcomes from a prospective multicenter trial. Endoscopy. 2010;42:781-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 153] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 91. | Shaheen NJ, Overholt BF, Sampliner RE, Wolfsen HC, Wang KK, Fleischer DE, Sharma VK, Eisen GM, Fennerty MB, Hunter JG, Bronner MP, Goldblum JR, Bennett AE, Mashimo H, Rothstein RI, Gordon SR, Edmundowicz SA, Madanick RD, Peery AF, Muthusamy VR, Chang KJ, Kimmey MB, Spechler SJ, Siddiqui AA, Souza RF, Infantolino A, Dumot JA, Falk GW, Galanko JA, Jobe BA, Hawes RH, Hoffman BJ, Sharma P, Chak A, Lightdale CJ. Durability of radiofrequency ablation in Barrett's esophagus with dysplasia. Gastroenterology. 2011;141:460-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 371] [Cited by in RCA: 336] [Article Influence: 22.4] [Reference Citation Analysis (1)] |
| 92. | Bulsiewicz WJ, Kim HP, Dellon ES, Cotton CC, Pasricha S, Madanick RD, Spacek MB, Bream SE, Chen X, Orlando RC, Shaheen NJ. Safety and efficacy of endoscopic mucosal therapy with radiofrequency ablation for patients with neoplastic Barrett's esophagus. Clin Gastroenterol Hepatol. 2013;11:636-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 93. | Qumseya BJ, Wani S, Desai M, Qumseya A, Bain P, Sharma P, Wolfsen H. Adverse Events After Radiofrequency Ablation in Patients With Barrett's Esophagus: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1086-1095.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 94. | Bollschweiler E, Schröder W, Hölscher AH, Siewert JR. Preoperative risk analysis in patients with adenocarcinoma or squamous cell carcinoma of the oesophagus. Br J Surg. 2000;87:1106-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 86] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 95. | Pech O, Bollschweiler E, Manner H, Leers J, Ell C, Hölscher AH. Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett's esophagus at two high-volume centers. Ann Surg. 2011;254:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 216] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 96. | Zehetner J, DeMeester SR, Hagen JA, Ayazi S, Augustin F, Lipham JC, DeMeester TR. Endoscopic resection and ablation vs esophagectomy for high-grade dysplasia and intramucosal adenocarcinoma. J Thorac Cardiovasc Surg. 2011;141:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 97. | Rice TW, Blackstone EH, Goldblum JR, DeCamp MM, Murthy SC, Falk GW, Ormsby AH, Rybicki LA, Richter JE, Adelstein DJ. Superficial adenocarcinoma of the esophagus. J Thorac Cardiovasc Surg. 2001;122:1077-1090. [RCA] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 115] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 98. | Gurusamy KS, Pallari E, Midya S, Mughal M. Laparoscopic vs open transhiatal oesophagectomy for oesophageal cancer. Cochrane Database Syst Rev. 2016;3:CD011390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 99. | Blazeby JM, Farndon JR, Donovan J, Alderson D. A prospective longitudinal study examining the quality of life of patients with esophageal carcinoma. Cancer. 2000;88:1781-1787. [PubMed] |
| 100. | Qumseya BJ, Wani S, Gendy S, Harnke B, Bergman JJ, Wolfsen H. Disease Progression in Barrett's Low-Grade Dysplasia With Radiofrequency Ablation Compared With Surveillance: Systematic Review and Meta-Analysis. Am J Gastroenterol. 2017;112:849-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 101. | Pouw RE, Klaver E, Phoa KN, van Vilsteren FG, Weusten BL, Bisschops R, Schoon EJ, Pech O, Manner H, Ragunath K, Fernández-Sordo JO, Fullarton G, Di Pietro M, Januszewicz W, O'Toole D, Bergman JJ. Radiofrequency ablation for low-grade dysplasia in Barrett's esophagus: long-term outcome of a randomized trial. Gastrointest Endosc. 2020;92:569-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 102. | Ganz RA, Overholt BF, Sharma VK, Fleischer DE, Shaheen NJ, Lightdale CJ, Freeman SR, Pruitt RE, Urayama SM, Gress F, Pavey DA, Branch MS, Savides TJ, Chang KJ, Muthusamy VR, Bohorfoush AG, Pace SC, DeMeester SR, Eysselein VE, Panjehpour M, Triadafilopoulos G; U. S. Multicenter Registry. Circumferential ablation of Barrett's esophagus that contains high-grade dysplasia: a U.S. Multicenter Registry. Gastrointest Endosc. 2008;68:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 175] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 103. | Okoro NI, Tomizawa Y, Dunagan KT, Lutzke LS, Wang KK, Prasad GA. Safety of prior endoscopic mucosal resection in patients receiving radiofrequency ablation of Barrett's esophagus. Clin Gastroenterol Hepatol. 2012;10:150-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 104. | Haidry RJ, Butt MA, Dunn JM, Gupta A, Lipman G, Smart HL, Bhandari P, Smith L, Willert R, Fullarton G, Di Pietro M, Gordon C, Penman I, Barr H, Patel P, Kapoor N, Hoare J, Narayanasamy R, Ang Y, Veitch A, Ragunath K, Novelli M, Lovat LB; UK RFA Registry. Improvement over time in outcomes for patients undergoing endoscopic therapy for Barrett's oesophagus-related neoplasia: 6-year experience from the first 500 patients treated in the UK patient registry. Gut. 2015;64:1192-1199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 105. | Li N, Pasricha S, Bulsiewicz WJ, Pruitt RE, Komanduri S, Wolfsen HC, Chmielewski GW, Corbett FS, Chang KJ, Shaheen NJ. Effects of preceding endoscopic mucosal resection on the efficacy and safety of radiofrequency ablation for treatment of Barrett's esophagus: results from the United States Radiofrequency Ablation Registry. Dis Esophagus. 2016;29:537-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 106. | Reed MF, Tolis G Jr, Edil BH, Allan JS, Donahue DM, Gaissert HA, Moncure AC, Wain JC, Wright CD, Mathisen DJ. Surgical treatment of esophageal high-grade dysplasia. Ann Thorac Surg. 2005;79:1110-5; discussion 1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 107. | Prasad GA, Wang KK, Buttar NS, Wongkeesong LM, Krishnadath KK, Nichols FC 3rd, Lutzke LS, Borkenhagen LS. Long-term survival following endoscopic and surgical treatment of high-grade dysplasia in Barrett's esophagus. Gastroenterology. 2007;132:1226-1233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 190] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 108. | Heitmiller RF, Redmond M, Hamilton SR. Barrett's esophagus with high-grade dysplasia. An indication for prophylactic esophagectomy. Ann Surg. 1996;224:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 213] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 109. | Wang VS, Hornick JL, Sepulveda JA, Mauer R, Poneros JM. Low prevalence of submucosal invasive carcinoma at esophagectomy for high-grade dysplasia or intramucosal adenocarcinoma in Barrett's esophagus: a 20-year experience. Gastrointest Endosc. 2009;69:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 110. | Edwards MJ, Gable DR, Lentsch AB, Richardson JD. The rationale for esophagectomy as the optimal therapy for Barrett's esophagus with high-grade dysplasia. Ann Surg. 1996;223:585-9; discussion 589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 131] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 111. | Ell C, May A, Pech O, Gossner L, Guenter E, Behrens A, Nachbar L, Huijsmans J, Vieth M, Stolte M. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett's cancer). Gastrointest Endosc. 2007;65:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 344] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 112. | Pech O, Behrens A, May A, Nachbar L, Gossner L, Rabenstein T, Manner H, Guenter E, Huijsmans J, Vieth M, Stolte M, Ell C. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett's oesophagus. Gut. 2008;57:1200-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 466] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 113. | Pouw RE, Gondrie JJ, Sondermeijer CM, ten Kate FJ, van Gulik TM, Krishnadath KK, Fockens P, Weusten BL, Bergman JJ. Eradication of Barrett esophagus with early neoplasia by radiofrequency ablation, with or without endoscopic resection. J Gastrointest Surg. 2008;12:1627-36; discussion 1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 114. | Pouw RE, Wirths K, Eisendrath P, Sondermeijer CM, Ten Kate FJ, Fockens P, Devière J, Neuhaus H, Bergman JJ. Efficacy of radiofrequency ablation combined with endoscopic resection for barrett's esophagus with early neoplasia. Clin Gastroenterol Hepatol. 2010;8:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 211] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 115. | van Vilsteren FG, Pouw RE, Seewald S, Alvarez Herrero L, Sondermeijer CM, Visser M, Ten Kate FJ, Yu Kim Teng KC, Soehendra N, Rösch T, Weusten BL, Bergman JJ. Stepwise radical endoscopic resection vs radiofrequency ablation for Barrett's oesophagus with high-grade dysplasia or early cancer: a multicentre randomised trial. Gut. 2011;60:765-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (1)] |
| 116. | Saligram S, Chennat J, Hu H, Davison JM, Fasanella KE, McGrath K. Endotherapy for superficial adenocarcinoma of the esophagus: an American experience. Gastrointest Endosc. 2013;77:872-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 117. | Pech O, May A, Manner H, Behrens A, Pohl J, Weferling M, Hartmann U, Manner N, Huijsmans J, Gossner L, Rabenstein T, Vieth M, Stolte M, Ell C. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology. 2014;146:652-660.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 323] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 118. | Agoston AT, Strauss AC, Dulai PS, Hagen CE, Muzikansky A, Fudman DI, Abrams JA, Forcione DG, Jajoo K, Saltzman JR, Odze RD, Lauwers GY, Gordon SR, Lightdale CJ, Rothstein RI, Srivastava A. Predictors Of Treatment Failure After Radiofrequency Ablation For Intramucosal Adenocarcinoma in Barrett Esophagus: A Multi-institutional Retrospective Cohort Study. Am J Surg Pathol. 2016;40:554-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 119. | Phoa KN, Pouw RE, Bisschops R, Pech O, Ragunath K, Weusten BL, Schumacher B, Rembacken B, Meining A, Messmann H, Schoon EJ, Gossner L, Mannath J, Seldenrijk CA, Visser M, Lerut T, Seewald S, ten Kate FJ, Ell C, Neuhaus H, Bergman JJ. Multimodality endoscopic eradication for neoplastic Barrett oesophagus: results of an European multicentre study (EURO-II). Gut. 2016;65:555-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 120. | Ngamruengphong S, Wolfsen HC, Wallace MB. Survival of patients with superficial esophageal adenocarcinoma after endoscopic treatment vs surgery. Clin Gastroenterol Hepatol. 2013;11:1424-1429.e2; quiz e81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 121. | Marino KA, Sullivan JL, Weksler B. Esophagectomy vs endoscopic resection for patients with early-stage esophageal adenocarcinoma: A National Cancer Database propensity-matched study. J Thorac Cardiovasc Surg. 2018;155:2211-2218.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 122. | Künzli HT, Belghazi K, Pouw RE, Meijer SL, Seldenrijk CA, Weusten B, Bergman J. Endoscopic management and follow-up of patients with a submucosal esophageal adenocarcinoma. United European Gastroenterol J. 2018;6:669-677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |