Published online Oct 15, 2022. doi: 10.4251/wjgo.v14.i10.1903
Peer-review started: June 26, 2022
First decision: July 25, 2022
Revised: August 5, 2022
Accepted: September 8, 2022
Article in press: September 8, 2022
Published online: October 15, 2022
Processing time: 110 Days and 6.3 Hours
Currently, 15 randomized controlled trials (RCTs) have been designed to investigate whether neoadjuvant therapy (NAT) benefits patients with resectable pancreatic adenocarcinoma (R-PA) compared to surgery alone. Five of them have acquired results so far; however, corresponding conclusions have not been obtained. We speculated that the reason for this phenomenon could be that some prognostic factors had proven to be adverse through upfront surgery curative patterns, but some of them were not regarded as independent baseline characteristics, which is important to obtaining comparability between the NAT and upfront surgery groups. This fact could cause bias and lead to the difference in the outcomes of RCTs. In this review, we collate data about risk factors (such as tumor size, resection margin, and lymph node status) influencing the prognoses of patients with R-PA from five RCTs and discuss the possible reasons for the varying outcomes.
Core Tip: In this review, we collate data about risk factors influencing the prognoses of patients with resectable pancreatic cancer from five randomized controlled trials (RCTs) and discuss the possible reasons for the varying outcomes. By comparing the overall survival of two RCTs, we speculated that neoadjuvant therapy might actually benefit patients with low-risk factors for long-term survival. Moreover, we address some suggestions for the RCTs in the future.
- Citation: Zhang HQ, Li J, Tan CL, Chen YH, Zheng ZJ, Liu XB. Neoadjuvant therapy in resectable pancreatic cancer: A promising curative method to improve prognosis. World J Gastrointest Oncol 2022; 14(10): 1903-1917
- URL: https://www.wjgnet.com/1948-5204/full/v14/i10/1903.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i10.1903
Pancreatic cancer (PC) is a malignancy with a very high mortality and poor prognosis. Patients usually die in a short time due to the progression of the tumor. To moderate progression, curative-intent surgery is necessary. Unfortunately, not all PC patients are eligible for resection, and only approximately 15% to 20% of patients have resectable PC[1]. To improve survival outcomes, adjuvant chemo
The conclusions have been inconsistent after analyzing the outcomes of all of the current randomized controlled trials (RCTs); for example, the results from the phase II FRENCH SFRO-FFCD 97-04 trial showed that NACRT is feasible for patients with R-PA[5], but in another previous meta-analysis, NACRT could not be recommended for patients with R-PA[6]. Some prognostic factors have been proved to be adverse through upfront surgery curative patterns, but some of them have not been regarded as independent baseline characteristics, which is important for obtaining comparability between the NAT and upfront surgery groups. This fact could cause bias and lead to the differences in the outcomes of RCTs. Moreover, the NAT regimens are out of date in some research, which could also lead to negative outcomes.
This review focuses on the deficiencies of and data from current high-quality RCTs and provides suggestions for future RCTs.
The RCTs on NAT in resectable PC included in this review are listed in Table 1. There are ten other registered RCTs comparing NAT and upfront surgery for R-PA patients: One is not yet recruiting, three are recruiting, two are active but not recruiting, two are terminated, and two are completed. Information about these RCTs is listed in Table 2.
| Ref. | Country | NCT number/Name | Treatment arm | N | mOS (mo) | pN0 rate | Resection rate | R0 rate | ITT R0 rate |
| Golcher et al[7] | Germany | NCT00335543 | Upfront surgery | 33 | 14.4 | 10/33 (30%) | 23/33 (70%) | 16/23 (70%) | 16/33 (48%) |
| GEM/CIS + RT | 33 | 17.4 | 13/33 (39%) | 19/33 (58%) | 17/19 (89%) | 17/33 (52%) | |||
| Versteijne et al[8] | Netherlands | PREOPANC | Upfront surgery | 68 | 14.6 | NM | 54/68 (79%) | 32/54 (59%) | 32/68 (47%) |
| GEM + RT | 65 | 15.6 | NM | 44/65 (68%) | 29/44 (66%) | 29/65 (45%) | |||
| Casadei et al[9] | Italy | - | Upfront surgery | 20 | 19.5 | 2/20 (10%) | 15/20 (75%) | 5/15 (33%) | 5/20 (25%) |
| GEM + RT | 18 | 22.4 | 5/18 (28%) | 11/18(61%) | 7/11 (64%) | 7/18 (39%) | |||
| Reni et al[10] | Italy | PACT-15 | Upfront surgery (GEM) | 26 | 20.4 | 6/22 (27%) | 22/26 (84%) | 6/22 (27%) | 6/26 (23%) |
| Upfront surgery (PEXG) | 30 | 26.4 | 7/27 (26%) | 27/30 (90%) | 10/27 (37%) | 10/30 (33%) | |||
| PEXG | 32 | 38.2 | 13/27 (48%) | 27/32 (84%) | 17/27 (63%) | 17/32 (53%) | |||
| Satoi et al[11] | Japan | Prep-02/JSAP-05 | Upfront surgery | 180 | 26.3 | NM | 72% | NM | NM |
| GEM + S-1 | 182 | 36.7 | NM | 77% | NM | NM |
| Trial name | NCT number | Phase | Status | Estimated enrollment | Treatment arm | Primary endpoint | Study start date | Estimated primary completion date | Estimated study completion date |
| ICI20-00047 | NCT05181605 | II/III | Not yet recruiting | 116 | FOLFIRINOX and SBRT with surgery | OS | January 2022 | January 2023 | December 2024 |
| Upfront surgery | |||||||||
| CISPD-1 | NCT03750669 | II | Recruiting | 416 | nPt/GEM and mFOLFIRINOX with surgery | DFS | October 2018 | October 2023 | October 2024 |
| Upfront surgery | |||||||||
| A021806 | NCT04340141 | III | Recruiting | 352 | mFOLFIRINOX with surgery | OS | July 2020 | January 2026 | November 2030 |
| Upfront surgery | |||||||||
| PREOPANC | NCT04927780 | III | Recruiting | 378 | mFOLFIRINOX with surgery | OS | September 2021 | February 2026 | July 2029 |
| Upfront surgery | |||||||||
| AIO-PAK-0313 | NCT02047513 | II | Active, not recruiting | 1271 | nPt/GEM with surgery | DFS | July 2015 | April 20212 | October 20223 |
| Upfront surgery | |||||||||
| NorPACT-1 | NCT02919787 | II/III | Active, not recruiting | 140 | FOLFIRINOX with surgery | 1-yr OS | September 2016 | October 2022 | April 2026 |
| Upfront surgery | |||||||||
| NEOPAC | NCT01314027 | III | Terminated | 381 | GEM/oxaliplatin with surgery | PFS | September 2009 | December 20182 | May 20193 |
| Upfront surgery | |||||||||
| NEOPA | NCT01900327 | III | Terminated | 321 | GEM and EBRT with surgery | 3-yr survival rate | February 2014 | November 20162 | July 20173 |
| Upfront surgery | |||||||||
| NEOPAC/IPC 2011-002 | NCT01521702 | III | Completed | 21 | GEM/Oxaliplatin with surgery | PFS | December 2011 | February 20152 | February 20153 |
| Upfront surgery | |||||||||
| NEPAFOX | NCT02172976 | II/III | Completed | 401 | FOLFIRINOX with surgery | mOS | November 2014 | January 20202 | May 20203 |
| Upfront surgery |
Five RCTs are introduced in detail as follows. The first is a German trial (NCT00335543), which was completed in 2013[7]. This study was the first prospective, randomized, phase II trial. A total of 254 patients were initially enrolled, but only 66 were actually enrolled until the trial was completed. Thirty-three patients were allocated to the NAT group, and another 33 were allocated to the upfront surgery group. The NAT regimen was gemcitabine and cisplatin plus radiotherapy. However, resectability was defined as no organ infiltration except the duodenum and maximal involvement of the peripancreatic vessels ≤ 180° confirmed by high-resolution CT. Thus, some patients who should have been regarded as BR-PA cases according to the latest NCCN guidelines were deemed to be R-PA cases.
The second is a Dutch randomized, phase III PREOPANC trial[8]. Notably, this trial included both R-PA and BR-PA patients, and only data about R-PA are discussed in this review. The largest difference in this study compared to three other trials was that it excluded T1 resectable tumors (< 2 cm, without vascular involvement). The planned number of subjects to be included was 244, but 246 eligible patients were ultimately enrolled, and 133 were eligible for resection. After random assignment, 68 R-PA patients were allocated to the upfront surgery group and another 65 to the NAT group. The NAT regimen was gemcitabine plus radiotherapy. Nevertheless, the resectability was also different from the latest NCCN guidelines. In this research, a tumor without arterial involvement and with venous involvement < 90° was considered resectable, and a tumor with arterial involvement < 90° and/or venous involvement between 90° and 270° without occlusion was considered borderline resectable. This categorization meant that some patients who should have been regarded as R-PA cases (venous involvement between 90° and 180°) were classified as BR-PA cases.
The third is an Italian trial that required 32 patients per group, but only 38 were eventually enrolled overall[9]. The protocol or the registration number of this trial was not found. Twenty patients were assigned to the upfront surgery group, and 18 were assigned to the NAT group. The regimen was gemcitabine plus radiotherapy. Resectability corresponded to the latest NCCN guidelines.
The fourth is a phase II Italian trial (PACT-15)[10]. The enrollment was also less than expected; only 98 eligible patients were included, while it was estimated that 1040 patients would be enrolled. Finally, only 88 patients were randomly assigned to three groups: 26 in Group A received postoperative adjuvant intravenous gemcitabine, 30 in Group B received postoperative adjuvant PEXG (intravenous cisplatin, epirubicin, gemcitabine, and oral capecitabine), and 32 in Group C received preoperative and postoperative PEXG. The resectability criteria were stricter than the latest NCCN guidelines; only tumors without contact with arteries or veins were regarded as resectable, so some R-PA cases (with venous invasion < 180° without vein contour irregularities) was deemed BR-PA.
The last is a Japanese phase III trial that has not been published but was reported at the 2019 ASCO annual meeting[11]. A total of 360 patients were needed, and a total of 362 eligible patients were randomly allocated to the NAT group (n = 182) or the upfront surgery group (n = 180). The NAT regimen was gemcitabine plus S-1. Patients with PV involvement and without arterial involvement were deemed resectable in this research; furthermore, those with PV involvement > 180° were regarded as BR-PA cases according to the latest NCCN guidelines. However, details such as baseline characteristics or the proportion of patients with BR-PA were not posted. Thus, the outcomes might not be authoritative.
Data about the median OS (mOS) of these RCTs are listed in Table 3. In the NCT00335543 trial[7], the mOS was 14.4 mo in the surgery group compared to 17.4 mo in the NAT group. In the Dutch trial[8], the mOS was comparable between the NAT group (15.6 mo) and the surgery group (14.6 mo). The outcome was similar in another RCT, in which the mOS was 19.5 and 22.4 mo in the upfront surgery group and the NAT group, respectively[9]. In the PACT-15 trial[10], the mOS in the NAT group (38.2 mo) was better than that in the upfront surgery groups (20.4 and 26.4 mo, respectively). However, data from the Prep-02/JSAP-05 study were inspiring; the mOS was 36.7 mo in the NAT group compared to 26.6 mo in the upfront surgery group (P = 0.015)[11].
| Ref. | Country | NCT number/Name | Variable | Upfront surgery | NAT plus surgery | P value |
| Golcher et al[7] | Germany | NCT00335543 | mOS; mo | 14.4 | 17.4 | 0.96 |
| Versteijne et al[8] | Netherlands | PREOPANC | mOS; mo | 14.6 | 15.6 | 0.83 |
| Casadei et al[9] | Italy | - | mOS; mo | 19.5 | 22.4 | 0.97 |
| Reni et al[10] | Italy | PACT-15 | mOS; mo | 20.4,26.4 | 38.2 | NM |
| Satoi et al[11] | Japan | Prep-02/JSAP-05 | mOS; mo | 26.6 | 36.7 | 0.015 |
The reasons for the different outcomes of these RCTs could include the following. First, three RCTs (the NCT00335543 trial, the Italian single-center trial, and the PACT-15 trial) obtained smaller enrollment than initially expected, and they terminated early due to the poor recruitment rates. The poor sample sizes could have caused bias and led to inaccurate outcomes.
Second, the five RCTs adopted different resectability criteria. Referring to the latest NCCN guidelines, some patients actually with BR-PA cases (tumor contact with peripancreatic artery of ≤ 180°) were regarded as R-PA cases in the NCT00335543 trial and the Prep-02/JSAP-05 study, and some patients actually with R-PA cases (no arterial involvement and venous involvement ≤ 180°) were deemed to be BR-PA cases in the Dutch trial and the PACT-15 trial. This difference might have influenced the outcomes and caused the differences in the results of these RCTs.
Third, the regimens for NAT were also different. Although the regimens of these five RCTs were based on gemcitabine, two adopted gemcitabine alone, while the other three used gemcitabine combined with cisplatin or other agents. In addition, all of these RCTs combined preoperative radiotherapy with chemotherapy except the PACT-15 trial and the Prep-02/JSAP-05 study. However, the regimens (based on FOLFIRINOX or modified FOLFIRINOX or gemcitabine plus albumin-bound paclitaxel) that were recommended by the NCCN guidelines were not adopted in these RCTs. The German trial and the PACT-15 trial adopted regimens of gemcitabine and cisplatin, which are recommended for patients with known BRCA1/2 or PALB2 mutations, but these mutations were not mentioned in these two trials. The different efficiencies of these regimens could be another factor leading to various outcomes.
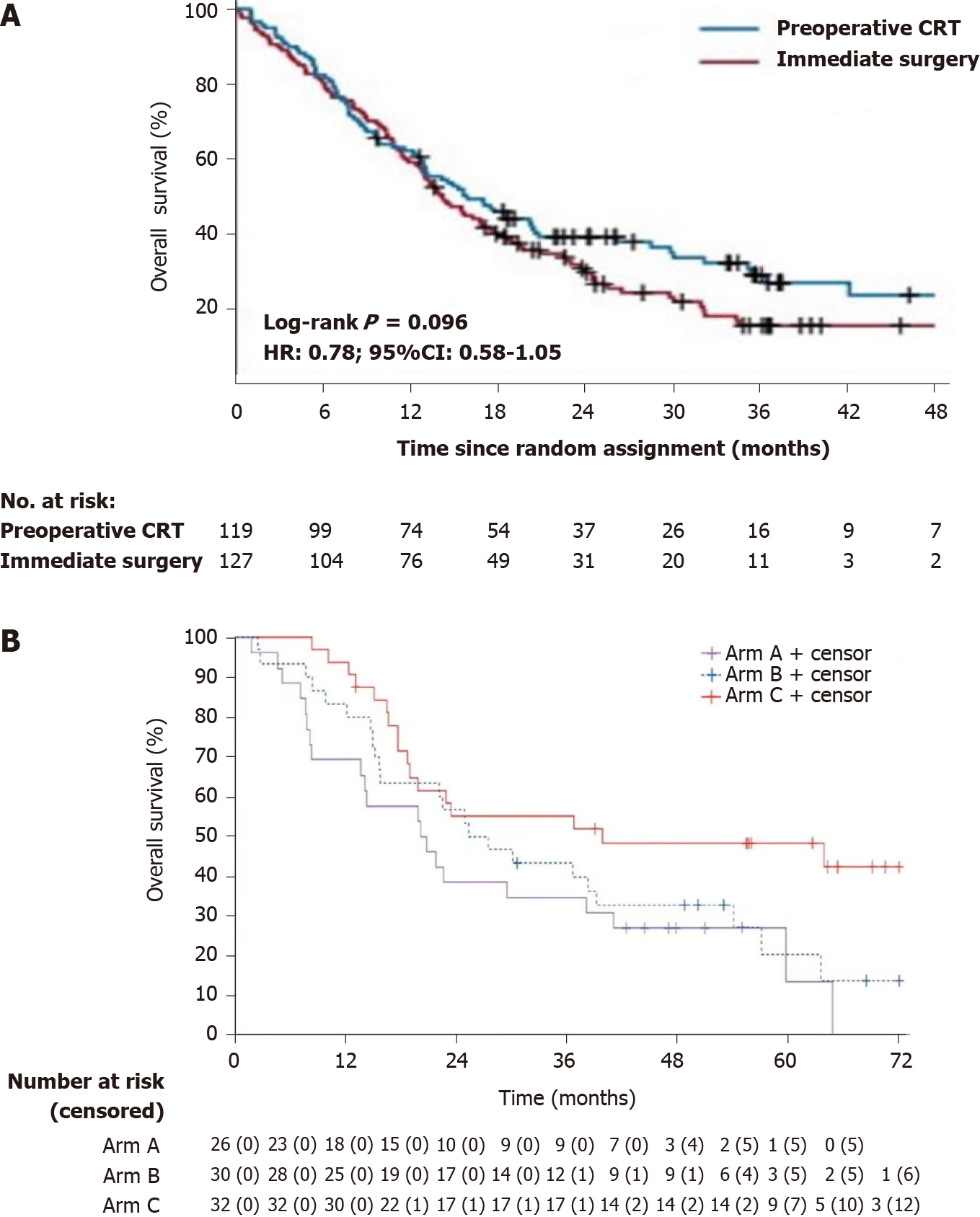
NAT might actually benefit patients with low-risk factors for long-term survival. According to the OS of the Dutch trial (Figure 1A), the 1-, 2-, 3-, and 4-year OS rates were comparable between the NAT group and the upfront surgery group[8]. The latest results of the PREOPANC trial showed that the hazard ratio (HR) of OS was 0.79 for patients with R-PA (P = 0.23)[12]. The reason for this outcome might be that this trial excluded T1 tumors (2 cm, without vascular involvement). Because patients with high-risk factors always die due to recurrence within 2 years, almost the only patients who were in the long-term follow-up were those with low-risk factors. Thus, when patients with low-risk factors were excluded, the benefit of NAT on long-term OS disappeared.
In the PACT-15 trial[10], the 1- and 2-year OS rates were similar between the NAT group and the upfront surgery group (Figure 1B). However, the NAT group showed better 3-year OS (55%) and 5-year OS (49%) than the surgery alone group (3-year OS: 35% and 43%; 5-year OS: 13% and 24%). The reason for this difference could be that T1 tumors were included. Tumors with low-risk factors could benefit from NAT and obtain better 3- and 5-year OS.
According to a recent meta-analysis, the NAT groups showed superior 1-, 2-, 3-, 4-, and 5-year survival rates compared to the upfront surgery group[13].
The lack of postoperative complications was an important prognostic factor of survival[14-16]. Lubrano et al[17] analyzed 942 patients with PDAC and found that the mOS and DFS decreased statistically when severe postoperative complications occurred[17]; the reason for this result could be that postoperative complications lead to failure of adjuvant therapy.
Data about postoperative complications are shown in Table 4.
| Ref. | Country | NCT number/Name | Variable | Upfront surgery | NAT plus surgery | P value |
| Golcher et al[7] | Germany | NCT00335543 | Clavien-Dindo I-II complications | 17/33 (52%) | 16/33 (48%) | NM |
| Clavien-Dindo III-V complications | 15/33 (45%) | 6/33 (18%) | NM | |||
| Versteijne et al[8] | Netherlands | PREOPANC | Postoperative complications | NM | NM | NM |
| Casadei et al[9] | Italy | - | Post-treatment morbidity | 9/20 (45%) | 10/18 (56%) | 0.746 |
| Reni et al[10] | Italy | PACT-15 | Minor complications (Clavien-Dindo I–II) | 21/49 (43%) | 13/27 (48%) | NM |
| Major complications (Clavien-Dindo III–IV) | 10/49 (20%) | 3/27 (11%) | NM |
In the German trial (NCT00335543)[7], postoperative complications were graded according to the Clavien-Dindo classification. In arm A (surgery alone), 17 patients suffered from grade I-II postoperative complications, and 15 patients suffered from grade III-V postoperative complications; in arm B (NACRT), 16 patients suffered from grade I-II postoperative complications, and 6 suffered from grade III-V postoperative complications.
Casadei et al[9] found that life-threatening complications were less frequent in the NAT group than in the surgery alone group. This could be attributed to pancreatic fibrosis as a result of NAT, which decreased the risk of pancreatic anastomotic leakage.
In the PACT-15 trial[10], minor complications (Clavien-Dindo I-II) in Group C (NACT) were observed in 13 of 27 (48%) patients, compared to 9 of 22 (41%) and 12 of 27 (44%) in Groups A and B, respectively. Major complications (Clavien-Dindo III-IV) in Group C were observed in 3 of 27 (11%) patients, compared to 5 of 22 (23%) and 5 of 27 (19%) in Groups A and B, respectively[10].
No significant difference in postoperative morbidity was found between the two groups in the Prep-02/JSAP-05 study[11].
Postoperative complications occurred in 31 of 63 (49%) patients in the Japanese trial[18], which seemed comparable to those in the RCTs.
No significant difference was observed in the comparison of postoperative complications, indicating that NAT did not increase the risk of postoperative complications. Moreover, Golcher et al[7] and Casadei et al[9] agreed that NAT could decrease severe complications by inducing fibrosis[7,9].
Another three studies supported that the probability of postoperative complications occurring in patients who received NAT was not different from that in patients who underwent upfront surgery[19-21]. One study found that patients with R-PA who underwent NAT obtained a lower rate of postoperative complications than those who did not (30% vs 63%, P = 0.001)[4].
In a recent retrospective study, Nipp et al[22] found that the absence of adjuvant therapy was correlated with a high risk of early mortality[22]. Additional adjuvant therapy was linked to a reduction in mortality[23]. The results from a randomized, phase III trial attributed prolonged DFS to adjuvant therapy[24].
In the NCT00335543 trial[7], 10 of 23 (43%) patients in the surgery alone group underwent adjuvant chemotherapy, which was comparable to 7 of 19 (37%) patients in the NAT group.
Although there are no data for the R-PA subgroup regarding the difference in this factor between the two groups in the Dutch trial, data based on R-PA plus BR-PA patients showed no significant difference[8].
According to the PACT-15 trial[10], one patient among 18 in Group A (postoperative gemcitabine) was unable to receive adjuvant gemcitabine because his clinical condition did not completely recover after surgery. Moreover, ten patients did not complete the whole adjuvant gemcitabine treatment. Factors were similar in Groups B (postoperative adjuvant PEXG) and C (preoperative and postoperative adjuvant PEXG), and some patients did not complete the adjuvant therapy. Thus, preoperative chemoradiotherapy appears to be particularly essential.
Regardless of whether we consider the surgery alone group or the NAT group, some patients lost the opportunity for adjuvant therapy, which is essential for controlling recurrence. Considering this point, NAT is necessary to guarantee that more patients receive perioperative adjuvant therapy.
One study indicated that patients with R-PA who received NACRT had fewer chances of undergoing postoperative adjuvant therapy than those who merely underwent surgery[25]. Compared to NACRT patients, NACT patients were more likely to receive adjuvant chemotherapy after resection (41.3% vs 24.7%, P < 0.001)[26].
Some studies have explored whether operative duration correlates with the OS of patients with R-PA. Garcea et al[27] found that operative duration is not associated with survival[27]. In contrast, the results from another study showed that a short operative duration improved long-term survival by reducing the presence of hypoalbuminemia at 1 mo postoperatively[28]. However, overall, it was believed that the increased operative time was associated with a poorer long-term survival[29].
Only the Prep-02/JSAP-05 study showed no significant difference in operation time between the two groups, while no other RCTs focused on this factor.
Two retrospective studies indicated that there was a significant difference between NAT and upfront surgery in patients deemed to have resectable PDAC[19,20]. Patients who received NAT had a longer operative time than those who underwent upfront surgery.
There have been limited studies of the relationship between nutritional status and survival of R-PA patients. A recent retrospective study indicated that the controlling nutritional status score, which is used to evaluate immune-nutritional status, had an independent correlation with survival in resected patients with PDAC[30]. No significant difference was shown between weight loss from the time of diagnosis and survival after resection[31]. Ueda et al[28] regarded hypoalbuminemia occurring within 1 mo after surgery as a predictor of survival in PDAC patients[28].
RCTs are needed to explore the correlation between nutritional status and NAT.
According to a retrospective study[21], the prognostic nutritional index (PNI), which was used to assess nutritional status, decreased in patients with R-PA from 48.2 ± 7.1 to 42.7 ± 6.0 (P < 0.0001) after NACRT. Poor preoperative PNI is a predictor of poor survival in PDAC patients.
In summary, NAT is safe and feasible for patients with R-PA. First, NAT did not increase postoperative complications, and it might even decrease severe complications by inducing pancreatic fibrosis. Furthermore, comparable postoperative morbidity indicates that NAT does not limit adjuvant therapy; in contrast, it provides systemic therapy for those who are ineligible for adjuvant therapy, mostly due to postoperative complications. Moreover, NAT did not prolong the operation time.
Tumor size is important for the assessment of prognosis. According to the 8th AJCC TNM staging of PC, tumors ≤ 2 cm in diameter are defined as T1, tumors > 2 cm and ≤ 4 cm are defined as T2, and tumors > 4 cm are classified as T3 when there is no arterial invasion. Phoa et al[32] found an HR of 1.375 [95% confidence interval (CI): 1.267-1.492] for the T1 stage compared with the T2 stage and 1.796 (95%CI: 1.629-1.982) with the T3 stage, indicating a significant prognostic advantage for smaller tumor sizes (P < 0.001). Survival was longest for patients with a tumor diameter < 2 cm, and poor survival was observed in patients with resected tumors > 3 cm in diameter[32]. In addition, tumor size was associated with an increased risk of lymph node metastases and influenced the rate of margin positivity following resection[33]. NAT can reduce tumor size[25,34].
Tumor size was decreased in patients who underwent NAT according to the data from three RCTs, and there was a statistically significant advantage for NAT in one study (Table 5).
| Ref. | Country | NCT number/Name | Variable | Upfront surgery | NAT plus surgery | P value |
| Golcher et al[7] | Germany | NCT00335543 | Tumoral diameter (cm) | NM | NM | NM |
| T1-2 stage | 2 | 4 | NM | |||
| T3-4 stage | 21 | 15 | NM | |||
| Versteijne et al[8] | Netherlands | PREOPANC | Tumoral diameter (cm) | NM | NM | NM |
| T1-2 stage | NM | NM | NM | |||
| T3-4 stage | NM | NM | NM | |||
| Casadei et al[9] | Italy | - | Tumoral diameter (cm) | NM | NM | NM |
| T1-2 stage | 0 | 9 | 0.016 | |||
| T3-4 stage | 20 | 8 | ||||
| Reni et al[10] | Italy | PACT-15 | Tumoral diameter (cm) | 2.1-2.5 | 2 | NM |
| T1-2 stage | 3 | 5 | NM | |||
| T3-4 stage | 46 | 22 | NM |
The results of the NCT00335543 trial showed that pT1-2 was more frequently observed in arm B (NACRT + surgery) than in those who underwent surgery only (arm A)[7]. Four of 19 (21%) patients in arm B were pT1-2 cases, while only 2 of 23 (9%) patients in arm A were at this stage.
The same conclusion was obtained in another RCT[9]. Pathologic T staging was significantly different between arm A (surgery alone) and arm B (NACRT plus surgery) (P = 0.016). Nine patients had pT1-2 stage disease in arm B, while none belonged to this stage in arm A.
In the PACT-15 trial, the tumor size in Group C (NACT) after resection was 2.0 cm, compared to 2.1 cm and 2.5 cm in Groups B and A, respectively[10].
In the Japanese phase II trial[18], the median tumor size before treatment was 24 mm, compared to 19 mm after receiving GSRT (gemcitabine and S-1 concurrently with radiation therapy).
A retrospective review showed that patients who underwent NACRT obtained smaller tumors in resection specimens (mean 2.5 cm) than those who underwent surgical exploration first (mean 3.1 cm, P = 0.04), although they had larger tumors prior to treatment (mean 2.5 cm vs 2.1 cm)[25].
NAT can reduce the tumor size and thus make it easier to obtain R0 resection. The anterior and posterior surfaces render it difficult to obtain R0 resection in large tumors.
According to the NCCN guidelines, resection margins are classified as R0 when the tumor is completely and microscopically removed within 1 mm. R1 denotes microscopic residual tumor, and R2 indicates macroscopic residual tumor. Most studies have supported that R1 resection leads to a worse outcome than R0 resection[29,34-36]. Positive margins can lead to local recurrence. The margins of the Whipple specimen include the SMA, portal vein, pancreatic neck, bile duct, and proximal (gastric or enteric) and distal enteric margins. The most important margin is the SMA margin, which consists of the soft tissue that connects the uncinate process to the right lateral border of the proximal 3–4 cm of the SMA.
The data about resection margins status are listed in Table 6.
| Ref. | Country | NCT number/Name | Variable | Upfront surgery | NAT plus surgery | P value |
| Golcher et al[7] | Germany | NCT00335543 | Resection rate | 23/33 (70%) | 19/33 (58%) | 0.31 |
| R0 rate | 16/23 (70%) | 17/19 (89%) | NM | |||
| ITT R0 rate | 16/33 (48%) | 17/33 (52%) | 0.81 | |||
| Versteijne et al[8] | Netherlands | PREOPANC | Resection rate | 54/68 (79%) | 44/65 (68%) | 0.17 |
| R0 rate | 32/54 (59%) | 29/44 (66%) | 0.54 | |||
| ITT R0 rate | 32/68 (47%) | 29/65 (45%) | NM | |||
| Casadei et al[9] | Italy | - | Resection rate | 15/20 (75%) | 11/18 (61%) | 0.489 |
| R0 rate | 5/15 (33%) | 7/11 (64%) | NM | |||
| ITT R0 rate | 5/20 (25%) | 7/18 (39%) | 0.489 | |||
| Reni et al[10] | Italy | PACT-15 | Resection rate | 49/56 (88%) | 27/32 (84%) | NM |
| R0 rate | 16/49 (33%) | 17/27 (63%) | NM | |||
| ITT R0 rate | 16/56 (29%) | 17/32 (53%) | NM |
In the NCT00335543 trial, 23 of 33 (70%) patients in arm A (upfront surgery group) underwent resection vs 19 of 33 (58%) in arm B (NAT group). R0 resection was obtained in 16 of 23 (70%) patients in arm A vs 17 of 19 (89%) in arm B. The intention-to-treat (ITT) R0 rate was 48% (16/33) in arm A compared to 52% (17/33) in arm B[7]. The time to progression was comparable between the two groups.
In the Dutch trial, 44 patients in the NACRT group (44/65, 68%) had successful resection, and 54 in the immediate surgery group (54/68, 79%) achieved resection[8]. The R0 resection rate was 66% (29 of 44) in patients who received NACRT vs 59% (32 of 54) in patients assigned to immediate surgery. The ITT R0 rate was 45% (29/65) in the NACRT group compared to 47% (32/68) in the immediate surgery group. The R0 rate increased in the NACRT group, while the resection rate decreased compared to the immediate surgery group. The locoregional failure-free interval (LFFI) was similar in the two groups.
In the Italian trial[9], R0 resection was defined as all PDAC with a clearance > 1 mm from each margin. R0 resection criteria were not mentioned in the other RCTs. Fifteen of 20 (75%) patients in arm A (upfront surgery group) underwent resection compared to 11 of 18 (61%) in arm B (NAT group). The R0 rate was 33% (5/15) in arm A vs 64% (7/11) in arm B. The ITT R0 resection rate was higher in arm B (7/18, 39%) than in arm A (5/20, 25%). No data about local recurrence were mentioned.
In the PACT-15 trial[10], the resection rates were 84% (22/26), 90% (27/30), and 84% (27/32) in Groups A (adjuvant gemcitabine), B (adjuvant PEXG), and C (neoadjuvant PEXG), respectively. The R0 resection rates were 27% (6/22), 37% (10/27), and 63% (17/27) in Groups A, B, and C, respectively. The ITT R0 resection rates were 23% (6/26), 33% (10/30), and 53% (17/32) in Groups A, B, and C, respectively. The rates of local failure were comparable between the NAT group and the two upfront surgery groups.
In the Prep-02/JSAP-05 study, no significant difference was observed in the R0 resection rate between the two groups[11].
In the Japanese trial[18], R0 resection was defined as the absence of cancer cells at the cut end of any specimen slice. Fifty-four of 63 patients underwent surgery, and all 54 patients who underwent resection achieved R0 resection.
An observational study showed that patients with R-PA who underwent NACRT obtained fewer positive microscopic resection margins than those who underwent upfront surgery (14% vs 30%; P = 0.042)[19].
Although the data from these five RCTs seem to indicate that the resection rates were lower in the NAT groups, and R0 resection rates were higher compared to the upfront surgery groups, there was no significant difference.
In the Prep-02/JSAP-05 study[11], a significant decrease in viable tumor cells was observed in primary tumors after NAT compared to upfront surgery (P < 0.01). Fewer viable tumor cells might be helpful in promoting response and limiting progression.
Although smaller tumors made it easier to obtain R0 resections, the margin status was also influenced by other factors. The SMA margin made it difficult to obtain R0 resection in tumors with vascular invasion, although they were small in size. NAT could reduce the tumor size and thus benefit the margin status, but it might be meaningless for improving the biological behavior of tumors (invasion of vessels, high differentiation grade, and so on); thus, R0 resection was not approved in some trials. Moreover, patients with BR-PA were deemed R-PA cases in the German trial, and the Prep-02/JSAP-05 study also influenced the rate of R0 resection. In addition, assessments of R0 were performed after surgery rather than after completing the whole treatment, and the benefit of systemic treatment could only be detected after all treatments were completed.
Lymph node metastasis is regarded as a factor for poor prognosis congruously, and lymph node status has been proved to be one of the most effective prognostic factors in some recent studies. Furthermore, the ratio of positive nodes to total examined nodes is more valuable for prognosis than merely evaluating the number of positive lymph nodes[29,35]. Elshaer et al[37] found that most studies indicated that lymph node ratio and number of positive nodes, but not total nodes examined, correlated with OS in PDAC[37]. Recently, Shi et al[38] proposed a modification of the 8th AJCC staging system for PDAC[38]. Compared to the 8th AJCC staging system, it weakens the influences of positive lymph nodes, and small tumors with positive nodes are divided into earlier stages.
Most studies have shown that lymph node positivity is less frequently observed in patients with R-PA who received NAT than in those who underwent surgery only[19-23,25,34,39]. Data about lymph node status are listed in Table 7. In the German trial, there were 22 of 33 (67%) patients with clinical N0 in the NAT group compared to 30 of 33 (91%) in the primary surgery group before treatment (P = 0.03), but a higher (y)pN0 rate was observed in the NAT group (39%, 13/33) than in the primary surgery group (30%, 10/33) after treatment[7]. The preoperative clinical N0 rate was not comparable, which might have caused the negativity of the difference in pN0. The rate of (y)pM0 was comparable. The Italian single-center RCT also indicated that the NAT group obtained a higher pN0 rate (28%, 5/18) than the surgery group (10%, 2/20)[9]. The rate of pM0 was also comparable in this research. In addition, in the PACT-15 trial, the pN0 rates were 27% (6/22), 26% (7/27), and 48% (13/27) in patients who underwent resection in Groups A (adjuvant intravenous gemcitabine), B (adjuvant PEXG), and C (preoperative and postoperative PEXG), respectively[10]. However, the number of patients with liver metastasis in the NAT group decreased by half compared to that in the upfront surgery groups (64%, 42%, and 33% in Groups A, B, and C, respectively). No data about this factor in the NACRT subgroup were mentioned in the Dutch trial[8]. However, the median distant metastasis-free intervals were similar between the two groups.
No significant difference was found in the data from the above four RCTs, indicating that NAT did not improve the lymph node status. However, in the Prep-02/JSAP-05 study, resected patients in the NAT group obtained a significantly decreased nodal positive rate compared with the upfront surgery group (P < 0.01)[11]. This finding could indicate that patients who completed NAT and resection obtained better nodal status. The reason for this phenomenon could be that some patients were ineligible for surgery due to progression during NAT, and node positivity was observed more frequently in these patients, leading to a nonsignificant difference in ITT analysis between the two groups. Regarding the subgroup analysis of resected patients, a significant decrease was observed in the NAT group. Moreover, hepatic recurrence after surgery was significantly decreased in the NAT group (30.0%) compared to the upfront surgery group (47.5%).
In the Japanese trial[18], 37 of 63 (59%) patients were in the N0 stage.
A randomized, phase II trial indicated that R-PA patients who received NACT with gemcitabine plus cisplatin exhibited less node positivity than those who received NACT with gemcitabine[40]. In another study[26], postoperative pathology showed that R-PA patients who received NACRT prior to surgery had a higher rate of node-negative pathology than those who received NACT (68.0% vs 42.7%, P < 0.001), indicating that neoadjuvant external beam radiation is beneficial for reducing the rates of node-positive pathology.
Tumors with poor biological behavior often invade vessels or nerves.
Direct invasion of the venous vasculature indicates distant spread and nodal or hematogenous metastasis, while perineural spread is associated with local recurrence[41]. At the same time, severe venous invasion leads to a poor prognosis[29].
The Dutch trial and the PACT-15 trial considered this factor, and they enrolled patients with less venous invasion (< 90° and no venous contact, respectively). The Italian trial specifically regarded the grade of superior mesenteric and portal vein involvement as independent baseline characteristics. However, most RCTs did not research this factor, and only the Dutch trial focused on it[8]. Perineural invasion was less frequent in patients treated with NACRT (39% vs 73%; P < 0.001), as was venous invasion (19% vs 36%; P = 0.024). Based on the latest results from the Dutch trial, vascular invasion was less frequently observed in the NAT group (36% vs 65%; P < 0.001)[12]. Nevertheless, the results were based on patients with R-PA and BR-PA, and there were no specific data about the venous or perineural invasion in the R-PA subgroup.
Barbier et al[34] found that there were fewer vascular and perineural invasions in PDAC patients who underwent NACRT than in those who underwent surgery first (P < 0.001)[34]. The same conclusion was shown in two other studies[19,22].
Tumors with elevated CA19-9 levels could indicate poor biological behavior and subclinical metastasis.
A national cancer database study enrolled 28074 PDAC patients with reported CA19-9[42]. Among early-stage (I/II) patients (n = 10806), there were 957 (8.8%) nonsecretors, 2708 (25.1%) with normal levels, and 7141 (66.1%) with elevated levels (CA19-9 > 37 U/mL). Survival was disappointing in patients with elevated CA-19-9, regardless of stage. Early-stage patients with elevated CA19-9 had worse survival at 1, 2, and 3 years than patients with normal levels. Nonsecretors and patients with normal levels had similar survival. High preoperative CA19-9 levels are regarded as a predictor of local and early (defined as relapse < 6 mo after resection) recurrence[25,43]. However, compared to preoperative CA19-9 levels, postoperative CA19-9 levels have more prognostic value because they can reflect the quality of tumor resection[44].
In the PACT-15 trial[10], the CA19-9 response was evaluated in 22 of 23 (96%) patients in the NACRT group. One patient obtained a decrease of more than 89%, 12 had decreases of 50% to 89%, and 9 had decreases less than 50%. In several previous studies, the median decrease in CA19-9 was 26%. The results indicated that NAT could decrease CA19-9 levels, but more authoritative evidence is needed to prove this deduction.
In the Japanese trial, median CA19-9 level decreased from 261 to 61 U/mL after patients received GSRT[18].
PDAC patients with CA19-9 levels that decreased during NAT had a better OS[45]. A phase I trial and a phase II trial demonstrated that PDAC patients had decreased median CA19-9 levels after achieving NAT[46,47].
The majority of studies have supported that there is a statistically significant correlation between well-differentiated tumors and improved prognosis. The degree of differentiation is inversely associated with tumor aggressiveness[29]. High-grade tumors usually demonstrate poor biological behavior.
The latest results of the PREOPANC trial showed that tumor differentiation was similar between the NAT group and the upfront surgery group (P = 0.91). The data were based on patients with R-PA and BR-PA, and there were no data about the R-PA subgroup.
Improved nodal status accompanied by less hepatic metastasis was shown in the PACT-15 trial and Prep-02/JSAP-05 study, but the other RCTs did not obtain significant differences. The probable reason for this finding is that, although NAT can improve nodal status, it does not influence the biological behavior of tumors. In fact, NAT could benefit patients by controlling micrometastasis and allowing for systemic therapy.
The primary factors influencing the outcomes were as follows. First, the actual sample sizes were less than expected in some trials. In addition, the criteria for resectability were different in the studies. Moreover, the regimens varied in the different RCTs. Finally, how NAT influences the biological behavior of PC is still unknown, and no research has regarded it as an independent baseline characteristic to assign. NAT might not be useful for improving it, and poor biological behavior would offset the benefits from NAT (such as improving R0 resection and nodal status), leading to negative outcomes.
NAT is feasible and safe because it does not increase postoperative complications or prolong the operation time, and some experts have supported that it decreases the occurrence of severe post
Some other advantages of NAT are as follows: (1) NAT might treat micrometastases and prevent local recurrence. However, in the Dutch trial, although the median LFFI was higher in the NAT group (17 mo) than in the immediate surgery group (13.5 mo), there was no significant difference (P = 0.067)[8]. In the PACT-15 trial[10], the local and distant recurrence rates were comparable between the NAT group and the surgery group. However, liver metastases were found less frequently in the NAT group (33%) than in the two adjuvant groups (64% and 42%, respectively); and (2) NAT provides sufficient time to expose metastases related to the progression of micrometastases that could not be detected previously and screens for patients who cannot benefit from surgery. Casadei et al[9] found that distant micrometastases had already occurred in some patients but were not detected. With the development of the diseases, they were found during NAT and avoided surgery, which was meaningless. Nevertheless, progression would occur if the NAT regimens were not effective. Currently, there is no RCT design for exploring these deductions, and more RCTs are needed to verify them in the future.
In general, the benefit of NAT outweighs the disadvantages. However, the majority of studies about NAT for R-PA patients have been retrospective; although there have been several prospective studies, their sample sizes have been too limited to obtain a conclusive outcome. In addition, more phase III trials and RCTs are needed to verify the conclusions.
It is essential to select patients who could mostly benefit from NAT. Some novel predictive factors in R-PA were addressed recently, such as molecular profiles, tumor microenvironments, immune cell infiltration, microRNAs, circulating tumor DNA, organoids, and the gut microbiome[48]. In the future, the RCT study design should likely be refined in patients with three types of resectable PC. The first group should include patients without risk factors for poor prognosis. The second category should include patients with a small tumor diameter who are prone to distant metastasis, as suggested by imaging or tumor biology-related examinations. The third group should include patients who could not easily obtain radical treatment of R0 disease by imaging evaluation.
| 1. | Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1481] [Cited by in RCA: 1557] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 2. | Golcher H, Brunner T, Grabenbauer G, Merkel S, Papadopoulos T, Hohenberger W, Meyer T. Preoperative chemoradiation in adenocarcinoma of the pancreas. A single centre experience advocating a new treatment strategy. Eur J Surg Oncol. 2008;34:756-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Murakami Y, Uemura K, Sudo T, Hashimoto Y, Kondo N, Nakagawa N, Takahashi S, Sueda T. Survival impact of neoadjuvant gemcitabine plus S-1 chemotherapy for patients with borderline resectable pancreatic carcinoma with arterial contact. Cancer Chemother Pharmacol. 2017;79:37-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Sho M, Akahori T, Tanaka T, Kinoshita S, Nagai M, Tamamoto T, Ohbayashi C, Hasegawa M, Kichikawa K, Nakajima Y. Importance of resectability status in neoadjuvant treatment for pancreatic cancer. J Hepatobiliary Pancreat Sci. 2015;22:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Mornex F, Girard N, Scoazec JY, Bossard N, Ychou M, Smith D, Seitz JF, Valette PJ, Roy P, Rouanet P, Ducreux M, Partensky C. Feasibility of preoperative combined radiation therapy and chemotherapy with 5-fluorouracil and cisplatin in potentially resectable pancreatic adenocarcinoma: The French SFRO-FFCD 97-04 Phase II trial. Int J Radiat Oncol Biol Phys. 2006;65:1471-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1243] [Cited by in RCA: 1193] [Article Influence: 74.6] [Reference Citation Analysis (3)] |
| 7. | Golcher H, Brunner TB, Witzigmann H, Marti L, Bechstein WO, Bruns C, Jungnickel H, Schreiber S, Grabenbauer GG, Meyer T, Merkel S, Fietkau R, Hohenberger W. Neoadjuvant chemoradiation therapy with gemcitabine/cisplatin and surgery versus immediate surgery in resectable pancreatic cancer: results of the first prospective randomized phase II trial. Strahlenther Onkol. 2015;191:7-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 250] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 8. | Versteijne E, Suker M, Groothuis K, Akkermans-Vogelaar JM, Besselink MG, Bonsing BA, Buijsen J, Busch OR, Creemers GM, van Dam RM, Eskens FALM, Festen S, de Groot JWB, Groot Koerkamp B, de Hingh IH, Homs MYV, van Hooft JE, Kerver ED, Luelmo SAC, Neelis KJ, Nuyttens J, Paardekooper GMRM, Patijn GA, van der Sangen MJC, de Vos-Geelen J, Wilmink JW, Zwinderman AH, Punt CJ, van Eijck CH, van Tienhoven G; Dutch Pancreatic Cancer Group. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J Clin Oncol. 2020;38:1763-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 788] [Article Influence: 131.3] [Reference Citation Analysis (0)] |
| 9. | Casadei R, Di Marco M, Ricci C, Santini D, Serra C, Calculli L, D'Ambra M, Guido A, Morselli-Labate AM, Minni F. Neoadjuvant Chemoradiotherapy and Surgery Versus Surgery Alone in Resectable Pancreatic Cancer: A Single-Center Prospective, Randomized, Controlled Trial Which Failed to Achieve Accrual Targets. J Gastrointest Surg. 2015;19:1802-1812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 10. | Reni M, Balzano G, Zanon S, Zerbi A, Rimassa L, Castoldi R, Pinelli D, Mosconi S, Doglioni C, Chiaravalli M, Pircher C, Arcidiacono PG, Torri V, Maggiora P, Ceraulo D, Falconi M, Gianni L. Safety and efficacy of preoperative or postoperative chemotherapy for resectable pancreatic adenocarcinoma (PACT-15): a randomised, open-label, phase 2-3 trial. Lancet Gastroenterol Hepatol. 2018;3:413-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 195] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 11. | Satoi S, Unno M, Motoi F, Matsuyama Y, Matsumoto I, Aosasa S, Shirakawa H, Wada K, Fujii T, Yoshitomi H, Takahashi S, Sho M, Ueno H, Yamamoto T, Kosuge T. The effect of neoadjuvant chemotherapy with gemcitabine and S-1 for resectable pancreatic cancer (randomized phase II/III trial; Prep-02/JSAP-05). J Clin Oncol. 2019;37:2. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Versteijne E, van Dam JL, Suker M, Janssen QP, Groothuis K, Akkermans-Vogelaar JM, Besselink MG, Bonsing BA, Buijsen J, Busch OR, Creemers GM, van Dam RM, Eskens FALM, Festen S, de Groot JWB, Groot Koerkamp B, de Hingh IH, Homs MYV, van Hooft JE, Kerver ED, Luelmo SAC, Neelis KJ, Nuyttens J, Paardekooper GMRM, Patijn GA, van der Sangen MJC, de Vos-Geelen J, Wilmink JW, Zwinderman AH, Punt CJ, van Tienhoven G, van Eijck CHJ; Dutch Pancreatic Cancer Group. Neoadjuvant Chemoradiotherapy Versus Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Long-Term Results of the Dutch Randomized PREOPANC Trial. J Clin Oncol. 2022;40:1220-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 506] [Article Influence: 126.5] [Reference Citation Analysis (1)] |
| 13. | Bradley A, Van Der Meer R. Upfront Surgery versus Neoadjuvant Therapy for Resectable Pancreatic Cancer: Systematic Review and Bayesian Network Meta-analysis. Sci Rep. 2019;9:4354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Evans DB, Varadhachary GR, Crane CH, Sun CC, Lee JE, Pisters PW, Vauthey JN, Wang H, Cleary KR, Staerkel GA, Charnsangavej C, Lano EA, Ho L, Lenzi R, Abbruzzese JL, Wolff RA. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496-3502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 552] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 15. | Howard TJ, Krug JE, Yu J, Zyromski NJ, Schmidt CM, Jacobson LE, Madura JA, Wiebke EA, Lillemoe KD. A margin-negative R0 resection accomplished with minimal postoperative complications is the surgeon's contribution to long-term survival in pancreatic cancer. J Gastrointest Surg. 2006;10:1338-45; discussion 1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 246] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 16. | Tzeng CW, Tran Cao HS, Lee JE, Pisters PW, Varadhachary GR, Wolff RA, Abbruzzese JL, Crane CH, Evans DB, Wang H, Abbott DE, Vauthey JN, Aloia TA, Fleming JB, Katz MH. Treatment sequencing for resectable pancreatic cancer: influence of early metastases and surgical complications on multimodality therapy completion and survival. J Gastrointest Surg. 2014;18:16-24; discussion 24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 17. | Lubrano J, Bachelier P, Paye F, Le Treut YP, Chiche L, Sa-Cunha A, Turrini O, Menahem B, Launoy G, Delpero JR. Severe postoperative complications decrease overall and disease free survival in pancreatic ductal adenocarcinoma after pancreaticoduodenectomy. Eur J Surg Oncol. 2018;44:1078-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Eguchi H, Takeda Y, Takahashi H, Nakahira S, Kashiwazaki M, Shimizu J, Sakai D, Isohashi F, Nagano H, Mori M, Doki Y. A Prospective, Open-Label, Multicenter Phase 2 Trial of Neoadjuvant Therapy Using Full-Dose Gemcitabine and S-1 Concurrent with Radiation for Resectable Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol. 2019;26:4498-4505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Fujii T, Satoi S, Yamada S, Murotani K, Yanagimoto H, Takami H, Yamamoto T, Kanda M, Yamaki S, Hirooka S, Kon M, Kodera Y. Clinical benefits of neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreatic head: an observational study using inverse probability of treatment weighting. J Gastroenterol. 2017;52:81-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Motoi F, Unno M, Takahashi H, Okada T, Wada K, Sho M, Nagano H, Matsumoto I, Satoi S, Murakami Y, Kishiwada M, Honda G, Kinoshita H, Baba H, Hishinuma S, Kitago M, Tajima H, Shinchi H, Takamori H, Kosuge T, Yamaue H, Takada T. Influence of preoperative anti-cancer therapy on resectability and perioperative outcomes in patients with pancreatic cancer: project study by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2014;21:148-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Sho M, Akahori T, Tanaka T, Kinoshita S, Tamamoto T, Nomi T, Yamato I, Hokuto D, Yasuda S, Kawaguchi C, Nishiofuku H, Marugami N, Enomonoto Y, Kasai T, Hasegawa M, Kichikawa K, Nakajima Y. Pathological and clinical impact of neoadjuvant chemoradiotherapy using full-dose gemcitabine and concurrent radiation for resectable pancreatic cancer. J Hepatobiliary Pancreat Sci. 2013;20:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Nipp RD, Zanconato A, Zheng H, Ferrone CR, Lillemoe KD, Wo JY, Hong TS, Clark JW, Ryan DP, Fernández-Del Castillo C. Predictors of Early Mortality After Surgical Resection of Pancreatic Adenocarcinoma in the Era of Neoadjuvant Treatment. Pancreas. 2017;46:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Mokdad AA, Minter RM, Zhu H, Augustine MM, Porembka MR, Wang SC, Yopp AC, Mansour JC, Choti MA, Polanco PM. Neoadjuvant Therapy Followed by Resection Versus Upfront Resection for Resectable Pancreatic Cancer: A Propensity Score Matched Analysis. J Clin Oncol. 2017;35:515-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 316] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 24. | Ueno H, Kosuge T, Matsuyama Y, Yamamoto J, Nakao A, Egawa S, Doi R, Monden M, Hatori T, Tanaka M, Shimada M, Kanemitsu K. A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br J Cancer. 2009;101:908-915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 348] [Cited by in RCA: 327] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 25. | Papalezova KT, Tyler DS, Blazer DG 3rd, Clary BM, Czito BG, Hurwitz HI, Uronis HE, Pappas TN, Willett CG, White RR. Does preoperative therapy optimize outcomes in patients with resectable pancreatic cancer? J Surg Oncol. 2012;106:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | Lutfi W, Talamonti MS, Kantor O, Wang CH, Stocker SJ, Bentrem DJ, Roggin KK, Winchester DJ, Marsh R, Prinz RA, Baker MS. Neoadjuvant external beam radiation is associated with No benefit in overall survival for early stage pancreatic cancer. Am J Surg. 2017;213:521-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Garcea G, Dennison AR, Ong SL, Pattenden CJ, Neal CP, Sutton CD, Mann CD, Berry DP. Tumour characteristics predictive of survival following resection for ductal adenocarcinoma of the head of pancreas. Eur J Surg Oncol. 2007;33:892-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Ueda M, Endo I, Nakashima M, Minami Y, Takeda K, Matsuo K, Nagano Y, Tanaka K, Ichikawa Y, Togo S, Kunisaki C, Shimada H. Prognostic factors after resection of pancreatic cancer. World J Surg. 2009;33:104-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Neuzillet C, Sauvanet A, Hammel P. Prognostic factors for resectable pancreatic adenocarcinoma. J Visc Surg. 2011;148:e232-e243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Kato Y, Yamada S, Suenaga M, Takami H, Niwa Y, Hayashi M, Iwata N, Kanda M, Tanaka C, Nakayama G, Koike M, Fujiwara M, Kodera Y. Impact of the Controlling Nutritional Status Score on the Prognosis After Curative Resection of Pancreatic Ductal Adenocarcinoma. Pancreas. 2018;47:823-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | Perini MV, Montagnini AL, Jukemura J, Penteado S, Abdo EE, Patzina R, Cecconello I, Cunha JE. Clinical and pathologic prognostic factors for curative resection for pancreatic cancer. HPB (Oxford). 2008;10:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Phoa SS, Tilleman EH, van Delden OM, Bossuyt PM, Gouma DJ, Laméris JS. Value of CT criteria in predicting survival in patients with potentially resectable pancreatic head carcinoma. J Surg Oncol. 2005;91:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Midha S, Chawla S, Garg PK. Modifiable and non-modifiable risk factors for pancreatic cancer: A review. Cancer Lett. 2016;381:269-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 197] [Article Influence: 19.7] [Reference Citation Analysis (3)] |
| 34. | Barbier L, Turrini O, Grégoire E, Viret F, Le Treut YP, Delpero JR. Pancreatic head resectable adenocarcinoma: preoperative chemoradiation improves local control but does not affect survival. HPB (Oxford). 2011;13:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 35. | Åkerberg D, Ansari D, Andersson R. Re-evaluation of classical prognostic factors in resectable ductal adenocarcinoma of the pancreas. World J Gastroenterol. 2016;22:6424-6433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Butler JR, Ahmad SA, Katz MH, Cioffi JL, Zyromski NJ. A systematic review of the role of periadventitial dissection of the superior mesenteric artery in affecting margin status after pancreatoduodenectomy for pancreatic adenocarcinoma. HPB (Oxford). 2016;18:305-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 37. | Elshaer M, Gravante G, Kosmin M, Riaz A, Al-Bahrani A. A systematic review of the prognostic value of lymph node ratio, number of positive nodes and total nodes examined in pancreatic ductal adenocarcinoma. Ann R Coll Surg Engl. 2017;99:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 38. | Shi S, Hua J, Liang C, Meng Q, Liang D, Xu J, Ni Q, Yu X. Proposed Modification of the 8th Edition of the AJCC Staging System for Pancreatic Ductal Adenocarcinoma. Ann Surg. 2019;269:944-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 39. | Moutardier V, Turrini O, Huiart L, Viret F, Giovannini MH, Magnin V, Lelong B, Bories E, Guiramand J, Sannini A, Giovannini M, Houvenaeghel G, Blache JL, Moutardier JC, Delpero JR. A reappraisal of preoperative chemoradiation for localized pancreatic head ductal adenocarcinoma in a 5-year single-institution experience. J Gastrointest Surg. 2004;8:502-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Palmer DH, Stocken DD, Hewitt H, Markham CE, Hassan AB, Johnson PJ, Buckels JA, Bramhall SR. A randomized phase 2 trial of neoadjuvant chemotherapy in resectable pancreatic cancer: gemcitabine alone versus gemcitabine combined with cisplatin. Ann Surg Oncol. 2007;14:2088-2096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 163] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 41. | Garcea G, Dennison AR, Pattenden CJ, Neal CP, Sutton CD, Berry DP. Survival following curative resection for pancreatic ductal adenocarcinoma. A systematic review of the literature. JOP. 2008;9:99-132. [PubMed] |
| 42. | Bichoo RA, Jha CK, Mishra A. Letter to editor in response to the article entitled "Successful Completion of the Pilot Phase of a Randomized Controlled Trial Comparing Sentinel Lymph Node Biopsy to No Further Axillary Staging in Patients with Clinical T1-T2 N0 Breast Cancer and Normal Axillary Ultrasound" by Cyr AE et al. in J Am Coll Surg 2016; 223 (2): 399-407. Am J Surg. 2017;214:979-980. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 43. | Osayi SN, Bloomston M, Schmidt CM, Ellison EC, Muscarella P. Biomarkers as predictors of recurrence following curative resection for pancreatic ductal adenocarcinoma: a review. Biomed Res Int. 2014;2014:468959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Ferrone CR, Finkelstein DM, Thayer SP, Muzikansky A, Fernandez-delCastillo C, Warshaw AL. Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol. 2006;24:2897-2902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 423] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 45. | Lim KH, Chung E, Khan A, Cao D, Linehan D, Ben-Josef E, Wang-Gillam A. Neoadjuvant therapy of pancreatic cancer: the emerging paradigm? Oncologist. 2012;17:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 46. | Tajima H, Kitagawa H, Tsukada T, Nakanuma S, Okamoto K, Sakai S, Makino I, Furukawa H, Nakamura K, Hayashi H, Oyama K, Inokuchi M, Nakagawara H, Miyashita T, Fujita H, Itoh H, Takamura H, Ninomiya I, Fushida S, Fujimura T, Ohta T. A phase I study of neoadjuvant chemotherapy with gemcitabine plus oral S-1 for resectable pancreatic cancer. Mol Clin Oncol. 2013;1:768-772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Talamonti MS, Small W Jr, Mulcahy MF, Wayne JD, Attaluri V, Colletti LM, Zalupski MM, Hoffman JP, Freedman GM, Kinsella TJ, Philip PA, McGinn CJ. A multi-institutional phase II trial of preoperative full-dose gemcitabine and concurrent radiation for patients with potentially resectable pancreatic carcinoma. Ann Surg Oncol. 2006;13:150-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 177] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 48. | Merz V, Mangiameli D, Zecchetto C, Quinzii A, Pietrobono S, Messina C, Casalino S, Gaule M, Pesoni C, Vitale P, Trentin C, Frisinghelli M, Caffo O, Melisi D. Predictive Biomarkers for a Personalized Approach in Resectable Pancreatic Cancer. Front Surg. 2022;9:866173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Merz V, Italy; Ryckman JM, Unite States S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR