Published online Jul 15, 2021. doi: 10.4251/wjgo.v13.i7.716
Peer-review started: March 21, 2021
First decision: April 30, 2021
Revised: June 6, 2021
Accepted: June 23, 2021
Article in press: June 23, 2021
Published online: July 15, 2021
Processing time: 111 Days and 1 Hours
Protein phosphatase 2 regulatory subunit B'' alpha (PPP2R3A) gene has been reported in other tumors, but the influence of PPP2R3A gene expression on the occurrence, development, and prognosis of hepatocellular carcinoma (HCC) remains unclear.
To investigate whether the PPP2R3A gene could be used to predict tumor recurrence and survival of HCC patients after liver transplantation (LT).
Diseased liver tissues of HCC patients after LT were collected as well as their clinical data and follow-up information. The immunohistochemical method was used to detect the expression of PPP2R3A protein in the tissues of 108 patients with primary liver cancer. The χ2 test was used to analyze the relationship between PPP2R3A protein expression levels and the clinicopathological features of tumors. The Kaplan-Meier method was used to analyze overall postoperative survival. The COX proportional hazard model was used to analyze adverse prognostic factors.
Immunohistochemistry showed that the PPP2R3A protein was mainly expressed in the cytoplasm of HCC cells. Compared to corresponding peritumoral tissues, expression was higher in HCC tissues (P ≤ 0.001). Correlation analysis showed that high PPP2R3A expression was correlated with preoperative serum alpha-fetoprotein (AFP) levels (P = 0.003), tumor-node-metastasis-t stage (P ≤ 0.001), and envelope invasion (P = 0.001). Univariate analysis showed that overall survival (P ≤ 0.001) and recurrence-free survival (P = 0.025) of patients with high PPP2R3A expression (≥ 4 points) were poor compared to those with low expression (< 4 points). The overall survival rates or recurrence-free survival rates at 1, 2, and 3 years with high PPP2R3A expression were 73%, 38%, and 23% or 31%, 23%, and 23%, respectively. Multivariate analysis showed that high PPP2R3A expression (hazard ratio = 2.900, 95% confidence interval: 1.411–5.960, P = 0.004) was an independent survival risk factor of HCC patients after LT, and it was also an independent predictor of postoperative tumor recurrence. This study also showed in patients with AFP ≥ 400 ng/mL, the overall survival (P ≤ 0.001) and recurrence-free survival (P = 0.023) of those with high PPP2R3A expression were significantly worse compared to those with low PPP2R3A expression. When PPP2R3A expression was low, the overall survival rate (P = 0.461) or recurrence-free survival rate (P = 0.072) after LT in patients with AFP < 400 ng/mL and ≥ 400 ng/mL was not significantly difference. The 1, 2, and 3 year survival rate of patients with low PPP2R3A expression and AFP < 400 ng/mL were 98%, 80%, and 69%, respectively, while patients who met Hangzhou criteria had a post-transplant 1, 2, and 3 years overall survival rate of 89%, 66%, and 55%, res
High expression of PPP2R3A might be a potential marker for predicting poor prognosis of HCC after LT. Combined with serum AFP levels, PPP2R3A might enhance the accuracy of predicting HCC outcome in patients after LT and supplement the efficacy of the Hangzhou criteria.
Core Tip: Protein phosphatase 2 regulatory subunit B''α (PPP2R3A) is an independent risk factor impacting the prognosis of liver transplantation (LT) in patients with hepatocellular carcinoma, with PPP2R3A being related to serum alpha-fetoprotein. PPP2R3A combined with alpha-fetoprotein can predict the prognosis of LT more accurately, supplementing the Hangzhou criteria, to a certain extent.
- Citation: He JJ, Shang L, Yu QW, Jiao N, Qiu S, Zhu WX, Wu DF, Tian YE, Zhang Q. High expression of protein phosphatase 2 regulatory subunit B'' alpha predicts poor outcome in hepatocellular carcinoma patients after liver transplantation. World J Gastrointest Oncol 2021; 13(7): 716-731
- URL: https://www.wjgnet.com/1948-5204/full/v13/i7/716.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i7.716
Hepatocellular carcinoma (HCC) is one of the most common and deadly malignant tumors globally. It has become the fourth leading cause of cancer-related death and the second leading cause of cancer death in China[1-4]. In China, around 383000 people die of HCC annually, representing 51% of all liver cancer related deaths globally. About 80% of the HCC patients are hepatitis B-virus (HBV) related liver cancer[5]. HCC is highly malignant, with high recurrence and mortality. Liver transplantation (LT) can completely cure tumors and liver cirrhosis, representing one of the effective methods for treating HCC. The Milan criteria[6] were first proposed for use as a global LT standard in 1996. The Milan criteria enhance the prognosis of LT recipients; however, their strict guidelines mean that many HCC patients suitable for LT are excluded. About 5 years later, the Milan criteria were gradually expanded, with examples including the University of California San Francisco criteria[7], the Shanghai Fudan criteria[8], "up-to-7"[9], the Hangzhou criteria[10], and the expanded Toronto criteria[11]. However, these criteria were based on the pathological characteristics of tumors, not the biological factors of tumors. Only the Hangzhou criteria include the molecular marker alpha-fetoprotein (AFP), and it is suitable for HCC patients undergoing LT in China[12]. The Hangzhou criteria contain three key specifications: (1) That the sum of tumor diameter is less than 8 cm; (2) That the sum of tumor diameter is more than 8 cm but AFP < 400 ng/mL with intermediate and well-differentiated histological grade; and (3) Intrahepatic macrovascular invasion and extrahepatic invasion transfer are absent.
The Metroticket Project, which is recognized by European Liver and Intestine Transplant Association and International Liver Transplantation Society, also includes AFP to evaluate better the prognostic survival of HCC patients after LT[13]. Although AFP helps with the diagnosis of HCC and can be used to predict the prognosis of LT for HCC, it still has limitations. For instance, 15%-30% of patients with advanced HCC have normal serum AFP levels (< 20 ng/mL), namely AFP-negative HCC (AFP-NHCC). AFP-NHCC accounts for about 30%-40% of liver cancer[14,15]. For these patients, AFP obviously cannot be used as an indicator of prognosis. Reports evaluating the predictive effect of genes on prognosis have gradually increased in recent years. The molecular markers that have been reported include osteopontin, β-catenin, Golgi protein-73, α-L-fucosidase, etc. However, most of the molecular markers that predict the recurrence of HCC after LT are still at the laboratory stage and are insufficient to guide clinical diagnosis and treatment[16-18]. Therefore, it is important to locate effective molecular markers able to predict the prognosis of LT more accurately and to supplement and expand LT standards.
Protein phosphatase 2 regulatory subunit B''α (PPP2R3A) gene is a regulatory subunit of protein phosphatase 2A (PP2A). PP2A is a serine/threonine phosphatase that is involved in regulating the activities of many cells, biological functions, and signal transduction pathways, including apoptosis, autophagy, cell proliferation, and DNA repair. Moreover, PP2A might act as a tumor promoter or suppressor. The active PP2A complex consists of a scaffolding subunit (PP2A-A), a regulatory subunit (PP2A-B), and a catalytic subunit (PP2A-C). Regulatory subunit B of PP2A can affect the activity of enzymes and contributes to substrate specificity and subcellular localization[19-24]. PPP2R3A contributes towards regulating PP2A activity and tumor-related signal proteins. Some previous studies found that PP2A has subunit mutations in many tumors, including the lung, rectum, breast, skin, and uterus, while PPP2R3A is involved in the occurrence and development of prostate cancer, breast cancer, kidney cancer, and lymphocytic leukemia. However, the relationship between the PPP2R3A gene and HCC remains unclear.
We previously demonstrated that the overexpression of PPP2R3A promotes the proliferation of HCC cells and, potentially, the invasion and migration of HCC cells. The down-regulation of PPP2R3A gene expression could inhibit the proliferation of HCC cells[25,26]. However, the relationship between the PPP2R3A gene and prognosis of HCC patients after LT remains unclarified, along with the prognostic value of PPP2R3A. Thus, the current study used relevant statistical methods to analyze the clinical significance and prognostic value of PPP2R3A expression in liver cancer patients who underwent LT in order to open up new ideas for further research on the prognosis of HCC.
Diseased liver tissues were collected from 108 patients with HCC who had undergone LT at the Third Medical Center of Chinese PLA General Hospital between January 2015 and December 2016, as well as their clinical data and follow-up information. The transplanted livers were donated from cardiac death donors. All patients were diagnosed with HCC by a histopathological examination. The collected clinical data included age, gender, tumor number, size, tumor-node-metastasis (TNM) stage, Child-Pugh grade, model for end-stage liver disease (MELD) score, AFP value, pathological stage, microsatellite foci, survival time, etc. The follow-up period was designated as January 2020 or the patient died or was lost to follow-up. The follow-up period was designated as 5-years, and all included patients had complete follow-up data. Among them, there were 29 patients who met the Milan criteria and 100 patients who met the Hangzhou criteria. All specimens were approved by the hospital ethics committee, and all patients gave informed consent.
Inclusion criteria included: (1) Preoperative clinical diagnosis or histopathological diagnosis as primary liver cancer; and (2) Cases with complete clinical, pathological, and follow-up data. Exclusion criteria included: (1) Non-HCC patients; (2) Clinicopathological data were missing; and (3) Patients who died for reasons other than tumors or were lost to follow-up after LT.
The antibody PPP2R3A kit was purchased from Sigma (St. Louis, MO, United States). The two-step detection kit and the secondary antibodies were purchased from ZhongShan Golden Bridge Biotechnology Co., LTD (Beijing, China).
One hundred and eight cases of HCC pathological tissues were selected for the immunohistochemical staining method. All specimens were formalin-fixed, paraffin-embedded, and serially sectioned to 4-μm thickness. Immunohistochemical staining was then performed according to the instructions of the two-step immunohistochemistry reagents. The primary antibody was PPP2R3A antibody (Sigma), used at a concentration of 1:200. The secondary antibody (Zhongshan Golden Bridge Biotechnology Co.) was antirabbit immunoglobulin G made in goat serum. Goat serum was used instead of the first antibody as the negative control.
Judgment criteria for staining results utilized a semiquantitative scoring system, which combined staining intensity and proportion of positive cells to evaluate the expression. The semi-quantitative method was used to determine the result because we observed a small amount of PPP2R3A protein expression on the cell membrane, and the quantitative method made the calculation of the result more difficult. No staining was 0 points, light yellow was 1 point, brown was 2 points, and tan was 3 points. The number of positive cells was scored as: 0 points, < 5% stained cells; 1 point, 5%–25%; 2 points, 26%–50%; and 3 points, > 50%. The staining result was calculated by multiplying the percentage score for positive cells with the score of staining intensity. A zero score was considered negative, score 1-3 was weak positive (+), score 4-6 was moderate positive (++), and score 7-9 was strongly positive (+++). In our analysis of the results, we found that there was no significant difference in scores of 0-3 points (P > 0.05). We defined a score of < 4 as the low expression, and ≥ 4 as the high expression. This classification was used to analyze the correlation and prognosis of HCC patients after LT.
Statistical analysis was completed using SPSS23 statistics software (Armonk, NY, United States). Measurement data were expressed as mean ± SD. The χ2 test was used to analyze the correlation. Survival curves were calculated and plotted using the Kaplan–Meier method. The log-rank test compared the difference in survival rates of the same index. Prognostic factors were analyzed using the Cox proportional hazard regression model. Significance was assumed for P < 0.05.
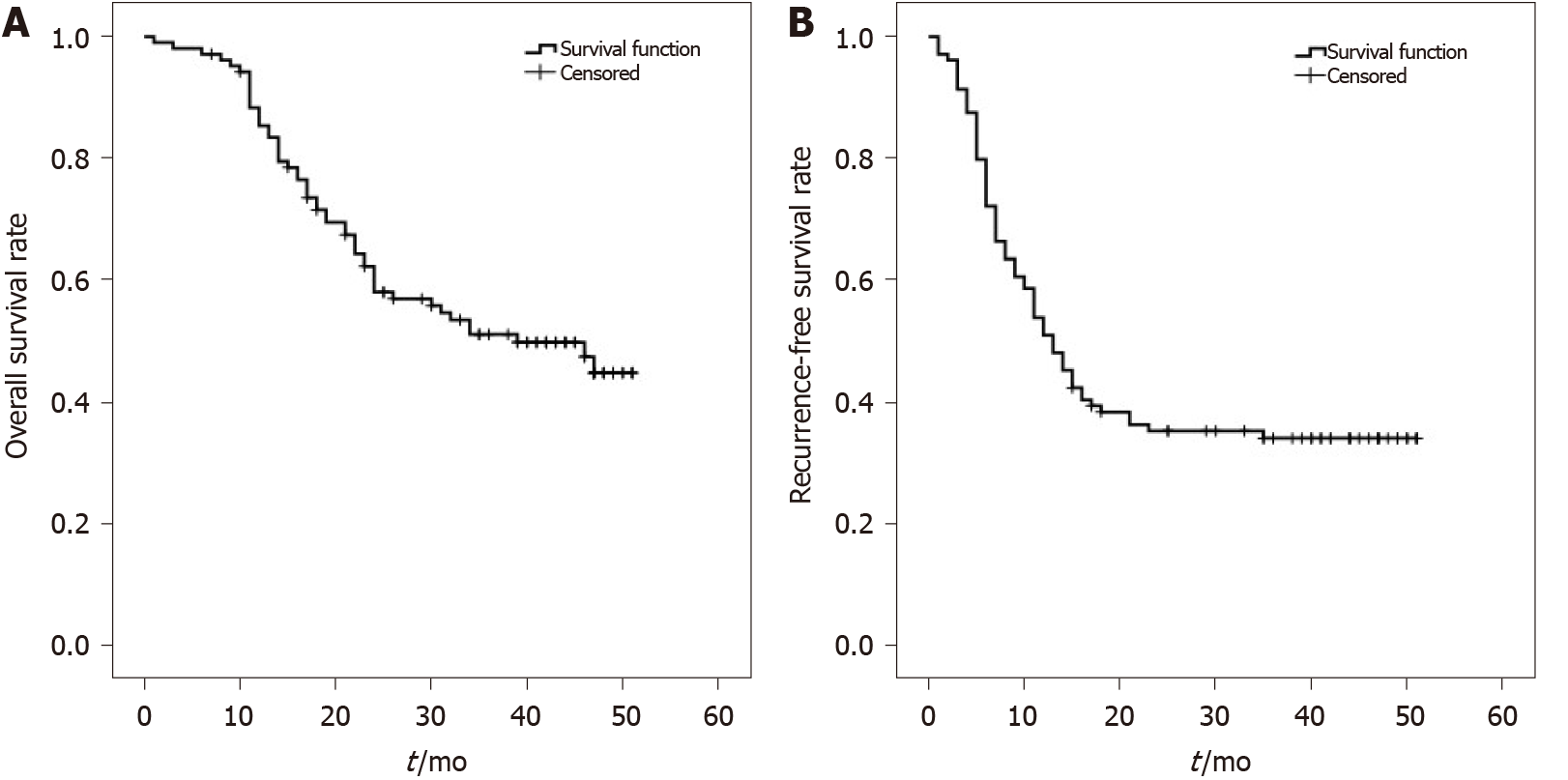
One hundred and eight HCC patients included in this study were mainly male (97.2%), aged between 30 and 71 years (median age 53.5 years). Among them, 44 patients (40.8%) had preoperative serum AFP values > 1000 ng/mL. TNM stage T2–T3 patients accounted for 57.4% of cases. Patients with tumor diameter ≥ 6 cm accounted for 36.1% of cases. Patients with tumor number > 1 accounted for 66.7% cases, see Table 1 for details. All patients had complete follow-up data, with the longest follow-up time being 60 mo. No cases were lost to follow-up. The overall survival for 108 HCC patients at 1, 2, and 3 years was 89%, 64%, and 53%, respectively (Figure 1A). Recurrence-free survival at 1, 2, and 3 years was 56%, 38%, and 35%, respectively (Figure 1B). Patients who met Hangzhou criteria had a post-transplant 1, 2, and 3-year overall survival rate or recurrence-free survival rate of 89%, 66%, and 55%, or 59%, 41%, and 38%, respectively. In 108 HCC patients, the average survival time was 34.332 ± 1.735 mo; median survival time was 39 mo. Average recurrence-free survival time was 23.480 ± 2.009 mo; median recurrence-free survival time was 13 mo.
| Clinicopathological variable | Patients, n (%) | PPP2R3A expression levels | Statistical results | |
| < 4 | ≥ 4 | |||
| Gender | ||||
| Male | 105 (97.2) | 79 (73.1) | 26 (24.1) | P = 0.195; X2 = 1.679 |
| Female | 3 (2.8) | 3 (2.8) | 0 (0) | |
| Age (yr) | ||||
| ≤ 50 | 45 (41.7) | 37 (34.2) | 8 (7.4) | P = 0.255 |
| > 50 | 63 (58.3) | 45 (41.7) | 18 (16.7) | X2 = 1.673 |
| Preoperative treatment | ||||
| No | 59 (54.6) | 47 (43.5) | 12 (11.1) | P = 0.370 |
| Yes | 49 (45.4) | 35 (32.4) | 14 (13) | X2 = 0.993 |
| AFP (ng/mL) | ||||
| < 13 | 24 (22.2) | 21 (19.4) | 3 (2.8) | P = 0.0031; X2 = 11.513 |
| 13-1000 | 40 (37.0) | 35 (32.4) | 5 (4.6) | |
| > 1000 | 44 (40.8) | 26 (24.1) | 18 (16.7) | |
| HBeAg | ||||
| Negative | 72 (66.7) | 56 (51.9) | 16 (14.8) | P = 0.634 |
| Positive | 36 (33.3) | 26 (24.1) | 10 (9.2) | X2 = 0. 405 |
| HBV-DNA (copy/mL) | ||||
| ≤ 1 | 75 (69.4) | 57 (52.8) | 18 (16.7) | P = 1.000 |
| > 1 | 33 (30.6) | 25 (23.1) | 8 (7.4) | X2 = 0.001 |
| MELD | ||||
| < 15 | 85 (78.7) | 65 (60.2) | 20 (18.5) | P = 1.000 |
| ≥ 15 | 23 (21.3) | 17 (15.7) | 6 (5.6) | X2 = 0.065 |
| Child-Pugh | ||||
| A | 65 (60.2) | 52 (48.3) | 13 (12.0) | P = 0.424; X2 = 3.952 |
| B | 27 (25.0) | 17 (15.7) | 10 (9.2) | |
| C | 16 (14.8) | 13 (12.0) | 3 (2.8) | |
| Pathology | ||||
| Well differentiation | 5 (4.6) | 5 (4.6) | 0 (0) | P = 0.296 |
| Moderate differentiation | 98 (90.8) | 74 (68.5) | 24 (22.2) | X2 = 3.382 |
| Poor differentiation | 5 (4.6) | 3 (2.8) | 2 (1.9) | |
| TNM-t | ||||
| T1 | 46 (42.6) | 38 (35.2) | 8 (7.4) | P = 0.0001; X2 = 16.601 |
| T2 | 21 (19.4) | 21 (19.4) | 0 (0) | |
| T3 | 41 (38.0) | 23 (21.3) | 18 (16.7) | |
| Length (cm) | ||||
| ≤ 3 | 31 (28.7) | 24 (22.2) | 7 (6.5) | P = 0.198; X2 = 3.294 |
| 4-5 | 38 (35.2) | 32 (29.6) | 6 (5.6) | |
| ≥ 6 | 39 (36.1) | 26 (24.1) | 13 (12.0) | |
| Tumor number | ||||
| 1 | 36 (33.3) | 30 (27.8) | 6 (5.6) | P = 0.240 |
| ≥ 2 | 72 (66.7) | 52 (48.1) | 20 (18.5) | X2 = 1.621 |
| Microsatellite stove | ||||
| No | 55 (50.9) | 44 (40.7) | 11 (10.2) | P = 0.371 |
| Yes | 53 (49.1) | 38 (35.2) | 15 (13.9) | X2 = 1.018 |
| Envelope invasion | ||||
| No | 75 (69.4) | 57 (52.8) | 18 (16.7) | P = 0.0011 |
| Yes | 33 (30.6) | 25 (23.1) | 8 (7.4) | X2 = 1.000 |
| Vascular invasion | ||||
| No | 69 (63.9) | 53 (49.1) | 16 (14.8) | P = 0.817 |
| Yes | 39 (36.1) | 29 (26.9) | 10 (9.2) | X2 = 0.082 |
| Lymph node metastasis | ||||
| No | 92 (85.2) | 72 (66.7) | 20 (18.5) | P = 0.207 |
| Yes | 16 (14.8) | 10 (9.2) | 6 (5.6) | X2 = 1.707 |
| Liver metastasis | ||||
| No | 67 (62.0) | 55 (50.9) | 12 (11.1) | P = 0.066 |
| Yes | 41 (38.0) | 27 (25) | 14 (13) | X2 = 3.668 |
| Chest metastasis | ||||
| No | 75 (69.4) | 61 (56.5) | 14 (13) | P = 0.055 |
| Yes | 33 (30.6) | 21 (19.4) | 12 (11.1) | X2 = 3.927 |
| Hepatitis B | ||||
| No recurrence | 102 (94.4) | 78 (72.9) | 24 (22.4) | P = 0.427 |
| Recurrence | 5 (4.6) | 3 (2.8) | 2 (1.9) | X2 = 0.632 |
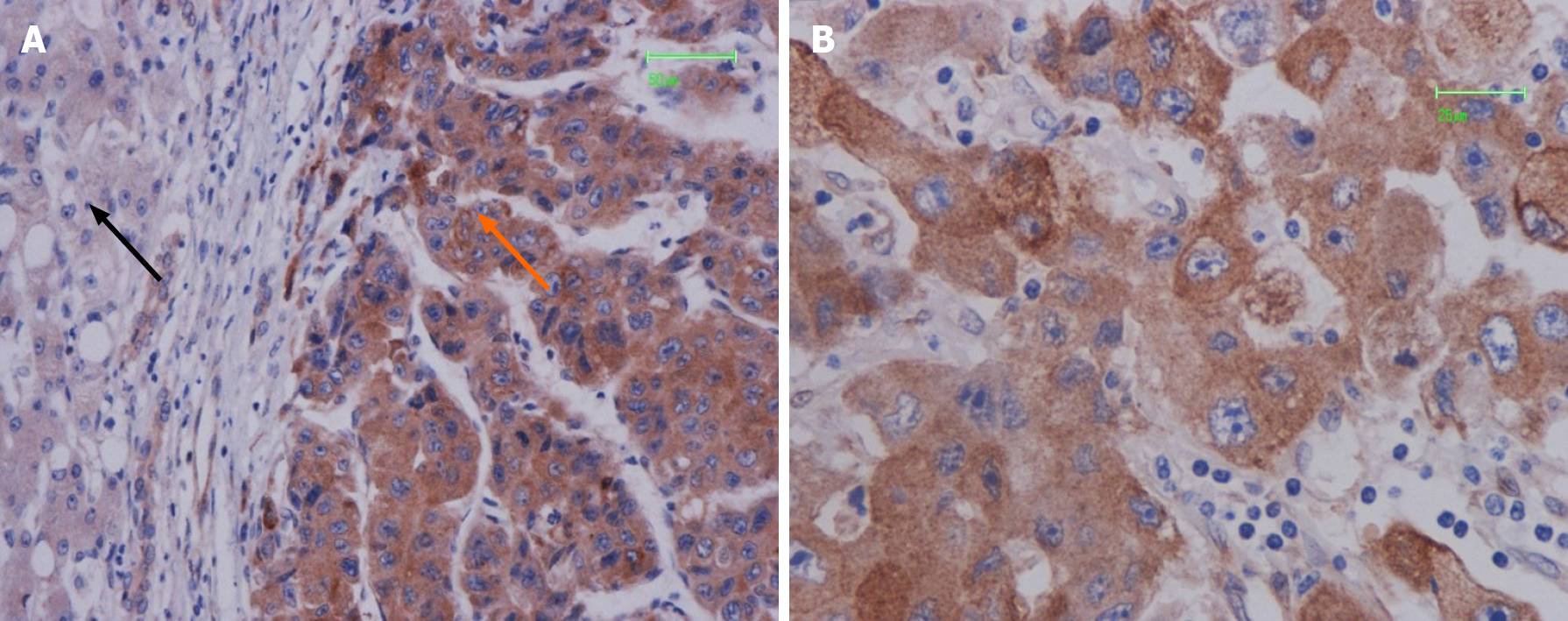
PPP2R3A was mainly located in the cytoplasm of HCC cells, with a small portion expressed on the cell membrane (Figure 2). The positive result generated brown-yellow particles or clumps. In 108 HCC tissues, PPP2R3A had 92.6% (100/108) positive expression, with high expression rates accounting for 63.9% (69/108). PPP2R3A protein was mostly negatively expressed or had low expression in pericarcinoma tissue. The expression rate in peritumoral tissue was only high in 18.5% (20/108) of cases. Statistical analysis showed that PPP2R3A protein expression in cancer tissue was significantly higher than that in corresponding peritumoral tissue (P ≤ 0.001).
The relationship between clinicopathological features of tumors and PPP2R3A protein expression was evaluated by χ2 tests. PPP2R3A expression was significantly correlated with AFP value (P = 0.003), TNM-t staging (P ≤ 0.001), and envelope invasion (P = 0.001). However, it was not associated with patient age, gender, tumor size, tumor number, microsatellite foci, vascular invasion, lymph node metastasis, pathological grade, preoperative anti-tumor therapy, hepatitis B e-antigen (HBeAg), HBV-DNA, Child-Pugh score, MELD score, or HBV recurrence (P > 0.05; Table 1).
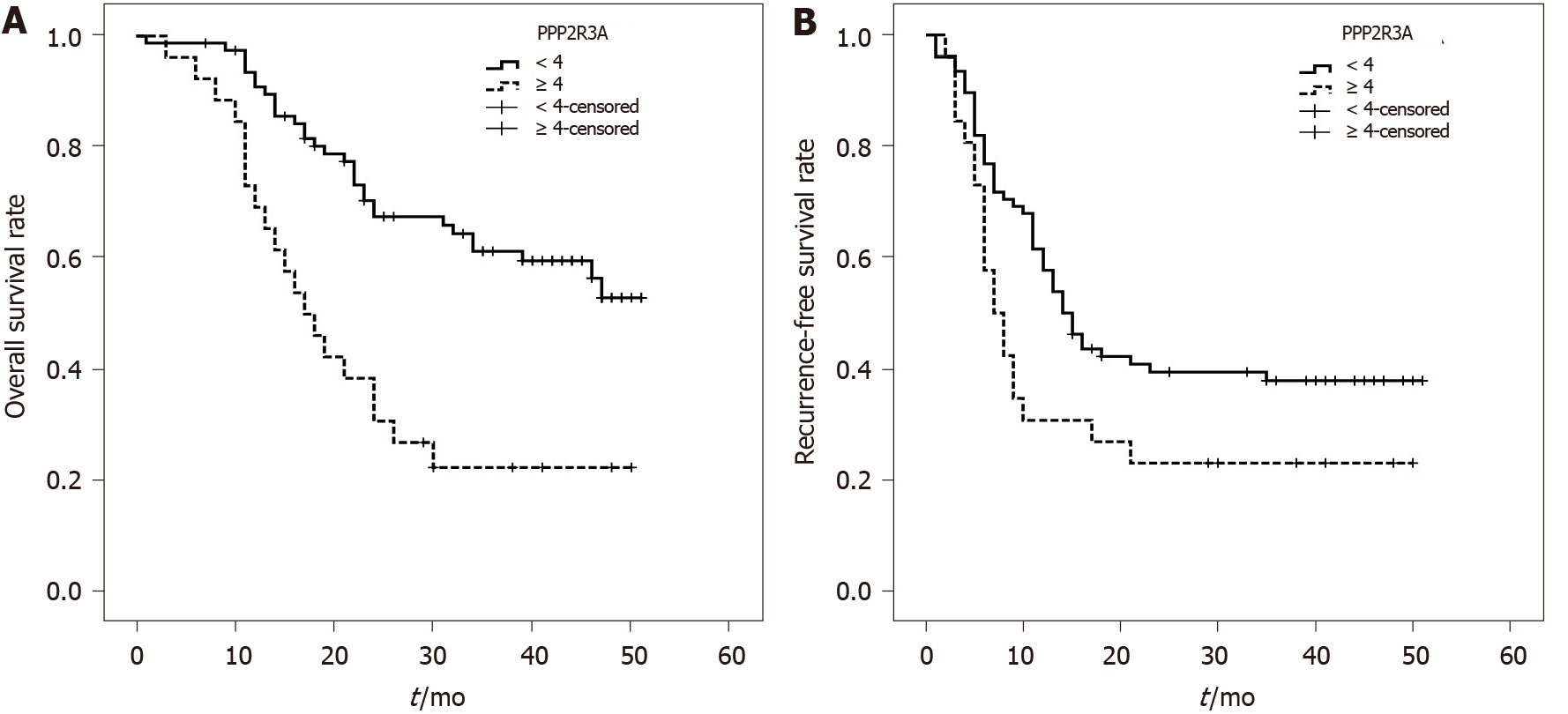
The overall survival rate of patients with high PPP2R3A expression (≥ 4 points) was 73%, 38%, and 23% at 1, 2, and 3 years, respectively. The overall survival rate of patients with low PPP2R3A expression (< 4 points) was 94%, 72%, and 63% at 1, 2, and 3 years, respectively. (Figure 3A). The survival rate of patients with high PPP2R3A expression was significantly worse than that of patients with low expression (P ≤ 0.001). Other indicators such as AFP > 1000 ng/mL (P ≤ 0.001), poorly differentiated tumors (P = 0.047), TNM-t stage T3 (P ≤ 0.001), number of tumors > 1 (P ≤ 0.001), tumor diameter ≥ 6 cm (P = 0.005), microsatellite foci (P ≤ 0.001), vascular invasion (P = 0.01), and chest metastasis (P = 0.001) were also associated with the overall survival of patients after surgery (Table 2).
| Variables | OS | RFS |
| Univariate | Univariate | |
| P value | P value | |
| PPP2R3A (< 4 vs ≥ 4) | 0.0001 | 0.0251 |
| Age (≤ 50 vs > 50) | 0.122 | 0.213 |
| Child-Pugh (≤ 6 vs 7-9 vs ≥ 10) | 0.375 | 0.206 |
| MELD (< 15 vs ≥ 15) | 0.827 | 0.809 |
| HBeAg (Negative vs Positive) | 0.461 | 0.120 |
| HBV-DNA (Negative vs Positive) | 0.581 | 0.090 |
| Pathology (Well vs Moderate vs Poor) | 0.0471 | < 0.051 |
| Tumor number (1 vs > 1) | 0.0001 | 0.0001 |
| Tumor size (cm) (≤ 3 vs 4-5 vs ≥ 6) | 0.0051 | < 0.051 |
| TNM-t (1 vs 2 vs 3) | 0.0001 | < 0.051 |
| Microsatellite stove (No vs Yes) | 0.0001 | 0.0001 |
| Vascular invasion (No vs Yes) | 0.011 | 0.0001 |
| Lymph node metastasis (No vs Yes) | 0.671 | 0.885 |
| Capsule invasion (No vs Yes) | 0.102 | 0.0051 |
| Liver metastasis (No vs Yes) | 0.124 | 0.0011 |
| Chest metastasis (No vs Yes) | 0.0011 | 0.0001 |
| AFP (ng/mL) (< 13 vs 13-1000 vs > 1000) | 0.0001 | 0.0001 |
| Preoperative treatment (No vs Yes) | 0.685 | 0.604 |
Univariate analysis showed that the recurrence-free survival rate of patients with high PPP2R3A expression was 31%, 23%, and 23% at 1, 2, and 3 years, respectively. The recurrence-free survival rate of patients with low PPP2R3A expression was 63%, 42%, and 39% at 1, 2, and 3 years, respectively (Figure 3B). The recurrence-free survival of patients with high PPP2R3A expression was significantly lower in patients with low expression (P = 0.025). Other indicators such as AFP > 1000 ng/mL (P ≤ 0.001), poorly differentiated tumors (P < 0.05), TNM-t stage T3 stage (P < 0.05), number of tumors > 1 (P ≤ 0.001), tumor diameter ≥ 6 cm (P < 0.05), microsatellite foci (P ≤ 0.001), vascular invasion (P ≤ 0.001), chest metastasis (P ≤ 0.001), envelopment invasion (P = 0.005), and intrahepatic metastasis (P = 0.001) were also related to the recurrence-free survival (Table 2).
The multivariate Cox model of overall survival included clinicopathological indicators of tumors such as age, gender, tumor number, tumor size, AFP, pathological grade, Child-Pugh score, MELD score, HBeAg, HBV-DNA, microsatellite foci, envelope invasion, vascular invasion, lymph node metastasis, HBV recurrence, preoperative anti-tumor therapy, and PPP2R3A classification (< 4 points, ≥ 4 points). The high expression of PPP2R3A (> 4 points) [hazard ratio (HR) = 2.900, 95% confidence interval (CI): 1.411-5.960, P = 0.004) represented an independent risk predictor of poor survival after LT. Tumor diameter ≥ 6 cm (HR = 2.760, 95%CI: 1.309–5.816, P = 0.008) and the number of tumors > 1 (HR = 4.707, 95%CI 2.088–10.612, P = 0.000) were also independent predictors of poor survival after LT (Table 3).
Above indicators and PPP2R3A classification (0-6 points) were included in the model (Table 4). COX multivariate analysis of the recurrence results showed that different expression intensities of PPP2R3A were independent risk factors affecting the recurrence of postoperative tumors (P = 0.024). Envelope invasion, AFP > 1000 ng/mL, and the number of tumors > 1 were also independent predictors of tumor recurrence in HCC patients after LT.
| Variables | B | SE | Wald | P value | OR | 95%CI for Exp (B) | |
| Lower | Upper | ||||||
| capsule invasion | 0.633 | 0.285 | 4.941 | 0.0261 | 1.883 | 1.078 | 3.290 |
| AFP (ng/mL) (< 13 vs > 1000) | 1.145 | 0.376 | 9.263 | 0.0021 | 3.143 | 1.503 | 6.571 |
| Tumor number (1 vs > 1) | 1.340 | 0.354 | 14.358 | 0.0001 | 3.819 | 1.910 | 7.638 |
| PPP2R3A | 14.562 | 0.0241 | |||||
| PPP2R3A (6 vs 0) | -1.527 | 0.489 | 9.775 | 0.0021 | 0.217 | 0.083 | 0.566 |
| PPP2R3A (6 vs 2) | -1.773 | 0.511 | 12.043 | 0.0011 | 0.170 | 0.062 | 0.462 |
| PPP2R3A (6 vs 3) | -1.117 | .515 | 4.714 | 0.0301 | 0.327 | 0.119 | 0.897 |
| PPP2R3A (6 vs 4) | -1.141 | 0.487 | 5.489 | 0.0191 | 0.320 | 0.123 | 0.830 |
| PPP2R3A (6 vs 5) | -0.630 | 0.697 | 0.817 | 0.366 | 0.532 | 0.136 | 2.088 |
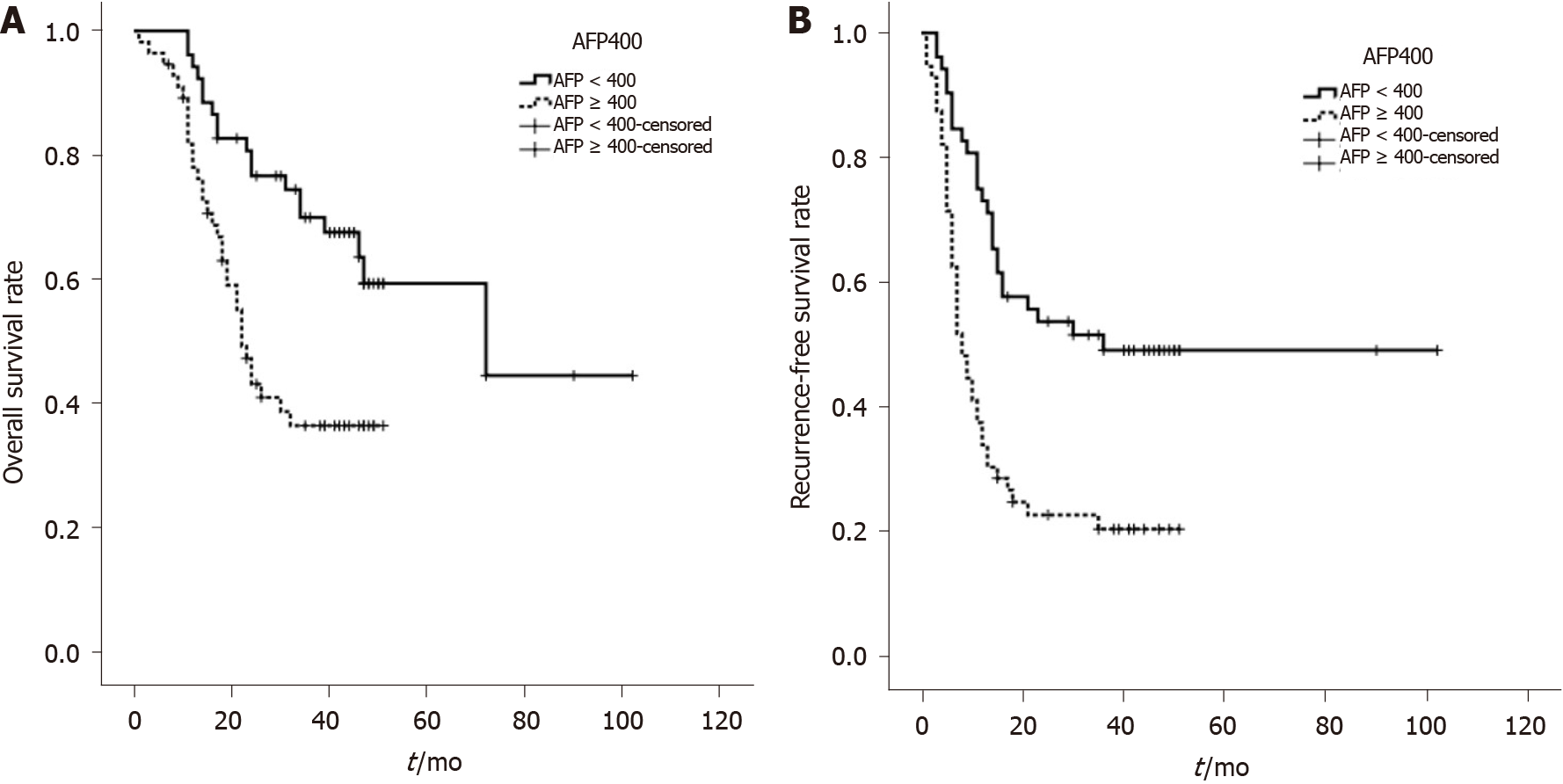
AFP was categorized into two groups (≥ 400 ng/mL and < 400 ng/mL) according to the Hangzhou criteria. The overall survival of the two groups was analyzed by a univariate factor. The overall survival of patients with AFP ≥ 400ng/mL was significantly lower than that of patients with AFP < 400 ng/mL, with this difference being statistically significant (P = 0.001; Figure 4A). The recurrence-free survival of patients with AFP ≥ 400 ng/mL was significantly lower in patients with AFP < 400 ng/mL (P = 0.000; Figure 4B).
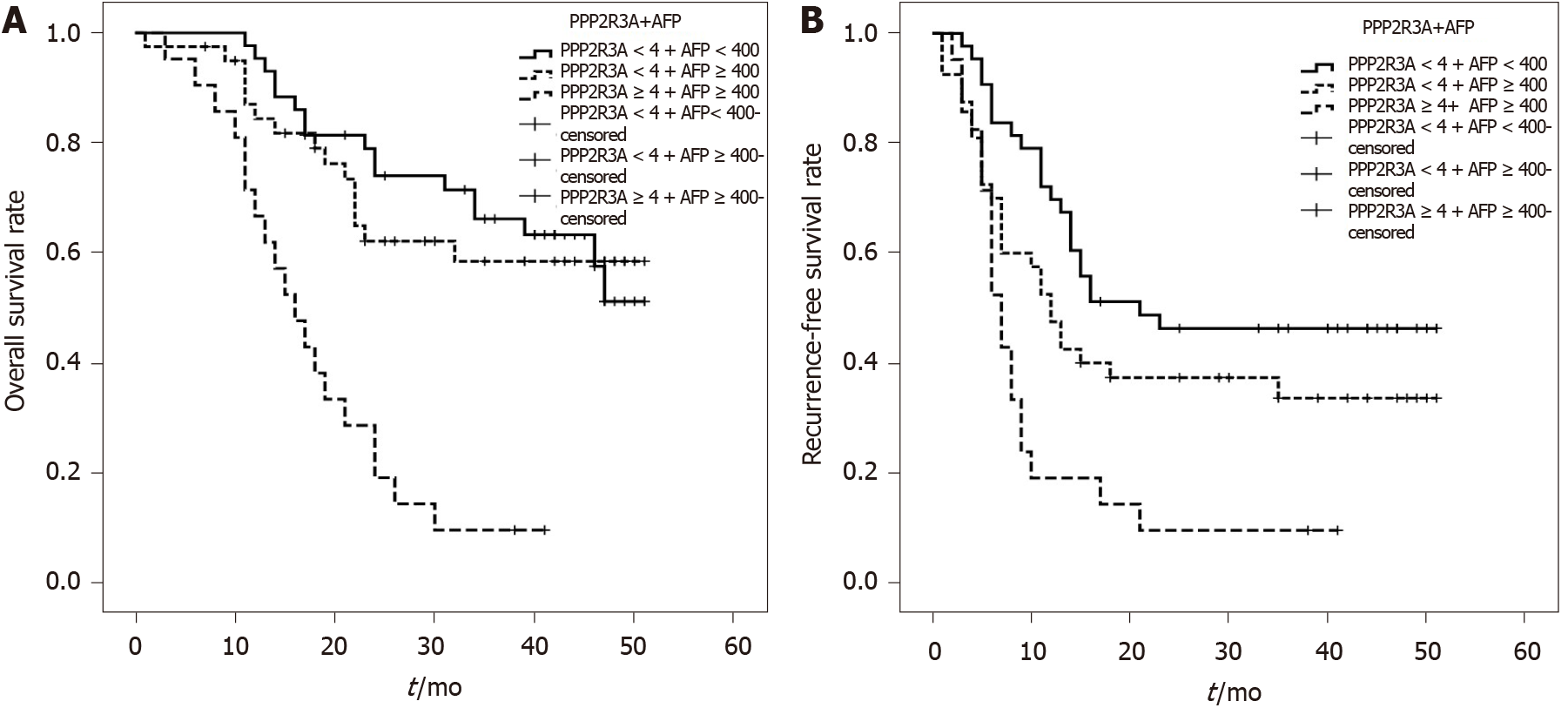
We comprehensively analyzed AFP values and the intensity of PPP2R3A expression together. Survival analysis showed that the 1, 2, and 3 years survival rates of patients with high PPP2R3A expression and AFP ≥ 400 ng/mL were 71%, 29%, and 10%, respectively. While the patients with low PPP2R3A expression and AFP ≥ 400 ng/mL were 88%, 59%, and 56%, respectively. The patients with low PPP2R3A expression and AFP < 400 ng/mL were 98%, 80%, and 69%, respectively. When AFP ≥ 400 ng/mL, the overall survival of patients with high PPP2R3A expression was significantly lower than that of patients with low expression (P ≤ 0.001). When PPP2R3A expression was low, patients with AFP < 400 ng/mL showed no significant difference in postoperative survival time compared with patients with AFP ≥ 400 ng/mL (P = 0.461; Figure 5A).
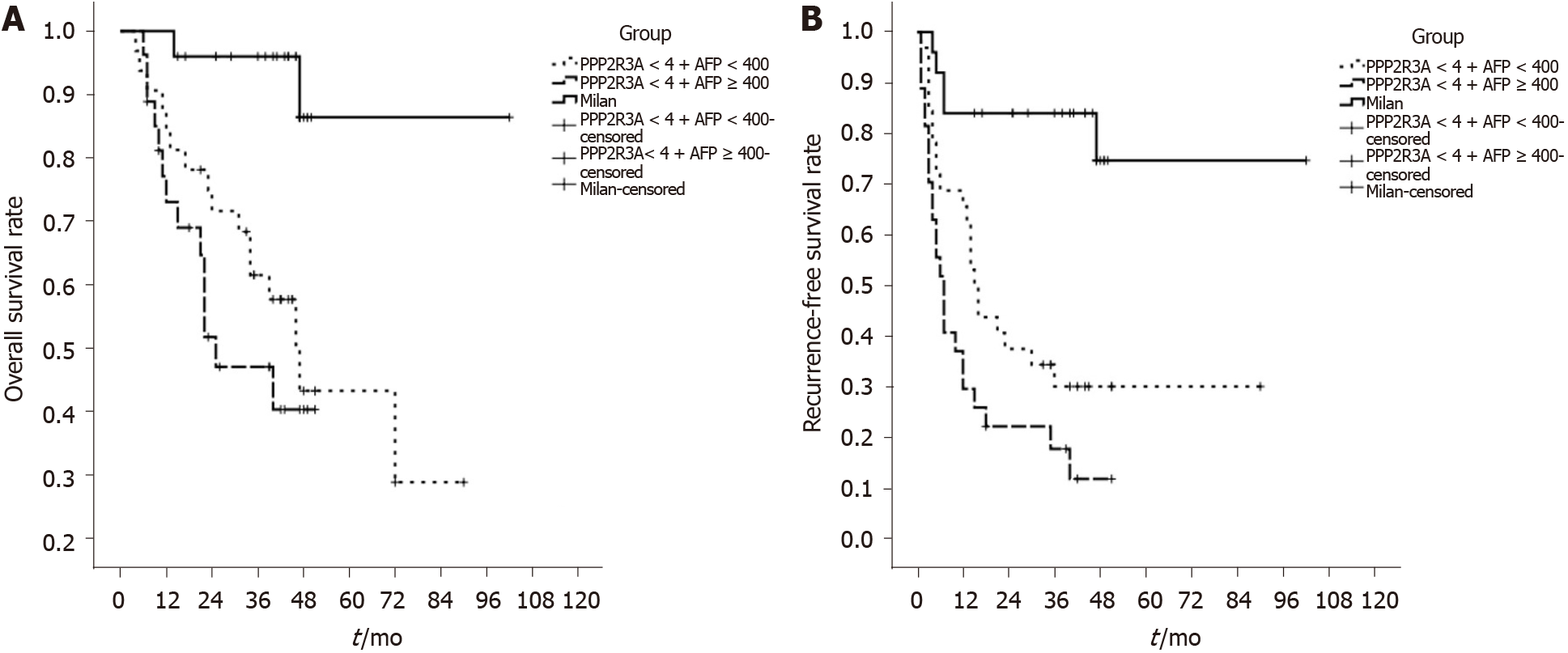
Recurrence-free survival analysis showed that the 1, 2, and 3 years recurrence-free survival rates of patients with high PPP2R3A expression and AFP ≥ 400 ng/mL were 19%, 10%, and 10%, respectively, while the patients with low PPP2R3A expression and AFP ≥ 400 ng/mL were 49%, 30%, and 27%, respectively. The patients with low PPP2R3A expression and AFP < 400 ng/mL were 74%, 51%, and 48%, respectively. When AFP ≥ 400 ng/mL, the recurrence-free survival of patients with high PPP2R3A expression was significantly worse compared to that of patients with low expression (P = 0.023). When PPP2R3A expression was low, the recurrence-free survival time of patients with AFP < 400 ng/mL was not significantly different to that of patients with AFP ≥ 400 ng/mL (P = 0.072; Figure 5B). We compared PPP2R3A combined with AFP with the Milan criteria. Survival analysis showed that patients who met Milan criteria had a post-transplant 1, 2, and 3-year overall survival rate of 100%, 96%, and 96%, respectively. When the expression of PPP2R3A was low, the overall survival rate of patients with AFP < 400ng/mL or ≥ 400 ng/mL was significantly different compared with the patients who met the Milan criteria (P < 0.05; Figure 6A). Similarly, recurrence-free survival analysis showed that patients who met Milan criteria had a post-transplant 1, 2, and 3-year recurrence-free survival rate of 86%, 86%, and 86%, respectively. The recurrence-free survival rate of these two groups of patients was also significantly different from that of patients who met the Milan criteria (P < 0.05; Figure 6B).
The PPP2R3A gene belongs to the B''/PR72 family and mainly encodes two members, namely PR130 and PR72[27]. The two domains contained in the same C-terminus provide an interactive interface with structural subunits, the nuclear localization of PR72, and stimulation of Ca2+-dependent phosphatase activity[19,20,27]. The PPP2R3A gene contributes to the occurrence and development of a variety of tumors; however, current studies are primarily focused on the molecular level, rather than evaluating how the PPP2R3A gene impacts tumor prognosis. Using univariate and multivariate analysis of 108 HCC patients after LT, the current study demonstrated that the overall survival and recurrence-free survival of patients with high PPP2R3A expression were worse than those with low PPP2R3A expression. Furthermore, high expression of PPP2R3A was an independent risk factor impacting survival and recurrence after LT. Thus, high expression of the PPP2R3A protein could be used as a predictor of poor prognosis for HCC patients after LT. PPP2R3A might be related to the occurrence and development of liver cancer.
Existing studies confirmed that the PPP2R3A gene is involved in the occurrence and development of prostate cancer, breast cancer, kidney cancer, and other tumors[21,28-31]. For example, Nam et al[21] showed that PPP2R3A promotes the occurrence of prostate cancer and is the direct target of miR-652 in prostate cancer cells[28]. We confirmed that the expression of PPP2R3A protein in HCC tissue is mainly located in the cell cytoplasm and is partly expressed in the cell membrane. In addition, we demonstrated that the expression of PPP2R3A in cancer tissue was significantly higher than that of adjacent tissues. PPP2R3A was mostly negatively expressed, or had low expression, in adjacent tissues. Previous studies showed that the subtype PR130 of PPP2R3A redistributes SH2-containing inositol phosphatase 2 in the cell membrane and inhibits the degradation of epidermal growth factor receptor after binding to it. The epidermal growth factor receptor has a tumor-promoting effect on a variety of tumors. Therefore, the cytoplasm and cell membrane are key components of the biological function of this gene[25]. Our previous research using Western blot also showed that PPP2R3A protein in HCC tissue had higher expression compared to the corresponding adjacent tissue[26]. Thus, the overexpression of PPP2R3A might be related to the malignant biological behavior of HCC, contributing to the occurrence and development of HCC.
Our study also demonstrated that high PPP2R3A expression was correlated to clinical (AFP values) and tumor pathological indicators of HCC (TNM-t stage and envelope invasion). AFP is a specific index for the clinical diagnosis of HCC. Previous studies showed that AFP impacted the proliferation of tumor cells and tumor escape immune function[32]. At cell proliferation and apoptosis stages of HCC, AFP positively regulated HCC cell proliferation by changing the p53/Bax/cytochrome c/caspase-3 signaling pathway[33]. AFP helped promote the invasion and distant metastasis of HCC by activating the phosphatidylinositol-3-kinase/AKT signaling pathway[34]. The TNM staging system is the most universally used globally. The seventh edition of the cancer staging manual of the American Joint Committee on Cancer[35] states that HCC samples range from stage I to stage IV with increasing malignancy. The primary tumor, regional lymph nodes, and distant metastasis (TNM) stage can be used to predict early recurrence[35,36]. The presence of envelope invasion indicates a higher degree of liver cancer, and liver cancer patients with envelope invasion are more likely to be accompanied by microvascular invasion[37]. Existing studies have confirmed that PR72 is a negative regulator of the classic Wnt signaling pathway[38], with PR130 being required for efficient cell migration through a mechanism that depends on lipoma-preferred partner and PP2A/C[31]. The PPP2R3A gene uses these mechanisms for tumor regulation and expression. β-catenin expression also increases in HCC cells overexpressing the PPP2R3A gene, with the activation of the Wnt/β-catenin signaling pathway usually being associated with the metastasis of HCC[39,40]. It was previously reported that more than 95% of HCC had an abnormal Wnt/β-catenin signaling pathway[41], with the upregulation of β-catenin promoting the proliferation and migration capabilities of HCC cells. Our group also previously demonstrated that the PPP2R3A gene promotes the proliferation and invasion of HCC cells by regulating the expression of p53 and β-catenin protein and inhibiting HCC cell apoptosis through the caspase apoptosis pathway[25,26]. After PPP2R3A is overexpressed, spliced PARP expression decreased in cells, with PARP protein representing a universal marker for promoting cell apoptosis, while PARP-1 is related to the tumor stage of liver cancer[25]. Therefore, we propose that the overexpression of the PPP2R3A impacts the malignant biological behavior of liver cancer through Wnt/β-catenin and other signaling pathways; however, the specific mechanism must be confirmed through further research.
Of note, we demonstrated that the expression intensity of PPP2R3A combined with AFP could impact the survival of HCC patients after LT. In patients with AFP ≥ 400 ng/mL, the overall survival and recurrence-free survival of patients with high PPP2R3A expression were significantly worse compared to patients with low expression. When PPP2R3A expression was low, the overall survival rate or recurrence-free survival rate after LT in patients with AFP < 400 ng/mL and ≥ 400 ng/mL was not significantly different. Hangzhou criteria[10] are one of the LT standards that include AFP. The Metroticket Project believes that AFP plays an important role in predicting the 5-year survival rate of LT patients. In addition, Toso et al[42] showed in a prospective study that HCC candidate selection for LT could be expanded to patients with total tumor volume ≤ 115 cm3 and AFP ≤ 400 ng/mL as the biological characteristics of tumors should be considered. Elevated serum AFP levels are correlated to the poor prognosis of HCC patients, with serum AFP concentrations ≥ 400 ng/mL consistently predicting poor prognosis across different clinical environments[43]. AFP response after treatment can be used to predict the survival of HCC patients[44]. AFP also has a predictive effect on the prognosis of HCC patients after LT[43]. The combination of pre-transplant AFP (critical value 200 ng/mL) and 18 F-fluorodeoxyglucose positron emission tomography has advantages in predicting the 5-year disease-free survival rate[45]. The current study also confirmed that tumor diameter and tumor number represent independent risk factors predicting the overall survival of HCC LT. Thus, the characteristics of tumors should be incorporated in the prognosis[46,47]. Above all, we suggest that PPP2R3A combined with AFP could help predict the prognosis of HCC patients after LT with greater accuracy. The inclusion of these parameters could help overcome the limitations of using AFP alone, supplementing and expanding the efficacy of the Hangzhou criteria.
According to reports, Milan criteria are currently the benchmark related to LT, and LT recipients who meet the Milan criteria have a higher postoperative survival rate[48]. Our research also confirmed this view. However, it is considered too strict and excludes many patients from the transplant list[48]. The current study showed that fewer patients met the Milan criteria and that patients with low PPP2R3A expression also benefited from LT, but the sample number needs to be expanded for further confirmation.
This study was a single-center study, and the number of patients was relatively small, which may have certain limitations. In the future, a multi-center study should be conducted in the next step to expand the sample size and add other types of liver disease for comparison, so as to improve the reliability of the research results. At the same time, the follow-up time can be extended, and the influencing factors on short-term and long-term prognosis of HCC patients after LT should be analyzed respectively.
In summary, the current study demonstrated that the PPP2R3A gene is potentially related to the occurrence and development of HCC, and could be used as an indicator of LT prognosis in HCC patients. By combining PPP2R3A with AFP, the prognosis of HCC patients after LT could be predicted with greater accuracy, expanding LT Hangzhou criteria. However, further randomized controlled clinical trials across multiple centers with large sample sizes are needed to verify whether PPP2R3A could be used as an indicator for predicting the survival and prognosis of HCC patients after LT.
Hepatocellular carcinoma (HCC) is one of the most common and deadly malignant tumors worldwide, and its incidence is increasing year by year. Liver transplantation (LT) is currently recognized as one of the effective methods for the treatment of HCC, but tumor recurrence and metastasis after LT restricts the long-term prognosis of patients. Therefore, it is necessary to find molecular indicators that can effectively predict the prognosis. The protein phosphatase 2 regulatory subunit B''α (PPP2R3A) gene has been found to be involved in the occurrence and development of tumors such as kidney cancer and prostate cancer. At present, it is not clear whether PPP2R3A is associated with the prognosis of HCC patients or whether it can be used as a prognostic indicator for HCC patients.
Effectively predicting the prognosis of LT is of great value for patients with liver cancer. PPP2R3A may be related to the occurrence and development of HCC. Our study aim is to explore the prognostic value of PPP2R3A for HCC patients after LT.
The main aim of the current study is to analyze the relationship between PPP2R3A and the clinicopathological characteristics of liver cancer and evaluate the prognostic value of PPP2R3A for HCC patients after LT.
The authors used immunohistochemical methods to observe the expression of PPP2R3A in liver cancer tissues. At the same time, we collected clinical data of patients and analyzed the relationship between PPP2R3A and liver cancer by χ2 test, then performed Cox regression analysis to investigate the prognostic value of PPP2R3A for HCC patients after LT.
In immunohistochemical experiments, we detected that the expression of PPP2R3A gene in liver cancer tissues was higher than that in adjacent tissues (P ≤ 0.001), and it was mainly located in the cytoplasm of cells. χ2 test indicated that the high expression of PPP2R3A was positively correlated with AFP, TNM-t staging, and envelope invasion. In multivariate logistic regression analysis and univariate Cox proportional hazards regression analysis, PPP2R3A could be used as an independent risk factor for predicting poor prognosis of HCC patients. In addition, it was also revealed that high PPP2R3A expression combined with AFP ≥ 400 ng/mL are linked to patients with poor overall survival and recurrence-free survival rates. The 1, 2, and 3 years survival rate of patients with low PPP2R3A expression and AFP < 400 ng/mL was 98%, 80%, and 69%, respectively, while patients who met Hangzhou criteria had a post-transplant 1, 2, and 3 years overall survival rate of 89%, 66%, and 55%, respectively.
PPP2R3A may be involved in the occurrence and development of liver cancer. The high expression of PPP2R3A may be a potential marker for predicting the poor prognosis and recurrence of LT for HCC patients. The combination of PPP2R3A and AFP can more accurately predict the prognosis of HCC patients after LT, supplementing and expanding the efficacy of the Hangzhou criteria.
This study is the first to explore the prognostic value of PPP2R3A gene in HCC patients after LT, but the sample of the current study was relatively limited. We expect large prospective randomized controlled trials to verify further our results. In addition, a prospective validation study should be performed to confirm further the prognostic value of PPP2R3A for HCC patients after LT.
| 1. | Long J, Zhang L, Wan X, Lin J, Bai Y, Xu W, Xiong J, Zhao H. A four-gene-based prognostic model predicts overall survival in patients with hepatocellular carcinoma. J Cell Mol Med. 2018;22:5928-5938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 2. | Aravalli RN, Cressman EN, Steer CJ. Cellular and molecular mechanisms of hepatocellular carcinoma: an update. Arch Toxicol. 2013;87:227-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 3. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3364] [Article Influence: 480.6] [Reference Citation Analysis (45)] |
| 4. | Craig AJ, von Felden J, Garcia-Lezana T, Sarcognato S, Villanueva A. Tumour evolution in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2020;17:139-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 596] [Article Influence: 99.3] [Reference Citation Analysis (1)] |
| 5. | Xu XL, Lu KJ, Zhu ML, Du YL, Zhu YF, Zhang NN, Wang XJ, Kang XQ, Xu DM, Ying XY, Yu RS, Lu CY, Ji JS, You J, Du YZ. Sialic Acid-Functionalized pH-Triggered Micelles for Enhanced Tumor Tissue Accumulation and Active Cellular Internalization of Orthotopic Hepatocarcinoma. ACS Appl Mater Interfaces. 2018;10:31903-31914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5388] [Article Influence: 179.6] [Reference Citation Analysis (7)] |
| 7. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1714] [Article Influence: 68.6] [Reference Citation Analysis (1)] |
| 8. | Fan J, Zhou J, Xu Y, Qiu SJ, Wu ZQ, Yu Y, Huang XW, Tang ZY, Wang YQ. [Indication of liver transplantation for hepatocellular carcinoma: Shanghai Fudan Criteria]. Zhonghua Yi Xue Za Zhi. 2006;86:1227-1231. [PubMed] |
| 9. | Takada Y, Ito T, Ueda M, Sakamoto S, Haga H, Maetani Y, Ogawa K, Ogura Y, Oike F, Egawa H, Uemoto S. Living donor liver transplantation for patients with HCC exceeding the Milan criteria: a proposal of expanded criteria. Dig Dis. 2007;25:299-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Zheng SS, Xu X, Wu J, Chen J, Wang WL, Zhang M, Liang TB, Wu LM. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation. 2008;85:1726-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 380] [Article Influence: 21.1] [Reference Citation Analysis (1)] |
| 11. | Sapisochin G, Goldaracena N, Laurence JM, Dib M, Barbas A, Ghanekar A, Cleary SP, Lilly L, Cattral MS, Marquez M, Selzner M, Renner E, Selzner N, McGilvray ID, Greig PD, Grant DR. The extended Toronto criteria for liver transplantation in patients with hepatocellular carcinoma: A prospective validation study. Hepatology. 2016;64:2077-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 284] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 12. | Zhan QF, Ling SB, Deng YN, Shan QN, Ye QW, Xu SJ, Jiang GJ, Lu D, Wei XY, Zhuang L, Zhang W, Shen T, Cen BN, Xie HY, Liu JM, Wu J, Zheng SS, Yang Y, Xu X. Hangzhou criteria as downstaging criteria in hepatocellular carcinoma before liver transplantation: A multicenter study from China. Hepatobiliary Pancreat Dis Int. 2020;19:349-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P; Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1616] [Article Influence: 89.8] [Reference Citation Analysis (1)] |
| 14. | Jing W, Peng R, Zhu M, Lv S, Jiang S, Ma J, Ming L. Differential Expression and Diagnostic Significance of Pre-Albumin, Fibrinogen Combined with D-Dimer in AFP-Negative Hepatocellular Carcinoma. Pathol Oncol Res. 2020;26:1669-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Luo P, Wu S, Yu Y, Ming X, Li S, Zuo X, Tu J. Current Status and Perspective Biomarkers in AFP Negative HCC: Towards Screening for and Diagnosing Hepatocellular Carcinoma at an Earlier Stage. Pathol Oncol Res. 2020;26:599-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 169] [Article Influence: 24.1] [Reference Citation Analysis (3)] |
| 16. | Gunsar F. Liver Transplantation for Hepatocellular Carcinoma Beyond the Milan Criteria. Exp Clin Transplant. 2017;15:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A; OLT for HCC Consensus Group. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 795] [Article Influence: 56.8] [Reference Citation Analysis (2)] |
| 18. | Luo FZ, Yang Z, Zhen SS. Progress in the study of molecular markers for the prognosis of liver transplantation for hepatocellular carcinoma. Gandanyi Waike Zazhi. 2019;31:385-391. |
| 19. | Ahn JH, Sung JY, McAvoy T, Nishi A, Janssens V, Goris J, Greengard P, Nairn AC. The B''/PR72 subunit mediates Ca2+-dependent dephosphorylation of DARPP-32 by protein phosphatase 2A. Proc Natl Acad Sci USA. 2007;104:9876-9881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Ruediger R, Pham HT, Walter G. Alterations in protein phosphatase 2A subunit interaction in human carcinomas of the lung and colon with mutations in the A beta subunit gene. Oncogene. 2001;20:1892-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 104] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Nam RK, Benatar T, Amemiya Y, Wallis CJD, Romero JM, Tsagaris M, Sherman C, Sugar L, Seth A. MicroRNA-652 induces NED in LNCaP and EMT in PC3 prostate cancer cells. Oncotarget. 2018;9:19159-19176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Song G, Han M, Li Z, Gan X, Chen X, Yang J, Dong S, Yan M, Wan J, Wang Y, Huang Z, Yin Z, Zheng F. Deletion of Pr72 causes cardiac developmental defects in Zebrafish. PLoS One. 2018;13:e0206883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Li M, Wen Y, Wen H, Gui C, Huang F, Zeng Z. Discovery of PPP2R3A and TMX3 pathogenic variants in a Zhuang family with coronary artery disease using whole-exome sequencing. Int J Clin Exp Pathol. 2018;11:3678-3684. [PubMed] |
| 24. | Dunwell TL, Hesson LB, Pavlova T, Zabarovska V, Kashuba V, Catchpoole D, Chiaramonte R, Brini AT, Griffiths M, Maher ER, Zabarovsky E, Latif F. Epigenetic analysis of childhood acute lymphoblastic leukemia. Epigenetics. 2009;4:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Chen HJ, Wang PX, Huang LL, Zhang HY, Chen XG, Zhang Q. [Overexpression of protein phosphatase 2 regulatory subunit B''α gene effect on proliferation and invasion of hepatoma cells]. Zhonghua Gan Zang Bing Za Zhi. 2019;27:872-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 26. | Chen H, Xu J, Wang P, Shu Q, Huang L, Guo J, Zhang X, Zhang H, Wang Y, Shen Z, Chen X, Zhang Q. Protein phosphatase 2 regulatory subunit B''Alpha silencing inhibits tumor cell proliferation in liver cancer. Cancer Med. 2019;8:7741-7753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Sangodkar J, Farrington CC, McClinch K, Galsky MD, Kastrinsky DB, Narla G. All roads lead to PP2A: exploiting the therapeutic potential of this phosphatase. FEBS J. 2016;283:1004-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 256] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 28. | Göder A, Emmerich C, Nikolova T, Kiweler N, Schreiber M, Kühl T, Imhof D, Christmann M, Heinzel T, Schneider G, Krämer OH. HDAC1 and HDAC2 integrate checkpoint kinase phosphorylation and cell fate through the phosphatase-2A subunit PR130. Nat Commun. 2018;9:764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 29. | Zwaenepoel K, Goris J, Erneux C, Parker PJ, Janssens V. Protein phosphatase 2A PR130/B''alpha1 subunit binds to the SH2 domain-containing inositol polyphosphate 5-phosphatase 2 and prevents epidermal growth factor (EGF)-induced EGF receptor degradation sustaining EGF-mediated signaling. FASEB J. 2010;24:538-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Dzulko M, Pons M, Henke A, Schneider G, Krämer OH. The PP2A subunit PR130 is a key regulator of cell development and oncogenic transformation. Biochim Biophys Acta Rev Cancer. 2020;1874:188453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Janssens V, Zwaenepoel K, Rossé C, Petit MM, Goris J, Parker PJ. PP2A binds to the LIM domains of lipoma-preferred partner through its PR130/B″ subunit to regulate cell adhesion and migration. J Cell Sci. 2016;129:1605-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Chen T, Dai X, Dai J, Ding C, Zhang Z, Lin Z, Hu J, Lu M, Wang Z, Qi Y, Zhang L, Pan R, Zhao Z, Lu L, Liao W, Lu X. AFP promotes HCC progression by suppressing the HuR-mediated Fas/FADD apoptotic pathway. Cell Death Dis. 2020;11:822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 33. | Wang S, Hu Y. Correlation analysis of AFP level and prognosis of patients with primary hepatocellular carcinoma surgery. Zhonghua Gandan Waike Zazhi. 2017;23:134-136. [DOI] [Full Text] |
| 34. | Rungsakulkij N, Suragul W, Mingphruedhi S, Tangtawee P, Muangkaew P, Aeesoa S. Prognostic role of alpha-fetoprotein response after hepatocellular carcinoma resection. World J Clin Cases. 2018;6:110-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 35. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5537] [Cited by in RCA: 6607] [Article Influence: 412.9] [Reference Citation Analysis (0)] |
| 36. | Liu H, Yan Y, Chen R, Zhu M, Lin J, He C, Shi B, Wen K, Mao K, Xiao Z. Integrated nomogram based on five stage-related genes and TNM stage to predict 1-year recurrence in hepatocellular carcinoma. Cancer Cell Int. 2020;20:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Song L, Li J, Luo Y. The importance of a nonsmooth tumor margin and incomplete tumor capsule in predicting HCC microvascular invasion on preoperative imaging examination: a systematic review and meta-analysis. Clin Imaging. 2020;76:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 38. | Creyghton MP, Roël G, Eichhorn PJ, Hijmans EM, Maurer I, Destrée O, Bernards R. PR72, a novel regulator of Wnt signaling required for Naked cuticle function. Genes Dev. 2005;19:376-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Lin J, Lin W, Ye Y, Wang L, Chen X, Zang S, Huang A. Kindlin-2 promotes hepatocellular carcinoma invasion and metastasis by increasing Wnt/β-catenin signaling. J Exp Clin Cancer Res. 2017;36:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 40. | Jin X, Liu Y, Liu J, Lu W, Liang Z, Zhang D, Liu G, Zhu H, Xu N, Liang S. The Overexpression of IQGAP1 and β-Catenin Is Associated with Tumor Progression in Hepatocellular Carcinoma In Vitro and In Vivo. PLoS One. 2015;10:e0133770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Bengochea A, de Souza MM, Lefrançois L, Le Roux E, Galy O, Chemin I, Kim M, Wands JR, Trepo C, Hainaut P, Scoazec JY, Vitvitski L, Merle P. Common dysregulation of Wnt/Frizzled receptor elements in human hepatocellular carcinoma. Br J Cancer. 2008;99:143-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 178] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 42. | Toso C, Meeberg G, Hernandez-Alejandro R, Dufour JF, Marotta P, Majno P, Kneteman NM. Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma: A prospective validation. Hepatology. 2015;62:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 239] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 43. | Galle PR, Foerster F, Kudo M, Chan SL, Llovet JM, Qin S, Schelman WR, Chintharlapalli S, Abada PB, Sherman M, Zhu AX. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019;39:2214-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 436] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 44. | He C, Peng W, Liu X, Li C, Li X, Wen TF. Post-treatment alpha-fetoprotein response predicts prognosis of patients with hepatocellular carcinoma: A meta-analysis. Medicine (Baltimore). 2019;98:e16557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 45. | Hong G, Suh KS, Suh SW, Yoo T, Kim H, Park MS, Choi Y, Paeng JC, Yi NJ, Lee KW. Alpha-fetoprotein and (18)F-FDG positron emission tomography predict tumor recurrence better than Milan criteria in living donor liver transplantation. J Hepatol. 2016;64:852-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 46. | Earl TM, Chapman WC. Tumor size remains key for prediction of hepatocellular carcinoma recurrence after liver transplantation. Ann Surg Oncol. 2011;18:1217-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Marelli L, Grasso A, Pleguezuelo M, Martines H, Stigliano R, Dhillon AP, Patch D, Davidson BR, Sharma D, Rolles K, Burroughs AK. Tumour size and differentiation in predicting recurrence of hepatocellular carcinoma after liver transplantation: external validation of a new prognostic score. Ann Surg Oncol. 2008;15:3503-3511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | Santopaolo F, Lenci I, Milana M, Manzia TM, Baiocchi L. Liver transplantation for hepatocellular carcinoma: Where do we stand? World J Gastroenterol. 2019;25:2591-2602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 83] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (3)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Akarsu M, Barauskas G S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Liu JH