Published online Oct 15, 2021. doi: 10.4251/wjgo.v13.i10.1475
Peer-review started: February 21, 2021
First decision: April 6, 2021
Revised: April 16, 2021
Accepted: July 21, 2021
Article in press: July 21, 2021
Published online: October 15, 2021
Processing time: 234 Days and 11.9 Hours
The differential diagnosis between benign and malignant lymph nodes (LNs) has a fundamental role in the characterization and staging of malignant conditions, as well as in subsequent patients’ management. All imaging modalities (i.e. computed tomography and magnetic resonance imaging) rely mainly on size; endoscopic ultrasound (EUS) criteria based on B-mode evaluation and Doppler features fail to adequately characterize with high specificity LNs nature. The introduction of EUS-elastography and contrast-enhanced harmonic EUS are useful techniques to increase the diagnostic yield in identifying metastatic LNs, to identify which suspicious LN should require pathological characterization and, finally, to target tissue acquisition. EUS-guided tissue acquisition (EUS-TA) is increasingly being used for diagnosing lymphadenopathy whenever the characterization modifies patients’ subsequent management and when no superficial LN is accessible. Since target therapy are currently available (i.e. lung cancer, breast cancer), EUS-TA of malignant LNs could be required to identify tumor biology. In this field, both fine needle aspiration and biopsy needles are able to guarantee accurate results with almost perfect specificity and sub-optimal sensitivity. We finally propose a diagnostic algorithm based on most recent, high-level evidence for the diagnostic approach to suspected LNs assessment.
Core Tip: The characterization of suspected mediastinal or abdominal lymph nodes (LNs) represents a crucial indication for endoscopic ultrasound (EUS) and tissue acquisition, since may significantly impact patients’ management and clinical out
- Citation: Tamanini G, Cominardi A, Brighi N, Fusaroli P, Lisotti A. Endoscopic ultrasound assessment and tissue acquisition of mediastinal and abdominal lymph nodes. World J Gastrointest Oncol 2021; 13(10): 1475-1491
- URL: https://www.wjgnet.com/1948-5204/full/v13/i10/1475.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i10.1475
Lymph nodes (LNs) are bean-shaped organs, with a variable diameter ranging bet
LNs may enlarge and change their architecture under different conditions, mainly due to acute or chronic inflammation, and neoplastic infiltration including hematological disorders[2].
The characterization of suspicious LNs has a pivotal role in clinical practice, mainly for the correct staging of malignant tumors. The N staging of Tumor-Node-Metastasis classification is essential to determine the prognosis of patients and to decide the correct medical, endoscopic or surgical approach to the disease[3-5]. However, the differential diagnosis between benign and malignant LNs through conventional imaging techniques has always been challenging. Imaging methods mostly rely on the size and shape of LNs, but these parameters seem not to be accurate, since a great part of metastatic LNs measure less than 5 mm in diameter[4-7]. Nowadays, thanks to the wide selection of therapeutic options for most thoracic and abdominal neoplasms, it is fundamental to find minimally invasive methods for more reliable LNs differential diagnosis.
Endoscopic ultrasonography (EUS) was introduced in the clinical practice about 40 years ago and it is now used in everyday hospital routine. It has become an important technique due to its high accuracy in diagnosis and staging of a variety of benign and malignant conditions[8]. The main advantage of EUS lies in its capability to visualize the gut wall as a multi-layer structure corresponding to histologic layers and to display very closely to organs and lesions surrounding the gut wall, in particular the pancreato-biliary area, mediastinal and abdominal masses and LNs. In 1992 fine needle aspiration (FNA) has been developed as an adjunct to EUS techniques, since then it has become indispensable in clinical practice making it possible to safely obtain tissue diagnosis of most masses and lesions under the reach of EUS[9-11].
Since its introduction, the indications and role of EUS have continued to expand and it has been validated in lots of diagnostic and staging algorithms. Staging of gastrointestinal neoplasms has dramatically changed. Many studies have demon
Ultrasound (US) is the imaging method of choice for the evaluation of LNs, because of its high resolution compared to CT and MRI, thanks to its cost-effectiveness and the possibility of a real-time evaluation. For anatomical reasons conventional transcutaneous US examination is difficult or not possible in deeper regions (abdominal and mediastinal LNs); endoscopic US should be considered in such cases. Current indications for conventional US B-mode techniques are: The detection of suspicious LNs, the characterization of palpable and peri-intestinal (EUS) LNs, the staging of malignancies and the guidance for LN puncture[1,2].
To differentiate LNs we often rely on their morphologic characteristics and to
Thanks to FNA/fine needle biopsy (FNB) EUS has undergone an impressive development but, nowadays, the use of EUS image enhancement techniques, such as Doppler-EUS, EUSreal-time tissue elastography (EUS-RTE) and contrast enhancement EUS (CE-EUS), can further improve the diagnostic value of EUS in the evaluation of lymphadenopathies, focusing on architectural and vascular changes of pathologic LNs and obtaining optimal results in terms of accuracy[7,14,16-18].
Doppler US was developed for the study of vessels and of the macrovascular ar
Doppler modes are useful in evaluating and diagnosing vascular pathologies or in assessing vascular characteristics of normal or pathologic tissues, although some of their capabilities are limited such as the assessment of flow in vascular beds, which is too slow to be detected with these techniques. In EUS, Doppler techniques are useful in guiding invasive procedure, such as FNA/FNB or injection[19,20]. However, color-Doppler hilar vascularization, peripheral signals and spectral analysis present perfect technical results in trans-abdominal US, while in EUS the use could be limited by scope instability, LNs size and limited probe capacity.
Strain imaging (“elastography”) is a real-time imaging technique for tissue characterization, which displays differences in hardness between tissues. It is based on the generation of external or internal forces (EUS elastography is based on the internal force concept) and measures compressioninduced tissue deformation (strain): Stiff tissues present lower strain and deform less under compression, while soft tissues deform more. Tissue deformation is evaluated within a region of interest (ROI) and assessed in a comparative fashion; it is then visualized using a transparent color overlay on the Bmode image. Different pathological processes, such as fibrosis, inflammation, and cancer, can alter tissue stiffness. Elastography was initially developed for the evaluation of organs and lesions accessible from the body surface but it was subsequently combined with conventional EUS (EUS real-time elastography) with promising results, in particular in the evaluation of pancreatic lesions and LNs[21-23].
No specific patient’s preparation is needed for EUSelastography (EUS-E) and, even if it’s not expected to replace bioptic assessment, it can give advantages to EUS thanks to its ease of use and its low cost[21,24]. Tissue stiffness is evaluated through the application of slight compression with an US transducer to the targeted tissue; the necessary strain is provided by physiologic vascular pulsation and respiratory movements. Displacements are measured only in an axial direction; resulting tissue displacement is recorded by the EUS probe and coded by a specific software. The region immediately in front of the transducer face could be subjected to more stress than the lateral portions because of the use of a curved array transducer but, to improve uniformity in strain image, the size of the ROI can be reduced[22,25].
EUS-E is performed by using a two-panel image, with both the conventional grey-scale B-mode EUS image and the elastography image. Qualitative EUSRTE uses a combination of color patterns to differentiate benign and malignant lesions. Elasticity (on a scale of 1–255) is shown as a superimposed color map on a conventional B-mode image; in this elasticity map hard tissues (minor strain) are visualized by dark blue, intermediate tissue elasticity results green and yellow, whereas soft tissues (distinct strain) are shown in red (color map may be changed by the operator). For a good reliable examination, a consistent color pattern in consecutive frames is necessary. The elastography ROI should include the targeted lesion as well as the surrounding tissues as a reference. The lesion of interest should cover 25%–50% of the ROI and, in the case of a large lesion, the ROI can be placed toward the edge of the lesion[21,25,26].
Using semi-quantitative elastography the operator can obtain better results, com
EUS-E has no formal contraindication. Over the past years EUS-E was used as a complementary method to other techniques for the evaluation of pancreatic lesions and lymph-nodes, but it has been progressively included in clinical evaluation of other lesions, such as subepithelial lesions of the GI tract and focal liver lesions[22].
Contrast-enhanced EUS is an EUS ancillary technique which combines the advantage of EUS with the administration of US contrast agents. Contrast agents consist of gas-filled microbubbles, covered with a phospholipid or lipid shell, of approximately 2 to 5 μm diameter, which resonate and are disrupted when receive US waves, producing a signal detected in the US image[28,29]. They present a pure intravascular distribution, so that they do not diffuse into the extravascular space; gases are then not metabolized in the human body and are eliminated by the lungs, while the stabilising shells are eliminated by the liver[30].
CE-EUS can be performed by using color or power Doppler as a generic signal intensifier in Contrast-Enhanced Color and Power Doppler EUS (CE-EUS) or by using a dedicated contrast harmonic in Contrast-Enhanced Harmonic EUS (CH-EUS). Doppler has been historically useful for evaluating large blood vessels with fast-flowing blood but is not sensitive enough to detect slow and low-volume flow. Microbubble contrast agents increase the intensity of backscattered USs and can increase the Doppler signal of small vessels (arterioles and venules) with a diameter of approximately 0.1–0.4 mm, but not the signal of capillaries. Unfortunately, CE-EUS has several disadvantages such as blooming artefacts, vulnerability to motion artefacts and low sensitivity to slow flow[26,31].
Contrast harmonic techniques permit to improve the resolution of this method filtering signals originating from different tissues by selectively detecting harmonic components. Harmonic imaging is based on non-linear acoustic effects of US in
During CH-EUS examination the echoendoscope is placed in front of the lesion of interest and, like in EUS-E, a reference B-mode image is kept beside the contrast enhanced image through a dual screen. In order to avoid breaking the microbubbles, a large intravenous catheter should be employed for the injection of the contrast agent, which should be administered slowly and followed by a saline flush. After the injection, the uptake and washout of the contrast agent are evaluated for at least 120 s: The microbubbles emit dynamically strong echo signals depicting large and small vessels and showing the microvasculature of the evaluated tissue, from the un
In recent years CH-EUS is achieving popularity, even if consensus is still lacking about its exact role in diagnostic workup of gastrointestinal lesions. It is commonly used in the investigation of pancreatic cystic and solid lesions, gallbladder abnor
The European Federation of Societies for US in Medicine and Biology guidelines recommend the use of elastography and US contrast agents to increase the ability to distinguish benign or malignant nature of solid pancreatic tumors, pancreatic cystic neoplasms, and LNs. On the other hand, these methods could be used to improve the diagnostic performance of tissue sampling[38-41].
The introduction of EUS-FNA and FNB allowed a definite pathology for thoracic and abdominal masses. EUS combines the high-resolution US imaging of lesions and a safe and effective fine needle-based tissue acquisition of these lesions[11,42].
Several types of needles have been developed to provide histological tissue examination. Anyway, also standard aspiration needles (mainly larger needles) may provide the capture of tissue suitable for histopathological evaluation[43-47]. His
The “fanning technique”, which permits to sample a large part of the target solid lesion or lymph node, represents the best EUS-sampling technique and is associated with a significant lower number of passes needed to establish the diagnosis[49]. “Suction technique” is used to facilitate EUS-guided tissue acquisition and consists in applying a negative suction pressure using a 5 mL or 10 mL syringe. On the other hand, the so-called “slow-pull technique”, consisting in the controlled pulling of the stylet allows a smooth negative pressure able to collect cytological specimens[50].
The amount of needle passes could be reduced based on on-site pathological evaluation (ROSE), where available, or should be based on gross visual inspection of the obtained material and the type of target lesion. A high number (≥ 7) of needle passes is thought to be a predictor of a high diagnostic sensitivity of EUS-FNA[51,52].
EUS-tissue acquisition is safe, with very low incidence of severe complications. No correlation among needle caliber and design and incidence of adverse events has been reported.
Perforation (0.03%–0.15%) is mainly due to the echoendoscope passage and not to the sampling procedure; limited data show that the perforation risk of EUS is similar to standard endoscopy.
The risk of acute pancreatitis following EUS-FNA is related to procedures which involve direct passage of the needle through the pancreas; the risk is higher in cystic than solid lesions sampling (0%-2%).
Severe bleeding following EUS-FNA is a rare event (0.13%), anyway an accurate evaluation between thromboembolic risk vs bleeding risk is mandatory before performing a EUS-guided intervention in patients on antiplatelet and/or anticoagulants therapy: Anticoagulant drug withdrawal should be considered based on patients’ risk assessment. A total platelet count lower than 50000/mmc and a prolonged prothrombin time (international normalized ratio greater than 1.5) should be considered a contraindication. No correlation among needle size, design, amount of passes has been related to an increased bleeding incidence[53,54].
Bacteremia and septic episodes following gastrointestinal EUS-FNA are rare events, comparable to that of standard diagnostic endoscopy; they are slightly more frequent in case of cystic lesions. In less than 1% of cases post-FNA fever was reported. Serious infectious complications have been reported following EUS-FNA of mediastinal cysts, ascites, perirectal cysts, pancreatic cystic lesions and pancreatic fluid collections. Guidelines recommend peri-interventional antibiotic treatment for EUS-FNA of pancreatic cystic lesions despite the absence of certain evidence, while the aspiration of mediastinal cysts is mainly contraindicated considering the potentially severe consequences of mediastinal infection[53-56].
EUS-guided sampling for malignant pancreatic tumors and for cholangiocarcinoma seems not to be a risk factor for the development of peritoneal seeding, tumor recurrence or decreased survival. The risk of peritoneal seeding following biopsy of pancreatic cancer appears to be significantly lower with EUS-FNA compared to percutaneous FNA[53,54].
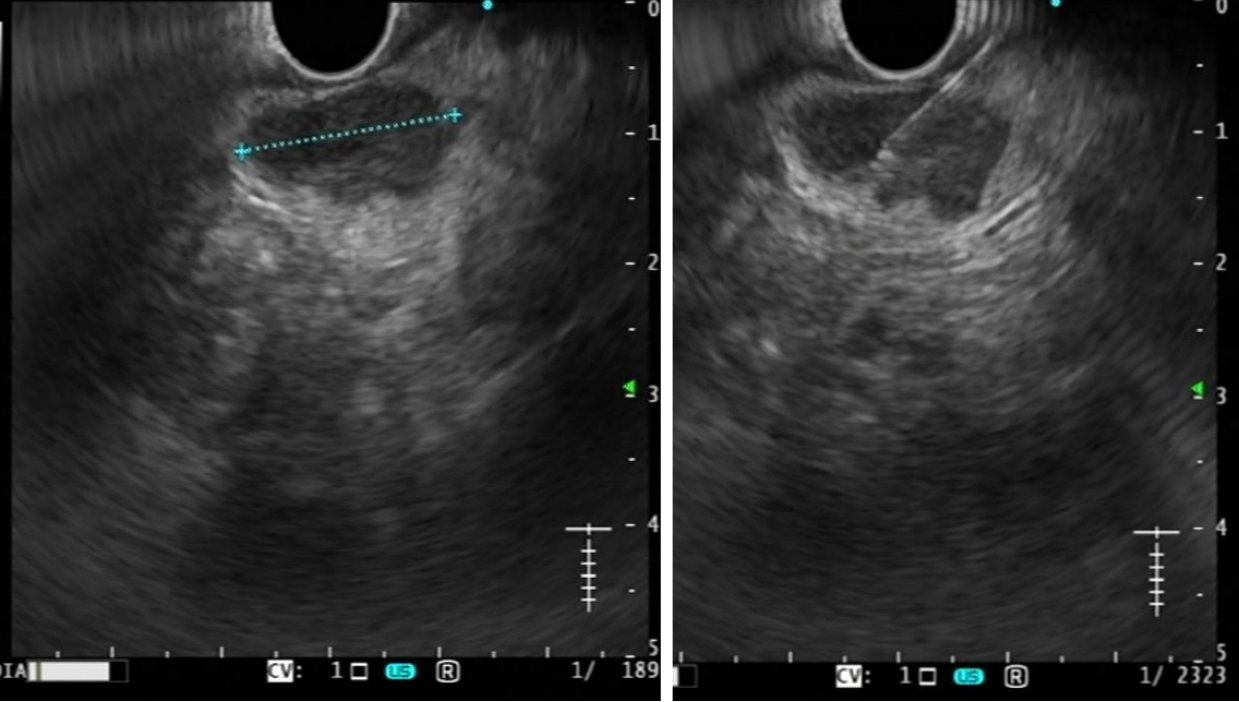
Normal LNs have a characteristic B-mode appearance, with a typical two-layer architecture: An echo-poor cortex and a hyperechoic inner zone (hilum), which consists of fat and connective tissue, blood and lymph vessels. When using high-frequency probe, echo-poor or cystic follicles can sometimes be seen within the hypoechoic cortex. LNs shape is typically oval; supplying vessels can sometimes be seen entering the hilum with tree-like branches departing from the hilum to the cortex at color-Doppler evaluation[1,2]. The most important ultrasonographical features for differential diagnosis are size, shape, border, presence or absence of a central hilum, and cortical homogeneity (Figure 1)[57].
Size: The nodal size is the most relevant parameter for LNs nature diagnosis; all imaging modalities use size for the LNs differential diagnosis. Normal LN diameter varies according to their different locations (from 1 to 40 mm) and the best size parameter to evaluate is debated. As a general rule, LNs with diameter < 15 mm seem to grow mainly roundish by most pathological processes (both in inflammation and neoplastic infiltration); in LNs > 15-20 mm the main growth is on the longitudinal diameter, instead. Van den Brekel et al[58] proved that the minor axial diameter is the most accurate in predicting tumor positive nodes[58]. Anyway, both inflammation and malignant infiltration cause LNs enlargement and the distinction seems to be almost impossible when based only on nodal size: Even LNs with small diameters (< 3−5 mm) might be malignant, thus a higher limit value leads to higher specificity but lower sensitivity in differential diagnosis and it is not possible to set a reference value[4-6]. In gastrointestinal and pancreatic cancer about 50% of metastatic infiltrations are found in small LNs, sized ≤ 5 mm[7].
Shape and border: As a rule, normal LNs are oblong, except in some sites of head and neck. They present a sharp border, often not clearly distinguishable from the sur
Most inflammatory LNs preserve their oval shape due to a homogeneous invo
Longitudinal-to-short axis ratio, the so-called Solbiati-Index (SI), has been often proposed for differentiation of reactive and malignant LNs. A SI of less than 2 is supposed to be a criterion of malignancy, even if it is more sensitive in the head and neck region than in other sites, such as in axillary and inguinal region, where it’s often false-negative[63].
Hilum: Homogeneously hypoechoic LNs without hyperechoic hilum are often considered malignant because of neoplastic hilar infiltration. However, in the head and neck region also some normal and inflammatory LNs could show no hyperechoic hilum and, by contrast, an echogenic hilum can often be seen even in neoplastic LNs, mostly in early phases. In some cases, squamous cell carcinoma metastases can cause inner inhomogeneities which appear hyperechoic and can resemble the hilum[2,64].
Echostructure: LNs cortex is typical uniform, even if slight width fluctuation is considered normal. Its thickness is only evaluable in the presence of a visible hilum and its uniformity can often be analyzed only in larger LNs. Cortical thickening occurs when cortex is > 50% of the main axis, and its thickness is considered asymmetric when it is at least double at one site than at the narrowest point. In inflammatory LNs with hypertrophic lymphatic follicles the cortex is typically concentrically widened, on the contrary eccentric cortical infiltration is an important sign of malignancy, even though cortex could be symmetrically involved in anaplastic carcinoma[2,63,65]. Lymphomas are diffuse diseases and often affect LNs cortex in a uniform way, even if it is not a rule.
Furthermore, the tendency toward necrotic and cystic degeneration of solid tumors can cause inhomogeneity of the internal structure of LNs, which is rare in lymphomas[2].
Faige et al[66] proposed a Visual analogue scale for the EUS assessment of LNs based on their B-mode appearance[66].
In normal LNs arteries and veins enter through the hilum and branch towards the cortex, ending in subcapsular sinuous capillaries. In most inflammatory processes there is no significant change in vascular architecture and, in early stages, also metastatic LNs preserve original vascularity, sometimes increased by related immune reactions. In advanced stages bulky neoplastic infiltration and desmoplastic reaction distort and encase the original vascular structure and, due to the production of angiogenetic factors, tumors recruit capsular vessels leading to peripheral and intra-tumoral hypervascularity. Anyway, the vessel density of malignant LNs often depends on the type of primary tumor and neoplastic LNs often show avascular areas due to necrotic or cystic degeneration[38,67,68]. On color- and power-Doppler US, the vascular pattern of normal LNs is characterized by hilar vascularity without pe
In summary, most remarkable Doppler-EUS findings suggesting malignancy are: Displacement of vascular hilum, focal avascular areas, aberrant and subcapsular vessels[69]. Esen et al[65] described a sensitivity of 50% but specificity of 97% of color Doppler US criterion alone for the detection of metastatic LNs[65].
On spectral analysis, normal LNs hilar arteries present a resistive index (RI) < 0.75 and a pulsatility index (PI) usually lower than 1.6. Inflammatory LNs usually show a higher vascularity with RI < 0.8 and PI < 1.6. In metastatic LNs blood vessels within the capsule are compressed by tumor infiltration, and vascular resistance may rise, usually with high RI (> 0.8) and high PI (> 1.6)[2,67]. It was hypothesized that the intranodal pressure increases as long as the capsule is intact, with increased resistance and relative ischemia. A relative drop in RI can follow the decrease of pressure within the LN due to malignant infiltration of the capsule or to the presence of arteriovenous shunts in advanced architecture distortion[1,70,71]. The RI in lymphomatous LNs often measures intermediate values between reactive and metastatic LNs[2]. Table 1 summarizes B-mode and color-Doppler criteria for LNs characterization.
| Benign LNs | Malignant LNs | |
| B-mode criteria | ||
| Shape | Oval | Round |
| Border | Irregular shape | Clear-cut, asymmetric |
| Echogenicity | Hyperechoic | Hypoechoic |
| Vascular hilum | Present, central | Absent |
| Echostructure | Heterogeneous | Homogeneous |
| Color-Doppler criteria | ||
| Hilar doppler sign | Present | Absent or displaced |
| Subcapsular vessels | Absent | Often present |
| Avascular areas | Absent | Often present |
| Resistance index | < 0.8 | > 0.8 |
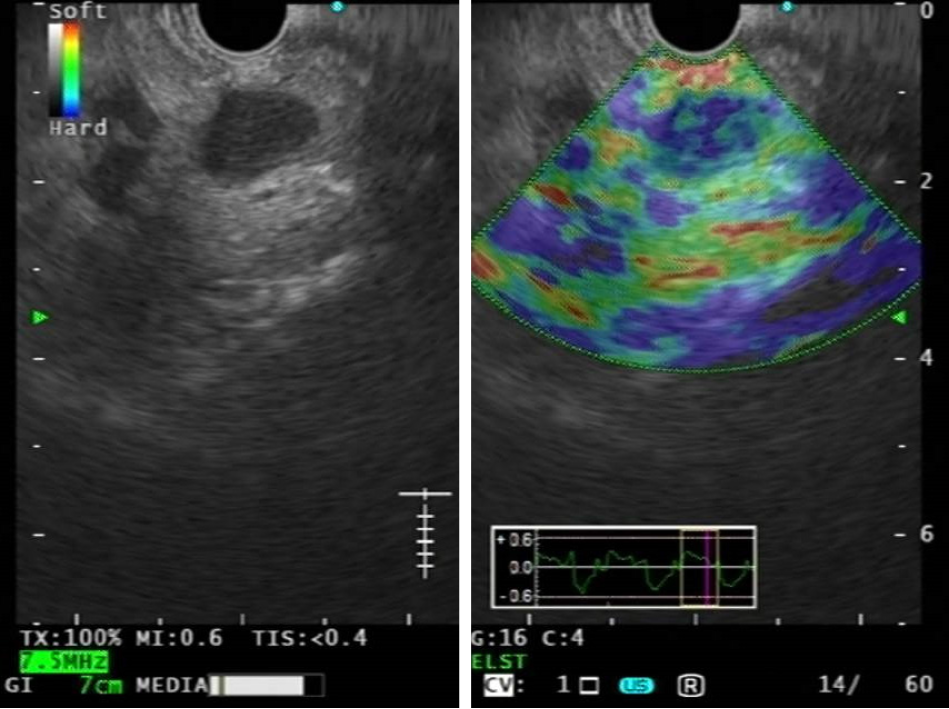
FNA/FNB remains the gold standard technique for the determination of LNs malignant infiltration, with a positive predictive value (PPV) up to 100%, in this context elastography could be useful in selecting most suspicious LNs for tissue sampling, reducing unnecessary biopsies (Figure 2). EUS-E can detect even early neoplastic infiltration or small metastatic nodes. Elastography can also offer an alternative for the differential diagnosis in cases of negative biopsy or if tissue acquisition is not possible[7,18,72,73].
Giovannini et al[16] studied the ability of EUS-E in the differential diagnosis between benign and malignant LNs, highlighting its importance for guiding biopsy and showing higher sensitivity and specificity compared to B-mode EUS (91.8% and 82.5% vs 78.6% and 50.0%). PPV and NPV were of 88.8% and 86.8%, respectively[16]. Săftoiu et al[18] showed high 91.7% sensitivity, 94.4% specificity and 92.9% diagnostic accuracy) in diagnostic differentiation using a qualitative analysis; even higher using quantitative histogram analysis, which is supposed to be less operator-dependent[18].
Malignant tissues are generally harder than normal tissues and elastography can give useful information for the distinction between benign and malignant LNs based on their stiffness. In most studies the elastography images have been scored according to different elastography patterns: LNs have been classified by EUS-E according to homogeneity and prevalent color, distinguishing homogeneous (blue or green), heterogeneous or honeycombed pattern[17,73,74]. In particular, Giovannini et al[16] classified LNs as benign when qualitative EUS-E showed an homogeneous green pattern or an heterogeneous soft tissue (green, yellow and red tissue), malignant when homogeneously blue or mainly blue with one or some central areas of soft tissue (representing necrotic areas), suspicious when it showed an honeycomb pattern, with mixed hard and soft tissue.
In conclusion, EUS-E is an ancillary imaging method for LNs characterization and with an optimal diagnostic yield; in particular, EUS-E seems to increase the diagnostic yield in case of small LNs, which are difficult to characterize through B-mode and tissue acquisition. EUS-E provides diagnostic information to conventional B-mode imaging and can be used for the selection of suspicious LNs worth FNA/FNB thanks to its high PPV; EUS-E alone cannot obviate the need of tissue acquisition in suspected LNs[18,73,75].
There are only few studies about the use of CE-EUS in the differential diagnosis of LNs.
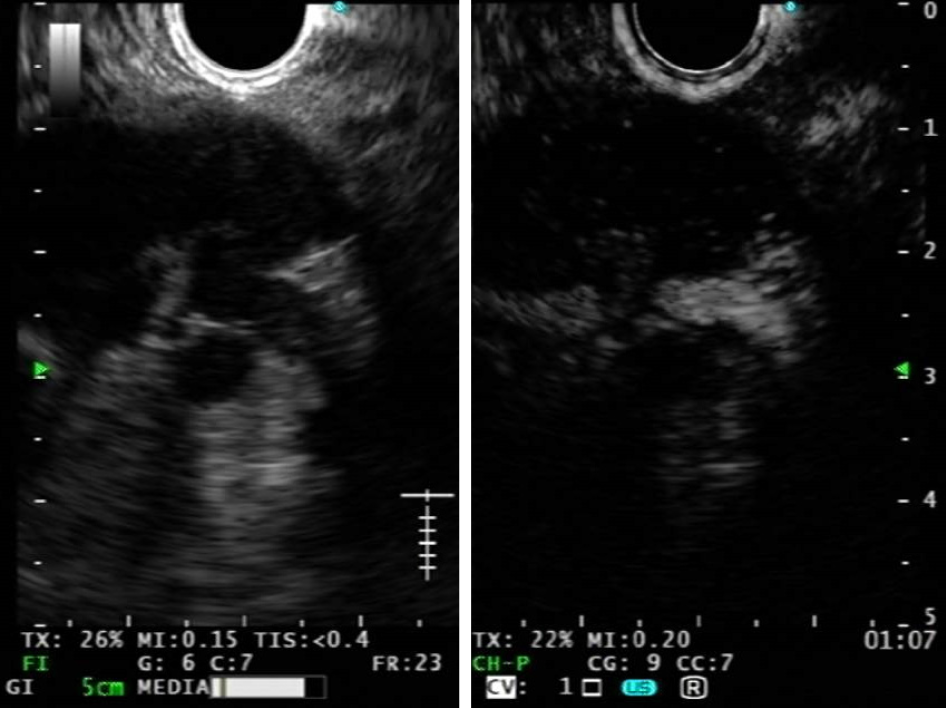
CE-EUS permits to analyze nodal microvasculature (Figure 3): contrast enhan
In metastatic LNs the normal capillary bed is destroyed by malignant infiltration, which also causes intranodal neoangiogenesis with development of pathological vessels. Thus, malignant LNs typically show a change in the perfusion pattern at CE-EUS with centripetal inhomogeneous enhancement due to abnormal neoplastic vessels, arteriovenous shunts and hypovascular areas, detected as focal hypoenhanced areas. For the same reasons malignant LNs also show longer contrast enhancement duration in comparison to benign ones. It’s possible to find avascular necrotic areas in granulomatous lymphadenopathies, such as tuberculosis and sarcoidosis leading to false positive results[32].
While color Doppler-EUS can study nodal vasculature only at the level of arterioles and venules, contrast harmonic mode can evaluate LNs microvasculature pattern. Thus, a massive lymph node involvement by malignancies could be detected even by CE-EUS, but tiny areas with abnormal capillary network within LNs can be revealed only at CH-EUS. In lymphomas CH-EUS nodal patterns are variable; the most common finding is intense homogeneous enhancement, not different from reactive LNs, because of preserved good vascularization in the capillary bed[32,37,77]. Finally, in case of CH-EUS non enhancement pattern, a colliquative necrosis could be deduced, suggesting the presence of inflammatory LN (i.e. tubercular LN with extensive necrosis).
Like EUS-E, contrast-enhanced EUS could be used for FNA targeting, in order to choose the most suspicious LNs ant to select vital nodal areas: the identification of signs of neoangiogenesis and the detection of hypoenhancing necrotic areas may be useful in detecting malignant infiltration and in guiding biopsy[32].
A recent meta-analysis showed that CE-EUS has a poor pooled sensitivity (82.1%) and an optimal specificity (90.7%) in LNs differential diagnosis[14]; the use of dedicated CH-EUS slightly increases the diagnostic accuracy, with a pooled sensitivity of 87.7% and a pooled specificity of 91.8%. Anyway, recent guidelines still do not recommend CH-EUS for this indication[11,42].
The concomitance of more than one LN is a clear CH-EUS limit; in fact, LNs characterization is focused on the arterial and early venous phase. Repeated contrast administrations should be necessary. The use of B-mode and EUS-E to identify suspected LNs, and then CH-EUS to characterize in detail could be performed.
Making the correct differential diagnosis of mediastinal and abdominal lymphadenopathies is still challenging and tissue acquisition becomes often necessary: EUS provides an easy access to these LNs (Figure 1) and allows a better evaluation and possibly FNA/FNB even for small LNs[78].
European guidelines recommend EUS-tissue acquisition as first-line histological assessment of mediastinal or abdominal LNs if the pathological result modifies the patient’s management and if percutaneous biopsy is not possible[11,42].
EUS-guided tissue acquisition was proved to increase the diagnostic accuracy in diagnosis and staging and to have a good safety profile, probably related with the high spatial resolution of EUS, the proximity of target lesions to the EUS probe and the possibility to identify interposed vessels[79-82].
There is no robust evidence supporting the choice of different techniques for EUS LN sampling. Indeed, EUS-TA techniques are mainly assessed in solid pancreatic tumors, that are usually fibrotic and poorly cellulated. As described before, LNs architecture is different to pancreatic one: Malignant LNs are usually poorly fibrotic and highly cellulated. The results observed in solid pancreatic tumors are not 100% reproducible in this field. Therefore, needle choice, number of needle passes, type and amount of suction could only be deduced from knowledge in the field of solid pancreatic tumors needle aspiration.
False-negative results are mainly related to the characteristics of the lesion (size and nature of the tumor) and to technical aspects of EUS-FNA (sampling errors and interpretative errors). The presence of small lesions or of an interposed vascular structure between the transducer and the biopsy target can lead to inadequate or nonrepresentative samples. Furthermore, the presence of severe inflammation can hide an infiltrating tumor and cause histopathological errors[80,83].
Causes for false-positive results are epithelial cell contamination, EUS sampling error and pathological misinterpretation. Tumor cells can be present in luminal fluid and can enter the FNA needle as it passes through the gut lumen to reach the target lymph node. Main contaminants originate from the duodenal and gastric mucosa, but malignant cells are commonly present even in the luminal fluid of patients with pancreatic cancer and not only with luminal cancers. Furthermore, it is advised not to pass through the primary tumor with the needle when performing EUS-tissue acquisition of LNs, in order to avoid contamination. It is also plausible that false-positive results occur when the interposing mucosa is inflamed or in premalig
Based on high-quality evidence, the rate of false-negative results is dramatically higher, compared to false-positive ones. This aspect could impact patients’ prognosis since the risk of under-staging with consequent under-treatment could not be ex
Cytopathologists and endoscopists should co-operate, sharing information about indications, clinical history and endoscopic technique, and increasing the level of their expertise, in order to reduce these kinds of errors and to increase the diagnostic value of this technique[82,85].
Okasha et al[78] reported a sensitivity and specificity of LNs EUS-FNA of 92% and 100%, respectively, with a very high PPV (100%) and a negative predictive value (NPV) of 88.1% in diagnosing malignant LNs[78].
A recent meta-analysis (26 studies, 2833 LNs) demonstrated that EUS-FNA present 87% pooled sensitivity with a 100% specificity, with an area under the curve of 0.99. The authors showed that sensitivity was slightly higher for abdominal (87%) than mediastinal (85%) LNs. Significant impact of ROSE was also observed (91% vs 85%)[86].
In 2020, another meta-analysis dealt with pooled diagnostic performance of EUS-FNA for abdominal LNs characterization[87]. The Authors included 12 studies (774 patients) and reported 94% pooled sensitivity and 98% specificity.
On the other hand, a meta-analysis conducted in 2008 was focused on the diagnostic accuracy of EUS-FNA on mediastinal LNs and showed a 88% pooled sensitivity and 96.4% pooled specificity[88]. Interestingly, this study clearly demonstrated that EUS-FNA diagnostic accuracy increased over time; indeed, studies conducted in 2000’s showed a pooled sensitivity of 91.7%[88].
To date, no randomized controlled study was designed to assess the difference between EUS-FNA and EUS-FNB for LNs characterization. A large trial comparing 20-gauge FNB needle to a standard 25-gauge FNA needle in 608 patients with solid lesions included a small proportion of LNs[89]. The Authors observed a trend toward a better accuracy in the FNB needle group (data on file; courtesy of Dr. Priscilla van Riet).
A large retrospective study enrolling 209 patients undergoing LN sampling. The Authors reported similar sensitivity (67% vs 75%) and diagnostic accuracy (79% vs 83%) for EUS-FNA and EUS-FNB, respectively, while a higher specificity was observed in EUS-FNB group (100% vs 94%). The Authors observed that ROSE availability increases the odd to obtain a correct diagnosis (Odd ratio 5.16 for accuracy)[90].
All studies reported a very low incidence of adverse events for EUS-guided LNs tissue acquisition. Pooled incidence was as low as 1.6% in published studies[86].
As demonstrated in the setting of solid pancreatic tumor, the diagnostic accuracy of EUS-guided tissue acquisition of LNs could be improved if used in combination to CH-EUS; the use of US contrast agent could be helpful not only in avoiding necrotic avascular areas, but also helping to identify the most suspicious LNs to be targeted[91].
High-quality evidence demonstrated that EUS-FNA is a sensitive, highly specific, and safe diagnostic tool for pathological characterization of mediastinal and ab
Table 2 summarizes the diagnostic performance of EUS (B-mode and Doppler), EUS-E, CH-EUS and EUS-guided tissue acquisition. While there is consensus on the low yield of B-mode EUS and Doppler criteria, the application of image enhancement techniques was not widely used, and final diagnosis was often based on EUS-guided tissue acquisition.
| Pooled sensitivity | Pooled specificity | Area under the curve | Ref. | |
| EUS (B-mode + Doppler) | 84.7% | 84.6% | 0.91 | Puli et al[88], 2008 |
| EUS-E | 88% | 85% | 0.95 | Xu et al[75], 2011 |
| CH-EUS | 87.7% | 91.8% | 0.97 | Lisotti et al[14], 2019 |
| Combined EUS-E + CH-EUS1 | 43.6% | 100% | 0.92 | Lisotti et al[92], 2019 |
| Combined EUS-E “or” CH-EUS2 | 93.6% | 87.5% | 0.91 | Lisotti et al[92], 2019 |
| EUS-FNA | 87% | 100% | 0.99 | Chen et al[86], 2020 |
However, even in this setting, EUS-tissue acquisition is burdened by a not-negligible false negative rate, leading to a pooled sensitivity about 90%.
Outside clinical trials and tertiary centers, EUS-E presents high rates of unde
On these bases, our study group is conducting a prospective study, aimed to assess the combination of EUS-E and CH-EUS[92]. We found that the combination of the two techniques was significantly more accurate: the concordance of the two techniques shows a specificity and PPV of 100%. Interestingly, the sensitivity raised to 93.6% when at least one technique resulted negative (EUS-E “or” CH-EUS positive for malignancy). Finally, no patient with both techniques negative had a malignant LN (specificity for benign LNs 100%)[92].
The integration of the two techniques was able to overcome single limit; for example, CH-EUS was able to identify malignant necrotic LNs that appear “soft” on EUS-E but inhomogeneously enhanced on CH-EUS. On the other hand, EUS-E was able to identify lymphomas as “hard” LNs, that could appear benign, as homogeneously hyperenhanced, on CH-EUS.
In conclusion, as LNs characterization represent a crucial point for patients’ ma
| 1. | Weskott HP, Ioanitescu ES. Diagnostic Approach to Lymph Node Diseases in Ultrasound. In: EFSUMB Course Book on Ultrasound. 1st ed. 2012: 189-224. |
| 2. | Cui XW, Hocke M, Jenssen C, Ignee A, Klein S, Schreiber-Dietrich D, Dietrich CF. Conventional ultrasound for lymph node evaluation, update 2013. Z Gastroenterol. 2014;52:212-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Cho JW. The Role of Endosonography in the Staging of Gastrointestinal Cancers. Clin Endosc. 2015;48:297-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Mönig SP, Zirbes TK, Schröder W, Baldus SE, Lindemann DG, Dienes HP, Hölscher AH. Staging of gastric cancer: correlation of lymph node size and metastatic infiltration. AJR Am J Roentgenol. 1999;173:365-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 91] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Mönig SP, Baldus SE, Zirbes TK, Schröder W, Lindemann DG, Dienes HP, Hölscher AH. Lymph node size and metastatic infiltration in colon cancer. Ann Surg Oncol. 1999;6:579-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Schröder W, Baldus SE, Mönig SP, Beckurts TK, Dienes HP, Hölscher AH. Lymph node staging of esophageal squamous cell carcinoma in patients with and without neoadjuvant radiochemotherapy: histomorphologic analysis. World J Surg. 2002;26:584-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Dietrich CF, Jenssen C, Arcidiacono PG, Cui XW, Giovannini M, Hocke M, Iglesias-Garcia J, Saftoiu A, Sun S, Chiorean L. Endoscopic ultrasound: Elastographic lymph node evaluation. Endosc Ultrasound. 2015;4:176-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Fujita A, Ryozawa S, Mizuide M, Tanisaka Y, Ogawa T, Suzuki M, Katsuda H, Saito Y, Tashima T, Miyaguchi K, Arai E, Kawasaki T, Mashimo Y. Diagnosis of Pancreatic Solid Lesions, Subepithelial Lesions, and Lymph Nodes Using Endoscopic Ultrasound. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Keter D, Melzer E. Endoscopic ultrasound in clinical practice. Acta Gastroenterol Latinoam. 2008;38:146-151. [PubMed] |
| 10. | Teshima CW, Sandha GS. Endoscopic ultrasound in the diagnosis and treatment of pancreatic disease. World J Gastroenterol. 2014;20:9976-9989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Dumonceau JM, Deprez PH, Jenssen C, Iglesias-Garcia J, Larghi A, Vanbiervliet G, Aithal GP, Arcidiacono PG, Bastos P, Carrara S, Czakó L, Fernández-Esparrach G, Fockens P, Ginès À, Havre RF, Hassan C, Vilmann P, van Hooft JE, Polkowski M. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated January 2017. Endoscopy. 2017;49:695-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 242] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 12. | Schizas D, Ntanasis-Stathopoulos I, Tsilimigras DI, Sioulas AD, Moris D, Spartalis E, Scotiniotis I, Papanikolaou IS. The Role of Endoscopic Ultrasound in the Diagnosis and Management of Primary Gastric Lymphoma. Gastroenterol Res Pract. 2017;2017:2397430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Labarca G, Aravena C, Ortega F, Arenas A, Majid A, Folch E, Mehta HJ, Jantz MA, Fernandez-Bussy S. Minimally Invasive Methods for Staging in Lung Cancer: Systematic Review and Meta-Analysis. Pulm Med. 2016;2016:1024709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Lisotti A, Ricci C, Serrani M, Calvanese C, Sferrazza S, Brighi N, Casadei R, Fusaroli P. Contrast-enhanced endoscopic ultrasound for the differential diagnosis between benign and malignant lymph nodes: a meta-analysis. Endosc Int Open. 2019;7:E504-E513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Takasaki Y, Irisawa A, Shibukawa G, Sato A, Abe Y, Yamabe A, Arakawa N, Maki T, Yoshida Y, Igarashi R, Yamamoto S, Ikeda T. New endoscopic ultrasonography criteria for malignant lymphadenopathy based on inter-rater agreement. PLoS One. 2019;14:e0212427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Giovannini M, Thomas B, Erwan B, Christian P, Fabrice C, Benjamin E, Geneviève M, Paolo A, Pierre D, Robert Y, Walter S, Hanz S, Carl S, Christoph D, Pierre E, Jean-Luc VL, Jacques D, Peter V, Andrian S. Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: a multicenter study. World J Gastroenterol. 2009;15:1587-1593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 204] [Cited by in RCA: 203] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 17. | Giovannini M, Hookey LC, Bories E, Pesenti C, Monges G, Delpero JR. Endoscopic ultrasound elastography: the first step towards virtual biopsy? Endoscopy. 2006;38:344-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 216] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 18. | Săftoiu A, Vilmann P, Hassan H, Gorunescu F. Analysis of endoscopic ultrasound elastography used for characterisation and differentiation of benign and malignant lymph nodes. Ultraschall Med. 2006;27:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Gettle LM, Revzin MV. Innovations in Vascular Ultrasound. Radiol Clin North Am. 2020;58:653-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Sung JJ. The use of color Doppler EUS in gastrointestinal diseases. Endoscopy. 1998;30 Suppl 1:A149-A151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 21. | Dietrich CF, Bibby E, Jenssen C, Saftoiu A, Iglesias-Garcia J, Havre RF. EUS elastography: How to do it? Endosc Ultrasound. 2018;7:20-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Iglesias-García J, Lariño-Noia J, Domínguez-Muñoz JE. New Imaging Techniques: Endoscopic Ultrasound-Guided Elastography. Gastrointest Endosc Clin N Am. 2017;27:551-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Iglesias-Garcia J, Lindkvist B, Lariño-Noia J, Domínguez-Muñoz JE. Endoscopic ultrasound elastography. Endosc Ultrasound. 2012;1:8-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Dietrich CF, Cantisani V. Current status and perspectives of elastography. Eur J Radiol. 2014;83:403-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Havre RF, Elde E, Gilja OH, Odegaard S, Eide GE, Matre K, Nesje LB. Freehand real-time elastography: impact of scanning parameters on image quality and in vitro intra- and interobserver validations. Ultrasound Med Biol. 2008;34:1638-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Knauth Meadows A, Ordovas K, Higgins CB, Reddy GP. Magnetic resonance imaging in the adult with congenital heart disease. Semin Roentgenol. 2008;43:246-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Iglesias-Garcia J, Lindkvist B, Lariño-Noia J, Abdulkader-Nallib I, Dominguez-Muñoz JE. Differential diagnosis of solid pancreatic masses: contrast-enhanced harmonic (CEH-EUS), quantitative-elastography (QE-EUS), or both? United European Gastroenterol J. 2017;5:236-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Kitano M, Yamashita Y. New Imaging Techniques for Endoscopic Ultrasonography: Contrast-Enhanced Endoscopic Ultrasonography. Gastrointest Endosc Clin N Am. 2017;27:569-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Dietrich CF, Sharma M, Hocke M. Contrast-enhanced endoscopic ultrasound. Endosc Ultrasound. 2012;1:130-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Baert A, Sartor K. Contrast media in ultrasonography. In: Quaia E, editor Basic principles and clinical applications. 1st ed. Berlin: Springer, 2005: 3-14. |
| 31. | Alvarez-Sánchez MV, Napoléon B. Contrast-enhanced harmonic endoscopic ultrasound imaging: basic principles, present situation and future perspectives. World J Gastroenterol. 2014;20:15549-15563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Hocke M, Ignee A, Dietrich C. Role of contrast-enhanced endoscopic ultrasound in lymph nodes. Endosc Ultrasound. 2017;6:4-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Lencioni R, Cioni D, Bartolozzi C. Tissue harmonic and contrast-specific imaging: back to gray scale in ultrasound. Eur Radiol. 2002;12:151-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 119] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Kitano M, Sakamoto H, Matsui U, Ito Y, Maekawa K, von Schrenck T, Kudo M. A novel perfusion imaging technique of the pancreas: contrast-enhanced harmonic EUS (with video). Gastrointest Endosc. 2008;67:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 35. | Dietrich CF, Ignee A, Frey H. Contrast-enhanced endoscopic ultrasound with low mechanical index: a new technique. Z Gastroenterol. 2005;43:1219-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Fusaroli P, Kypraios D, Caletti G, Eloubeidi MA. Pancreatico-biliary endoscopic ultrasound: a systematic review of the levels of evidence, performance and outcomes. World J Gastroenterol. 2012;18:4243-4256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Cui XW, Jenssen C, Saftoiu A, Ignee A, Dietrich CF. New ultrasound techniques for lymph node evaluation. World J Gastroenterol. 2013;19:4850-4860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 89] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 38. | Piscaglia F, Nolsøe C, Dietrich CF, Cosgrove DO, Gilja OH, Bachmann Nielsen M, Albrecht T, Barozzi L, Bertolotto M, Catalano O, Claudon M, Clevert DA, Correas JM, D'Onofrio M, Drudi FM, Eyding J, Giovannini M, Hocke M, Ignee A, Jung EM, Klauser AS, Lassau N, Leen E, Mathis G, Saftoiu A, Seidel G, Sidhu PS, ter Haar G, Timmerman D, Weskott HP. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med. 2012;33:33-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 700] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 39. | Cosgrove D, Piscaglia F, Bamber J, Bojunga J, Correas JM, Gilja OH, Klauser AS, Sporea I, Calliada F, Cantisani V, D'Onofrio M, Drakonaki EE, Fink M, Friedrich-Rust M, Fromageau J, Havre RF, Jenssen C, Ohlinger R, Săftoiu A, Schaefer F, Dietrich CF; EFSUMB. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med. 2013;34:238-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 640] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 40. | Kamata K, Takenaka M, Kitano M, Omoto S, Miyata T, Minaga K, Yamao K, Imai H, Sakurai T, Watanabe T, Nishida N, Chikugo T, Chiba Y, Imamoto H, Yasuda T, Lisotti A, Fusaroli P, Kudo M. Contrast-enhanced harmonic endoscopic ultrasonography for differential diagnosis of submucosal tumors of the upper gastrointestinal tract. J Gastroenterol Hepatol. 2017;32:1686-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 41. | Fusaroli P, Eloubeidi MA. Diagnosis of pancreatic cancer by contrast-harmonic endoscopic ultrasound (EUS): complementary and not competitive with EUS-guided fine-needle aspiration. Endoscopy. 2014;46:380-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Fusaroli P, Jenssen C, Hocke M, Burmester E, Buscarini E, Havre RF, Ignee A, Saftoiu A, Vilmann P, Nolsøe CP, Nürnberg D, D'Onofrio M, Gilja OH, Lorentzen T, Piscaglia F, Sidhu PS, Dietrich CF. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part V - EUS-Guided Therapeutic Interventions (short version). Ultraschall Med. 2016;37:412-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 43. | Panic N, Larghi A. Techniques for endoscopic ultrasound-guided fine-needle biopsy. Gastrointest Endosc Clin N Am. 2014;24:83-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Dietrich CF, Jenssen C. Endoscopic ultrasound-guided sampling in gastroenterology: European society of gastrointestinal endoscopy technical guidelines. Endosc Ultrasound. 2013;2:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 45. | Varadarajulu S, Fraig M, Schmulewitz N, Roberts S, Wildi S, Hawes RH, Hoffman BJ, Wallace MB. Comparison of EUS-guided 19-gauge Trucut needle biopsy with EUS-guided fine-needle aspiration. Endoscopy. 2004;36:397-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 168] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 46. | Varadarajulu S, Hawes RH. The changing paradigm in EUS-guided tissue acquisition. Gastrointest Endosc Clin N Am. 2014;24:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Polkowski M, Jenssen C, Kaye P, Carrara S, Deprez P, Gines A, Fernández-Esparrach G, Eisendrath P, Aithal GP, Arcidiacono P, Barthet M, Bastos P, Fornelli A, Napoleon B, Iglesias-Garcia J, Seicean A, Larghi A, Hassan C, van Hooft JE, Dumonceau JM. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline - March 2017. Endoscopy. 2017;49:989-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 261] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 48. | Hébert-Magee S. How can an endosonographer assess for diagnostic sufficiency and options for handling the endoscopic ultrasound-guided fine-needle aspiration specimen and ancillary studies. Gastrointest Endosc Clin N Am. 2014;24:29-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 49. | Bang JY, Magee SH, Ramesh J, Trevino JM, Varadarajulu S. Randomized trial comparing fanning with standard technique for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic mass lesions. Endoscopy. 2013;45:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 197] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 50. | Tarantino I, Di Mitri R, Fabbri C, Pagano N, Barresi L, Granata A, Liotta R, Mocciaro F, Maimone A, Baccarini P, Fabio T, Curcio G, Repici A, Traina M. Is diagnostic accuracy of fine needle aspiration on solid pancreatic lesions aspiration-related? Dig Liver Dis. 2014;46:523-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 51. | Dumonceau JM, Koessler T, van Hooft JE, Fockens P. Endoscopic ultrasonography-guided fine needle aspiration: Relatively low sensitivity in the endosonographer population. World J Gastroenterol. 2012;18:2357-2363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 52. | Lisotti A, Frazzoni L, Fuccio L, Serrani M, Cominardi A, Bazzoli F, Fusaroli P. Repeat EUS-FNA of pancreatic masses after nondiagnostic or inconclusive results: systematic review and meta-analysis. Gastrointest Endosc. 2020;91:1234-1241.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 53. | ASGE Standards of Practice Committee; Early DS, Acosta RD, Chandrasekhara V, Chathadi KV, Decker GA, Evans JA, Fanelli RD, Fisher DA, Fonkalsrud L, Hwang JH, Jue TL, Khashab MA, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Sharaf RN, Shergill AK, Cash BD. Adverse events associated with EUS and EUS with FNA. Gastrointest Endosc. 2013;77:839-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 54. | Polkowski M, Larghi A, Weynand B, Boustière C, Giovannini M, Pujol B, Dumonceau JM; European Society of Gastrointestinal Endoscopy (ESGE). Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy. 2012;44:190-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 218] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 55. | Voss M, Hammel P, Molas G, Palazzo L, Dancour A, O'Toole D, Terris B, Degott C, Bernades P, Ruszniewski P. Value of endoscopic ultrasound guided fine needle aspiration biopsy in the diagnosis of solid pancreatic masses. Gut. 2000;46:244-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 249] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 56. | Guarner-Argente C, Shah P, Buchner A, Ahmad NA, Kochman ML, Ginsberg GG. Use of antimicrobials for EUS-guided FNA of pancreatic cysts: a retrospective, comparative analysis. Gastrointest Endosc. 2011;74:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 57. | Tregnaghi A, De Candia A, Calderone M, Cellini L, Rossi CR, Talenti E, Blandamura S, Borsato S, Muzzio PC, Rubaltelli L. Ultrasonographic evaluation of superficial lymph node metastases in melanoma. Eur J Radiol. 1997;24:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | van den Brekel MW, Stel HV, Castelijns JA, Nauta JJ, van der Waal I, Valk J, Meyer CJ, Snow GB. Cervical lymph node metastasis: assessment of radiologic criteria. Radiology. 1990;177:379-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 619] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 59. | Shozushima M, Suzuki M, Nakasima T, Yanagisawa Y, Sakamaki K, Takeda Y. Ultrasound diagnosis of lymph node metastasis in head and neck cancer. Dentomaxillofac Radiol. 1990;19:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Ahuja A, Ying M, King A, Yuen HY. Lymph node hilus: gray scale and power Doppler sonography of cervical nodes. J Ultrasound Med. 2001;20:987-992; quiz 994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 61. | Ahuja AT, Ying M. Sonographic evaluation of cervical lymph nodes. AJR Am J Roentgenol. 2005;184:1691-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 189] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 62. | Barreiros AP, Braden B, Schieferstein-Knauer C, Ignee A, Dietrich CF. Characteristics of intestinal tuberculosis in ultrasonographic techniques. Scand J Gastroenterol. 2008;43:1224-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 63. | Vassallo P, Wernecke K, Roos N, Peters PE. Differentiation of benign from malignant superficial lymphadenopathy: the role of high-resolution US. Radiology. 1992;183:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 351] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 64. | Gritzmann N, Hollerweger A, Macheiner P, Rettenbacher T. Sonography of soft tissue masses of the neck. J Clin Ultrasound. 2002;30:356-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 65. | Esen G, Gurses B, Yilmaz MH, Ilvan S, Ulus S, Celik V, Farahmand M, Calay OO. Gray scale and power Doppler US in the preoperative evaluation of axillary metastases in breast cancer patients with no palpable lymph nodes. Eur Radiol. 2005;15:1215-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 66. | Faige DO. EUS in patients with benign and malignant lymphadenopathy. Gastrointest Endosc. 2001;53:593-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 67. | Steinkamp HJ, Wissgott C, Rademaker J, Felix R. Current status of power Doppler and color Doppler sonography in the differential diagnosis of lymph node lesions. Eur Radiol. 2002;12:1785-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 68. | Kamata K, Takenaka M, Kitano M, Omoto S, Miyata T, Minaga K, Yamao K, Imai H, Sakurai T, Nishida N, Kashida H, Chikugo T, Chiba Y, Nakai T, Takeyama Y, Lisotti A, Fusaroli P, Kudo M. Contrast-enhanced harmonic endoscopic ultrasonography for differential diagnosis of localized gallbladder lesions. Dig Endosc. 2018;30:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 69. | Tschammler A, Ott G, Schang T, Seelbach-Goebel B, Schwager K, Hahn D. Lymphadenopathy: differentiation of benign from malignant disease--color Doppler US assessment of intranodal angioarchitecture. Radiology. 1998;208:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 103] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 70. | Chang DB, Yuan A, Yu CJ, Luh KT, Kuo SH, Yang PC. Differentiation of benign and malignant cervical lymph nodes with color Doppler sonography. AJR Am J Roentgenol. 1994;162:965-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 86] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 71. | Yamashita Y, Shimokawa T, Napoléon B, Fusaroli P, Gincul R, Kudo M, Kitano M. Value of contrast-enhanced harmonic endoscopic ultrasonography with enhancement pattern for diagnosis of pancreatic cancer: A meta-analysis. Dig Endosc. 2019;31:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 72. | Fusaroli P, D'Ercole MC, De Giorgio R, Serrani M, Caletti G. Contrast harmonic endoscopic ultrasonography in the characterization of pancreatic metastases (with video). Pancreas. 2014;43:584-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 73. | Janssen J, Dietrich CF, Will U, Greiner L. Endosonographic elastography in the diagnosis of mediastinal lymph nodes. Endoscopy. 2007;39:952-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 74. | Altonbary AY, Hakim H, El-Shamy AM. Endoscopic Ultrasound Elastography for Evaluation of Lymph Nodes: A Single Center Experience. Diagn Ther Endosc. 2018;2018:7186341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 75. | Xu W, Shi J, Zeng X, Li X, Xie WF, Guo J, Lin Y. EUS elastography for the differentiation of benign and malignant lymph nodes: a meta-analysis. Gastrointest Endosc. 2011;74:1001-1009; quiz 1115.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 76. | Hocke M, Menges M, Topalidis T, Dietrich CF, Stallmach A. Contrast-enhanced endoscopic ultrasound in discrimination between benign and malignant mediastinal and abdominal lymph nodes. J Cancer Res Clin Oncol. 2008;134:473-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 77. | Jin Y, He YS, Zhang MM, Parajuly SS, Chen S, Zhao HN, Peng YL. Value of contrast-enhanced ultrasonography in the differential diagnosis of enlarged lymph nodes: a meta-analysis of diagnostic accuracy studies. Asian Pac J Cancer Prev. 2015;16:2361-2368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 78. | Okasha H, Elkholy S, Sayed M, Salman A, Elsherif Y, El-Gemeie E. Endoscopic ultrasound-guided fine-needle aspiration and cytology for differentiating benign from malignant lymph nodes. Arab J Gastroenterol. 2017;18:74-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 79. | Stelow EB, Lai R, Bardales RH, Mallery S, Linzie BM, Crary G, Stanley MW. Endoscopic ultrasound-guided fine-needle aspiration of lymph nodes: the Hennepin County Medical Center experience. Diagn Cytopathol. 2004;30:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 80. | Bardales RH, Stelow EB, Mallery S, Lai R, Stanley MW. Review of endoscopic ultrasound-guided fine-needle aspiration cytology. Diagn Cytopathol. 2006;34:140-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 81. | Williams DB, Sahai AV, Aabakken L, Penman ID, van Velse A, Webb J, Wilson M, Hoffman BJ, Hawes RH. Endoscopic ultrasound guided fine needle aspiration biopsy: a large single centre experience. Gut. 1999;44:720-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 438] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 82. | Chin YK, Iglesias-Garcia J, de la Iglesia D, Lariño-Noia J, Abdulkader-Nallib I, Lázare H, Rebolledo Olmedo S, Dominguez-Muñoz JE. Accuracy of endoscopic ultrasound-guided tissue acquisition in the evaluation of lymph nodes enlargement in the absence of on-site pathologist. World J Gastroenterol. 2017;23:5755-5763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 83. | Eloubeidi MA, Jhala D, Chhieng DC, Chen VK, Eltoum I, Vickers S, Mel Wilcox C, Jhala N. Yield of endoscopic ultrasound-guided fine-needle aspiration biopsy in patients with suspected pancreatic carcinoma. Cancer. 2003;99:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 249] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 84. | van Hemel BM, Lamprou AA, Weersma R, Plukker JT, Suurmeijer AJ, van Dullemen HM. Procedure-related, false-positive cytology results during EUS-guided FNA in patients with esophageal cancer. Gastrointest Endosc. 2010;71:1130-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 85. | Gleeson FC, Kipp BR, Caudill JL, Clain JE, Clayton AC, Halling KC, Henry MR, Rajan E, Topazian MD, Wang KK, Wiersema MJ, Zhang J, Levy MJ. False positive endoscopic ultrasound fine needle aspiration cytology: incidence and risk factors. Gut. 2010;59:586-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 86. | Chen L, Li Y, Gao X, Lin S, He L, Luo G, Li J, Huang C, Wang G, Yang Q, Shan H. High Diagnostic Accuracy and Safety of Endoscopic Ultrasound-Guided Fine-Needle Aspiration in Malignant Lymph Nodes: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 87. | Li C, Shuai Y, Zhou X. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of intra-abdominal lymphadenopathy: a systematic review and meta-analysis. Scand J Gastroenterol. 2020;55:114-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 88. | Puli SR, Batapati Krishna Reddy J, Bechtold ML, Ibdah JA, Antillon D, Singh S, Olyaee M, Antillon MR. Endoscopic ultrasound: it's accuracy in evaluating mediastinal lymphadenopathy? World J Gastroenterol. 2008;14:3028-3037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 89. | van Riet PA, Larghi A, Attili F, Rindi G, Nguyen NQ, Ruszkiewicz A, Kitano M, Chikugo T, Aslanian H, Farrell J, Robert M, Adeniran A, Van Der Merwe S, Roskams T, Chang K, Lin F, Lee JG, Arcidiacono PG, Petrone M, Doglioni C, Iglesias-Garcia J, Abdulkader I, Giovannini M, Bories E, Poizat F, Santo E, Scapa E, Marmor S, Bucobo JC, Buscaglia JM, Heimann A, Wu M, Baldaque-Silva F, Moro CF, Erler NS, Biermann K, Poley JW, Cahen DL, Bruno MJ. A multicenter randomized trial comparing a 25-gauge EUS fine-needle aspiration device with a 20-gauge EUS fine-needle biopsy device. Gastrointest Endosc. 2019;89:329-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 90. | de Moura DTH, McCarty TR, Jirapinyo P, Ribeiro IB, Farias GFA, Ryou M, Lee LS, Thompson CC. Endoscopic Ultrasound Fine-Needle Aspiration versus Fine-Needle Biopsy for Lymph Node Diagnosis: A Large Multicenter Comparative Analysis. Clin Endosc. 2020;53:600-610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 91. | Facciorusso A, Mohan BP, Crinò SF, Ofosu A, Ramai D, Lisotti A, Chandan S, Fusaroli P. Contrast-enhanced harmonic endoscopic ultrasound-guided fine-needle aspiration vs standard fine-needle aspiration in pancreatic masses: a meta-analysis. Expert Rev Gastroenterol Hepatol. 2021;1-8. [DOI] [Full Text] |
| 92. | Lisotti A, Fusaroli P. Contrast-enhanced EUS for the differential diagnosis of lymphadenopathy: technical improvement with defined indications. Gastrointest Endosc. 2019;90:995-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen L, Wang G S-Editor: Fan JR L-Editor: Filipodia P-Editor: Yuan YY