Published online Oct 15, 2021. doi: 10.4251/wjgo.v13.i10.1397
Peer-review started: March 1, 2021
First decision: June 16, 2021
Revised: April 20, 2021
Accepted: August 3, 2021
Article in press: August 3, 2021
Published online: October 15, 2021
Processing time: 226 Days and 1.2 Hours
Hepatocellular carcinoma (HCC) is one of most common cancers that cause death in the world. Thermal ablation (TA) is an important alternative treatment method for HCC patients who are not appropriate for surgery or liver transplantation. Particularly for small and early HCCs, TA can be considered as the first-line curative treatment. However, local and distant recurrence rates are still high even though the TA equipment and technology develop rapidly. Immunotherapy is a novel systemic treatment method to enhance the anti-tumor immune response of HCC patients, which has the potential to reduce the tumor recurrence and metastasis. The combination of local TA and systemic immunotherapy for HCCs may be an ideal treatment for enhancing the efficacy of TA and controlling the recurrence. Herein we summarize the latest progress in TA, immunotherapy, and their combination for the treatment of patients with HCC and discuss the limitations and future research directions of the combined therapy.
Core Tip: Hepatocellular carcinoma (HCC) is one of the most leading causes of death in the world. Thermal ablation (TA) is an important treatment method for HCC patients. The main disadvantage of TA for HCC is the high local and distant recurrence rates after treatment. Immunotherapy is a novel systemic treatment that presents potential efficacy to inhibit the tumor recurrence. The combination of local TA and immunotherapy for HCC may be an ideal treatment for enhancing the efficacy of TA and controlling the recurrence. We herein summarize the latest progress in TA, immunotherapy, and the combination of TA with immunotherapy for HCC and discuss the future directions of the combined therapy.
- Citation: Bo XW, Sun LP, Yu SY, Xu HX. Thermal ablation and immunotherapy for hepatocellular carcinoma: Recent advances and future directions. World J Gastrointest Oncol 2021; 13(10): 1397-1411
- URL: https://www.wjgnet.com/1948-5204/full/v13/i10/1397.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i10.1397
Hepatocellular carcinoma (HCC) is one of the most common cancers and leading causes of death in the world[1-4]. Liver resection is the primary treatment method for patients who are eligible for surgery[5-8]. However, most of the patients are not eligible for surgical resection[8]. Thermal ablation (TA), mainly radiofrequency ablation (RFA) and microwave ablation (MWA), is applicable for patients who are not appropriate candidates for liver resection or transplantation[6-8]. TA has been widely used for the treatment of HCC and is considered as a curative treatment method for early and small HCCs[5,7-9].
TA is recommended as the first-line treatment strategy for patients with very early-stage or early-stage HCC according to the American Association for the Study of Liver Diseases guidelines[5,7]. However, TA seems to be less effective for lesions bigger than 3 cm or lesions that are near to the adjacent structures such as liver capsule or blood vessels in the liver. There are many controversies regarding the ablation treatment for HCCs with a diameter of 3-5 cm[10-12]. The main obstacle for TA application for HCC is the relatively high local tumor progression rate and lower long overall survival (OS) rate after ablation[9,12-16]. The combination of TA with percutaneous ethanol ablation (EA), transarterial chemo-embolization (TACE), nanoparticle-mediated therapy, targeted molecular therapy, and immunotherapy seems to be more effective than ablation alone, with better local tumor control and overall and recurrence-free survival rates[16].
Immunotherapy is considered one of the most promising novel therapies for cancer, which can stimulate specific immune responses and enhance the self-regulation ability of the immune system, thereby delaying and reducing tumor recurrence[15,17,18]. Compared with other treatment strategies such as surgery, liver transplantation, and TA, immunotherapy can be applied theoretically for various stages of HCC. In addition, immunotherapy can induce systemic and durable responses by immunological memory, which is beneficial for controlling tumor recurrence[17].
Previous studies have found that TA could enhance the body’s anti-tumor immune response through releasing more tumor-related antigens (TAAs) and thereby inducing infiltration of T-cells and dendritic cells (DCs)[16,19-22]. However, the ablation-induced immune enhancement effect is not lasting[19]. The combination of TA and immunotherapy seems to be a promising method for the treatment of HCC in the future. Accordingly, this review seeks to analyze the current studies of combined TA with immunotherapy for HCC, and discuss the further research directions on this topic.
TA is a minimally invasive treatment method for patients suffering from HCC, which is usually guided by ultrasound or computed tomography[6,23]. TA usually includes RFA, MWA, high intensity focused ultrasound (HIFU), laser ablation, and cryoablation. And the most common TA techniques are RFA and MWA[6,23].
Ten years ago, Peng et al[24] found that the efficacy and safety of percutaneous RFA were better than surgical resection (SR) in patients suffering from HCC measuring ≤ 2 cm in a retrospective comparative study with a 5-year follow-up. Recently, Lee et al[13] also suggested that the efficacy of percutaneous RFA as the first-line therapy for single nodular HCC < 3 cm was excellent in a 10-year follow-up study. In a randomized controlled trial (RCT) study comparing SR with RFA for early-stage HCC (solitary tumor ≤ 5 cm; or multiple tumors ≤ 3, each ≤ 3 cm), RFA showed similar disease-free survival (DFS) rates, overall tumor recurrence rates, and OS rates at 1, 3, 5, and 10 years after treatment, whereas RFA had less blood loss and shorter treatment time and hospital stay than the SR group[10]. In addition, RFA had the same 1-, 3-, and 5-year OS and progression-free survival (PFS) as repeat resection for the recurrent HCC patients in a multicenter retrospective study, but with a lower rate of complications[25].
However, SR showed higher long-term PFS and OS rates than RFA in patients with small perivascular HCCs[26]. In addition, patients with HCCs > 2 cm after RFA as first-line therapy had a greater risk of recurrence beyond Milan criteria[14]. Xu et al[27] also concluded that RFA and SR had similar OS at 1 year (P = 0.63) and 3 years (P = 0.29), whereas RFA had shorter OS at 5 years (P = 0.001) after treatment for small HCCs in a systematic review of RCTs.
No-touch multibipolar RFA and MWA were also recommended for HCCs within the Milan criteria in some studies[11,28,29]. Nevertheless, Zhuang et al[30] showed that SR had better survival benefit than RFA for HCC within Milan criteria in a population-based study. Ryu et al[11] also reported that the SR group had a better recurrence-free survival than the MWA group for HCC patients with tumor size > 3 cm. Recently, Gui et al[31] reported that TACE plus RFA offers comparable oncologic outcomes in patients with HCC as compared with SR, with added benefit of lower morbidity from a meta-analysis with eight retrospective studies and one RCT. However, in another meta-analysis with 7 RCTs and 18 matched nonrandomized trials, Shin et al[12] concluded that SR was better than MWA or RFA plus TACE in terms of OS and recurrence free survival for HCCs within Milan criteria.
Accordingly, TA as the first-line treatment for patients with HCC is still controversial and more multicenter clinical studies and RCTs are needed in the future. However, many patients with HCCs are not the ideal candidates for SR or liver transplantation. The main obstacle for the application of TA is the relatively higher local and distant recurrence compared with SR. Therefore, there is an urgent need for combined treatment with other methods to decrease the recurrence after ablation.
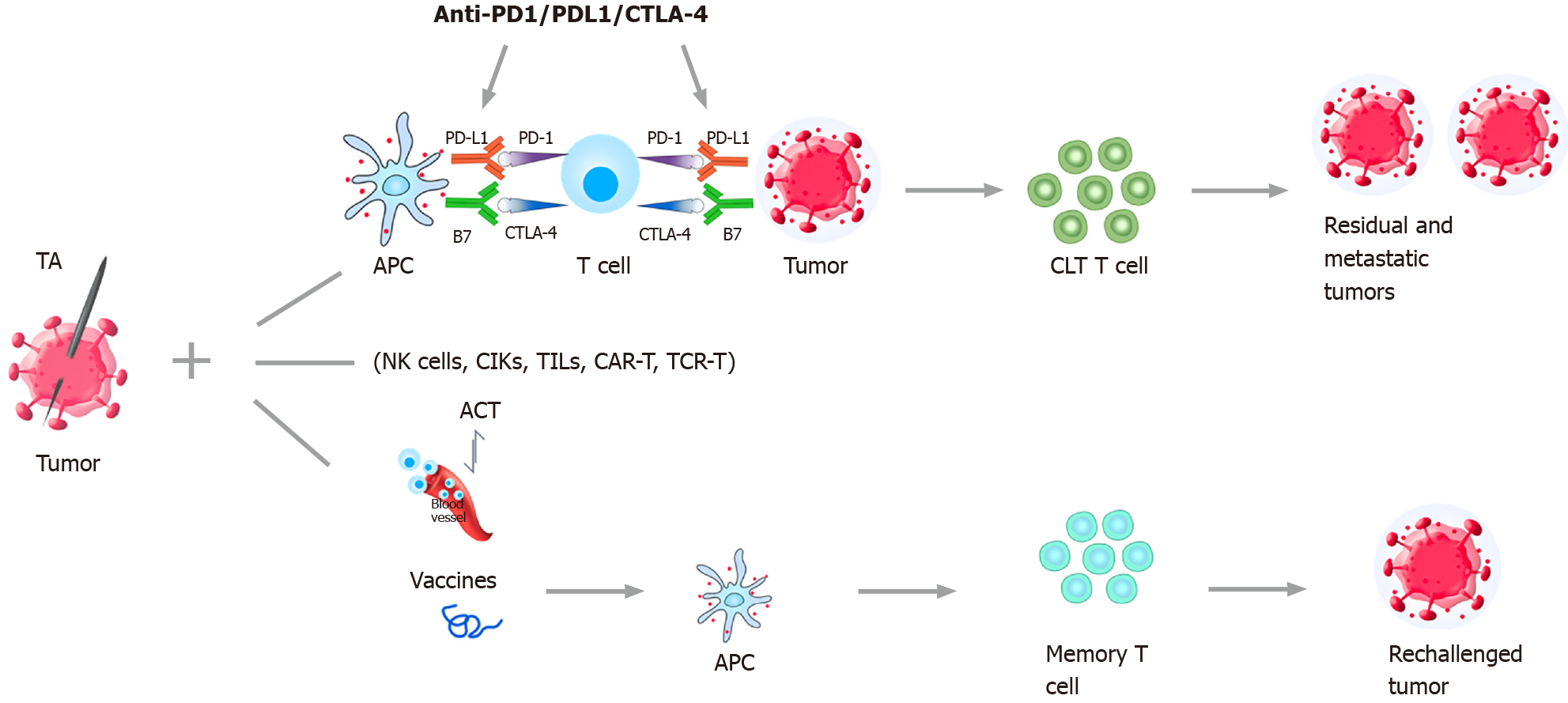
The main immunotherapeutic strategies include immune checkpoint inhibitors (ICIs) therapy, cell-based therapies such as adoptive cell transfer (ACT), and tumor vaccine therapy[18,32] (Figure 1).
ICIs can block tumor-induced immunosuppression, and thereby reactivate and enhance the body’s anti-tumor immune response[17,18,33]. Programmed cell death protein-1 (PD-1), programmed cell death 1 ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) are the main targets of ICIs for HCC treatment[18,34,35]. Nivolumab and pembrolizumab had been approved by the United States Food and Drug Administration (FDA) as the second-line treatment for HCC after progression of disease on first-line therapy with sorafenib, which were validated in some previous clinical studies[18,36-39]. The combination of atezolizumab/ bevacizumab had also been approved by the FDA as the first-line therapy in previously untreated, unresectable HCC[18,40]. More ICIs, such as tislelizumab, camrelizumab, durvalumab, and avelumab, are currently being evaluated in some ongoing clinical studies[18,41,42] (Table 1). ICIs were considered as a safe and effective method for advanced-stage HCC in a recent systematic review and pooled analysis of 2402 patients[43]. The results showed that the disease control rate, objective response rate, and mean OS rate were 60.7%, 22.7%, and 15.8 mo, respectively. However, 14.9% of patients discontinued because of adverse events. ICIs were not recommended for the liver transplant setting because of the high fatal graft rejection (40.0%) and mortality (80.0%).
| Drugs | Trade name | Targets | Approved/ongoing | Company | Indications |
| Nivolumab | Opdivo | PD-1 | Approved by FDA in 2017; Approved by China NMPA in 2018 | Bristol-Myers Squibb | HCC progression after first-line therapy with sorafenib (second-line treatment) |
| Pembrolizumab | Keytruda | PD-1 | Approved by FDA in 2018; Approved by China NMPA in 2018 | Merck Sharp & Dohme | HCC progression or high toxicity after first-line therapy with sorafenib (second-line treatment) |
| Atezolizumab | Tecentriq | PD-L1 | Approved by China NMPA in 2020 | Roche Pharmaceutical Ltd | Advanced HCC |
| Camrelizumab | Erika | PD-1 | Approved by China NMPA in 2020 | Jiangsu Hengrui medicine | Advanced HCC after therapy with sorafenib or oxaliplatin |
| Tislelizumab | Baizean | PD-1 | Ongoing | BeiGene (Shanghai) | / |
| Sintilimab | Daboshu | PD-1 | Ongoing | Innovent | / |
| Toripalimab | Toi | PD-1 | Ongoing | Shanghai Junshi Biosciences | / |
| Durvalumab | Imfinzi | PD-L1 | Ongoing | AstraZeneca | / |
| Avelumab | BAVENCIO | PD-L1 | Ongoing | Pfizer & Merck | / |
| Tremelimumab | Tremelimumab | CTLA-4 | Ongoing | AstraZeneca | / |
ACT refers to a method of passive immunotherapy in which immune cells with tumor-killing effects are expanded and cultured in vitro, and then returned to tumor patients to achieve anti-tumor purposes[44]. ACT mainly includes cytokine-induced killer cell (CIK), natural killer (NK) cell, tumor-infiltrating lymphocyte, T cell receptor-engineered T cell, and chimeric antigen receptor T cell (CAR-T) therapy[18,44,45]. Twenty years ago, Takayama et al[46] conducted a RCT study in which 150 patients who had undergone curative resection for HCC were allocated to no adjuvant treatment (n = 74) or adoptive immunotherapy (n = 76). The results showed that adoptive immunotherapy decreased the recurrence rate by 18% compared with the control group. Patients in the immunotherapy group had significantly longer disease-specific survival (P = 0.04) and recurrence-free survival (P = 0.01) than the control group. However, the OS between the two groups had no significant difference (P = 0.09).
Many clinical studies, including some RCTs, have demonstrated that CIK cell therapy was useful in prolonging the OS, PFS, and time to recurrence for patients with HCC after curative resection or RFA[47-52]. Jia et al[51] conducted a retrospective study to compare the efficacy of autologous transplantation of CIK cells as an adjuvant therapy for patients with HCC (165 cases) with the control group who only received surgery or TACE (99 cases). The results showed that patients in the surgery + CIK group had a significantly longer OS compared with those in the CIK group, TACE + CIK group, and TACE-based comprehensive treatment + CIK group. In addition, the surgery + CIK group likely had an improved PFS but a similar OS compared with the patients in the surgery-alone group. Cao et al[53] performed a meta-analysis to investigate the effectiveness of cellular immunotherapy (CIT), involving DCs and CIKs, combined with various conventional treatments for HCC. The results showed that immunotherapy with DCs and/or CIKs improved the OS significantly at 6 mo (P = 0.02), 1 year (P < 0.00001), 3 years (P < 0.00001), and 5 years (P < 0.00001). The CIT also significantly reduced the recurrence rate at 6 mo (P < 0.0001) and 1 year (P < 0.00001) after treatment. However, the adverse effect rate in immunotherapy groups was higher than conventional treatments while symptoms relieved within 24 h. CAR-T therapy is a promising novel ACT technique for advanced HCCs, which was proved to be safe in some phase I or II clinical studies[45,54]. However, the efficacy should be evaluated in future phase III studies.
Tumor vaccine therapy refers to the introduction of tumor cells, tumor-related peptides, or TAA coding genes into patients[17,18]. By inducing and perpetuating the tumor specific immune response, activated effector T cells can specifically reduce tumor burden and control tumor recurrence.
Some tumor vaccines, such as peptide vaccine, DCs, and oncolytic viruses, have been used for the treatment of advanced HCC in some phase I or II clinical studies, which presented potential efficacy in controlling tumor recurrence[55-59]. However, oncolytic immunotherapy seemed not effective for advanced stage HCC[60]. Recently, Moehler et al[60] conducted a randomized, open-label phase IIb trial in 129 HCC patients who failed in sorafenib therapy to compare the OS between vaccinia virus-based oncolytic immunotherapy plus best supportive care (BSC) (86 patients) and BSC alone (43 patients). There was no significant difference between the two groups in OS (median 4.2 mo vs 4.4 mo, P = 0.428), response rate, or time to progression. The main adverse events included pyrexia (8%) and hypotension (8%).
Based on the previous clinical studies, ICIs and ACT are the most common used immunotherapy strategies for the treatment of patients with HCC. And the combination with other inhibitors, chemotherapy, RFA, or TACE is more effective than immunotherapy alone[18]. Tumor vaccine therapy is safe for HCC patients according to the phase I and II studies. The efficacy of tumor vaccine therapy should be evaluated in more clinical studies in the future. Furthermore, immunotherapy alone for HCC is less effective than the combination with other treatments.
The coagulation of tumor tissue caused by TA leads to the release and exposure of tumor antigens, which can stimulate the immune response[19,20,61-63]. The peripheral blood cytotoxic T lymphocyte (CTL) population, CTL/Treg ratios, and NK cells increased significantly in HCC patients undergoing TA therapy[64,65]. Accordingly, long-lasting anti-tumor immune response can be obtained after the combination therapy, and the residual and metastatic tumors will be eliminated or inhibited[20] (Figure 1).
RFA is the most commonly used TA method for the treatment of HCC. In 2003, Wissniowski et al[66] found that RFA induced a tumor-specific T-cell reaction when destroying tumor tissue in tumor-bearing VX2 hepatoma rabbits. The combination of RFA and anti-PD-1 antibodies significantly enhanced tumor antigen-specific T cell responses, increased intratumoral Teff to Treg ratio, and synergistically inhibited growth of the distant tumor[67].
Previous studies also demonstrated that RFA could enhance T-cell-mediated immune response in HCC patients after treatment. Zerbini et al[68] found that RFA activated and enhanced the tumor specific T-cell response in 20 HCC patients 1 mo after treatment. They[69] also showed that RFA enhanced the expression of costimulatory molecules, lymph node homing chemokine receptor, antigen presentation, and cytokine secretion and induced tumor-specific T-cell responses by creating an antigenic source along with stimuli appropriate for maturation of APCs. Nobuoka et al[70] validated that RFA induced stronger glypican-3-specific T-cell-mediated immune response than SR in HCC patients and tumor-bearing mice for the first time. Compared RFA, SR removes all TAAs and thereby weakens tumor-related immune response. Accordingly, RFA combined with immunotherapy has more potential than SR for HCC therapy. However, although RFA can enhance various TAA-specific T cell responses, the memory phenotype and lifetime of TAA-specific T cells are not sufficient to prevent HCC recurrence completely[71]. Thus, the combination of RFA and immunotherapy presents a promising novel treatment method to inhibit tumor recurrence[22].
As the most commonly used immunotherapy strategy, ICI combined with TA seems to be a promising therapy. In 2017, Duffy et al[72] conducted a clinical trial to evaluate the safety and feasibility of tremelimumab (CTLA-4) combined with ablation (RFA and chemoablation) in 32 patients with HCC. The results showed that 26.3% (5/19) of the evaluable patients achieved a confirmed partial response. The tumor PFS rates at 6 and 12 mo for these refractory HCCs were 57.1% and 33.1%, respectively. The median OS and time to tumor progression were 12.3 mo (95%CI: 9.3 to 15.4 mo) and 7.4 mo (95%CI: 4.7 to 19.4 mo), respectively. In addition, the viral load decreased in 85.7% (12/14) of those patients with quantifiable HCV. No dose-limiting toxicities were found and the most common toxicity was pruritus. Nevertheless, the small sample of the study only proposed a new and promising treatment method for advanced HCC, which should be further verified in future studies.
TA combined with ACT immunotherapy is more commonly used in the treatment of HCC patients. In 2008, Weng et al[73] preformed a RCT study to test the efficacy of RFA and TACE combined with CIK cells in 85 HCC patients. The results showed that the 12-mo and 18-mo recurrence rates in the combination group were lower than those of the control group after treatment (12-mo: 8.9% vs 30.0%; 18-mo: 15.6% vs 40.0%; P < 0.05 for both). In the combination group, the percentages of CD3+, CD4+, CD56+, and CD3+CD56+ cells and CD4+/CD8+ cell ratio increased and the percentage of CD8+ cells decreased after CIK cell infusions. In addition, the most common adverse events were mild fever and shiver (24.4%, 11/45) in the combination group. Huang et al[74] also reported that the overall response rate and disease control rate were similar in the TACE + RFA + CIK group (85 HCC patients) and the TACE + RFA group (89 HCC patients). However, the patients in the TACE + RFA + CIK group had a significantly longer OS (56 mo vs 31 mo, P = 0.001) and PFS (17 mo vs 10 mo, P = 0.001) than those in the TACE + RFA group through the Kaplan-Meier analysis. No severe side effects occurred in the CIK cell transfusion. Ma et al[75] found that the percentage of CD3+CD8+ cells and IFNγ concentration in the peripheral blood significantly increased and CD4+/CD8+ cell ratio decreased after combination of RFA with autologous RetroNectin activated killer cells for the treatment of seven HCC patients with tumor size < 4 cm. During a 7-mo follow-up period, no severe adverse events, tumor recurrences, or deaths were observed. Cui et al[76] carried out a control study to compare the efficacy of CIT combined with RFA (30 patients) or RFA alone (32 patients) in 62 HCC patients. After a median follow-up time of 12 (10-28) mo, the PFS and OS in the RFA/CIT group were significantly longer than those of the RFA group (P < 0.05). The percentages of CIK and NK cells were also higher in the RFA/CIT group. Moreover, the hepatic function of patients in the RFA/CIT group maintained and only one patient developed a mild fever (38.5℃) after one infusion and recovered 2 h later. However, Lee et al[58] reported that the combination of RFA and DC vaccine adjuvant immunotherapy increased the risk of tumor recurrence in HCC patients in a phase II trial. And the specific mechanism remains unclear and further investigation is needed.
The combination of RFA and radioimmunotherapy was also applied for HCC. Bian et al[77] performed a RCT study to compare the tumor recurrence rates of 127 patients with Barcelona Clinic Liver Cancer (BCLC) classifications of stage 0-B after either RFA alone (n = 65) or RFA followed by (131I) metuximab (n = 62). The results showed that the 1- and 2-year recurrence rates in the combination group were 31.8% and 58.5%, whereas those in the RFA group were 56.3% and 70.9%, respectively. The median time to overall tumor recurrence was 10 mo in the RFA group and 17 mo in the combination group (P = 0.03). In those metuximab target (i.e., CD147)-positive patients, the combination group showed better anti-recurrence benefit than RFA alone (P = 0.007). Tu et al[78] conducted a retrospective study to compare the efficacy of the combination of monoclonal antibody (131I-chTNT, Iodine-131-labeled chimeric tumor necrosis therapy) with RFA therapy (12 cases) and RFA alone (22 cases) in treating middle-advanced stage HCC. The results showed that the PFS rate of patients in the combination group was significantly longer than that of the RFA group (6-mo: 72.7% vs 50%, 1-year: 45.5% vs 0%). The 1-, 2-, and 3-year OS rates of the patients in the combination group were higher than those in the RFA group (100%, 91.7%, and 75 % vs 81.6%, 56.5%, and 42.4%). The counts of white blood cells significantly increased in the combination group (P < 0.05) at 1 wk and were reversed to normal at 2 wk and 1 mo after therapy. The blood levels of thyroxine (T4), triiodothyronine (T3), TSH (thyroid-stimulating hormone), free thyroxine (FT4), and free triiodothyronine (FT3) of patients in the combination therapy group did not differ significantly at 1 mo after treatment. However, more local recurrences were found in patients with HCCs measuring > 4 cm.
MWA is also a commonly used TA technique for HCC treatment. MWA combined with anti-PD-1/anti-CTLA-4 not only increased the survival time and protected the mice against tumor recurrence, but also enhanced the intratumoral infiltration of CTL and systemic T-cell immune responses induced by MWA through activation of synergistically specific antitumor immunity[79]. Recently, Leuchte et al[80] performed a prospective study to observe the immune responses of 23 HCC patients undergoing MWA. The results showed that only moderate effects on circulating immune cells were found after MWA. Enhanced tumor-specific immune responses were found in 30% of patients. Interferon-y and interleukin-5 T cell responses against TAAs were higher in patients with a long-time remission (> 1 year) after MWA (7/16 of patients) than those suffering from an early relapse (0/13 of patients). The PFS in patients with a long-time remission was also longer (27.5 mo vs 10.0 mo). Accordingly, immune-related effects of MWA in HCC patients were related to tumor control, thus providing additional evidence for the combination of MWA and immunotherapy.
Several previous studies also evaluated the efficacy of the combination therapy of MWA and immunotherapy. In 2011, Zhou et al[81] performed a phase I clinical study of combination therapy with MWA and CIT in ten HCC patients. The results showed that 57.14% (4/7) of patients had a decrease in viral load. The percentage of CD8+CD28- effector cells increased significantly and the percentage of CD4+CD25high regulatory T lymphocytes decreased significantly at 1 and 3 mo after therapy. Yu et al[82] compared the therapeutic efficacy of immunotherapy after MWA in 14 patients received multiple courses of immunotherapy after MWA (immunotherapy group) and 15 patients received MWA alone (control group). The results showed that more intrahepatic and extrahepatic recurrence was identified in the control group (40.0%) than in the immunotherapy group (7.1%). However, there was no significant difference in the rate of DFS or OS within 16 mo between the two groups (DFS: 92.86% vs 66.67%, P = 0.076; OS: 100% vs 80%, P = 0.126). The mean absolute lymphocyte count and the mean albumin level were significantly higher in the immunotherapy group (P < 0.05). Recently, Huang et al[83] performed a retrospective study to compare the efficacy and safety of TACE + MWA + CIK therapy (43 cases) and TACE + MWA therapy (57 cases) in 100 unresectable HCCs. The results showed that the patients in the TACE + MWA + CIK group have similar overall response rate [74.42% (32/43) vs 77.19% (44/57), P = 0.243], and higher disease control rate (87.72% vs 79.07%, P = 0.748) compared with those in the TACE + MWA group, though there was no statistical difference. The OS (41 mo vs 24 mo, P = 0.002) and PFS (17 mo vs 10 mo, P = 0.023) of patients in the combined CIK cell treatment group were also longer than those in the TACE + MWA therapy group. In addition, no procedure-related mortality or severe complications were reported during follow-up.
Until now, no studies have been reported on HIFU or laser ablation combined with immunotherapy for the treatment of HCC. Only one study[84] was documented with respect to the combination therapy of cryotherapy and DC-CIK cell immunotherapy. In 2013, Niu et al[84] conducted a retrospective study to investigate the effect of comprehensive cryosurgery plus DC-CIK cell immunotherapy in 45 patients with recurrent HCC (intra- and extra-hepatic tumors). After an 8-year follow-up, the median OS in the cryo-immunotherapy, immunotherapy, cryotherapy, and untreated groups was 32, 4, 17.5, and 3 mo, respectively. The median OS of patients in the cryo-immunotherapy group after multiple treatments was higher than that after a single treatment (36.5 mo vs 21 mo, P < 0.05).
The published clinical studies on the combination of TA and immunotherapy are presented in Table 2. From previous clinical studies, the combination of RFA, MWA, or cryoablation with immunotherapy were all effective in reducing or delaying tumor recurrence and improving OS for patients with HCC. RFA combined with cell-based therapy was the most commonly used combination therapy. In addition, no serious adverse events or treatment-related deaths were observed in these studies. The most common adverse events were mild fever, chills, pruritus, and increased white blood cell count in the combination therapy group. However, the combination of DC vaccine and RFA seems not to be the ideal treatment for patients with HCC[58]. Moreover, HIFU, laser ablation, and cryotherapy were seldom used in the combination group. Considering that previous studies are all single-center and small sample studies, more multi-center, large-scale RCTs are needed to validate the effectiveness of combination therapy in the future.
| Ref. | Location | No. (combination group vs control group) | Tumor size (cm) | Number of tumor nodules (single/multiple) | Combination group | Control group | Follow-up period (mo) | Recurrence rates | OS, RFS, PFS or DFS | Adverse events in the combination group |
| Weng et al[73], 2008 | China | 45 vs 40 | 2-13 | NR | RFA + TACE + CIK cells | RFA + TACE | 18 | 1-year (8.9% vs 30.0%), 18-mo (15.6% vs 40.0%) (both P < 0.05) | RFS rates: 68.9% (31/45) vs 20.0% (8/40) | A light feverand shiver (24.4%, 11/45) |
| Ma et al[75], 2010 | China | 7 | 1-3.5 | 6/1 | RFA + RAK cells | / | 7 | No recurrence | NR | A slight fever (14.3%, 1/7) |
| Huang et al[74], 2013 | China | 85 vs 89 | 1-26 | 41/44 vs 40/49 | RFA + TACE + CIK cells | RFA + TACE | median 78 (5–173) | NR | OS: 56 mo vs 31 mo (P = 0.001); PFS: 17 mo vs 10 mo (P = 0.001) | Fever (37.5–39.3℃) and chills (3.5%) |
| Cui et al[76], 2014 | China | 30 vs 32 | 2-8 | 12/18 vs 18/14 | RFA + CIT | RFA | Median 12 (10–28) | NR | OS rates: 1-year (100% vs 92.6%), 2-yr (100% vs 76.6%); PFS: not reached vs 12 mo (P < 0.0001) | Fever (38.5℃) (3.3%) |
| Bian et al[77] , 2014 | China | 62 vs 65 | BCLC stage 0-B | 35/27 | RFA + 131I metuximab | RFA | NR | 1-year (31.8% vs 56.3%), 2-year (58.5% vs 70.9%) | NR | No serious adverse events |
| Tu et al[78], 2015 | China | 12 vs 22 | < 5 (66.7%) | 10/2 vs 16/6 | RFA + 131I-chTNT | RFA | Median 31 (5–48) | 83.3% (10/12) vs 86.4% (19/22) | OS: 43 mo vs 35 mo (P = 0.052); PFS: 23 mo vs 7 mo (P = 0.047) | The counts of white blood cells increased |
| Yu et al[82], 2015 | China | 14 vs 15 | 2.4 ± 0.7 vs 2.5 ± 0.8 | NR | MWA + immunotherapy | MWA | 16 vs 19 | 7.1% vs 40% | OS rates: 100% vs 80% (P = 0.126); DFS rates: 92.86% vs 66.67% (P = 0.076) | Fever (< 39℃) (42.9%) |
| Duffy et al[72], 2017 | United States | 32 | BCLC stage C or B | NR | RFA + anti-CTLA-4 (tremelimumab) | / | NR | median time to tumor progression: 7.4 mo | Median OS: 12.3 mo; PFS rate: 6-m (57.1%), 12-mo (33.1%) | Pruritus |
| Huang et al[83] , 2020 | China | 43 vs 57 | Median 9 (5–26) | 19/24 vs 30/27 | TACE + MWA + CIK | TACE + MWA | Median 31 (1–163) | NR | OS: 41 mo vs 24 mo (P = 0.002); DFS: 17 mo vs 10 mo (P = 0.023) | No severe adverse events |
TA can completely eliminate the tumor and simultaneously enhance the body’s anti-tumor immune response. The relatively weak specific T-cell-mediated immune response is not sufficient to prevent tumor recurrence[61]. Immunotherapy aims to improve the body’s immune system against TAAs and induce tumor cell death. Thereafter, the tumor can be completely ablated and the local and remote recurrence can be alleviated after the combination therapy. From the results of present studies, TA combined with immunotherapy shows higher OS and PFS than ablation or immunotherapy alone. However, there are some unanswered questions in these fields.
First, the specific mechanism of synergy of the combination of TA and immunotherapy is still unclear. There are so many immunotherapeutic strategies and TA therapy methods. TA induces the release of more TAAs, then increases the infiltration of T-cells and DCs, and facilitates the activation of CD4+ and CD8+ T cells, which strengthens the body’s systemic anti-tumor immune response[16,20-22]. Additionally, the sub-lethal zone can also activate the anti-tumor immune response by generating inflammatory cytokines [such as heat shock proteins, interleukin (IL)-6, IL-1, and tumor necrosis factor-α] and make tumor cells more sensitive to immunotherapy by modulating the immunogenicity[16,20,22,85]. Therefore, the sustained anti-tumor immune response can be achieved after the combination of TA and immunotherapy[86].
Second, the sequence of TA and immunotherapy is unclear. Most studies reported that TA was performed before[76-78,82,83] or during immunotherapy[72]. Accordingly, TA performed before or during immunotherapy are all effective to prevent local and distant tumor progression. In clinical practice, for tumors that can be completely eliminated after using ablation, immunotherapy may be applied thereafter. However, for patients with HCC that are difficult to obtain the complete response, immunotherapy might be conducted first to reduce tumor size or number.
Third, HCC can induce tumor immune tolerance through multiple mechanisms to evade the body’s immune killing, and eventually cause tumor recurrence and metastasis. Accordingly, single-agent immunotherapy combined with ablation is difficult to obtain a high clinical response rate. Previous studies reported that TACE or hepatic artery embolization could also enhance immune function by decreasing Treg populations after treatment for patients with HCC[87,88]. Combined immunotherapy and targeted molecular therapies resulted in higher OS and PFS in patients with unresectable HCC[40,89]. Therefore, comprehensive treatment, combination of ablation, immunotherapy, chemotherapy, TACE, and targeted therapy, or combining multiple targets immunotherapy may be more effective[90,91].
Fourth, the criteria for certain patients included in the combination therapy are also unknown. RFA conducted for early-stage HCCs might be more effective. Immunotherapy is now mainly performed for advanced HCC patients. Currently, no uniform standard is found for those patients included in the combination therapy. Few studies were carried out to compare the efficacy of the combination therapy for HCCs with different BCLC stages. In addition, more studies are still needed to obtain the specific dose and time of immunotherapy in the combination for different stages of HCC.
With the development of immunotherapy, more targets and mechanism of immunotherapy are found, thus more new immunotherapy drugs will appear. However, immunotherapy alone is less effective than the combination therapy. Comprehensive treatment and personalized treatment are the future development trends of HCC treatment. Further studies are needed to focus on the following directions. First, in order to select the appropriate immunotherapy and the combination therapy method for each patient, there is an urgent need for more accurate means to evaluate the effectiveness of immunotherapy drugs. Compared with tumor immunohistochemical test and genomic sequencing, functional diagnosis might be the ideal method for testing the effectiveness of immunotherapy[92]. Second, more clinical studies are needed to evaluate the efficacy of combination therapy. High-level clinical studies are also needed to evaluate the sequence of immunotherapy and TA, the efficacy of combination therapy in various BCLC stages of HCC, and the combination with other novel therapies, such as molecular targeted therapy, nanoparticle-mediated therapy, and VEGF inhibitors[93].
Based on previous clinical studies, the combination of TA with immunotherapy could effectively control the tumor recurrence and prolong the PFS and OS in patients with HCC compared with TA therapy or immunotherapy alone. Patients could obtain higher and sustained anti-tumor immune response after the combination therapy. However, more high evidence-based studies are needed to confirm the efficacy of the combination therapy.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68587] [Article Influence: 13717.4] [Reference Citation Analysis (201)] |
| 2. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 12201] [Article Influence: 2440.2] [Reference Citation Analysis (7)] |
| 3. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56666] [Article Influence: 7083.3] [Reference Citation Analysis (135)] |
| 4. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13324] [Article Influence: 1332.4] [Reference Citation Analysis (4)] |
| 5. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3435] [Article Influence: 429.4] [Reference Citation Analysis (3)] |
| 6. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4362] [Article Influence: 545.3] [Reference Citation Analysis (6)] |
| 7. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3143] [Article Influence: 392.9] [Reference Citation Analysis (3)] |
| 8. | Roayaie S, Jibara G, Tabrizian P, Park JW, Yang J, Yan L, Schwartz M, Han G, Izzo F, Chen M, Blanc JF, Johnson P, Kudo M, Roberts LR, Sherman M. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology. 2015;62:440-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 338] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 9. | Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1933] [Cited by in RCA: 1954] [Article Influence: 195.4] [Reference Citation Analysis (4)] |
| 10. | Ng KKC, Chok KSH, Chan ACY, Cheung TT, Wong TCL, Fung JYY, Yuen J, Poon RTP, Fan ST, Lo CM. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br J Surg. 2017;104:1775-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 262] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 11. | Ryu T, Takami Y, Wada Y, Hara T, Sasaki S, Saitsu H. Hepatic resection versus operative microwave ablation for single hepatocellular carcinoma ≤5 cm: A propensity score-matched analysis. Surgery. 2019;166:254-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Shin SW, Ahn KS, Kim SW, Kim TS, Kim YH, Kang KJ. Liver Resection Versus Local Ablation Therapies for Hepatocellular Carcinoma Within the Milan Criteria: A Systematic Review and Meta-analysis. Ann Surg. 2021;273:656-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 13. | Lee MW, Kang D, Lim HK, Cho J, Sinn DH, Kang TW, Song KD, Rhim H, Cha DI, Lu DSK. Updated 10-year outcomes of percutaneous radiofrequency ablation as first-line therapy for single hepatocellular carcinoma < 3 cm: emphasis on association of local tumor progression and overall survival. Eur Radiol. 2020;30:2391-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 14. | Doyle A, Gorgen A, Muaddi H, Aravinthan AD, Issachar A, Mironov O, Zhang W, Kachura J, Beecroft R, Cleary SP, Ghanekar A, Greig PD, McGilvray ID, Selzner M, Cattral MS, Grant DR, Lilly LB, Selzner N, Renner EL, Sherman M, Sapisochin G. Outcomes of radiofrequency ablation as first-line therapy for hepatocellular carcinoma less than 3 cm in potentially transplantable patients. J Hepatol. 2019;70:866-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 15. | Brown ZJ, Greten TF, Heinrich B. Adjuvant Treatment of Hepatocellular Carcinoma: Prospect of Immunotherapy. Hepatology. 2019;70:1437-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 16. | Chen L, Sun J, Yang X. Radiofrequency ablation-combined multimodel therapies for hepatocellular carcinoma: Current status. Cancer Lett. 2016;370:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Nakano S, Eso Y, Okada H, Takai A, Takahashi K, Seno H. Recent Advances in Immunotherapy for Hepatocellular Carcinoma. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 18. | Kole C, Charalampakis N, Tsakatikas S, Vailas M, Moris D, Gkotsis E, Kykalos S, Karamouzis MV, Schizas D. Immunotherapy for Hepatocellular Carcinoma: A 2021 Update. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 19. | van den Bijgaart RJ, Eikelenboom DC, Hoogenboom M, Fütterer JJ, den Brok MH, Adema GJ. Thermal and mechanical high-intensity focused ultrasound: perspectives on tumor ablation, immune effects and combination strategies. Cancer Immunol Immunother. 2017;66:247-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 205] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 20. | den Brok MH, Sutmuller RP, van der Voort R, Bennink EJ, Figdor CG, Ruers TJ, Adema GJ. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004;64:4024-4029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 342] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 21. | Dromi SA, Walsh MP, Herby S, Traughber B, Xie J, Sharma KV, Sekhar KP, Luk A, Liewehr DJ, Dreher MR, Fry TJ, Wood BJ. Radiofrequency ablation induces antigen-presenting cell infiltration and amplification of weak tumor-induced immunity. Radiology. 2009;251:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 184] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 22. | Fagnoni FF, Zerbini A, Pelosi G, Missale G. Combination of radiofrequency ablation and immunotherapy. Front Biosci. 2008;13:369-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, Chen MH, Choi BI, de Baère T, Dodd GD 3rd, Dupuy DE, Gervais DA, Gianfelice D, Gillams AR, Lee FT Jr, Leen E, Lencioni R, Littrup PJ, Livraghi T, Lu DS, McGahan JP, Meloni MF, Nikolic B, Pereira PL, Liang P, Rhim H, Rose SC, Salem R, Sofocleous CT, Solomon SB, Soulen MC, Tanaka M, Vogl TJ, Wood BJ, Goldberg SN; International Working Group on Image-guided Tumor Ablation; Interventional Oncology Sans Frontières Expert Panel; Technology Assessment Committee of the Society of Interventional Radiology,; Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology. 2014;273:241-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 954] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 24. | Peng ZW, Lin XJ, Zhang YJ, Liang HH, Guo RP, Shi M, Chen MS. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: a retrospective comparative study. Radiology. 2012;262:1022-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 25. | Feng Y, Wu H, Huang DQ, Xu C, Zheng H, Maeda M, Zhao X, Wang L, Xiao F, Lv H, Liu T, Qi J, Li J, Zhong N, Wang C, Feng H, Liang B, Ren W, Qin C, Nguyen MH, Zhu Q. Radiofrequency ablation versus repeat resection for recurrent hepatocellular carcinoma (≤ 5 cm) after initial curative resection. Eur Radiol. 2020;30:6357-6368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 26. | Lee S, Kang TW, Cha DI, Song KD, Lee MW, Rhim H, Lim HK, Sinn DH, Kim JM, Kim K. Radiofrequency ablation vs. surgery for perivascular hepatocellular carcinoma: Propensity score analyses of long-term outcomes. J Hepatol. 2018;69:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 27. | Xu XL, Liu XD, Liang M, Luo BM. Radiofrequency Ablation versus Hepatic Resection for Small Hepatocellular Carcinoma: Systematic Review of Randomized Controlled Trials with Meta-Analysis and Trial Sequential Analysis. Radiology. 2018;287:461-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 178] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 28. | Mohkam K, Dumont PN, Manichon AF, Jouvet JC, Boussel L, Merle P, Ducerf C, Lesurtel M, Rode A, Mabrut JY. No-touch multibipolar radiofrequency ablation vs. surgical resection for solitary hepatocellular carcinoma ranging from 2 to 5 cm. J Hepatol. 2018;68:1172-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 29. | Liu W, Zou R, Wang C, Qiu J, Shen J, Liao Y, Yang Z, Zhang Y, Wang Y, Yuan Y, Li K, Zuo D, He W, Zheng Y, Li B. Microwave ablation versus resection for hepatocellular carcinoma within the Milan criteria: a propensity-score analysis. Ther Adv Med Oncol. 2019;11:1758835919874652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Zhuang BW, Li W, Wang W, Li B, Lu MD, Kuang M, Xie XH, Xie XY. Treatment effect of radiofrequency ablation versus liver transplantation and surgical resection for hepatocellular carcinoma within Milan criteria: a population-based study. Eur Radiol. 2021;31:5379-5389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Gui CH, Baey S, D'cruz RT, Shelat VG. Trans-arterial chemoembolization + radiofrequency ablation versus surgical resection in hepatocellular carcinoma - A meta-analysis. Eur J Surg Oncol. 2020;46:763-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 32. | Pinter M, Jain RK, Duda DG. The Current Landscape of Immune Checkpoint Blockade in Hepatocellular Carcinoma: A Review. JAMA Oncol. 2021;7:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 272] [Article Influence: 54.4] [Reference Citation Analysis (1)] |
| 33. | Xu W, Liu K, Chen M, Sun JY, McCaughan GW, Lu XJ, Ji J. Immunotherapy for hepatocellular carcinoma: recent advances and future perspectives. Ther Adv Med Oncol. 2019;11:1758835919862692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 34. | Greten TF, Sangro B. Targets for immunotherapy of liver cancer. J Hepatol. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 35. | Webb ES, Liu P, Baleeiro R, Lemoine NR, Yuan M, Wang YH. Immune checkpoint inhibitors in cancer therapy. J Biomed Res. 2018;32:317-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 36. | El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3278] [Cited by in RCA: 3451] [Article Influence: 383.4] [Reference Citation Analysis (2)] |
| 37. | Finkelmeier F, Waidmann O, Trojan J. Nivolumab for the treatment of hepatocellular carcinoma. Expert Rev Anticancer Ther. 2018;18:1169-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 38. | Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, Kudo M, Han KH, Harding JJ, Merle P. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2019;30:v874-v875. [RCA] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 433] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 39. | Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M; KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1979] [Article Influence: 247.4] [Reference Citation Analysis (0)] |
| 40. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 5304] [Article Influence: 884.0] [Reference Citation Analysis (29)] |
| 41. | Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, Bai Y, Yang L, Zhu H, Fang W, Lin X, Chen X, Li E, Wang L, Chen C, Zou J. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21:571-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 455] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 42. | Iyer RV, Li D, Dayyani F, Phan AT, Abrams TA. DEDUCTIVE: A study of tivozanib in combination with durvalumab in subjects with untreated advanced hepatocellular carcinoma—Phase Ib results. J Clin Oncol. 2021;39:294-294. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Ziogas IA, Evangeliou AP, Giannis D, Hayat MH, Mylonas KS, Tohme S, Geller DA, Elias N, Goyal L, Tsoulfas G. The Role of Immunotherapy in Hepatocellular Carcinoma: A Systematic Review and Pooled Analysis of 2,402 Patients. Oncologist. 2021;26:e1036-e1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 44. | Zhang L, Ding J, Li HY, Wang ZH, Wu J. Immunotherapy for advanced hepatocellular carcinoma, where are we? Biochim Biophys Acta Rev Cancer. 2020;1874:188441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 45. | Rochigneux P, Chanez B, De Rauglaudre B, Mitry E, Chabannon C, Gilabert M. Adoptive Cell Therapy in Hepatocellular Carcinoma: Biological Rationale and First Results in Early Phase Clinical Trials. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 46. | Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y, Kakizoe T. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356:802-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 658] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 47. | Hui D, Qiang L, Jian W, Ti Z, Da-Lu K. A randomized, controlled trial of postoperative adjuvant cytokine-induced killer cells immunotherapy after radical resection of hepatocellular carcinoma. Dig Liver Dis. 2009;41:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 48. | Pan K, Li YQ, Wang W, Xu L, Zhang YJ, Zheng HX, Zhao JJ, Qiu HJ, Weng DS, Li JJ, Wang QJ, Huang LX, He J, Chen SP, Ke ML, Wu PH, Chen MS, Li SP, Xia JC, Zeng YX. The efficacy of cytokine-induced killer cell infusion as an adjuvant therapy for postoperative hepatocellular carcinoma patients. Ann Surg Oncol. 2013;20:4305-4311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 49. | Ma Y, Xu YC, Tang L, Zhang Z, Wang J, Wang HX. Cytokine-induced killer (CIK) cell therapy for patients with hepatocellular carcinoma: efficacy and safety. Exp Hematol Oncol. 2012;1:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 50. | Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW, Yoon JH. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383-1391.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 401] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 51. | Jia CC, Chen YH, Cai XR, Li Y, Zheng XF, Yao ZC, Zhao LY, Qiu DB, Xie SJ, Chen WJ, Liu C, Liu QL, Wu XY, Wang TT, Zhang Q. Efficacy of cytokine-induced killer cell-based immunotherapy for hepatocellular carcinoma. Am J Cancer Res. 2019;9:1254-1265. [PubMed] |
| 52. | Yoon JS, Song BG, Lee JH, Lee HY, Kim SW, Chang Y, Lee YB, Cho EJ, Yu SJ, Sinn DH, Kim YJ, Yoon JH. Adjuvant cytokine-induced killer cell immunotherapy for hepatocellular carcinoma: a propensity score-matched analysis of real-world data. BMC Cancer. 2019;19:523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Cao J, Kong FH, Liu X, Wang XB. Immunotherapy with dendritic cells and cytokine-induced killer cells for hepatocellular carcinoma: A meta-analysis. World J Gastroenterol. 2019;25:3649-3663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (2)] |
| 54. | Guo J, Tang Q. Recent updates on chimeric antigen receptor T cell therapy for hepatocellular carcinoma. Cancer Gene Ther. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 55. | Palmer DH, Midgley RS, Mirza N, Torr EE, Ahmed F, Steele JC, Steven NM, Kerr DJ, Young LS, Adams DH. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology. 2009;49:124-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 218] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 56. | Mizukoshi E, Nakagawa H, Kitahara M, Yamashita T, Arai K, Sunagozaka H, Iida N, Fushimi K, Kaneko S. Phase I trial of multidrug resistance-associated protein 3-derived peptide in patients with hepatocellular carcinoma. Cancer Lett. 2015;369:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 57. | Sawada Y, Yoshikawa T, Ofuji K, Yoshimura M, Tsuchiya N, Takahashi M, Nobuoka D, Gotohda N, Takahashi S, Kato Y, Konishi M, Kinoshita T, Ikeda M, Nakachi K, Yamazaki N, Mizuno S, Takayama T, Yamao K, Uesaka K, Furuse J, Endo I, Nakatsura T. Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology. 2016;5:e1129483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 58. | Lee JH, Tak WY, Lee Y, Heo MK, Song JS, Kim HY, Park SY, Bae SH, Lee JH, Heo J, Kim KH, Bae YS, Kim YJ. Adjuvant immunotherapy with autologous dendritic cells for hepatocellular carcinoma, randomized phase II study. Oncoimmunology. 2017;6:e1328335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 59. | Rizell M, Sternby Eilard M, Andersson M, Andersson B, Karlsson-Parra A, Suenaert P. Phase 1 Trial With the Cell-Based Immune Primer Ilixadencel, Alone, and Combined With Sorafenib, in Advanced Hepatocellular Carcinoma. Front Oncol. 2019;9:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 60. | Moehler M, Heo J, Lee HC, Tak WY, Chao Y, Paik SW, Yim HJ, Byun KS, Baron A, Ungerechts G, Jonker D, Ruo L, Cho M, Kaubisch A, Wege H, Merle P, Ebert O, Habersetzer F, Blanc JF, Rosmorduc O, Lencioni R, Patt R, Leen AM, Foerster F, Homerin M, Stojkowitz N, Lusky M, Limacher JM, Hennequi M, Gaspar N, McFadden B, De Silva N, Shen D, Pelusio A, Kirn DH, Breitbach CJ, Burke JM. Vaccinia-based oncolytic immunotherapy Pexastimogene Devacirepvec in patients with advanced hepatocellular carcinoma after sorafenib failure: a randomized multicenter Phase IIb trial (TRAVERSE). Oncoimmunology. 2019;8:1615817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 61. | Slovak R, Ludwig JM, Gettinger SN, Herbst RS, Kim HS. Immuno-thermal ablations - boosting the anticancer immune response. J Immunother Cancer. 2017;5:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 152] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 62. | Mehta A, Oklu R, Sheth RA. Thermal Ablative Therapies and Immune Checkpoint Modulation: Can Locoregional Approaches Effect a Systemic Response? Gastroenterol Res Pract. 2016;2016:9251375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 63. | Cirincione R, Di Maggio FM, Forte GI, Minafra L, Bravatà V, Castiglia L, Cavalieri V, Borasi G, Russo G, Lio D, Messa C, Gilardi MC, Cammarata FP. High-Intensity Focused Ultrasound- and Radiation Therapy-Induced Immuno-Modulation: Comparison and Potential Opportunities. Ultrasound Med Biol. 2017;43:398-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 64. | Zerbini A, Pilli M, Laccabue D, Pelosi G, Molinari A, Negri E, Cerioni S, Fagnoni F, Soliani P, Ferrari C, Missale G. Radiofrequency thermal ablation for hepatocellular carcinoma stimulates autologous NK-cell response. Gastroenterology. 2010;138:1931-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 65. | Takaki H, Imai N, Thomas CT, Yamakado K, Yarmohammadi H, Ziv E, Srimathveeravalli G, Sofocleous CT, Solomon SB, Erinjeri JP. Changes in peripheral blood T-cell balance after percutaneous tumor ablation. Minim Invasive Ther Allied Technol. 2017;26:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 66. | Wissniowski TT, Hänsler J, Neureiter D, Frieser M, Schaber S, Esslinger B, Voll R, Strobel D, Hahn EG, Schuppan D. Activation of tumor-specific T lymphocytes by radio-frequency ablation of the VX2 hepatoma in rabbits. Cancer Res. 2003;63:6496-6500. [PubMed] |
| 67. | Shi L, Chen L, Wu C, Zhu Y, Xu B, Zheng X, Sun M, Wen W, Dai X, Yang M, Lv Q, Lu B, Jiang J. PD-1 Blockade Boosts Radiofrequency Ablation-Elicited Adaptive Immune Responses against Tumor. Clin Cancer Res. 2016;22:1173-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 244] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 68. | Zerbini A, Pilli M, Penna A, Pelosi G, Schianchi C, Molinari A, Schivazappa S, Zibera C, Fagnoni FF, Ferrari C, Missale G. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res. 2006;66:1139-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 198] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 69. | Zerbini A, Pilli M, Fagnoni F, Pelosi G, Pizzi MG, Schivazappa S, Laccabue D, Cavallo C, Schianchi C, Ferrari C, Missale G. Increased immunostimulatory activity conferred to antigen-presenting cells by exposure to antigen extract from hepatocellular carcinoma after radiofrequency thermal ablation. J Immunother. 2008;31:271-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 70. | Nobuoka D, Motomura Y, Shirakawa H, Yoshikawa T, Kuronuma T, Takahashi M, Nakachi K, Ishii H, Furuse J, Gotohda N, Takahashi S, Nakagohri T, Konishi M, Kinoshita T, Komori H, Baba H, Fujiwara T, Nakatsura T. Radiofrequency ablation for hepatocellular carcinoma induces glypican-3 peptide-specific cytotoxic T lymphocytes. Int J Oncol. 2012;40:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 71. | Mizukoshi E, Yamashita T, Arai K, Sunagozaka H, Ueda T, Arihara F, Kagaya T, Fushimi K, Kaneko S. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2013;57:1448-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 217] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 72. | Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T, ElGindi M, Uppala A, Korangy F, Kleiner DE, Figg WD, Venzon D, Steinberg SM, Venkatesan AM, Krishnasamy V, Abi-Jaoudeh N, Levy E, Wood BJ, Greten TF. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 661] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 73. | Weng DS, Zhou J, Zhou QM, Zhao M, Wang QJ, Huang LX, Li YQ, Chen SP, Wu PH, Xia JC. Minimally invasive treatment combined with cytokine-induced killer cells therapy lower the short-term recurrence rates of hepatocellular carcinomas. J Immunother. 2008;31:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 74. | Huang ZM, Li W, Li S, Gao F, Zhou QM, Wu FM, He N, Pan CC, Xia JC, Wu PH, Zhao M. Cytokine-induced killer cells in combination with transcatheter arterial chemoembolization and radiofrequency ablation for hepatocellular carcinoma patients. J Immunother. 2013;36:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 75. | Ma H, Zhang Y, Wang Q, Li Y, He J, Wang H, Sun J, Pan K, Chen M, Xia J. Therapeutic safety and effects of adjuvant autologous RetroNectin activated killer cell immunotherapy for patients with primary hepatocellular carcinoma after radiofrequency ablation. Cancer Biol Ther. 2010;9:903-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 76. | Cui J, Wang N, Zhao H, Jin H, Wang G, Niu C, Terunuma H, He H, Li W. Combination of radiofrequency ablation and sequential cellular immunotherapy improves progression-free survival for patients with hepatocellular carcinoma. Int J Cancer. 2014;134:342-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 77. | Bian H, Zheng JS, Nan G, Li R, Chen C, Hu CX, Zhang Y, Sun B, Wang XL, Cui SC, Wu J, Xu J, Wei D, Zhang X, Liu H, Yang W, Ding Y, Li J, Chen ZN. Randomized trial of [131I] metuximab in treatment of hepatocellular carcinoma after percutaneous radiofrequency ablation. J Natl Cancer Inst. 2014;106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 78. | Tu J, Ji J, Wu F, Wang Y, Zhang D, Zhao Z, Ying X. Effectiveness of combined (131)I-chTNT and radiofrequency ablation therapy in treating advanced hepatocellular carcinoma. Cell Biochem Biophys. 2015;71:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 79. | Duan X, Wang M, Han X, Ren J, Huang G, Ju S, Zhang Q. Combined use of microwave ablation and cell immunotherapy induces nonspecific immunity of hepatocellular carcinoma model mice. Cell Cycle. 2020;19:3595-3607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 80. | Leuchte K, Staib E, Thelen M, Gödel P, Lechner A, Zentis P, Garcia-Marquez M, Waldschmidt D, Datta RR, Wahba R, Wybranski C, Zander T, Quaas A, Drebber U, Stippel DL, Bruns C, von Bergwelt-Baildon M, Wennhold K, Schlößer HA. Microwave ablation enhances tumor-specific immune response in patients with hepatocellular carcinoma. Cancer Immunol Immunother. 2021;70:893-907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 81. | Zhou P, Liang P, Dong B, Yu X, Han Z, Xu Y. Phase Ⅰ clinical study of combination therapy with microwave ablation and cellular immunotherapy in hepatocellular carcinoma. Cancer Biol Ther. 2011;11:450-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 82. | Yu MA, Liang P, Yu XL, Han ZY, Dong XJ, Wang YU, Cheng C, Li X. Multiple courses of immunotherapy with different immune cell types for patients with hepatocellular carcinoma after microwave ablation. Exp Ther Med. 2015;10:1460-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 83. | Huang ZM, Lai CX, Zuo MX, An C, Wang XC, Huang JH, Ning E. Adjuvant cytokine-induced killer cells with minimally invasive therapies augmented therapeutic efficacy of unresectable hepatocellular carcinoma. J Cancer Res Ther. 2020;16:1603-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 84. | Niu LZ, Li JL, Zeng JY, Mu F, Liao MT, Yao F, Li L, Liu CY, Chen JB, Zuo JS, Xu KC. Combination treatment with comprehensive cryoablation and immunotherapy in metastatic hepatocellular cancer. World J Gastroenterol. 2013;19:3473-3480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 85. | Gameiro SR, Higgins JP, Dreher MR, Woods DL, Reddy G, Wood BJ, Guha C, Hodge JW. Combination therapy with local radiofrequency ablation and systemic vaccine enhances antitumor immunity and mediates local and distal tumor regression. PLoS One. 2013;8:e70417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 86. | Ali MY, Grimm CF, Ritter M, Mohr L, Allgaier HP, Weth R, Bocher WO, Endrulat K, Blum HE, Geissler M. Activation of dendritic cells by local ablation of hepatocellular carcinoma. J Hepatol. 2005;43:817-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 87. | Liao J, Xiao J, Zhou Y, Liu Z, Wang C. Effect of transcatheter arterial chemoembolization on cellular immune function and regulatory T cells in patients with hepatocellular carcinoma. Mol Med Rep. 2015;12:6065-6071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 88. | Takaki H, Imai N, Contessa TT, Srimathveeravalli G, Covey AM, Getrajdman GI, Brown KT, Solomon SB, Erinjeri JP. Peripheral Blood Regulatory T-Cell and Type 1 Helper T-Cell Population Decrease after Hepatic Artery Embolization. J Vasc Interv Radiol. 2016;27:1561-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 89. | Lee MS, Ryoo BY, Hsu CH, Numata K, Stein S, Verret W, Hack SP, Spahn J, Liu B, Abdullah H, Wang Y, He AR, Lee KH; GO30140 investigators. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21:808-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 437] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 90. | Ghavimi S, Apfel T, Azimi H, Persaud A, Pyrsopoulos NT. Management and Treatment of Hepatocellular Carcinoma with Immunotherapy: A Review of Current and Future Options. J Clin Transl Hepatol. 2020;8:168-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 91. | Tagliamonte M, Mauriello A, Cavalluzzo B, Ragone C, Manolio C, Petrizzo A, Buonaguro L. Tackling hepatocellular carcinoma with individual or combinatorial immunotherapy approaches. Cancer Lett. 2020;473:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 92. | Pauli C, Hopkins BD, Prandi D, Shaw R, Fedrizzi T, Sboner A, Sailer V, Augello M, Puca L, Rosati R, McNary TJ, Churakova Y, Cheung C, Triscott J, Pisapia D, Rao R, Mosquera JM, Robinson B, Faltas BM, Emerling BE, Gadi VK, Bernard B, Elemento O, Beltran H, Demichelis F, Kemp CJ, Grandori C, Cantley LC, Rubin MA. Personalized In Vitro and In Vivo Cancer Models to Guide Precision Medicine. Cancer Discov. 2017;7:462-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 675] [Cited by in RCA: 746] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 93. | Kudo M. Scientific Rationale for Combined Immunotherapy with PD-1/PD-L1 Antibodies and VEGF Inhibitors in Advanced Hepatocellular Carcinoma. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 160] [Article Influence: 26.7] [Reference Citation Analysis (2)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen BB S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Li X