Published online Oct 15, 2021. doi: 10.4251/wjgo.v13.i10.1367
Peer-review started: April 6, 2021
First decision: June 23, 2021
Revised: July 6, 2021
Accepted: August 13, 2021
Article in press: August 13, 2021
Published online: October 15, 2021
Processing time: 190 Days and 9.5 Hours
Gastric cancer (GC) is the fifth most diagnosed cancer and the third leading cause of cancer-related death worldwide. Although progress has been made in diag
Core Tip: FOXOs perform diverse roles in the occurrence and development of gastric cancer, the fifth most diagnosed type of cancer and third leading cause of cancer-related death worldwide. This article reviews the cellular functions of FOXOs in gastric cancer and provides potential therapeutic targets for patients with gastric cancer.
- Citation: Chen YH, Li CL, Chen WJ, Liu J, Wu HT. Diverse roles of FOXO family members in gastric cancer. World J Gastrointest Oncol 2021; 13(10): 1367-1382
- URL: https://www.wjgnet.com/1948-5204/full/v13/i10/1367.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i10.1367
Gastric cancer (GC) is the fifth most diagnosed cancer and the third leading cause of cancer-related death worldwide[1]. Upper gastrointestinal series and endoscopy, which have been demonstrated to be effective for screening, have not been widely adopted worldwide because of their invasive nature and high cost. Moreover, the lack of universal guidelines for screening has increased the difficulty of early diagnosis of GC[2-4]. It is estimated that more than 700000 cancer-related deaths are caused by GC, which is primarily because the cancer is already at an advanced stage at initial diagnosis[5,6]. Unsurprisingly, although great progress has been made in diagnosis, surgical resection, systemic chemotherapy, and immunotherapy in recent decades, patients with advanced GC still exhibit a very poor prognosis, with a median overall survival (OS) of 10-12 mo and an overall 5-year survival rate of less than 5%[7-9]. To improve the availability of accurate diagnostic tests for the early detection of GC and to identify more specific therapeutic targets for GC patients, it is important to explore the molecular mechanism of GC. This will help overcome the critical limitations in diagnostics and therapeutics in patients with GC.
FOXOs, the O subfamily of the forkhead box (FOX) family of transcription factors, comprise four members, FOXO1, FOXO3, FOXO4, and FOXO6. This subfamily has been reported to be involved in the cell cycle, cell growth, apoptosis, autophagy, stress resistance, protection from aggregate toxicity, DNA repair, tumor suppression, and metabolism[10,11]. Importantly, FOXOs are involved in the pathological processes of malignant tumors, as well as in the physiological processes of development[12]. However, the functions of FOXOs in malignant tumors vary under different con
It is well known that transcription factors regulate the expression of target genes by identifying and binding to specific DNA sequences, after which they participate in the formation of a complex signaling network to maintain cell homeostasis[16]. Dysregulation of transcription factors leads to a variety of pathological changes in cells, results in the occurrence of various diseases, and determines the various behaviors of malignant tumors[17,18]. Among various transcription factors, FOX transcription factors are widely distributed in organisms from yeasts to humans. They are characterized by a forkhead domain (FHD) and a highly conserved DNA binding domain (DBD) that is composed of 100 amino acid residues folded into a helix-turn-helix motif with two characteristic large loops and three α helices[19].
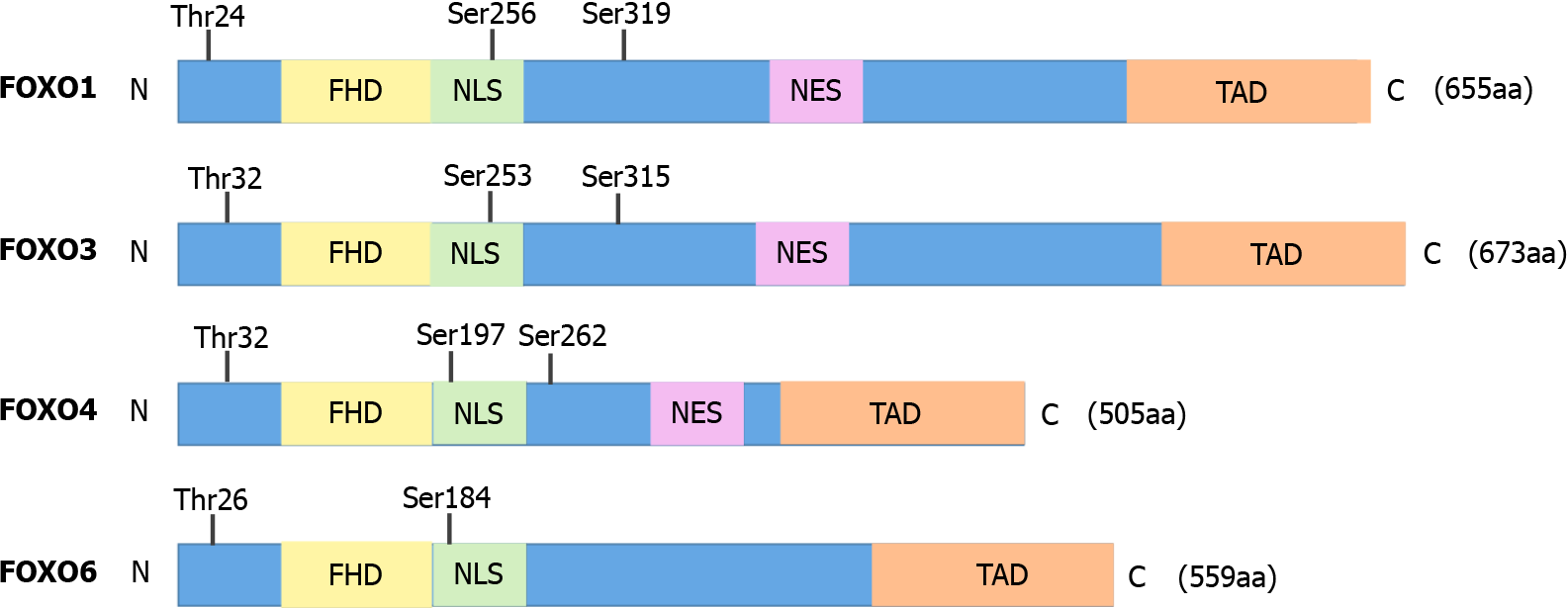
Among the different types of FOX transcription factors, the four FOXO isoforms, FOXO1, FOXO3, FOXO4, and FOXO6, in mammals belong to the O subfamily of the FOX family of transcription factors[20]. FOXOs have four common domains, including a FHD, a nuclear export sequence (NES) domain, a nuclear localization signal (NLS), and a C-terminal transactivation domain (TAD), although FOXO6 lacks the NES domain (Figure 1). All FOXOs can recognize and bind to two sequences: the Daf-16 family member-binding element (DEB), 5′-GTAAA(T/C)AA-3′, and the insulin-responsive sequence (IRE), 5′-(C/A)(A/C)AAA(C/T)AA-3′[21,22].
FOXO1, FOXO3, and FOXO4 are widely expressed in almost all tissues, and their transcriptional activity changes as they shuttle between different subcellular localizations[22,23]. FOXO6, a novel member of the FOXO class reported by Jacobs et al[24], was originally only observed in the central nervous system, but subsequent investigations have confirmed that FOXO6 is also expressed in peripheral tissues, including the lungs, liver, kidneys, intestine, muscle, and adipose tissue[25]. Interestingly, the expression pattern of FOXO6 is different from that of other FOXO isoforms in its evolution, and it is the least characterized member of the FOXO family. Due to the lack of an NES sequence, FOXO6 does not shuttle between the nucleus and cytoplasm and is located only in the nucleus[26].
FOXOs function as central transcription factors that regulate many cellular processes through transcriptional activity. Unsurprisingly, FOXOs are also regulated by multiple signaling pathways involving synthesis, phosphorylation, acetylation, and ubiquitination, which mainly determine subcellular localization, transcriptional activity, and protein stability[11,22,27]. As transcription factors, FOXOs usually exist in the nuclei of quiescent or growth factor (GF)-deficient cells. When GFs are absent, FOXOs shuttle into and accumulate in the nucleus to promote cell cycle arrest, stress resistance, and apoptosis, by upregulating the transcription of a series of target genes. However, in the presence of cell GFs, FOXOs relocate to the cytoplasm for degradation by the ubiquitin-proteasome pathway[23].
Phosphorylation via the classical PI3K-AKT pathway: Except for FOXO6, the regulation of FOXO-dependent transcription primarily depends on shuttling between the nucleus and cytoplasm. More specifically, negative regulation by the PI3K-AKT pathway is dependent on activation by GF receptor tyrosine kinases (RTKs)[28]. Under normal physiological conditions, RTKs are activated by autophosphorylation after binding GFs or insulin, which is followed by recruitment and activation of PI3K. Then, activated PI3K catalyzes phosphatidylinositol-4,5-bisphosphate (PIP2) to phospha
According to a previously defined mechanism, FOXOs enter the nucleus, bind to a variety of transcription cofactors, and regulate the transcription of target genes related to the cell cycle, apoptosis, the antioxidant state, metabolism, and angiogenesis[28]. For FOXO6, phosphorylation of two residues (threonine 26 and serine 184) by AKT results in inactivation. Unlike other FOXOs, the PI3K-AKT pathway cannot affect the subcellular localization of FOXO6 due to the lack of carboxy-terminal AKT-dependent phosphorylation sites in FOXO6[11,25,29].
AKT-independent phosphorylation: Inhibition of FOXOs by the PI3K-AKT pathway is believed to enhance tumor development, while stress-activated kinases, such as c-Jun N-terminal kinase (JNK), mammalian sterile 20like kinase 1 (MST1), and protein kinase RNA-like endoplasmic reticulum kinase (PERK), play a tumor inhibitory role by promoting FOXO function in an AKT-independent manner[11].
Essers et al[30] illustrated that in contrast to insulin-mediated regulation, under oxidative stress, FOXO4 is phosphorylated by JNK on threonine 447 and threonine 451 in a GTPase-dependent manner, which leads to the nuclear translocation of p-FOXO4. Specifically, the regulatory effect of JNK on FOXO activity involves phosphorylation of 14-3-3 on serine 184 to block 14-3-3 proteins from binding to FOXOs[11,31].
Lehtinen et al[32] extended the molecular mechanism by which oxidative stress influences cell survival and homeostasis, by demonstrating the role of the protein kinase MST1 in oxidative stress-induced cell death. In the case of increased cellular oxidative stress, MST1 phosphorylates FOXO proteins at a conserved site to disrupt their interaction with 14-3-3 proteins, which results in FOXO nuclear translocation, and induces neuronal cell death[32]. Soon after, Yuan et al[33] also found that MST1-induced phosphorylation of FOXO1 at serine 212, which corresponds to serine 207 in FOXO3, disrupts the association between FOXO1 and 14-3-3 proteins. The above findings indicate that MST1-FOXO1 signaling is an important link to serum-deprivation-induced neuronal cell death.
Recently, PERK was found to be involved in endoplasmic reticulum (ER) stress related to the onset of type 2 diabetes[34]. Imbalances between protein synthesis and folding lead to ER stress, which partially enhances FOXO activity through the PERK pathway. Interestingly, although three target sites serine 298, serine 301, and serine 303 on FOXO1 can be phosphorylated by PERK, PERK-mediated phosphorylation preferentially occurs on serine 298, which is not a target site for AKT[34]. Phosphorylation by PERK enhances the transcriptional activity of FOXOs and counteracts the effect of Akt phosphorylation[34,35].
In addition, extracellular signalregulated kinase (ERK), p38, cyclin-dependent kinases (CDKs), adenosine monophosphate-activated protein kinase (AMPK), and IκB kinase (IκK) regulate FOXOs in an AKT-independent manner. For example, mitogen-activated protein kinases (MAPKs), ERK, and p38 jointly phosphorylate FOXO1, which results in p-FOXO1 serving as a coactivator for Ets-1[36]. Additionally, ERK mediates the phosphorylation of FOXO3 at serine 294, serine 344, and serine 425, which permits the association of p-FOXO3 with the E3 ubiquitin ligase MDM2 (murine double minute 2). This in turn results in the ubiquitination and degradation of p-FOXO3 to promote cell proliferation and tumorigenesis[37]. CDK2 binds to and phosphorylates FOXO1 at serine 249 in a glucose-dependent manner, and loss of CDK2 may mediate persistent insulin secretion defects through this pathway[38,39]. Lu et al[40] proposed FO1–6nls, a FOXO1-derived peptide inhibitor of CDK1/2-mediated phosphorylation of FOXO1 at serine 249, as a potential therapeutic for the treatment of prostate cancers. AMPK phosphorylates FOXO1 and forms the AMPK/ FOXO1 axis, which is involved in multiple pathological processes, such as liver fibrosis[41], cardiac hypertrophy[42], and epithelial-mesenchymal transition (EMT)[43]. The phosphorylation of FOXO3 at serine 644 by IκK normally leads to ubiquitin-dependent proteasomal degradation[44], but causes cytoplasmic retention in acute myeloid leukemia[45].
Acetylation: Histone acetylation is an epigenetic modification that regulates numerous genes essential for various biological processes, including development and stress responses[46]. It has been reported that calcium response element-binding protein (CBP)/p300 acetylates FOXOs to promote their phosphorylation by AKT and allows FOXOs to be retained in the cytoplasm[47]. However, stress-induced FOXO1 acety
Other posttranslational modifications: In addition to phosphorylation, acetylation, and polyubiquitination, the activity of FOXOs is regulated by other posttranslational modifications, including mono-ubiquitination, methylation, and glycosylation.
In contrast to degradation induced by polyubiquitination, mono-ubiquitination enhances FOXO activity. Interestingly, under oxidative stress, MDM2, which promotes the degradation of p-FOXO3, can induce mono-ubiquitination of FOXO4 to increase FOXO4 nuclear entry and transcriptional activity[52]. Methylation of FOXO1 by protein arginine methyltransferase 1 (PRMT1) inhibits AKT-induced phosphorylation, and thus, promotes FOXO1 retention in the nucleus and increases the expression of downstream target genes[53]. However, methylation of FOXO3 by the Set9 methyltransferase reduces the DNA-binding and transcriptional activities of FOXO3[54]. O-glycosylation improves the transcriptional activity of FOXO1 without influencing its subcellular localization[55]. Recently, N6-methyladenosine modifications of FOXO1 mRNA, reported by Jian et al[56], were demonstrated to mediate METTL14-induced endothelial inflammation and atherosclerosis. Shin et al[57] identified a novel post
Of course, other posttranslational modifications may exist and remain to be discovered. The transcriptional activities of FOXOs are involved in regulating the cell cycle, oxidative stress, apoptosis, and autophagy, as well as metabolic and immunoregulatory factors. Moreover, FOXO3 is closely related to longevity in humans[58-60]. The biological function of FOXO6 has not been well studied, and most research has indicated its participation in glucose and lipid metabolism[26]. Unsurprisingly, FOXOs are involved in many aspects of malignant tumors.
It is well known that FOXOs are tumor suppressors in many types of malignant tumors[29]. Usually, in cancers, the PI3K-PKB/AKT signaling pathway is enhanced, and FOXOs are negatively regulated downstream molecules in the pathway. Spe
In terms of cell cycle control, Baugh and Sternberg[63] found that the induced expression of cell cycle kinase inhibitors (CKIs) by FOXOs leads to the inhibition of cyclin/CDK complexes, which are responsible for cell cycle progression at different phases. This causes cell cycle arrest in G0/G1 and G2 phases and even senescence and promotes developmental arrest via transcriptional regulation of numerous target genes that control various aspects of development[63].
Moreover, in both normal and cancer cells, FOXOs are reported to induce the expression of proapoptotic genes, resulting in apoptosis. Wang et al[64] showed that activation of AMPK-FOXO is upstream of the KLF2 pathway and contributes to the induction of apoptosis and differentiation by DT-13 (Liriope muscari baily saponins C) in acute myelocytic leukemia. Laporte et al[65] revealed that HDAC inhibition-induced apoptosis and decreased tumor burden in synovial sarcoma are related to reactive oxygen species (ROS)-mediated FOXO activation and the subsequent increase in the expression of the proapoptotic factors BIK, BIM, and BMF. Interestingly, in the case of detachment from the extracellular matrix, FOXOs induce anoikis and prevent metastasis by promoting BMF expression, whereas under anchorage-independent conditions, cyclin D1 induces an antagonistic effect on FOXO-regulated anoikis[66].
It is widely accepted that ROS abnormally accumulate in cancer cells due to the reprogramming of redox metabolism, which plays opposite roles in various aspects of occurrence and development of malignant tumors[67]. Upon AKT activation, FOXOs become phosphorylated and translocate from the nucleus, which results in reduced expression of superoxide dismutase 2 (SOD2) and an increase in ROS and mito
Based on a previous mechanism, a series of investigations reported a significant relationship between FOXO expression and the clinical parameters of malignant tumors. Xu et al[69] found that a low level of FOXO4 expression in non-small cell lung cancer patients is significantly correlated with TNM stage and lymph node metastasis, which suggests an inhibition of FOXO4 function during the process of EMT. In CRC tissues, the expression of FOXO3 is also significantly lower than that in normal tissues, and interestingly, the progressive downregulation of FOXO3 is correlated with the progression of pathological stage in patients with CRC. Moreover, the mean disease-free survival (DFS) of CRC patients with low FOXO3 expression is significantly shorter compared with that of CRC patients with high FOXO3 expression[70]. Wu et al[71] conducted multivariate analyses and revealed that FOXO1 expression is an in
For further study, knockout techniques have provided additional methods by which the function and molecular mechanism of FOXO in tumors can be investigated. Renault et al[73] revealed that FOXO3 is a direct target of the p53 tumor suppressor gene. However, no association was observed between FOXO3 loss and p53 loss in tumor development. Paik et al[74] established a FOXO1/FOXO3/FOXO4 triple knock
However, every coin has two sides. The expression of FOXO3 has been found to be increased in glioblastoma (GBM), and a high level of FOXO3 is associated with a poor prognosis in GBM patients. In addition, FOXO3 knockout significantly reduces, whereas FOXO3 overexpression enhances, the proliferation and invasiveness of GBM cells[75]. Yu et al[76] demonstrated that the expression of FOXO3 can be upregulated by SP1, which promotes CRC cell progression in vitro and in vivo. FOXO3 was found to promote tumor growth, under hypoxic conditions, and angiogenesis in aggressive neuroblastoma, which predicts adverse clinical outcomes[77]. In addition, FOXO3 acts as a conditional chemoprotection factor in late-stage neuroblastoma, enhancing tumor cell survival under chemotherapy[78]. The above reports reveal the complicated roles of FOXOs in cancer. As Hornsveld et al[28] suggested, FOXOs may function to support resilience in both healthy and cancer cells, rather than as typical tumor suppressors.
Unsurprisingly, the expression level of FOXOs is often altered in GC. Decreased levels of FOXO1/FOXO3/FOXO4 and increased expression of FOXO6 in GC have been reported. By examining 50 pairs of samples, Zang et al[79] found that the mRNA level of FOXO1 is downregulated in GC tissues compared with corresponding noncancerous tissues. Lower levels of FOXO3 mRNA and protein have also been found in GC tissues compared with peritumoral tissues[80]. Similarly, FOXO4 expression is consistently lower in GC tissues than in adjacent normal tissues[81]. However, FOXO6 has been reported to be overexpressed in GC. Elevated FOXO6 expression was demonstrated to promote the proliferation, invasiveness, and migration of GC cells, and is associated with a poor prognosis in GC patients[82,83]. Although FOXO1/ FOXO3/FOXO4 are often downregulated in GC, and mainly play a tumor inhibitory role, FOXOs possess tumor-promoting functions in certain conditions, and these functions are associated with different underlying molecular mechanisms.
Tumorigenesis and proliferation: Tumorigenesis begins with one or more genetic or epigenetic changes in a single cell, followed by subsequent changes that promote tumor development and progression of the tumor to a more aggressive phenotype. Following the accumulation of multiple genetic and epigenetic changes, when a cell has adapted enough to escape cellular homeostasis, cancer processes are initiated[84]. The expression level of FOXO4 is controlled by methylation of its promoter, and Zhou et al[85] showed that hypermethylation of FOXO4, which is induced by ubiquitin-like containing PHD ring finger 1, is involved in GC carcinogenesis.
Regulating the tumorigenic ability of GC cells by FOXOs involves their ability to inhibit GC cell self-renewal. Negative crosstalk between FOXO1 and leucine-rich repeat-containing G-protein-coupled receptor 5 (LGR5) was found in GC, and down
After tumor formation, cancer cell proliferation controlled by FOXOs is related to cell cycle arrest and induction of autophagy. FOXO1 inhibition by activation of the upstream c-Myc/NAMPT/SIRT1 signaling pathway or upregulation of downstream HER2, which results from FOXO1 Loss, promotes GC cell growth[89,90]. Su et al[81] found that FOXO4 induces cell cycle arrest in G1 phase and also reported a shortened S phase in GC cells. Activation of FOXO1 induces the expression of CDKI, p21Cip1, and p27Kip1, which can suppress GC cell proliferation by triggering cell cycle arrest[91,92]. MiR-96–5p and miR-1274a directly target the 3’-untranslated regions of FOXO3 and FOXO4 mRNA, respectively, and promote GC cell proliferation[93,94]. Moreover, in an acidic microenvironment, FOXO3 enhances autophagy by increasing the expression of autophagy proteins, such as LC3I, LC3II, and Beclin-1, to inhibit GC cell growth[95].
Apoptosis: The ability to escape apoptosis is a hallmark of cancer cells[96]. Identification of the mechanism of apoptosis induction provides potential therapeutic strategies for malignant tumors. In an α-fetoprotein (AFP)-producing GC (AFPGC) model, miR-122-5p inhibited apoptosis and promoted tumor progression by directly targeting FOXO3[97]. The transcription factor RUNX3 binds to two RUNX binding elements (RBE1 and RBE2) in the promoter region of the Bim gene, which encodes a pro-apoptotic protein. FOXO3 binds upstream of RBE1, which triggers apoptosis by activating Bim transcription through a physical interaction with RUNX3[98,99]. The induced Bim protein promotes the release of cytochrome c into the cytoplasm to initiate the formation of the apoptosome, which activates caspase-3 and leads to the execution phase of apoptosis[100]. Shahbazi et al[101] revealed the molecular me
Angiogenesis: Angiogenesis-dependent tumor growth is an important characteristic of cancers[96]. Vascular endothelial GF (VEGF) and hypoxia-inducible factor-1α (HIF-1α) are critical in promoting tumor angiogenesis[104]. Under hypoxic conditions, HIF-1α and HIF-1β subunits form heterodimers that activate the transcription of many target genes to adapt to the hypoxic environment of human cancer cells[105]. However, under anoxic conditions, inhibition of FOXO1 causes upregulated expression of HIF-1α and VEGF in GC cells and increases microvessel areas in GC tissue, thus promoting angiogenesis[106,107]. In GC cells, miR-135b can be delivered via exosomes to human umbilical vein endothelial cells (HUVECs) and can then directly bind to and down
Drugs can also affect angiogenesis in GC through FOXO-related signaling path
Metastasis: Metastasis is the leading cause of cancer-related death[96], and is related to EMT, which is characterized by loss of polarity of epithelial cells, decreased expression of epithelial markers, such as E-cadherin and β-catenin, and increased expression of mesenchymal markers, such as N-cadherin and vimentin. These characteristics endow tumor cells with metastatic properties by enhancing cell motility, invasiveness, and resistance to apoptosis. In addition, EMT-associated transcription factors, including Snail and Zeb, are involved in core EMT programs[110,111].
FOXO1 silencing results in upregulation of HER2 expression, which induces a mesenchymal cell phenotype, including decreased E-cadherin levels, increased Snail levels, and the presence of many filamentous processes with abundant actin bundles in GC cells, thus promoting the migration and invasiveness of GC cells[89]. Human telomerase reverse transcriptase cooperates with MDM2 to enhance FOXO3 degra
Chemoresistance: Studies that have focused on the role of FOXOs in GC chemoresistance are limited. Park et al[113] investigated resistance of GC cells to lapatinib in GC cells and showed that FOXO1 serves as an important link between the HER2 and MET signaling pathways by negatively regulating HER2 and MET expression at the transcriptional level, which could reverse resistance to lapatinib. Moreover, rosmarinic acid (RA) was found to increase FOXO4 expression by downregulating miR-6785–5p and miR-642a–3p levels and enhancing the sensitivity of drug-resistant GC cells to 5-fluorouracil[114].
Although many studies support the inhibitory effect of FOXOs in cancers, several recent studies have provided solid evidence of the opposite effect, whereby FOXOs can promote GC progression, including proliferation, invasion, migration, and chemo
Park et al[115] reported that treatment with cisplatin increases the mRNA level of FOXO1 and promotes the accumulation and activation of the FOXO1 protein to confer protection against cisplatin-induced cytotoxicity in GC cells. Interestingly, in addition to their findings of the suppressive role of FOXO1 in acquired lapatinib-resistance in HER2-positive GC cells, Park et al[115] also investigated the role of FOXO1 in cisplatin-resistant GC cells. They showed that constitutive activation of FOXO1 increases resistance to cisplatin, whereas FOXO1 silencing enhances cisplatin-induced cy
High FOXO6 expression promotes the proliferation of GC cells by binding to the transcription factor hepatic nuclear factor 4, which mediates histone acetylation and leads to subsequent induction of c-Myc expression after removal of HDAC3 from the c-Myc gene promoter[82]. Noncoding RNA activated by DNA damage, an lncRNA with potential carcinogenic effects in bladder and colon cancers, was found to be downregulated in GC cells, which could reduce the targeted inhibition of FOXO6 by miR-608 through competitive inhibition[118].
Therefore, it can be inferred that the changing microenvironment of GC at different stages of development may be one of the reasons why studies on the role of FOXOs in GC have reached opposite conclusions as to whether FOXOs participate in tumor progression.
As mentioned above, phosphorylation results in the translocation of FOXO1 to the cytoplasm, which prevents FOXO-dependent transcription and loss of FOXO-dependent regulation of downstream target genes. High levels of phosphorylated FOXO1 are associated with vascular invasion, lymph node metastasis, distant metastasis, and higher pTNM stage in colon cancer and are indicative of a poor prognosis in astrocytomas[119,120]. In prostate cancer, the traditional Chinese medicines CFF-1 (alcohol extract from an anticancer compound Chinese medicine) and ISO (isorhapontigenin) inhibit cell growth and induce cell apoptosis by decreasing p-FOXO1 and regulating the expression of apoptosis-related and cycle-related genes[121,122]. These findings are consistent with the antitumor effect of FOXO1 in GC. However, Kim et al[123] reported that p-FOXO1 is expressed in 84.6% of GC tissues and that its expression is higher in early stage GC and is correlated with better outcomes. These findings further confirm that the role of FOXO1 is dependent on cancer stage.
Yang et al[80] reported a significant correlation between low FOXO3 levels and large tumor size, poor histopathological classification, greater depth of invasion, local lymph node metastasis, distant metastasis, and high AJCC stage. Upregulation and activation of FOXO3 in GC are closely associated with a good outcome in GC patients[124], which suggests that FOXO3 is a potential prognostic marker as well as a therapeutic target in GC patients. Li et al[125] demonstrated that a low FOXO4 level is an independent prognostic factor for poor OS and DFS in GC patients, while a high FOXO6 level promotes tumor invasiveness and predicts a poor prognosis in GC patients[83].
Some potential GC chemotherapeutic agents antagonize tumors by targeting FOXOs and related proteins to inhibit cell growth and proliferation, and induce cell differentiation and apoptosis. Endogenous proteins, such as sphingosine kinase 1 (SPHK1) and PRMT1, microRNAs, and circular RNAs also affect FOXOs and their related signaling pathways, and change the biological characteristics of GC cells. All of these molecules are potential therapeutic targets for the treatment of GC (Table 1).
| Molecule | Targets | Mechanism | Effects | Ref. |
| Luteolin | FOXO1 | Increases FOXO1 expression | Represses GC cell growth | Ding et al[127] |
| Celecoxib | Akt, GSK3b, FOXO1, and caspase-9 | Downregulates Akt, GSK3b, and FOXO1 and upregulates caspase-9 in the mitochondrial apoptotic pathway | Represses GC cell growth | Kim et al[128] |
| 4-Amino-2-trifluoromethyl-phenyl retinate | 14-3-3ε | Downregulates expression of 14-3-3ε, resulting in increased expression of FOXO1 and P27kip1, decreased expression of CDK2 and cyclin E, and decreased activity of AKP and LDH. Blocks the cell cycle at G0/G1 phase | Inhibits cell proliferation and induces cell differentiation | Xia et al[129] |
| Gramicidin | FOXO1 | Decreases phosphorylation of FOXO1 and down-regulates the expression of cyclinD1 and Bcl-2, leading to G2/M cell cycle arrest | Inhibits cell proliferation | Chen et al[130] |
| Olaparib | PARP1 | Inhibits PARP1 and thus induces G2/M cell cycle arrest by activating FOXO3 | Inhibits cell proliferation | Park et al[126] |
| Bacillomycind-C16 | Akt and FOXO3 | Inhibits phosphorylation of Akt and increases the level of FOXO3 protein | Induces apoptosis | Lin et al[131] |
| Protein arginine methyltransferase 1 | FOXO1 and BAD | Activates FOXO1 and BAD | Induces chemosensitivity | Altan et al[132] |
| Sphingosine kinase 1 | FOXO1 and FOXO3 | Attenuates the transcriptional activity of FOXO1 and FOXO3 via promoting PI3K/Akt-mediated phosphorylation | Enhances proliferation (targeting FOXO1) and resistance to apoptosis (targeting FOXO3) | Xia et al[91] and Xiong et al[133] |
| Hsa_circ_0001368 | miR-6506-5p | Acts as a competing endogenous RNA for miR-6506-5p and inhibits the downregulation by miR-6506-5p on FOXO3 | Inhibits tumor growth | Lu et al[134] |
| miR-1274a | FOXO4 | Inhibits FOXO4 expression | Promotes tumor growth and migration | Wang et al[94] |
It is worth noting that the effect of some drugs is influenced by oncogene ex
Additionally, miR-633 enhances the chemoresistance of GC cells by downregulating FADD expression. Doxorubicin-induced nuclear accumulation of FOXO3 inhibits miR-633 transcription. Inhibition of miR-633 by an antagomir increases the FADD level and enhances doxorubicin/cisplatin-induced apoptosis. A miR-633 antagomir combined with doxorubicin significantly reduces GC cell growth[103].
Overall, FOXOs are promising prognostic markers and therapeutic targets in GC. However, recent studies have primarily focused on the molecular mechanism and are limited to the cell level, which indicates a huge gap between recent research findings and clinical applications. Therefore, the clinical implications of FOXOs still require clarification by additional studies.
FOXOs have historically been regarded as tumor suppressors, but recent studies have suggested that FOXOs support resiliency in healthy and cancer cells. In GC, the antitumor effect of FOXO4 and the tumor-promoting effect of FOXO6 are relatively clear. FOXO1 and FOXO3 play dual roles in many types of cancers, including GC. Whether they promote or inhibit GC may be related to changes in the tumor microenvironment caused by tumor progression and drug treatment. In advanced GC, the effect of changes in the expression level or activity of FOXOs on GC treatment has not been investigated. Therefore, caution should be exercised when FOXO1 and FOXO3 are used as targets for cancer treatment.
We are thankful to Professor Lin S for his English editing and Shen L for his valuable advice and English editing.
| 1. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 3308] [Article Influence: 551.3] [Reference Citation Analysis (6)] |
| 2. | Hamashima C; Systematic Review Group and Guideline Development Group for Gastric Cancer Screening Guidelines. Update version of the Japanese Guidelines for Gastric Cancer Screening. Jpn J Clin Oncol. 2018;48:673-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 297] [Article Influence: 37.1] [Reference Citation Analysis (3)] |
| 3. | Kim GH, Liang PS, Bang SJ, Hwang JH. Screening and surveillance for gastric cancer in the United States: Is it needed? Gastrointest Endosc. 2016;84:18-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 154] [Article Influence: 15.4] [Reference Citation Analysis (2)] |
| 4. | Necula L, Matei L, Dragu D, Neagu AI, Mambet C, Nedeianu S, Bleotu C, Diaconu CC, Chivu-Economescu M. Recent advances in gastric cancer early diagnosis. World J Gastroenterol. 2019;25:2029-2044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 307] [Cited by in RCA: 313] [Article Influence: 44.7] [Reference Citation Analysis (3)] |
| 5. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68637] [Article Influence: 13727.4] [Reference Citation Analysis (201)] |
| 6. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 12202] [Article Influence: 2440.4] [Reference Citation Analysis (7)] |
| 7. | Johnston FM, Beckman M. Updates on Management of Gastric Cancer. Curr Oncol Rep. 2019;21:67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 336] [Article Influence: 48.0] [Reference Citation Analysis (1)] |
| 8. | Charalampakis N, Economopoulou P, Kotsantis I, Tolia M, Schizas D, Liakakos T, Elimova E, Ajani JA, Psyrri A. Medical management of gastric cancer: a 2017 update. Cancer Med. 2018;7:123-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 9. | Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v38-v49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1150] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 10. | Murtaza G, Khan AK, Rashid R, Muneer S, Hasan SMF, Chen J. FOXO Transcriptional Factors and Long-Term Living. Oxid Med Cell Longev. 2017;2017:3494289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 135] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 11. | Yadav RK, Chauhan AS, Zhuang L, Gan B. FoxO transcription factors in cancer metabolism. Semin Cancer Biol. 2018;50:65-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 12. | Calissi G, Lam EW, Link W. Therapeutic strategies targeting FOXO transcription factors. Nat Rev Drug Discov. 2021;20:21-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 235] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 13. | Dumitrascu GR, Bucur O. Critical physiological and pathological functions of Forkhead Box O tumor suppressors. Discoveries (Craiova). 2013;1:e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Dong T, Zhang Y, Chen Y, Liu P, An T, Zhang J, Yang H, Zhu W, Yang X. FOXO1 inhibits the invasion and metastasis of hepatocellular carcinoma by reversing ZEB2-induced epithelial-mesenchymal transition. Oncotarget. 2017;8:1703-1713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 15. | van der Vos KE, Coffer PJ. The extending network of FOXO transcriptional target genes. Antioxid Redox Signal. 2011;14:579-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 16. | Lambert SA, Jolma A, Campitelli LF, Das PK, Yin Y, Albu M, Chen X, Taipale J, Hughes TR, Weirauch MT. The Human Transcription Factors. Cell. 2018;172:650-665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2883] [Cited by in RCA: 2170] [Article Influence: 271.3] [Reference Citation Analysis (0)] |
| 17. | Lambert M, Jambon S, Depauw S, David-Cordonnier MH. Targeting Transcription Factors for Cancer Treatment. Molecules. 2018;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 264] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 18. | Ulasov AV, Rosenkranz AA, Sobolev AS. Transcription factors: Time to deliver. J Control Release. 2018;269:24-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Jin Y, Liang Z, Lou H. The Emerging Roles of Fox Family Transcription Factors in Chromosome Replication, Organization, and Genome Stability. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Jiramongkol Y, Lam EW. FOXO transcription factor family in cancer and metastasis. Cancer Metastasis Rev. 2020;39:681-709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 206] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 21. | Kim CG, Lee H, Gupta N, Ramachandran S, Kaushik I, Srivastava S, Kim SH, Srivastava SK. Role of Forkhead Box Class O proteins in cancer progression and metastasis. Semin Cancer Biol. 2018;50:142-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 22. | Link W. Introduction to FOXO Biology. Methods Mol Biol. 2019;1890:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 23. | Farhan M, Wang H, Gaur U, Little PJ, Xu J, Zheng W. FOXO Signaling Pathways as Therapeutic Targets in Cancer. Int J Biol Sci. 2017;13:815-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 406] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 24. | Jacobs FM, van der Heide LP, Wijchers PJ, Burbach JP, Hoekman MF, Smidt MP. FoxO6, a novel member of the FoxO class of transcription factors with distinct shuttling dynamics. J Biol Chem. 2003;278:35959-35967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 262] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 25. | Lee S, Dong HH. FoxO integration of insulin signaling with glucose and lipid metabolism. J Endocrinol. 2017;233:R67-R79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 260] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 26. | Moon KM, Lee B, Kim DH, Chung HY. FoxO6 inhibits melanogenesis partly by elevating intracellular antioxidant capacity. Redox Biol. 2020;36:101624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Klotz LO, Sánchez-Ramos C, Prieto-Arroyo I, Urbánek P, Steinbrenner H, Monsalve M. Redox regulation of FoxO transcription factors. Redox Biol. 2015;6:51-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 424] [Cited by in RCA: 596] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 28. | Hornsveld M, Dansen TB, Derksen PW, Burgering BMT. Re-evaluating the role of FOXOs in cancer. Semin Cancer Biol. 2018;50:90-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 29. | Coomans de Brachène A, Demoulin JB. FOXO transcription factors in cancer development and therapy. Cell Mol Life Sci. 2016;73:1159-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 241] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 30. | Essers MA, Weijzen S, de Vries-Smits AM, Saarloos I, de Ruiter ND, Bos JL, Burgering BM. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 2004;23:4802-4812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 651] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 31. | Wang Z, Yu T, Huang P. Post-translational modifications of FOXO family proteins (Review). Mol Med Rep. 2016;14:4931-4941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 32. | Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villén J, Becker EB, DiBacco S, de la Iglesia N, Gygi S, Blackwell TK, Bonni A. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 690] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 33. | Yuan Z, Lehtinen MK, Merlo P, Villén J, Gygi S, Bonni A. Regulation of neuronal cell death by MST1-FOXO1 signaling. J Biol Chem. 2009;284:11285-11292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 34. | Zhang W, Hietakangas V, Wee S, Lim SC, Gunaratne J, Cohen SM. ER stress potentiates insulin resistance through PERK-mediated FOXO phosphorylation. Genes Dev. 2013;27:441-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 35. | Alasiri G, Jiramongkol Y, Zona S, Fan LY, Mahmud Z, Gong G, Lee HJ, Lam EW. Regulation of PERK expression by FOXO3: a vulnerability of drug-resistant cancer cells. Oncogene. 2019;38:6382-6398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Asada S, Daitoku H, Matsuzaki H, Saito T, Sudo T, Mukai H, Iwashita S, Kako K, Kishi T, Kasuya Y, Fukamizu A. Mitogen-activated protein kinases, Erk and p38, phosphorylate and regulate Foxo1. Cell Signal. 2007;19:519-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 188] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 37. | Yang JY, Zong CS, Xia W, Yamaguchi H, Ding Q, Xie X, Lang JY, Lai CC, Chang CJ, Huang WC, Huang H, Kuo HP, Lee DF, Li LY, Lien HC, Cheng X, Chang KJ, Hsiao CD, Tsai FJ, Tsai CH, Sahin AA, Muller WJ, Mills GB, Yu D, Hortobagyi GN, Hung MC. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol. 2008;10:138-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 553] [Cited by in RCA: 582] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 38. | Huang H, Regan KM, Lou Z, Chen J, Tindall DJ. CDK2-dependent phosphorylation of FOXO1 as an apoptotic response to DNA damage. Science. 2006;314:294-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 279] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 39. | Kim SY, Lee JH, Merrins MJ, Gavrilova O, Bisteau X, Kaldis P, Satin LS, Rane SG. Loss of Cyclin-dependent Kinase 2 in the Pancreas Links Primary β-Cell Dysfunction to Progressive Depletion of β-Cell Mass and Diabetes. J Biol Chem. 2017;292:3841-3853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 40. | Lu H, Liu P, Pan Y, Huang H. Inhibition of cyclin-dependent kinase phosphorylation of FOXO1 and prostate cancer cell growth by a peptide derived from FOXO1. Neoplasia. 2011;13:854-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Mohseni R, Alavian SM, Sadeghabadi ZA, Heiat M. Therapeutic effects of Chlorella vulgaris on carbon tetrachloride induced liver fibrosis by targeting Hippo signaling pathway and AMPK/FOXO1 axis. Mol Biol Rep. 2021;48:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Qiu J, Xiao H, Zhou S, Du W, Mu X, Shi G, Tan X. Bone marrow mesenchymal stem cells inhibit cardiac hypertrophy by enhancing FoxO1 transcription. Cell Biol Int. 2021;45:188-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Xiao Q, Liu H, Wang HS, Cao MT, Meng XJ, Xiang YL, Zhang YQ, Shu F, Zhang QG, Shan H, Jiang GM. Histone deacetylase inhibitors promote epithelial-mesenchymal transition in Hepatocellular Carcinoma via AMPK-FOXO1-ULK1 signaling axis-mediated autophagy. Theranostics. 2020;10:10245-10261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 44. | Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, Kobayashi R, Hung MC. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 673] [Cited by in RCA: 749] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 45. | Chapuis N, Park S, Leotoing L, Tamburini J, Verdier F, Bardet V, Green AS, Willems L, Agou F, Ifrah N, Dreyfus F, Bismuth G, Baud V, Lacombe C, Mayeux P, Bouscary D. IκB kinase overcomes PI3K/Akt and ERK/MAPK to control FOXO3a activity in acute myeloid leukemia. Blood. 2010;116:4240-4250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 46. | Kumar V, Thakur JK, Prasad M. Histone acetylation dynamics regulating plant development and stress responses. Cell Mol Life Sci. 2021;78:4467-4486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 47. | Mortuza R, Chen S, Feng B, Sen S, Chakrabarti S. High glucose induced alteration of SIRTs in endothelial cells causes rapid aging in a p300 and FOXO regulated pathway. PLoS One. 2013;8:e54514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 48. | Pomiès P, Blaquière M, Maury J, Mercier J, Gouzi F, Hayot M. Involvement of the FoxO1/MuRF1/Atrogin-1 Signaling Pathway in the Oxidative Stress-Induced Atrophy of Cultured Chronic Obstructive Pulmonary Disease Myotubes. PLoS One. 2016;11:e0160092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 49. | Morshneva A, Gnedina O, Svetlikova S, Pospelov V, Igotti M. Time-dependent modulation of FoxO activity by HDAC inhibitor in oncogene-transformed E1A+Ras cells. AIMS Genet. 2018;5:41-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Morshneva A, Gnedina O, Marusova T, Igotti M. Expression of Adenoviral E1A in Transformed Cells as an Additional Factor of HDACi-Dependent FoxO Regulation. Cells. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 51. | Hui B, Hou X, Liu R, Liu XH, Hu Z. Gypenoside inhibits ox-LDL uptake and foam cell formation through enhancing Sirt1-FOXO1 mediated autophagy flux restoration. Life Sci. 2021;264:118721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 52. | Brenkman AB, de Keizer PL, van den Broek NJ, Jochemsen AG, Burgering BM. Mdm2 induces mono-ubiquitination of FOXO4. PLoS One. 2008;3:e2819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 53. | Bayen S, Saini S, Gaur P, Duraisamy AJ, Kumar Sharma A, Pal K, Vats P, Singh SB. PRMT1 promotes hyperglycemia in a FoxO1-dependent manner, affecting glucose metabolism, during hypobaric hypoxia exposure, in rat model. Endocrine. 2018;59:151-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 54. | Calnan DR, Webb AE, White JL, Stowe TR, Goswami T, Shi X, Espejo A, Bedford MT, Gozani O, Gygi SP, Brunet A. Methylation by Set9 modulates FoxO3 stability and transcriptional activity. Aging (Albany NY). 2012;4:462-479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 55. | Zhu J, Wang P, Yu Z, Lai W, Cao Y, Huang P, Xu Q, Yu M, Xu J, Huang Z, Zeng B. Advanced glycosylation end product promotes forkhead box O1 and inhibits Wnt pathway to suppress capacities of epidermal stem cells. Am J Transl Res. 2016;8:5569-5579. [PubMed] |
| 56. | Jian D, Wang Y, Jian L, Tang H, Rao L, Chen K, Jia Z, Zhang W, Liu Y, Chen X, Shen X, Gao C, Wang S, Li M. METTL14 aggravates endothelial inflammation and atherosclerosis by increasing FOXO1 N6-methyladeosine modifications. Theranostics. 2020;10:8939-8956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 218] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 57. | Shin H, Cha HJ, Na K, Lee MJ, Cho JY, Kim CY, Kim EK, Kang CM, Kim H, Paik YK. O-GlcNAcylation of the Tumor Suppressor FOXO3 Triggers Aberrant Cancer Cell Growth. Cancer Res. 2018;78:1214-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 58. | Liu Y, Ao X, Ding W, Ponnusamy M, Wu W, Hao X, Yu W, Wang Y, Li P, Wang J. Critical role of FOXO3a in carcinogenesis. Mol Cancer. 2018;17:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 368] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 59. | Xing YQ, Li A, Yang Y, Li XX, Zhang LN, Guo HC. The regulation of FOXO1 and its role in disease progression. Life Sci. 2018;193:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 312] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 60. | Liu W, Li Y, Luo B. Current perspective on the regulation of FOXO4 and its role in disease progression. Cell Mol Life Sci. 2020;77:651-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 61. | Roy SK, Srivastava RK, Shankar S. Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of FOXO transcription factor, leading to cell cycle arrest and apoptosis in pancreatic cancer. J Mol Signal. 2010;5:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 314] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 62. | Chung CY, Park YL, Song YA, Myung E, Kim KY, Lee GH, Ki HS, Park KJ, Cho SB, Lee WS, Jung YD, Kim KK, Joo YE. Knockdown of RON inhibits AP-1 activity and induces apoptosis and cell cycle arrest through the modulation of Akt/FoxO signaling in human colorectal cancer cells. Dig Dis Sci. 2012;57:371-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 63. | Baugh LR, Sternberg PW. DAF-16/FOXO regulates transcription of cki-1/Cip/Kip and repression of lin-4 during C. elegans L1 arrest. Curr Biol. 2006;16:780-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 198] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 64. | Wang C, He H, Liu G, Ma H, Li L, Jiang M, Lu Q, Li P, Qi H. DT-13 induced apoptosis and promoted differentiation of acute myeloid leukemia cells by activating AMPK-KLF2 pathway. Pharmacol Res. 2020;158:104864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 65. | Laporte AN, Poulin NM, Barrott JJ, Wang XQ, Lorzadeh A, Vander Werff R, Jones KB, Underhill TM, Nielsen TO. Death by HDAC Inhibition in Synovial Sarcoma Cells. Mol Cancer Ther. 2017;16:2656-2667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 66. | Gan L, Liu P, Lu H, Chen S, Yang J, McCarthy JB, Knudsen KE, Huang H. Cyclin D1 promotes anchorage-independent cell survival by inhibiting FOXO-mediated anoikis. Cell Death Differ. 2009;16:1408-1417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 67. | Wang Y, Qi H, Liu Y, Duan C, Liu X, Xia T, Chen D, Piao HL, Liu HX. The double-edged roles of ROS in cancer prevention and therapy. Theranostics. 2021;11:4839-4857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 449] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 68. | El Maï M, Marzullo M, de Castro IP, Ferreira MG. Opposing p53 and mTOR/AKT promote an in vivo switch from apoptosis to senescence upon telomere shortening in zebrafish. Elife. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 69. | Xu MM, Mao GX, Liu J, Li JC, Huang H, Liu YF, Liu JH. Low expression of the FoxO4 gene may contribute to the phenomenon of EMT in non-small cell lung cancer. Asian Pac J Cancer Prev. 2014;15:4013-4018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 70. | Bullock MD, Bruce A, Sreekumar R, Curtis N, Cheung T, Reading I, Primrose JN, Ottensmeier C, Packham GK, Thomas G, Mirnezami AH. FOXO3 expression during colorectal cancer progression: biomarker potential reflects a tumour suppressor role. Br J Cancer. 2013;109:387-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 71. | Wu Y, Elshimali Y, Sarkissyan M, Mohamed H, Clayton S, Vadgama JV. Expression of FOXO1 is associated with GATA3 and Annexin-1 and predicts disease-free survival in breast cancer. Am J Cancer Res. 2012;2:104-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 72. | Zhang H, Pan Y, Zheng L, Choe C, Lindgren B, Jensen ED, Westendorf JJ, Cheng L, Huang H. FOXO1 inhibits Runx2 transcriptional activity and prostate cancer cell migration and invasion. Cancer Res. 2011;71:3257-3267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 73. | Renault VM, Thekkat PU, Hoang KL, White JL, Brady CA, Kenzelmann Broz D, Venturelli OS, Johnson TM, Oskoui PR, Xuan Z, Santo EE, Zhang MQ, Vogel H, Attardi LD, Brunet A. The pro-longevity gene FoxO3 is a direct target of the p53 tumor suppressor. Oncogene. 2011;30:3207-3221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 74. | Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, Jiang S, Gilliland DG, Chin L, Wong WH, Castrillon DH, DePinho RA. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 788] [Cited by in RCA: 876] [Article Influence: 46.1] [Reference Citation Analysis (1)] |
| 75. | Qian Z, Ren L, Wu D, Yang X, Zhou Z, Nie Q, Jiang G, Xue S, Weng W, Qiu Y, Lin Y. Overexpression of FoxO3a is associated with glioblastoma progression and predicts poor patient prognosis. Int J Cancer. 2017;140:2792-2804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 76. | Yu Y, Peng K, Li H, Zhuang R, Wang Y, Li W, Yu S, Liang L, Xu X, Liu T. SP1 upregulated FoxO3a promotes tumor progression in colorectal cancer. Oncol Rep. 2018;39:2235-2242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 77. | Hagenbuchner J, Rupp M, Salvador C, Meister B, Kiechl-Kohlendorfer U, Müller T, Geiger K, Sergi C, Obexer P, Ausserlechner MJ. Nuclear FOXO3 predicts adverse clinical outcome and promotes tumor angiogenesis in neuroblastoma. Oncotarget. 2016;7:77591-77606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 78. | Rupp M, Hagenbuchner J, Rass B, Fiegl H, Kiechl-Kohlendorfer U, Obexer P, Ausserlechner MJ. FOXO3-mediated chemo-protection in high-stage neuroblastoma depends on wild-type TP53 and SESN3. Oncogene. 2017;36:6190-6203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 79. | Zang Y, Wang T, Pan J, Gao F. miR-215 promotes cell migration and invasion of gastric cancer cell lines by targeting FOXO1. Neoplasma. 2017;64:579-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 80. | Yang XB, Zhao JJ, Huang CY, Wang QJ, Pan K, Wang DD, Pan QZ, Jiang SS, Lv L, Gao X, Chen HW, Yao JY, Zhi M, Xia JC. Decreased expression of the FOXO3a gene is associated with poor prognosis in primary gastric adenocarcinoma patients. PLoS One. 2013;8:e78158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 81. | Su L, Liu X, Chai N, Lv L, Wang R, Li X, Nie Y, Shi Y, Fan D. The transcription factor FOXO4 is down-regulated and inhibits tumor proliferation and metastasis in gastric cancer. BMC Cancer. 2014;14:378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 82. | Qinyu L, Long C, Zhen-dong D, Min-min S, Wei-ze W, Wei-ping Y, Cheng-hong P. FOXO6 promotes gastric cancer cell tumorigenicity via upregulation of C-myc. FEBS Lett. 2013;587:2105-2111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 83. | Wang JH, Tang HS, Li XS, Zhang XL, Yang XZ, Zeng LS, Ruan Q, Huang YH, Liu GJ, Wang J, Cui SZ. Elevated FOXO6 expression correlates with progression and prognosis in gastric cancer. Oncotarget. 2017;8:31682-31691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 84. | Foo J, Leder K, Michor F. Stochastic dynamics of cancer initiation. Phys Biol. 2011;8:015002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 85. | Zhou L, Shang Y, Jin Z, Zhang W, Lv C, Zhao X, Liu Y, Li N, Liang J. UHRF1 promotes proliferation of gastric cancer via mediating tumor suppressor gene hypermethylation. Cancer Biol Ther. 2015;16:1241-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 86. | Choi Y, Park J, Ko YS, Kim Y, Pyo JS, Jang BG, Kim MA, Lee JS, Chang MS, Lee BL. FOXO1 reduces tumorsphere formation capacity and has crosstalk with LGR5 signaling in gastric cancer cells. Biochem Biophys Res Commun. 2017;493:1349-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 87. | Huang Y, Zhang J, Hou L, Wang G, Liu H, Zhang R, Chen X, Zhu J. LncRNA AK023391 promotes tumorigenesis and invasion of gastric cancer through activation of the PI3K/Akt signaling pathway. J Exp Clin Cancer Res. 2017;36:194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 88. | Choi Y, Park J, Choi Y, Ko YS, Yu DA, Kim Y, Pyo JS, Jang BG, Kim MA, Kim WH, Lee BL. c-Jun N-terminal kinase activation has a prognostic implication and is negatively associated with FOXO1 activation in gastric cancer. BMC Gastroenterol. 2016;16:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 89. | Ko YS, Cho SJ, Park J, Kim Y, Choi YJ, Pyo JS, Jang BG, Park JW, Kim WH, Lee BL. Loss of FOXO1 promotes gastric tumour growth and metastasis through upregulation of human epidermal growth factor receptor 2/neu expression. Br J Cancer. 2015;113:1186-1196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 90. | Liu H, Liu N, Zhao Y, Zhu X, Wang C, Liu Q, Gao C, Zhao X, Li J. Oncogenic USP22 supports gastric cancer growth and metastasis by activating c-Myc/NAMPT/SIRT1-dependent FOXO1 and YAP signaling. Aging (Albany NY). 2019;11:9643-9660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 91. | Xia J, Wu Z, Yu C, He W, Zheng H, He Y, Jian W, Chen L, Zhang L, Li W. miR-124 inhibits cell proliferation in gastric cancer through down-regulation of SPHK1. J Pathol. 2012;227:470-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 92. | Fan C, Liu S, Zhao Y, Han Y, Yang L, Tao G, Li Q, Zhang L. Upregulation of miR-370 contributes to the progression of gastric carcinoma via suppression of FOXO1. Biomed Pharmacother. 2013;67:521-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 93. | He X, Zou K. MiRNA-96-5p contributed to the proliferation of gastric cancer cells by targeting FOXO3. J Biochem. 2020;167:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 94. | Wang GJ, Liu GH, Ye YW, Fu Y, Zhang XF. The role of microRNA-1274a in the tumorigenesis of gastric cancer: accelerating cancer cell proliferation and migration via directly targeting FOXO4. Biochem Biophys Res Commun. 2015;459:629-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 95. | Gao Y, Qi W, Sun L, Lv J, Qiu W, Liu S. FOXO3 Inhibits Human Gastric Adenocarcinoma (AGS) Cell Growth by Promoting Autophagy in an Acidic Microenvironment. Cell Physiol Biochem. 2018;49:335-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 96. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 48784] [Article Influence: 3252.3] [Reference Citation Analysis (12)] |
| 97. | Maruyama S, Furuya S, Shiraishi K, Shimizu H, Saito R, Akaike H, Hosomura N, Kawaguchi Y, Amemiya H, Kawaida H, Sudo M, Inoue S, Kono H, Ichikawa D. Inhibition of apoptosis by miR-22-p in α-rotein-ducing gastric cancer. Oncol Rep. 2019;41:2595-2600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 98. | Vogiatzi P, De Falco G, Claudio PP, Giordano A. How does the human RUNX3 gene induce apoptosis in gastric cancer? Cancer Biol Ther. 2006;5:371-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 99. | Yamamura Y, Lee WL, Inoue K, Ida H, Ito Y. RUNX3 cooperates with FoxO3a to induce apoptosis in gastric cancer cells. J Biol Chem. 2006;281:5267-5276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 100. | Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1328] [Cited by in RCA: 2008] [Article Influence: 133.9] [Reference Citation Analysis (0)] |
| 101. | Shahbazi R, Baradaran B, Khordadmehr M, Safaei S, Baghbanzadeh A, Jigari F, Ezzati H. Targeting ROCK signaling in health, malignant and non-malignant diseases. Immunol Lett. 2020;219:15-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 102. | Wang YZ, Feng ZQ. Induction of apoptosis by L-NMMA, via FKHRL1/ROCK pathway in human gastric cancer cells. Biomed Environ Sci. 2006;19:285-291. [PubMed] |
| 103. | Pang X, Zhou Z, Yu Z, Han L, Lin Z, Ao X, Liu C, He Y, Ponnusamy M, Li P, Wang J. Foxo3a-dependent miR-633 regulates chemotherapeutic sensitivity in gastric cancer by targeting Fas-associated death domain. RNA Biol. 2019;16:233-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 104. | Jiang S, Fu R, Shi J, Wu H, Mai J, Hua X, Chen H, Liu J, Lu M, Li N. CircRNA-Mediated Regulation of Angiogenesis: A New Chapter in Cancer Biology. Front Oncol. 2021;11:553706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 105. | Nienhüser H, Schmidt T. Angiogenesis and Anti-Angiogenic Therapy in Gastric Cancer. Int J Mol Sci. 2017;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 106. | Kim SY, Yoon J, Ko YS, Chang MS, Park JW, Lee HE, Kim MA, Kim JH, Kim WH, Lee BL. Constitutive phosphorylation of the FOXO1 transcription factor in gastric cancer cells correlates with microvessel area and the expressions of angiogenesis-related molecules. BMC Cancer. 2011;11:264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 107. | Kim SY, Ko YS, Park J, Choi Y, Park JW, Kim Y, Pyo JS, Yoo YB, Lee JS, Lee BL. Forkhead Transcription Factor FOXO1 Inhibits Angiogenesis in Gastric Cancer in Relation to SIRT1. Cancer Res Treat. 2016;48:345-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 108. | Bai M, Li J, Yang H, Zhang H, Zhou Z, Deng T, Zhu K, Ning T, Fan Q, Ying G, Ba Y. miR-135b Delivered by Gastric Tumor Exosomes Inhibits FOXO1 Expression in Endothelial Cells and Promotes Angiogenesis. Mol Ther. 2019;27:1772-1783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 109. | Zhang L, Liu L, Zhan S, Chen L, Wang Y, Zhang Y, Du J, Wu Y, Gu L. Arsenic Trioxide Suppressed Migration and Angiogenesis by Targeting FOXO3a in Gastric Cancer Cells. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 110. | Xu W, Yang Z, Lu N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adh Migr. 2015;9:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 502] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 111. | Mittal V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu Rev Pathol. 2018;13:395-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 1087] [Article Influence: 155.3] [Reference Citation Analysis (0)] |
| 112. | Hu C, Ni Z, Li BS, Yong X, Yang X, Zhang JW, Zhang D, Qin Y, Jie MM, Dong H, Li S, He F, Yang SM. hTERT promotes the invasion of gastric cancer cells by enhancing FOXO3a ubiquitination and subsequent ITGB1 upregulation. Gut. 2017;66:31-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 113. | Park J, Choi Y, Ko YS, Kim Y, Pyo JS, Jang BG, Kim MA, Lee JS, Chang MS, Park JW, Lee BL. FOXO1 Suppression is a Determinant of Acquired Lapatinib-Resistance in HER2-Positive Gastric Cancer Cells Through MET Upregulation. Cancer Res Treat. 2018;50:239-254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 114. | Yu C, Chen DQ, Liu HX, Li WB, Lu JW, Feng JF. Rosmarinic acid reduces the resistance of gastric carcinoma cells to 5-fluorouracil by downregulating FOXO4-targeting miR-6785-5p. Biomed Pharmacother. 2019;109:2327-2334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 115. | Park J, Ko YS, Yoon J, Kim MA, Park JW, Kim WH, Choi Y, Kim JH, Cheon Y, Lee BL. The forkhead transcription factor FOXO1 mediates cisplatin resistance in gastric cancer cells by activating phosphoinositide 3-kinase/Akt pathway. Gastric Cancer. 2014;17:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 116. | Yu S, Yu Y, Zhang W, Yuan W, Zhao N, Li Q, Cui Y, Wang Y, Li W, Sun Y, Liu T. FOXO3a promotes gastric cancer cell migration and invasion through the induction of cathepsin L. Oncotarget. 2016;7:34773-34784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 117. | Li Z, Zhang H, Chen Y, Fan L, Fang J. Forkhead transcription factor FOXO3a protein activates nuclear factor κB through B-cell lymphoma/leukemia 10 (BCL10) protein and promotes tumor cell survival in serum deprivation. J Biol Chem. 2012;287:17737-17745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 118. | Miao Z, Guo X, Tian L. The long noncoding RNA NORAD promotes the growth of gastric cancer cells by sponging miR-608. Gene. 2019;687:116-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 119. | Chen C, Xu T, Zhou J, Yan Y, Li W, Yu H, Hu G, Ding X, Chen J, Lu Y. High cytoplasmic FOXO1 and pFOXO1 expression in astrocytomas are associated with worse surgical outcome. PLoS One. 2013;8:e69260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 120. | Ko YS, Kim NY, Pyo JS. Clinicopathological significance and angiogenic role of the constitutive phosphorylation of the FOXO1 transcription factor in colorectal cancer. Pathol Res Pract. 2020;216:153150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 121. | Wu Z, Zhu Q, Yin Y, Kang D, Cao R, Tian Q, Zhang Y, Lu S, Liu P. Traditional Chinese Medicine CFF-1 induced cell growth inhibition, autophagy, and apoptosis via inhibiting EGFR-related pathways in prostate cancer. Cancer Med. 2018;7:1546-1559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 122. | Zhu C, Zhu Q, Wu Z, Yin Y, Kang D, Lu S, Liu P. Isorhapontigenin induced cell growth inhibition and apoptosis by targeting EGFR-related pathways in prostate cancer. J Cell Physiol. 2018;233:1104-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 123. | Kim JH, Kim MK, Lee HE, Cho SJ, Cho YJ, Lee BL, Lee HS, Nam SY, Lee JS, Kim WH. Constitutive phosphorylation of the FOXO1A transcription factor as a prognostic variable in gastric cancer. Mod Pathol. 2007;20:835-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 124. | Yu S, Yu Y, Sun Y, Wang X, Luo R, Zhao N, Zhang W, Li Q, Cui Y, Wang Y, Li W, Liu T. Activation of FOXO3a suggests good prognosis of patients with radically resected gastric cancer. Int J Clin Exp Pathol. 2015;8:2963-2970. [PubMed] |
| 125. | Li J, Jiang Z, Han F, Liu S, Yuan X, Tong J. FOXO4 and FOXD3 are predictive of prognosis in gastric carcinoma patients. Oncotarget. 2016;7:25585-25592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 126. | Park SH, Jang KY, Kim MJ, Yoon S, Jo Y, Kwon SM, Kim KM, Kwon KS, Kim CY, Woo HG. Tumor suppressive effect of PARP1 and FOXO3A in gastric cancers and its clinical implications. Oncotarget. 2015;6:44819-44831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 127. | Ding J, Li Q, He S, Xie J, Liang X, Wu T, Li D. Luteolin-loading of Her-2-poly (lactic-co-glycolic acid) nanoparticles and proliferative inhibition of gastric cancer cells via targeted regulation of forkhead box protein O1. J Cancer Res Ther. 2020;16:263-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 128. | Kim N, Kim CH, Ahn DW, Lee KS, Cho SJ, Park JH, Lee MK, Kim JS, Jung HC, Song IS. Anti-gastric cancer effects of celecoxib, a selective COX-2 inhibitor, through inhibition of Akt signaling. J Gastroenterol Hepatol. 2009;24:480-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 129. | Xia Q, Zhao Y, Wang J, Qiao W, Zhang D, Yin H, Xu D, Chen F. Proteomic analysis of cell cycle arrest and differentiation induction caused by ATPR, a derivative of all-trans retinoic acid, in human gastric cancer SGC-7901 cells. Proteomics Clin Appl. 2017;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 130. | Chen T, Wang Y, Yang Y, Yu K, Cao X, Su F, Xu H, Peng Y, Hu Y, Qian F, Wang Z. Gramicidin inhibits human gastric cancer cell proliferation, cell cycle and induced apoptosis. Biol Res. 2019;52:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 131. | Lin F, Yang J, Muhammad U, Sun J, Huang Z, Li W, Lv F, Lu Z. Bacillomycin D-C16 triggers apoptosis of gastric cancer cells through the PI3K/Akt and FoxO3a signaling pathways. Anticancer Drugs. 2019;30:46-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 132. | Altan B, Yokobori T, Ide M, Mochiki E, Toyomasu Y, Kogure N, Kimura A, Hara K, Bai T, Bao P, Suzuki M, Ogata K, Asao T, Nishiyama M, Oyama T, Kuwano H. Nuclear PRMT1 expression is associated with poor prognosis and chemosensitivity in gastric cancer patients. Gastric Cancer. 2016;19:789-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 133. | Xiong H, Wang J, Guan H, Wu J, Xu R, Wang M, Rong X, Huang K, Huang J, Liao Q, Fu Y, Yuan J. SphK1 confers resistance to apoptosis in gastric cancer cells by downregulating Bim via stimulating Akt/FoxO3a signaling. Oncol Rep. 2014;32:1369-1373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 134. | Lu J, Zhang PY, Li P, Xie JW, Wang JB, Lin JX, Chen QY, Cao LL, Huang CM, Zheng CH. Circular RNA hsa_circ_0001368 suppresses the progression of gastric cancer by regulating miR-6506-5p/FOXO3 axis. Biochem Biophys Res Commun. 2019;512:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mohamed SY S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Yuan YY