Published online Aug 15, 2020. doi: 10.4251/wjgo.v12.i8.903
Peer-review started: December 30, 2019
First decision: February 20, 2020
Revised: May 29, 2020
Accepted: June 14, 2020
Article in press: June 14, 2020
Published online: August 15, 2020
Processing time: 225 Days and 16.9 Hours
Cytoreductive surgery (CRS) in combination with hyperthermic intraperitoneal chemotherapy (HIPEC) improves patient survival in colorectal cancer (CRC) with peritoneal carcinomatosis (PC). Commonly used cytotoxic agents include mitomycin C (MMC) and oxaliplatin. Studies have reported varying results, and the evidence for the choice of the HIPEC agent and uniform procedure protocols is limited.
To evaluate therapeutic benefits and complications of CRS + MMC vs oxaliplatin HIPEC in patients with peritoneal metastasized CRC as well as prognostic factors.
One hundred and two consecutive patients who had undergone CRS and HIPEC for CRC PC between 2007 and 2019 at the Medical Center of the University Freiburg regarding interdisciplinary cancer conference decision were retrospectively analysed. Oxaliplatin and MMC were used in 68 and 34 patients, respectively. Each patient’s demographics and tumour characteristics, operative details, postoperative complications and survival were noted. Complications were stratified and graded using Clavien/Dindo analysis. Prognostic outcome factors were identified using univariate and multivariate analysis of survival.
The two groups did not differ significantly regarding baseline characteristics. We found no difference in median overall survival between MMC and oxaliplatin HIPEC. Regarding postoperative complications, patients treated with oxaliplatin HIPEC suffered increased complications (66.2% vs 35.3%; P = 0.003), particularly intestinal atony, intraabdominal infections and urinary tract infection, and had a prolonged intensive care unit stay compared to the MMC group (7.2 d vs 4.4 d; P = 0.035). Regarding univariate analysis of survival, we found primary tumour factors, nodal positivity and resection margins to be of prognostic value as well as peritoneal cancer index (PCI)-score and the completeness of cytoreduction regarding peritoneal carcinomatosis. Multivariate analysis of survival confirmed primary distant metastasis and primary tumour resection status to have a significant impact on survival and likewise peritoneal cancer index-scoring regarding peritoneal carcinomatosis.
In this single-institution retrospective review of patients undergoing CRS with either oxaliplatin or MMC HIPEC, overall survival was not different, though oxaliplatin was associated with a higher postoperative complication rate, indicating treatment favourably with MMC. Further studies comparing HIPEC regimens would improve evidence-based decision-making.
Core tip: We evaluated the therapeutic efficiency of cytoreductive surgery in combination with two different hyperthermic intraperitoneal chemotherapy (HIPEC) regimens, comparing mitomycin C HIPEC vs oxaliplatin HIPEC. We therefore retrospectively evaluated 102 patients undergoing cytoreductive surgery and HIPEC and statistically analysed demographics, perioperative complication and survival outcome. We found no difference in median overall survival between mitomycin C and oxaliplatin HIPEC. Regarding postoperative complications, patients treated with oxaliplatin HIPEC suffered an increased complication rate. Regarding multivariate analysis of survival, primary distant metastasis and primary tumour resection seem to have a significant impact on survival and likewise peritoneal cancer index-scoring regarding peritoneal carcinomatosis. Further prospective studies comparing HIPEC regimens would improve therapeutic decision-making.
- Citation: Spiegelberg J, Neeff H, Holzner P, Runkel M, Fichtner-Feigl S, Glatz T. Comparison of hyperthermic intraperitoneal chemotherapy regimens for treatment of peritoneal-metastasized colorectal cancer. World J Gastrointest Oncol 2020; 12(8): 903-917
- URL: https://www.wjgnet.com/1948-5204/full/v12/i8/903.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i8.903
Among patients with resected colorectal cancer (CRC), approximately 50% develop distant metastases either synchronously or metachronously. Most common locations are liver (35%-55%), lungs (10%-20%) and peritoneal carcinomatosis (PC) (10%-25%)[1]. In the past, the median overall survival (OS) of patients diagnosed with PC of CRC origin was 4-7 mo, for patients undergoing palliative surgery or 5-fluorouracil (5-FU)-based systemic chemotherapy[2-4]. Improvement in systemic chemotherapy, using chemotherapeutic agents such as oxaliplatin and irinotecan, along with anti-angiogenesis molecular targeting agents cetuximab and bevacizumab, led to an increased OS of about 12 mo[5].
The introduction of multimodal treatment strategies including systemic chemotherapy and cytoreductive surgery (CRS) plus hyperthermic intraperitoneal chemotherapy (HIPEC) showed promising progress in long-term survival. The HIPEC procedure is intended to destroy any remaining tumour cells after surgical tumour debulking by local administration of chemotherapy to the peritoneal cavity for homogeneous drug distribution and enhanced cytotoxicity induced by heat[6]. Depending on the extent of intraabdominal tumour load, remarkable survival benefits have been reported compared to systemic chemotherapy with 5-FU/leucovorin alone in a randomized controlled trial[7]. Median OS of selected patients with CRC PC improved to 21-63 mo with a 5-year survival rate up to approximately 58%[8]. The most frequently used cytotoxic drugs for HIPEC in CRC are mitomycin C (MMC) and oxaliplatin combined with systemic 5-FU and leucovorin[9].
Initially, HIPEC regimen was most commonly conducted with MMC but subsequently the addition of oxaliplatin became the standard systemic treatment in CRC[10-12]. This brought about a change of regimen for HIPEC with MMC being only used as salvage treatment[13]. The combination of cisplatin and MMC is also frequently used and seems to be a valid HIPEC protocol in peritoneal metastases of CR origin. Recent studies evaluating this protocol demonstrated prolonged survival with limited toxicity[14,15].
Upfront CRS with HIPEC (CRS-HIPEC) is currently the standard treatment for colorectal peritoneal metastases in eligible patients due to the proven superiority to palliative chemotherapy alone[16,17]. Nevertheless, therapeutic efficacy of this treatment strategy for CRC PC patients remains controversial due to contradicting evidence, especially regarding the value of HIPEC.
The first formal randomized controlled trial for CRC assessing the benefit of a 30 min oxaliplatin-based HIPEC added to surgery failed to show survival improvement[18]. Leung et al[19] demonstrated that patients with CRC treated with oxaliplatin HIPEC had better OS than those receiving MMC-based HIPEC (median survival: 56 mo vs 26 mo, respectively). In contrast, Prada-Villaverde et al[20] reported that HIPEC with MMC may be superior to oxaliplatin-based HIPEC when patients have favourable histology or a low burden of PC (median survival: 54.3 mo vs 30.4 mo, respectively). At present there is no prospective study that compares these two HIPEC regimens for treatment of peritoneal metastasized CRC. Thus, a reassessment of HIPEC and the need for structured treatment protocols should be addressed. In this retrospective clinical analysis, we evaluated the outcome of patients undergoing CRS HIPEC at the university medical centre of Freiburg.
This study evaluated the outcome of 102 consecutive patients with PC of colorectal origin, who underwent CRS and HIPEC between January 2007 and March 2019 at the Medical Center of the University Freiburg (MCUF). Patients receiving HIPEC with either palliative or CRS were included.
Patients with appendiceal tumours/pseudomyxoma peritonei and PC of other origin (non-colorectal) were excluded as well as patients who were planned for HIPEC but had not received HIPEC treatment due to surgeon’s intraoperative decision. HIPEC regimens were chosen regarding current available data with MMC or oxaliplatin.
From 2007 until 2014, MMC was used, and from 2014 to 2018 it changed to oxaliplatin. Analogous to PRODIGE7 trial, HIPECs since 2018 were conducted with MMC.
Informed consent was obtained from all patients before their inclusion in the cancer registry. The study was approved by the Medical Ethics Committee of the University of Freiburg (EK-FR 4/20). The analysed data was extracted from the anaesthetic protocols and the electronic health records.
Preoperative work-up started in the outpatient setting of MCUF. Previous oncological therapies and comorbidities were recorded, and pulmonary and cardiac check-ups were routinely performed in high-risk patients. Pretherapeutic diagnostics included thoraco-abdominal computerized tomography in all patients and endoscopy or diagnostic laparoscopy with biopsies when appropriate. All patients were discussed in our interdisciplinary cancer conference, and decision for CRS with HIPEC was made if a complete resection seemed achievable. Extensive liver metastases as well as extra abdominal or retroperitoneal metastases were seen as contraindication for surgical intervention.
Depending on the treating physician’s protocol and interdisciplinary consensus as well as timing of diagnosis and previous chemotherapy courses, perioperative systemic therapy consisted of either neoadjuvant and adjuvant cycles of capecitabine with oxaliplatin, neoadjuvant and adjuvant cycles of 5-FU/leucovorin with oxaliplatin, or neoadjuvant cycles of 5-FU/leucovorin with irinotecan followed by capecitabine or adjuvant cycles of fluoropyrimidine monotherapy.
For patients with intestinal obstruction, palliative resections and palliative HIPEC were considered according to interdisciplinary cancer conference decision.
The operative procedure was chosen according to the extent and location of the primary tumour and the peritoneal metastases. After explorative midline laparotomy, the complete abdominal cavity was inspected to assess the extent of peritoneal carcinomatosis, defined by the peritoneal cancer index (PCI). According to Sugarbaker’s original work, the PCI system divides the abdomen and the pelvis into 13 regions. The lesions are graded according to size (0 through 3) in each abdominopelvic region and are added as a numerical score[21].
Afterwards, the Sugarbaker protocol (Sugarbaker et al[6], 1995) was adhered, which assessed tumour resection and resection of visceral organs and peritoneum. Here, resections were classified and subdivided into large intestine, small intestine, liver, diaphragm, omentum and peritoneum.
The Completeness of Cytoreduction (CC) Score, which quantifies the completion of CRS, was assessed after resection. Before closure of the abdominal cavity at least four 24CH silicon-drainages and a temperature probe for the HIPEC were placed.
Simultaneous application of cytotoxic drugs both intraperitoneal and intravenously (i.v.) was used when performing an oxaliplatin based HIPEC with 5-FU + leukovorin i.v. (bidirectional HIPEC). Cytotoxic drugs were prepared by our clinic pharmacy using saline solution as carrier solution in a 50 mL syringe. Dosage level was 30 mg/m² body surface for MMC, 300 mg/m² for oxaliplatin, 400 mg/m² for 5-FU and 20 mg/m² for leukovorin.
The chemo infusion was performed in a closed abdominal system using an extra corporal roller pump system with heat exchanger. Three silicon-drainages were used as fluid inlets and one as outlet. After establishing a stable circulation of saline solution, the cytotoxic drug was added. The degree of hyperthermia ranged between 39 °C to 43 °C using 42 °C as target level. The intraperitoneal circulating time of oxaliplatin was 30 min, respectively 90 min for MMC. After completing the circulation time, the roller pump was used to aspirate the intraabdominal fluids. Silicon drainages were left in the early postoperative setting to allow drainage of remaining accumulated fluids. All patients were transferred postoperatively to the intensive care unit (ICU) for further monitoring.
Perioperative complications were recorded up to 90 d after surgery and were graded according to Clavien/Dindo-Classification[22]. Grade 1 complications (minor deviation) were not recorded. Discharged patients were followed up at least once in the surgical outpatient department and referred back either to the oncology department or to a resident oncologist for further follow-up. The survival data were systematically obtained from the cancer registry of the MCUF Cancer Center. Data regarding postoperative chemotherapy were directly obtained from the resident oncologist or general physician.
The results of our study were gained by retrospective analysis of our prospective CRC databases. SPSS 22 for WindowsTM was used for statistical analysis (SPSS, Armonk, NY, United States). Categorical variables were given in absolute and relative frequencies; differences were evaluated by Fisher’s exact test. Quantitative values were expressed as mean ± standard deviation and medians with range, as appropriate, and differences were measured using the Kruskal-Wallis test. A Mann-Whitney-U-test was added to compare groups. Survival was univariately analysed by the Kaplan-Meier method with a log-rank test for the comparison of subgroups. Multivariate survival analysis was performed by the Cox proportional hazard model (forward selection strategy using a likelihood ratio statistic) including the report of relative risks and their 95%-confidential intervals. A P value < 0.05 was considered statistically significant.
From January 2007 to March 2019, 102 patients underwent CRS-HIPEC or palliative resections and HIPEC. The cohort contained 60 male patients and 42 female patients. Sixty-eight patients were treated with oxaliplatin/5-FU HIPEC and 34 patients with MMC HIPEC. Three patients in the MMC-group received early postoperative intraperitoneal chemotherapy during the first 48 hours after CRS.
The groups were balanced regarding baseline characteristics, besides a higher American Society of Anesthesiologists (ASA) (P = 0.002) score and a higher rate of T4b (P = 0.027) tumours in the Oxaliplatin group. Median PCI-score was not statistically different across groups but was lower by trend in the Oxaliplatin group [8 (range 0-30) vs 12 (range 0-39) in the MMC-group; P = 0.312].
Palliative resections without cytoreduction were performed in one patient receiving oxaliplatin/5-FU HIPEC and in two patients treated with MMC-HIPEC (Table 1).
| All, n = 102 | MMC, n = 34 | Oxaliplatin/5-FU, n = 68 | P value1 | |
| Male sex | 60 (59) | 24 (40) | 36 (60) | 0.135 |
| Age in yr, median (range) | 57.2 (23-80) | 56.3 (23-73) | 57.7 (40-80) | 0.884 |
| BMI in kg/m² | 25.3 (15.9-39.6) | 25.5 (19.1-33.6) | 25.2 (15.9-39.6) | 0.266 |
| ASA-score | 0.002b | |||
| 1-2 | 49 (48) | 24 (71) | 25 (37) | |
| 3-4 | 53 (52) | 10 (29) | 43 (63) | |
| Tumour location | 1.000 | |||
| Colon | 91 (89) | 30 (88) | 61 (90) | |
| Rectum | 11 (11) | 4 (12) | 7 (10) | |
| Surgical approach | 0.257 | |||
| Complete cytoreduction | 99 (97) | 32 (94) | 67 (99) | |
| Palliative resection | 3 (3) | 2 (6) | 1 (2) | |
| Resection | ||||
| Peritoneum | 81 (80) | 29 (85) | 52 (77) | 0.437 |
| Omentum | 66 (65) | 26 (77) | 40 (59) | 0.123 |
| Colon/rectum | 55 (54) | 18 (53) | 37 (54) | 1.000 |
| Small intestine | 49 (48) | 15 (44) | 34 (50) | 0.675 |
| Liver | 42 (41) | 13 (38) | 29 (43) | 0.831 |
| Diaphragm | 16 (16) | 9 (27) | 7 (10) | 0.045a |
| Other | 63 (64) | 21 (68) | 42 (62) | 0.655 |
| Pretherapeutic T stage | 0.027a | |||
| T1 | 2 (2) | 0 | 2 (3) | |
| T2 | 2 (2) | 0 | 2 (3) | |
| T3 | 34 (34) | 13 (41) | 21 (31) | |
| T4a | 40 (40) | 17 (53) | 23 (34) | |
| T4b | 22 (22) | 2 (6) | 20 (30) | |
| Pretherapeutic N stage | 1.000 | |||
| N0 | 26 (26) | 8 (25) | 18 (27) | |
| N+ | 73 (74) | 24 (75) | 49 (73) | |
| Pretherapeutic M stage | 1.000 | |||
| M0 | 36 (37) | 12 (35) | 24 (38) | |
| M+ | 62 (63) | 22 (65) | 40 (63) | |
| Tumour grading | 1.000 | |||
| G1 | 0 | 0 | 0 | |
| G2 | 59 (63) | 22 (65) | 37 (63) | |
| G3 | 34 (37) | 12 (35) | 22 (37) | |
| PCI score (0-39) | 9.4 (0-39) | 12.0 (0-39) | 8.1 (0-30) | 0.312 |
| Postop CC-level | 0.350 | |||
| CC0 | 89 (87) | 28 (82) | 61 (90) | |
| CC1 | 8 (8) | 3 (9) | 5 (7) | |
| CC2/3 | 5 (5) | 3 (9) | 2 (3) | |
| Mucinous cells | 21 (21) | 6 (18) | 15 (22) | 0.796 |
We had a loss to follow-up rate of 3.9 % (four patients). All of them were treated with MMC-HIPEC.
There was no difference in the overall length of hospital stay [11.4 d (4-35)] for MMC vs 12.4 (2-46) for oxaliplatin; however, the oxaliplatin based HIPEC showed a significantly longer ICU stay [7.2 d (2-50) vs 4.4 d (2-9); P = 0.035].
Our data showed a total complication rate of 56%, with a statistically significant higher complication rate associated with oxaliplatin compared to MMC: 35% vs 66% (P = 0.003).
In further subgroup analysis we found an increased rate of intestinal atony (9% vs 29%; P = 0.015), abdominal infections (3% vs 21%; P = 0.013) and urinary tract infections (0% vs 9%; P = 0.034) for oxaliplatin HIPEC. The severity of complications, stratified according to the Clavien-Dindo classification, was also higher in the Oxaliplatin group (P = 0.029).
No patients died perioperatively, and 11 patients died during the first 90 d after surgery due to oncological or other medical reasons (Table 2).
| Parameter | Total, n = 102 | Mitomycin, n = 34 | Oxaliplatin/5-FU, n = 68 | P value1 |
| Median operative time in min | 379 (95-774) | 410 (95-774) | 363 (96-722) | 0.260 |
| Median blood substitution in mL | 105 (0-1800) | 185 (0 -1800) | 66 (0-1200) | 0.068 |
| Hospitalization in d | 12 (2-46) | 11,4 (4-35) | 12,4 (2-46) | 0.315 |
| ICU stay in d | 6.3 (2-50) | 4.4 (2-9) | 7.2 (2-50) | 0.035a |
| In-hospital mortality | ||||
| Rate of complications | 57 (56) | 12 (35) | 45 (66) | 0.003b |
| Cardio-pulmonary morbidity | ||||
| Pneumonia | 5 (5) | 2 (6) | 3 (4) | 0.542 |
| Re-intubation | 2 (2) | 0 | 2 (3) | 0.442 |
| Pulmonary embolism/thrombosis | 2 (2) | 0 | 2 (3) | 0.442 |
| Hematoma | 2 (2) | 1 (3) | 1 (2) | 0.558 |
| Postoperative haemorrhage | 4 (4) | 1 (3) | 3 (4) | 0.593 |
| Surgical morbidity | ||||
| Intestinal atony | 23 (23) | 3 (9) | 20 (30) | 0.015a |
| Wound infection | 15 (15) | 5 (15) | 10 (15) | 0.608 |
| Abdominal abscess | 13 (13) | 5 (15) | 8 (12) | 0.448 |
| Abdominal infection | 15 (15) | 1 (3) | 14 (21) | 0.013a |
| Burst abdomen | 8 (8) | 1 (3) | 7 (10) | 0.184 |
| Peritonitis | 6 (6) | 0 | 6 (9) | 0.081 |
| Sepsis | 6 (6) | 0 | 6 (9) | 0.081 |
| Renal complications | ||||
| Urinary retention | 4 (4) | 0 | 4 (6) | 0.192 |
| Renal failure | 7 (7) | 2 (6) | 5 (7) | 0.344 |
| Urinary tract infections | 8 (8) | 0 | 8 (12) | 0.034a |
| Severity of complicationsb | 0.029a | |||
| Grade 0/I | 45 (44) | 22 (65) | 23 (34) | |
| Grade II | 23 (23) | 3 (9) | 20 (30) | |
| Grade IIIa | 16 (16) | 5 (15) | 11 (16) | |
| Grade IIIb | 10 (10) | 3 (9) | 7 (10) | |
| Grade IV | 8 (8) | 1 (3) | 7 (10) | |
| Grade V (in-hospital mortality) | 0 | 0 | 0 | |
| Mortality | 0.139 | |||
| 30 d | 5 (5) | 4 (12) | 1 (1) | |
| 90 d | 11 (10) | 5 (15) | 6 (9) |
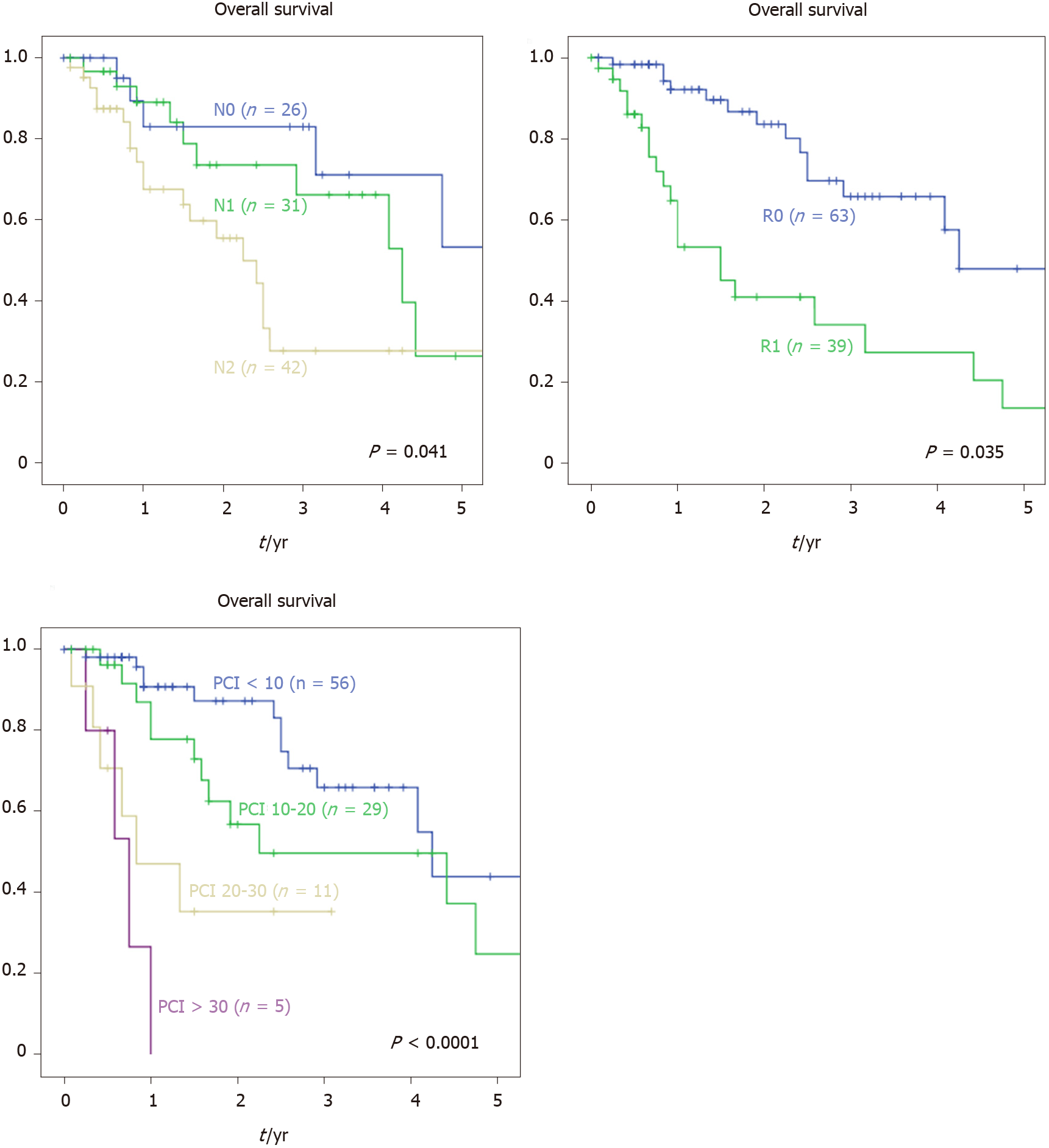
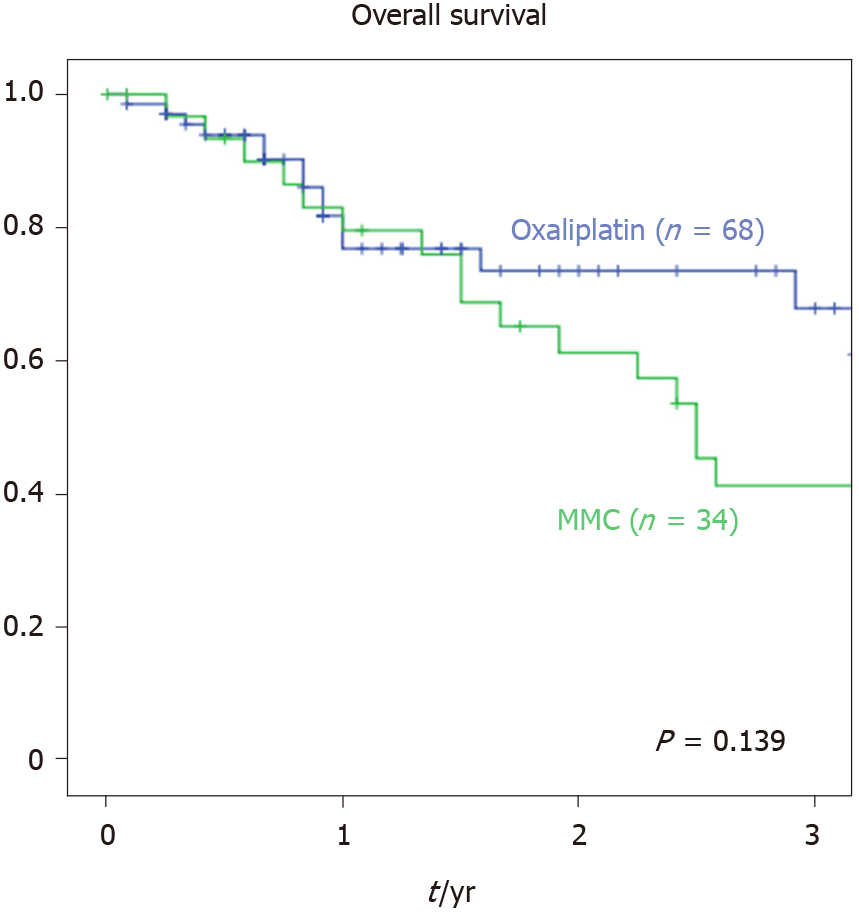
Mean follow-up was 23.3 mo. There was no statistically significant difference recording median OS (P = 0.139). We performed a univariate survival analysis to compare potential prognostic factors. No differences in survival rates were found comparing sex, age, body mass index (BMI) and ASA-scoring (Table 3). Likewise, primary tumour location (colon vs rectum) did not affect survival rate in our cohort (P = 1.0). Our data showed no difference in median survival when comparing primary T-stage (49 mo for T1-3 vs 30 mo for T4a vs not reached for T4b) but a significant influence of primary nodal stage (88 mo for N0 vs 51 mo for N1 vs 30 mo for N2a and 18 mo for N2b; P = 0.013). Likewise, according to our data, synchronous diagnosis of the PC or other distant metastasis was associated with a worse median survival (57 mo for M0 vs 35 mo for M+; P = 0.046). Furthermore, tumour grading and primary resection level also affected median survival (Figure 1).
| Predictor | n | Median survival in mo | P value1 |
| Sex | 0.884 | ||
| Male | 60 | 49 | |
| Female | 42 | 57 | |
| Age | 0.147 | ||
| < 50 yr | 27 | 38 | |
| ≥ 50 yr | 75 | 49 | |
| Preoperative BMI | 0.423 | ||
| < 18.5 | 4 | 4 | |
| 18.5-25 | 49 | 53 | |
| 25-30 | 35 | 51 | |
| > 30 | 14 | 49 | |
| ASA score | 0.457 | ||
| 1-2 | 49 | 49 | |
| 3-4 | 53 | 57 | |
| Primary tumour location | 0.620 | ||
| Colon | 91 | 49 | |
| Rectum | 11 | 23 | |
| Primary T-stage | 0.669 | ||
| T1-3 | 38 | 49 | |
| T4a | 40 | 30 | |
| T4b | 22 | Not reached | |
| Primary nodal stage | 0.013a | ||
| N0 | 26 | 88 | |
| N1 | 31 | 51 | |
| N2a | 19 | 30 | |
| N2b | 23 | 18 | |
| Primary distant metastasis | 0.046a | ||
| M0 | 36 | 57 | |
| M+ | 62 | 35 | |
| Primary tumour grading | 0.010a | ||
| G2 | 59 | 51 | |
| G3 | 34 | 29 | |
| Primary tumour resection | 0.035a | ||
| R0 | 76 | 51 | |
| R1 | 20 | 30 | |
| R2 | 4 | 16 | |
| Cytoreduction level | < 0.001b | ||
| CC0 | 89 | 49 | |
| CC1 | 10 | 12 | |
| CC2-3 + palliative resections | 3 | 3 | |
| PCI-score | < 0.001b | ||
| < 10 | 56 | 51 | |
| 10-20 | 29 | 27 | |
| 20-30 | 11 | 10 | |
| > 30 | 5 | 9 | |
| Operation extent | |||
| Partial colectomy | 55 | 53 | 0.189 |
| No colon resection | 47 | 31 | |
| Small bowel resection | 49 | 30 | 0.355 |
| No small bowel resection | 53 | 51 | |
| Liver metastasis resection | 42 | 27 | 0.024a |
| No liver resection | 60 | 51 | |
| HIPEC regimen | 0.139 | ||
| MMC | 34 | 30 | |
| Oxaliplatin/5-FU | 68 | Not reached |
In addition, lower PCI-score and a CC0- resection were associated with higher median survival. Patients undergoing a simultaneous liver metastasis resection during CRS had a worse survival prognosis (51 mo vs 27 mo for liver metastasis resection; P = 0.024).
To analyse further survival outcome factors, we performed multivariate analysis (Cox regression) with forward selection strategy using a likelihood ratio statistic. Synchronous distant metastasis (P = 0.029) and primary tumour resection status (P = 0.016) were confirmed to have a significant impact on survival as well as PCI-scoring regarding PC (P = 0.001). After carrying out a separate multivariate analysis, adapting the cut-off P value for inclusion to include HIPEC regimen into the analysis, HIPEC regimen failed to prove significance regarding OS at a P value of 0.144 (Figure 2).
With varying evidence for the therapeutic value of CRS-HIPEC in metastatic colon cancer, attention has refocused upon standardization and optimization of this procedure. However, there is a severe lack of evidence regarding comparison of survival benefits for the most commonly utilized chemotherapeutic agents for HIPEC oxaliplatin and MMC. This study is one of a few to focus on prognostic factors and treatment strategies after the development of peritoneal metastasis. Furthermore, the two most commonly used cytotoxic agents were compared regarding survival benefits and outcome rates.
Oxaliplatin and MMC, both interfering with DNA and DNA-synthesis, can reach high intraperitoneal drug concentrations during HIPEC with simultaneous limited systemic absorption[23,24]. Furthermore, they have elevated cytotoxicity under hyperthermia with a concordant tissue penetration depth of 2 mm[9]. The most commonly used intraperitoneal dose for oxaliplatin is 460 mg/m² with a perfusion time limited to 30 min. In contrast, the recommended intraperitoneal dose for MMC is 35 mg/m² with a prolonged perfusion duration of 90 min[9,25,26]. With the objective of potentiating the oxaliplatin activity, patients in the Oxaliplatin group received intravenous 5-FU and folinic acid approximately 1 hour before starting intraperitoneal HIPEC circulation.
Our study shows a 3-year-survival rate of 43% after CRS/HIPEC for peritoneal metastasized CRC. We could not show any statistically significant survival benefit comparing HIPEC regimens with oxaliplatin/5-FU vs MMC. Nevertheless, a statistical trend towards the oxaliplatin/5-FU group was noticed (Figure 2; median survival 30 mo for MMC vs not reached for oxaliplatin/5-FU). In our cohort, MMC group had a trend towards a higher PCI-scoring and a smaller number of CC-0 resections, which could possibly be responsible for the observed trend towards a prolonged survival in the Oxaliplatin group as well as differences in systemic preoperative treatments regarding multi-agent and targeted systemic therapy and surgical approach.
Regarding PRODIGE 7 trial, subgroup analysis showed a significant survival benefit for CRS + oxaliplatin HIPEC vs CRS for a subgroup with PCI 10-15[15]. Thus, there is a need of further studies, stratifying patients by PCI and prospectively examining the relative therapeutic effectiveness of MMC and oxaliplatin.
On the other hand, our study demonstrates significant differences between the two regimes regarding postoperative morbidity and complication rates. In our collective, patients treated with oxaliplatin/5-FU suffered increased rates of postoperative complications, especially intraperitoneal infections, urinary tract infections and intestinal atony.
Postoperative morbidity has to be taken into account when selecting an appropriate cytotoxic agent. Oxaliplatin has been suggested to cause higher morbidity rates with Grade II and III complication compared to MMC[27], as confirmed in this study. Reported complications in oxaliplatin trials include fistula formation, pneumonia or intraabdominal abscess formation[28]. The PRODIGE 7 trial likewise reported enhanced complication rates for CRS + oxaliplatin HIPEC vs CRS. A similar study design focusing on hematologic changes after CRS and HIPEC with either MMC or oxaliplatin was not able to show an increased complication rate after oxaliplatin HIPEC but a different complication scheme[29]. Contrary to this study, our analysis focuses on surgical complications in the postoperative phase. Therefore, the difference in the results can be explained.
Increased postoperative complication rates, especially severe complications (grade IIIb and IV according to Clavien-Dindo analysis), were also associated with prolonged ICU stay for the Oxaliplatin group compared to MMC (7.2 d vs 4.4 d; P = 0.035), which adds to evidence supporting MC for CRS-HIPEC.
Furthermore, we were able to identify different primary tumour factors affecting OS in this collective of peritoneal metastasized CRC. Interestingly, clinical factors such as age, sex, BMI or even ASA-scoring at CRS-HIPEC operation time have no influence on OS. Literature describes poorly differentiated carcinoma, venous invasion, lymphatic invasion, T4 disease, lymph node metastasis, malignant bowel obstruction and adjuvant chemotherapy as having negative impact on OS[30].
Even though primary T-stage and tumour location (colon/rectum) had no influence on survival outcome, primary nodal positivity and poor differentiation grade seem to affect tumour recurrence and lower survival rates in our patients with peritoneal carcinomatosis. This agrees with numerous other studies[31-33].
In our cohorts, 21% of tumours (18% in the MMC group and 22% in the Oxaliplatin group) were mucinous carcinoma. Regarding univariate analysis, we found no survival benefits for mucinous carcinoma vs adenocarcinoma. Our cohort contains no patients with adenosquamous or squamous carcinoma. As both groups contain a similar percentage of mucinous carcinoma, we expect no selection bias due to this histopathological criterion.
We also found R1-resections of primary tumours to be a prognostic factor after peritoneal metastasis, as well as synchronous metastatic spread. Two studies[34,35] analysed the prognostic influence of disease-free resection margins on survival and also found this to have independent prognostic value. These results are useful to identify optimal subgroups for high risk of recurrent PC.
An important prognostic factor of survival is the concept of tumour burden correlated with PCI-scoring. Oncologic results seem to be significantly better when PCI is < 10[36] or ≤ 13[37]. However, PCI ≥ 20 is associated with decreased survival according to many different studies[38-40]. This agrees with our results from univariate and multivariate analysis of survival. Patients with distant metastasis, especially liver metastasis, were included in this analysis. Current literature suggests that patients with distant metastasis amendable to resection should not be excluded from CRS and HIPEC[38,41]. Concordant to the literature, univariate analysis of survival of our data shows a significant reduced survival for patients undergoing liver resections during CRS and HIPEC (27 mo vs 51 mo without liver resection; P = 0.024).
There are several limitations in this study that should be considered. Mainly, the retrospective non-randomized study design lowers comparability between the groups. Furthermore, the retrospective database lacks complete information regarding Tumour Node Metastasis staging, preoperative treatments especially chemotherapy as well as varying follow-up duration. The patients were treated over a time period of 10 years with changes in perioperative management and systemic chemotherapy. Different surgeons performed HIPECs at the university hospital of Freiburg. Therefore, an individual learning curve cannot be assessed. Nevertheless, the learning curve of the complete surgical department could influence postoperative outcome depending on operation timing.
For this special collective of patients with PC based on a colorectal primary tumour, several outcome predictors were identified. We were also able to show comparable outcome results for CRS/HIPEC with oxaliplatin and MMC. Nevertheless, increased complication rates for oxaliplatin were demonstrated, which, according to the literature, significantly affects OS[42] indicating that patients should be treated favourably with MMC-HIPEC. As we could not show any survival benefit for patients treated with MMC or oxaliplatin HIPEC, it remains to be determined whether there is enough evidence for HIPEC. However, the importance of complete cytoreduction has been established, which has been broadly discussed in the literature and is consistent with our data.
Further studies, in particular a phase III clinical trial comparing both HIPEC regimens, would improve evidence-based decision-making.
Cytoreductive Surgery (CRS) in combination with hyperthermic intraperitoneal chemotherapy (HIPEC) improves patient survival in colorectal cancer (CRC) with peritoneal carcinomatosis (PC). Commonly used cytotoxic agents nowadays include mitomycin C (MMC) and oxaliplatin. Evidence for the choice of the HIPEC agent and uniform procedure protocols is scarce, with studies reporting varying results.
There’s a severe lack of evidence regarding comparison of survival benefits for most commonly utilized chemotherapeutic agents for HIPEC oxaliplatin and MMC. At present there is no prospective study that compares these two HIPEC regimens for treatment of peritoneal metastasized CRC, thus leading to the reassessment of HIPEC and the need for structured treatment protocols. In this retrospective clinical analysis, we evaluated the outcome of patients undergoing CRS HIPEC at the university medical centre of Freiburg. Furthermore, this study is one of a few to focus on prognostic factors and treatment strategies after the development of peritoneal metastasis.
The aim of the study was to evaluate therapeutic benefits and operative and postoperative complications of CRS + MMC vs oxaliplatin HIPEC in patients with peritoneal metastasized CRC as well as prognostic factors for overall survival (OS).
One hundred two patients who had undergone CRS and HIPEC for CRC PC between 2007 and 2019 at the Medical Center of the University Freiburg regarding interdisciplinary cancer conference decision were retrospectively analysed. Oxaliplatin and MMC were used in 68 and 34 patients, respectively. Each patient’s demographics and tumour characteristics, operative details, postoperative complications and survival were noted and compared. Complications were stratified and graded using Clavien/Dindo analysis. Prognostic outcome factors were identified using univariate and multivariate analysis of survival.
The two groups did not differ significantly regarding baseline characteristics. We found no difference in median OS. Patients treated with oxaliplatin HIPEC suffered increased postoperative complications (66.2% vs 35.3%; P = 0.003), particularly intestinal atony, intraabdominal infections and urinary tract infections, and had a prolonged intensive care unit (ICU) stay compared to the MMC group (7.2 d vs 4.4 d; P = 0.035). Regarding univariate analysis of survival, we found primary tumour factors, nodal positivity and resection margins to be of prognostic value as well as PC index (PCI)-score and the completeness of cytoreduction regarding peritoneal carcinomatosis. Multivariate analysis of survival confirmed primary distant metastasis and primary tumour resection status to have a significant impact on survival and likewise PCI-scoring regarding peritoneal carcinomatosis.
We could not show any survival advantage for neither HIPEC regimens. Oxaliplatin showed an increased complication rate. Increased postoperative complication rates, especially severe complications (grade IIIb and IV according to Clavien-Dindo analysis), were also associated with prolonged ICU stay for the Oxaliplatin group compared to MMC (7.2 d vs 4.4 d; P = 0.035), which improves evidence to choose MMC for CRS-HIPEC.
Primary distant metastasis and primary tumour resection seem to have a significant impact on survival and likewise PCI-scoring regarding peritoneal carcinomatosis.
For this special collective of patients with PC based on a colorectal primary tumour, several outcome predictors could be identified. We were also able to show comparable outcome results for CRS/HIPEC with oxaliplatin and MMC. Nevertheless, increased complication rates for oxaliplatin were demonstrated, which, according to literature, significantly affects OS, indicating that patients should be treated favourably with MMC-HIPEC. Further studies, in particular a phase III clinical trial comparing both HIPEC regimens would improve evidence-based decision-making.
| 1. | Sánchez-Hidalgo JM, Rodríguez-Ortiz L, Arjona-Sánchez Á, Rufián-Peña S, Casado-Adam Á, Cosano-Álvarez A, Briceño-Delgado J. Colorectal peritoneal metastases: Optimal management review. World J Gastroenterol. 2019;25:3484-3502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 2. | Chu DZ, Lang NP, Thompson C, Osteen PK, Westbrook KC. Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer. 1989;63:364-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 3. | Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89:1545-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 605] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 4. | Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL, Faure JL, Porcheron J, Peix JL, François Y, Vignal J, Gilly FN. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88:358-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 5. | Adachi T, Hinoi T, Egi H, Shimomura M, Ohdan H. Oxaliplatin and molecular-targeted drug therapies improved the overall survival in colorectal cancer patients with synchronous peritoneal carcinomatosis undergoing incomplete cytoreductive surgery. Surg Today. 2015;45:986-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Sugarbaker PH. Management of peritoneal carcinomatosis. Acta Med Austriaca. 1989;16:57-60. [PubMed] |
| 7. | Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737-3743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1542] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 8. | Esquivel J, Lowy AM, Markman M, Chua T, Pelz J, Baratti D, Baumgartner JM, Berri R, Bretcha-Boix P, Deraco M, Flores-Ayala G, Glehen O, Gomez-Portilla A, González-Moreno S, Goodman M, Halkia E, Kusamura S, Moller M, Passot G, Pocard M, Salti G, Sardi A, Senthil M, Spilioitis J, Torres-Melero J, Turaga K, Trout R. The American Society of Peritoneal Surface Malignancies (ASPSM) Multiinstitution Evaluation of the Peritoneal Surface Disease Severity Score (PSDSS) in 1,013 Patients with Colorectal Cancer with Peritoneal Carcinomatosis. Ann Surg Oncol. 2014;21:4195-4201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 9. | Kusamura S, Dominique E, Baratti D, Younan R, Deraco M. Drugs, carrier solutions and temperature in hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2008;98:247-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938-2947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3005] [Cited by in RCA: 2838] [Article Influence: 109.2] [Reference Citation Analysis (1)] |
| 11. | Makatsoris T, Kalofonos HP, Aravantinos G, Papadimitriou C, Kastritis E, Rigatos SK, Xiros N, Petsas T, Economopoulos T, Sakadamis AK, Fountzilas G; Hellenic Cooperative Oncology Group. A phase II study of capecitabine plus oxaliplatin (XELOX): a new first-line option in metastatic colorectal cancer. Int J Gastrointest Cancer. 2005;35:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Soulié P, Raymond E, Brienza S, Cvitkovic E. [Oxaliplatin: the first DACH platinum in clinical practice]. Bull Cancer. 1997;84:665-673. [PubMed] |
| 13. | Alkis N, Demirci U, Benekli M, Yilmaz U, Isikdogan A, Sevinc A, Ozdemir NY, Koca D, Yetisyigit T, Kaplan MA, Uncu D, Unek T, Gumus M. Mitomycin-C in combination with fluoropyrimidines in the treatment of metastatic colorectal cancer after oxaliplatin and irinotecan failure. J BUON. 2011;16:80-83. [PubMed] |
| 14. | Macrì A, Arcoraci V, Belgrano V, Caldana M, Carbonari L, Cioppa T, De Cian F, De Manzoni G, De Simone M, Giardina C, Muffatti F, Orsenigo E, Robella M, Roviello F, Saladino E, Sammartino P, Vaira M. Short-term outcome of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy used as treatment of colo-rectal carcinomatosis: a multicentric study. Updates Surg. 2020;72:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Pinto A, Pocard M. Hyperthermic intraperitoneal chemotherapy with cisplatin and mitomycin C for colorectal cancer peritoneal metastases: A systematic review of the literature. Pleura Peritoneum. 2019;4:20190006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Lord AC, Shihab O, Chandrakumaran K, Mohamed F, Cecil TD, Moran BJ. Recurrence and outcome after complete tumour removal and hyperthermic intraperitoneal chemotherapy in 512 patients with pseudomyxoma peritonei from perforated appendiceal mucinous tumours. Eur J Surg Oncol. 2015;41:396-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Mulier S, Claes JP, Dierieck V, Amiel JO, Pahaut JP, Marcelis L, Bastin F, Vanderbeeken D, Finet C, Cran S, Velu T. Survival benefit of adding Hyperthermic IntraPEritoneal Chemotherapy (HIPEC) at the different time-points of treatment of ovarian cancer: review of evidence. Curr Pharm Des. 2012;18:3793-3803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Quenet F, Elias D, Roca L, Goere D, Ghouti L, Pocard M, Facy O, Arvieux C, Lorimier G, Pezet D. A UNICANCER phase III trial of hyperthermic intra-peritoneal chemotherapy (HIPEC) for colorectal peritoneal carcinomatosis (PC): PRODIGE 7. J Clin Oncol. 2018;36:1. [RCA] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 228] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 19. | Leung V, Huo YR, Liauw W, Morris DL. Oxaliplatin versus Mitomycin C for HIPEC in colorectal cancer peritoneal carcinomatosis. Eur J Surg Oncol. 2017;43:144-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 20. | Prada-Villaverde A, Esquivel J, Lowy AM, Markman M, Chua T, Pelz J, Baratti D, Baumgartner JM, Berri R, Bretcha-Boix P, Deraco M, Flores-Ayala G, Glehen O, Gomez-Portilla A, González-Moreno S, Goodman M, Halkia E, Kusamura S, Moller M, Passot G, Pocard M, Salti G, Sardi A, Senthil M, Spiliotis J, Torres-Melero J, Turaga K, Trout R. The American Society of Peritoneal Surface Malignancies evaluation of HIPEC with Mitomycin C versus Oxaliplatin in 539 patients with colon cancer undergoing a complete cytoreductive surgery. J Surg Oncol. 2014;110:779-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 21. | Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M, Barone R, Yonemura Y, Cavaliere F, Quenet F, Gutman M, Tentes AA, Lorimier G, Bernard JL, Bereder JM, Porcheron J, Gomez-Portilla A, Shen P, Deraco M, Rat P. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22:3284-3292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 860] [Cited by in RCA: 891] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 22. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 9205] [Article Influence: 541.5] [Reference Citation Analysis (1)] |
| 23. | Lambert LA, Armstrong TS, Lee JJ, Liu S, Katz MH, Eng C, Wolff RA, Tortorice ML, Tansey P, Gonzalez-Moreno S, Lambert DH, Mansfield PF. Incidence, risk factors, and impact of severe neutropenia after hyperthermic intraperitoneal mitomycin C. Ann Surg Oncol. 2009;16:2181-2187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15:2426-2432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 765] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 25. | Elias D, Pocard M, Goere D. HIPEC with oxaliplatin in the treatment of peritoneal carcinomatosis of colorectal origin. Cancer Treat Res. 2007;134:303-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | van Ruth S, Mathôt RA, Sparidans RW, Beijnen JH, Verwaal VJ, Zoetmulder FA. Population pharmacokinetics and pharmacodynamics of mitomycin during intraoperative hyperthermic intraperitoneal chemotherapy. Clin Pharmacokinet. 2004;43:131-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Rouers A, Laurent S, Detroz B, Meurisse M. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal peritoneal carcinomatosis: higher complication rate for oxaliplatin compared to Mitomycin C. Acta Chir Belg. 2006;106:302-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Elias DM, Ouellet JF. Intraperitoneal chemohyperthermia: rationale, technique, indications, and results. Surg Oncol Clin N Am. 2001;10:915-933, xi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 71] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Votanopoulos K, Ihemelandu C, Shen P, Stewart J, Russell G, Levine EA. A comparison of hematologic toxicity profiles after heated intraperitoneal chemotherapy with oxaliplatin and mitomycin C. J Surg Res. 2013;179:e133-e139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Nagata H, Ishihara S, Hata K, Murono K, Kaneko M, Yasuda K, Otani K, Nishikawa T, Tanaka T, Kiyomatsu T, Kawai K, Nozawa H, Watanabe T. Survival and Prognostic Factors for Metachronous Peritoneal Metastasis in Patients with Colon Cancer. Ann Surg Oncol. 2017;24:1269-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Königsrainer I, Horvath P, Struller F, Forkl V, Königsrainer A, Beckert S. Risk factors for recurrence following complete cytoreductive surgery and HIPEC in colorectal cancer-derived peritoneal surface malignancies. Langenbecks Arch Surg. 2013;398:745-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Lemmens VE, Klaver YL, Verwaal VJ, Rutten HJ, Coebergh JW, de Hingh IH. Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: a population-based study. Int J Cancer. 2011;128:2717-2725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 263] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 33. | Segelman J, Akre O, Gustafsson UO, Bottai M, Martling A. Individualized prediction of risk of metachronous peritoneal carcinomatosis from colorectal cancer. Colorectal Dis. 2014;16:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | Chafai N, Chan CL, Bokey EL, Dent OF, Sinclair G, Chapuis PH. What factors influence survival in patients with unresected synchronous liver metastases after resection of colorectal cancer? Colorectal Dis. 2005;7:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Harris GJ, Senagore AJ, Lavery IC, Church JM, Fazio VW. Factors affecting survival after palliative resection of colorectal carcinoma. Colorectal Dis. 2002;4:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Goéré D, Malka D, Tzanis D, Gava V, Boige V, Eveno C, Maggiori L, Dumont F, Ducreux M, Elias D. Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann Surg. 2013;257:1065-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 204] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 37. | Yan TD, Chu F, Links M, Kam PC, Glenn D, Morris DL. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal carcinoma: non-mucinous tumour associated with an improved survival. Eur J Surg Oncol. 2006;32:1119-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Cavaliere F, De Simone M, Virzì S, Deraco M, Rossi CR, Garofalo A, Di Filippo F, Giannarelli D, Vaira M, Valle M, Pilati P, Perri P, La Pinta M, Monsellato I, Guadagni F. Prognostic factors and oncologic outcome in 146 patients with colorectal peritoneal carcinomatosis treated with cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy: Italian multicenter study S.I.T.I.L.O. Eur J Surg Oncol. 2011;37:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 39. | Quenet F, Goéré D, Mehta SS, Roca L, Dumont F, Hessissen M, Saint-Aubert B, Elias D. Results of two bi-institutional prospective studies using intraperitoneal oxaliplatin with or without irinotecan during HIPEC after cytoreductive surgery for colorectal carcinomatosis. Ann Surg. 2011;254:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 40. | Van Sweringen HL, Hanseman DJ, Ahmad SA, Edwards MJ, Sussman JJ. Predictors of survival in patients with high-grade peritoneal metastases undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Surgery. 2012;152:617-24; discussion 624-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Cao C, Yan TD, Black D, Morris DL. A systematic review and meta-analysis of cytoreductive surgery with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol. 2009;16:2152-2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 193] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 42. | Lee L, Alie-Cusson F, Dubé P, Sideris L. Postoperative complications affect long-term outcomes after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal peritoneal carcinomatosis. J Surg Oncol. 2017;116:236-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cloyd JM, Ding JX, Macri A S-Editor: Dou Y L-Editor: Filipodia P-Editor: Li X