Published online Aug 15, 2020. doi: 10.4251/wjgo.v12.i8.893
Peer-review started: March 4, 2020
First decision: April 26, 2020
Revised: May 26, 2020
Accepted: July 19, 2020
Article in press: July 19, 2020
Published online: August 15, 2020
Processing time: 161 Days and 7.8 Hours
Neuroendocrine tumors (NETs) frequently occur in the gastrointestinal tract, lung, and pancreas, and the rectum and appendix are the sites with the highest incidence. Epidemiology statistics show that an estimated 8000 people every year in the United States are diagnosed with NETs occurring in the gastrointestinal tract, including the stomach, intestine, appendix, colon, and rectum. The pathological changes and clinical symptoms of NETs are not specific, and therefore they are frequently misdiagnosed.
To investigate the clinical symptoms, pathological characteristics, treatment, and prognosis of rectal neuroendocrine tumors (RNETs) by analyzing the clinical and pathological data of 132 RNET cases at our hospital.
All RNETs were graded according to Ki-67 positivity and mitotic events. The tumors were staged as clinical stages I, II, III, and IV according to infiltrative depth and tumor size. COX proportional hazard model was used to assess the main risk factors for survival.
These 132 RNETs included 83 cases of G1, 21 cases of G2, and 28 cases of G3 (neuroendocrine carcinoma) disease. Immunohistochemical staining showed that 89.4% of RNETs were positive for synaptophysin and 39.4% positive for chromogranin A. There were 19, 85, 23, and 5 cases of clinical stages I, II, III, and IV, respectively. The median patient age was 52.96 years. The diameter of tumor, depth of invasion, and pathological grade were the main reference factors for the treatment of RNETs. The survival rates at 6, 12, 36, and 60 mo after operation were 98.5%, 94.6%, 90.2%, and 85.6%, respectively. Gender, tumor size, tumor grade, lymph node or distant organ metastasis, and radical resection were the main factors associated with prognosis of RNETs. Multivariate analysis showed that tumor size and grade were independent prognostic factors.
The clinical symptoms of RNETs are not specific, and they are easy to misdiagnose. Surgery is the main treatment method. The grade and stage of RNETs are the main indices to evaluate prognosis.
Core tip: Tumor size and grade were the most significantly associated factors, and tumor size was the sole factor that was independently related to survival in a multivariate analysis. Patients with tumors larger than 2 cm had a ten-fold higher risk of death. Patients with advanced neuroendocrine carcinomas had a significantly decreased 5-year overall survival compared to patients with grades 1 and 2 disease.
- Citation: Yu YJ, Li YW, Shi Y, Zhang Z, Zheng MY, Zhang SW. Clinical and pathological characteristics and prognosis of 132 cases of rectal neuroendocrine tumors. World J Gastrointest Oncol 2020; 12(8): 893-902
- URL: https://www.wjgnet.com/1948-5204/full/v12/i8/893.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i8.893
Neuroendocrine tumors (NETs) frequently occur in the gastrointestinal tract, lung, and pancreas, and the rectum and appendix are the sites with the highest incidence[1,2]. Epidemiology statistics show that an estimated 8000 people every year in the United States are diagnosed with NETs occurring in the gastrointestinal tract, including the stomach, intestine, appendix, colon, and rectum. Most NETs follow a benign course. However, some display malignant characteristics. NETs are believed to arise from various neuroendocrine cells and are graded histologically according to markers of cellular proliferation[3].
Rectal NETs (RNETs) only account for 1% to 2% of rectal tumors, but represent a high proportion of gastrointestinal NETs. Compared to gastrointestinal NETs in other locations, RNETs have a relatively small average volume and unique biological behavior[4,5]. RNETs are more common in patients from 40-60 years of age, most occur as a single tumor, and the clinical symptoms and signs of RNETs are not typically observed in RNETs[6,7]. Although surgical treatment is still the first choice for the treatment of RNETs, with the continuous development of endoscopic technology, as well as endoscopic treatment with less trauma, faster recovery, and decreased cost, more people choose endoscopic treatment[8].
In this study, we retrospectively analyzed the pathological characteristics and prognosis of 132 patients with RNETs at our hospital according to the classification and nomenclature of NETs of the digestive system, and investigated the clinicopathological characteristics and treatment of RNETs.
The study was approved by the Ethics Committee of Tianjin Union Medical Center in January 2015. The Ethics Committee approved related screening, treatment, data collection, and follow-up of these patients, and all subjects signed a written informed consent form. All research was undertaken following the provisions of the Declaration of Helsinki. Patients with the following criteria were included: (1) Confirmed RNETs according to the 2006 European NET Association gastrointestinal NETs grading recommendations and 2010 World Health Organization (WHO) digestive system tumor classification criteria; (2) Complete clinical examination and case data; (3) Age at least 18 years; and (4) Available resected samples.
NETs were graded histologically according to Ki-67 IHC staining and mitosis[9]. The grades were defined as follows: G1, < 2 mitotic events per 10 high power fields (HPFs) and Ki-67 index < 3%; G2, ≥ 2 and ≤ 20 mitotic events per 10 HPFs, and Ki-67 index ≥ 3% and ≤ 20%; G3: > 20 mitotic events per 10 HPFs, and Ki-67 index > 20%. G3 is also sometimes referred to as neuroendocrine carcinoma (NEC).
Currently, there is no staging system for NETs of all locations. Based on anatomical location, RNETs were staged as clinical stages I, II, III, or IV according to infiltrative depth and tumor size as follows[10-13]: Stage I, invasion into the lamina propria or submucosa with the greatest dimension ≤ 2 cm; stage II, invasion into the muscularis propria, or the greatest dimension > 2 cm with invasion of the lamina propria or submucosa, or invasion through the muscularis propria into the subserosal tissue without penetration of the overlying serosa; stage III, invasion into the visceral peritoneum (serosal) or other organs or adjacent structures, or lymph node involvement[10]; and stage IV, distant metastasis.
Resected samples were fixed in 4% formaldehyde solution. After conventional dehydration and paraffin embedding, samples were sectioned at a thickness of 4 μm. Hematoxylin and eosin (HE) staining and immunohistochemical staining were performed. Primary antibodies against synaptophysin (Syn) (Zhongshan Golden Bridge Biotechnology, Beijing, China; ZM-0246, dilution 1:100), chromogranin A (CgA) (Zhongshan Golden Bridge Biotechnology; ZA-0076, dilution 1:100), and Ki-67 (Zhongshan Golden Bridge Biotechnology; ZM-0167, dilution 1:200) were used. Positive and negative controls were used for each antibody. Ten unique fields of each section under a light microscope (400 ×) were selected for image analysis, and the numbers of positive cells and total cells were counted. In this study, immunohistochemical staining for Syn and CgA was used to validate the diagnosis of RNET. Positive expression of Syn and CgA was defined as > 30% of positive cells. The percentage of Ki-67 positive cells was defined as the number of positive cells in 100 tumor cells.
Patient data, including tumor size, lymph node and distant metastasis, endoscopic morphology characteristics, treatment, pathological diagnosis, postoperative complications, and the survival rate, were retrospectively analyzed. All patients were followed by outpatient visits or telephone, and June 30, 2012 was employed as the end of the follow-up.
SPSS 19.0 statistical software (IBM Corporation, United States) was used for data analyses. Count data are expressed as ratios. COX proportional hazard model was used for analyzing tumor prognostic factors. P < 0.05 was considered statistically significant. Survival time of patients with RNETs was analyzed using the Kaplan-Meier method and differences were assessed using the log-rank test.
From December 2005 to May 2012, a total of 132 patients were diagnosed with RNETs at our hospital. The median patient age was 52.96 years. In addition, 62.9% (83) patients were male and 37.1% (49) were female. The main clinical symptoms included anal bulge discomfort, blood in the stool, and bowel habit change. Approximately 1/3 of the patients did not exhibit any symptoms, and RNETs in these patients were discovered by colorectal cancer screening.
One hundred and eighteen (89.4%) tumors occurred mainly in the middle and upper rectum (the distal margin of the tumor was more than 8 cm from the anal margin) and four (3%) occurred near the anal dentate line. All of the tumors were located in the rectum and were 15 cm from the anal verge. Tumor diameter was 0.2-1.0 cm in 60 cases, 1.1-2.0 cm in 45 cases, and > 2.0 cm in 27 cases. Most G1 and G2 RNETs displayed as a protruded mass on colonoscopy (Figure 1A, a and b), and ulcers were detected in some cases, as is common in NECs (Figure 1A, c). There were 19, 85, 23, and 5 cases of clinical stages I, I, III, and IV disease, respectively, according to the 2006 European NET Society of Gastrointestinal NET classification and the 2010 WHO digestive system tumor classification. All 132 patients received surgical treatment, including 113 patients who underwent endoscopic local tumor resection, in whom pathological results confirmed that 12 cases were basal positive. Eight cases of laparotomy and 11 cases of endoscopic surgery were performed. Miles surgery was performed in 4 patients, 14 patients underwent anterior resection, and 1 patient received combined rectal and metastatic liver resection.
In the treatment of RNETs, the diameter of tumor, depth of invasion, and pathological grade were the main factors used for comprehensive evaluation. Ultrasound endoscopy was often used to detect the tumor size and depth of invasion. The tumor less than 1 cm in diameter had a lower probability of metastasis, and most of them were G1/G2 RNETs. Endoscopic resection of tumor was used if the tumor has not invaded the muscularis propria. If the tumor infiltrated into the muscularis propria, local surgical removal was required. For patients with RNETs more than 2 cm in diameter, the probability of distant metastasis was greatly increased. Imaging examination was used to exclude distant metastasis. Presacral resection or total mesorectal excision was feasible for those patients without distant metastasis. For tumors with a diameter of 1-2 cm, local resection of tumor was used for those patients whose tumor did not metastasize and the invasive depth did not reach the muscularis propria. For patients whose tumor invasive depth reached or exceeded the muscularis propria, presacral resection or total mesorectal excision should be used. For NEC patients without distant metastasis, it should be treated as adenocarcinoma regardless of the diameter of the tumor. After surgery, adjuvant radiotherapy and chemotherapy were given according to the pathological stage. For RNET patients with distant metastasis, surgery was only used for relieving local symptoms, such as obstruction and bleeding.
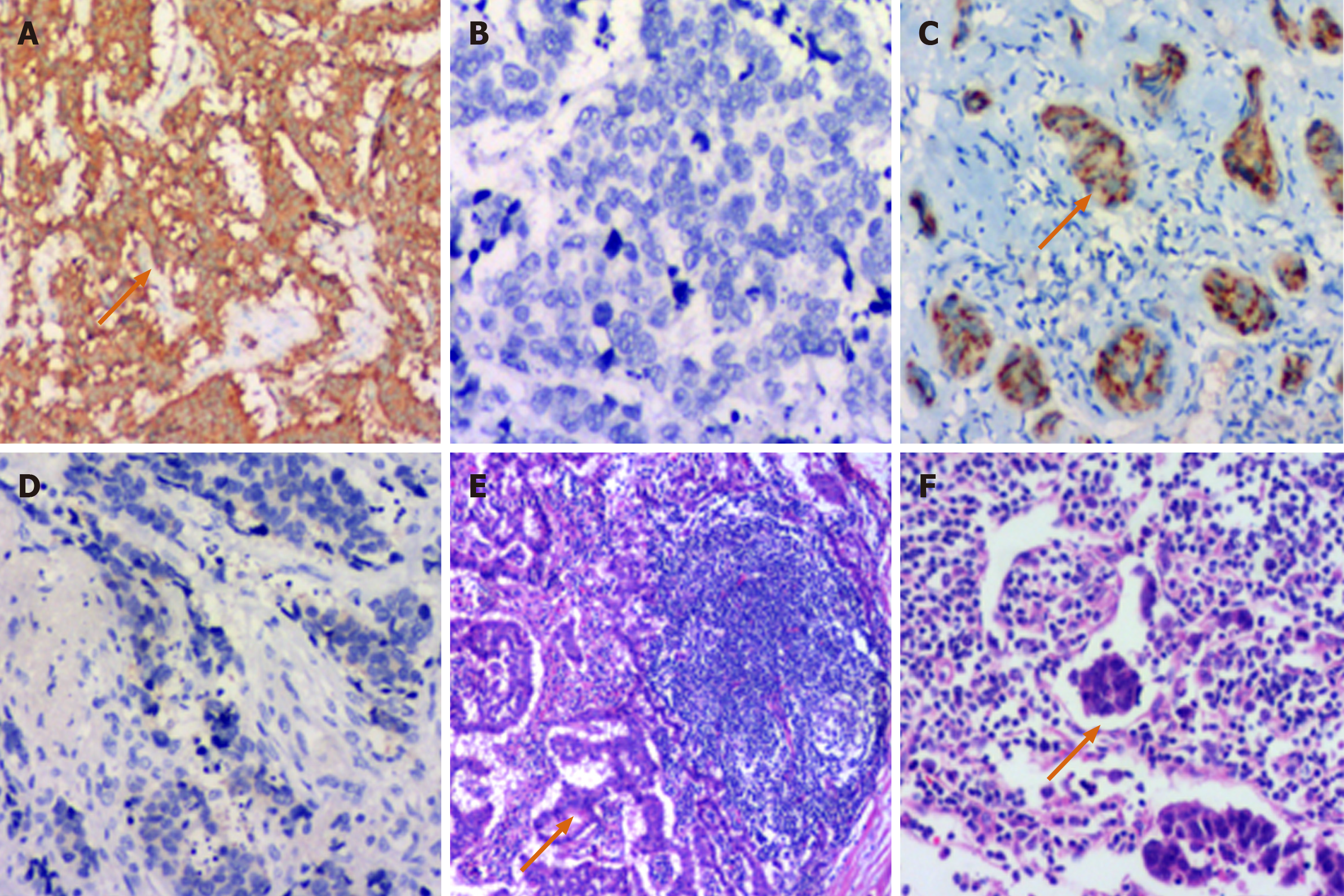
The typical morphological characteristics of RNETs included adenoid, trabecular, and papillary structures. The tumor cells were small and regular based on the HE staining (Figure 1B, a-c). Based on Ki-67 index and mitotic events per 10 HPFs, it was showed that 83 cases were G1 (Figure 1B, d), 21 were G2 (Figure 1B, e), and 28 were NEC (Figure 1B, f). One hundred and eighteen (89.4%) cases were positive for Syn by immunohistochemical staining (Figure 2A and B), and 52 were positive for CgA (Figure 2C and D). Four cases were found to have a single distant organ metastasis, and 1 case had multiple distant organ metastases. Thirteen cases were postoperatively confirmed to have lymph node metastases (Figure 2E), and intravascular tumor thrombus was found in 2 cases (Figure 2F).
The follow-up period of the 132 patients ranged from 3 to 60 mo, and effective follow-up was completed in 102 (77.3%) patients. Thirty patients were lost to follow-up. The survival rates at 6, 12, 36, and 60 mo after operation were 98.5%, 94.6%, 90.2%, and 85.6%, respectively. The 5-year survival rates of patients with NETs and neuroendocrine carcinoma were 96.2% and 25.0%, respectively. Twenty-eight patients with NECs received postoperative adjuvant therapy, 9 received postoperative chemotherapy alone, 4 received radiotherapy alone, and 15 received both radiotherapy and chemotherapy. At least one follow-up was performed in 25 patients with NECs and the follow-up rate was 89.28%. The average follow-up time was 38 mo. As of June 30, 2012, 19 patients died of NECs. Metastasis including 9 cases of liver metastasis occurred in 13 patients.
Univariate COX regression analysis showed that gender, tumor size, lymph node metastasis, radical resection, and pathological grade influenced the prognosis of RNETs (P < 0.05), while age, distant metastasis, and Syn and CgA expression had no influence on the prognosis of RNETs (P > 0.05) (Table 1). Of these independent variables, age, gender, tumor size, lymph node metastasis, radical resection, and pathological grade (P < 0.01) were considered as potential independent variables that can be used for multivariate COX regression analysis. When controlling for factors such as age and gender, tumor size was an independent factor for prognosis, the risk of death in patients with tumors ≥ 2 cm was 10.173 times that of patients with tumors < 2 cm (P > 0.05, Table 2). In addition, Kaplan-Meier survival analysis was used to compare the differences of 5-year survival rate in 132 cases of patients with RNETs, which showed that gender, tumor size, lymph node metastasis, radical excision, and pathological grade had statistical significance. Male patients (χ2 = 4.327, P = 0.038), tumors with a diameter more than 2 cm (χ2 = 64.98, P = 0.000), positive lymph node metastasis (χ2 = 22.37, P = 0.000), non-radical excision (χ2 = 25.89, P = 0.000), and patients with NECs (χ2 = 71.79, P = 0.000) were associated with poor 5-year survival (Table 3).
| B | SE | Wald | df | P value | RR | 95%CI | |||
| Age (yr) | < 55 | 0.847 | 0.458 | 3.415 | 1 | 0.065 | 2.332 | 0.950 | 5.723 |
| ≥ 55 | |||||||||
| Gender | Male | 0.850 | 0.429 | 3.918 | 1 | 0.048 | 2.340 | 1.008 | 5.429 |
| Female | |||||||||
| Tumor size | < 2 cm | 3.177 | 0.748 | 18.046 | 1 | 0.000 | 23.984 | 5.537 | 103.895 |
| ≥ 2cm | |||||||||
| Lymph node metastasis | No | 2.290 | 0.480 | 22.743 | 1 | 0.000 | 9.878 | 3.854 | 25.320 |
| Yes | |||||||||
| Distant metastasis | No | 0.806 | 0.552 | 2.129 | 1 | 0.144 | 2.239 | 0.758 | 6.608 |
| Yes | |||||||||
| Radical excision | No | -1.970 | 0.424 | 21.612 | 1 | 0.000 | 0.139 | 0.061 | 0.320 |
| Yes | |||||||||
| Pathological grade | G1 + G2 | 2.648 | 0.630 | 17.657 | 1 | 0.000 | 14.121 | 4.107 | 48.552 |
| NEC | |||||||||
| Syn | Negative | -0.295 | 0.547 | 0.291 | 1 | 0.589 | 0.744 | 0.255 | 2.175 |
| Positive | |||||||||
| CgA | Negative | -0.049 | 0.456 | 0.012 | 1 | 0.914 | 0.952 | 0.389 | 2.326 |
| Positive | |||||||||
| B | SE | Wald | df | P value | RR | 95%CI | ||
| Gender | 0.331 | 0.476 | 0.481 | 1 | 0.488 | 1.392 | 0.547 | 3.541 |
| Tumor size | 2.320 | 1.151 | 4.065 | 1 | 0.044 | 10.173 | 1.067 | 97.012 |
| Lymph node metastasis | 0.613 | 0.628 | 0.954 | 1 | 0.329 | 1.846 | 0.539 | 6.320 |
| Radical excision | -0.581 | 0.488 | 1.416 | 1 | 0.234 | 0.559 | 0.215 | 1.456 |
| Pathological grade | -0.048 | 1.009 | 0.002 | 1 | 0.962 | 0.953 | 0.132 | 6.889 |
| n | 5-yr survival rate | χ2 | P value | ||
| Age (yr) | < 55 | 73 | 83.6 | 0.554 | 0.457 |
| ≥ 55 | 59 | 88.1 | |||
| Gender | Male | 83 | 80.7 | 4.327 | 0.038 |
| Female | 49 | 93.9 | |||
| Tumor size | < 2 cm | 105 | 98.1 | 64.98 | 0.000 |
| ≥ 2cm | 27 | 37 | |||
| Lymph node metastasis | No | 111 | 91.9 | 22.37 | 0.000 |
| Yes | 21 | 52.4 | |||
| Distant metastasis | No | 127 | 86.6 | 2.765 | 0.096 |
| Yes | 5 | 60 | |||
| Radical excision | No | 16 | 43.8 | 25.89 | 0.000 |
| Yes | 116 | 91.4 | |||
| Pathological grade | G1 + G2 | 104 | 99 | 71.79 | 0.000 |
| NEC | 28 | 35.7 | |||
| Syn | Negative | 14 | 78.6 | 0.629 | 0.428 |
| Positive | 118 | 86.4 | |||
| CgA | Negative | 80 | 83.8 | 0.568 | 0.451 |
| Positive | 52 | 88.5 |
NETs occur in various parts of the body, and the rectum is a high incidence area of NETs. The incidence of RNETs has risen in recent years[13,14]. The incidence of rectal NETs (carcinoid tumors) in an Asian population, including Chinese patients, is 4.99 times that of non-Asian populations[15-17]. In our study, the occurrence of RNETs was higher in men than in women. Moreover, RNETs are more common in patients 40-60 years old, and the median age of our study participants was 52.96 years old, which is consistent with literature reports[18].
The pathological changes and clinical symptoms of NETs are not specific, and therefore they are frequently misdiagnosed. However, despite the heterogeneity of NECs, advanced tumors are often accompanied by metastasis and high mortality[12,19,20].
In our study, there was a significant difference in prognosis in patients with NETs and NECs. Pathological morphological observation and immunohistochemical staining are the most accurate methods to identify RNETs. The typical morphological characteristics of G1 and G2 NETs in the gastrointestinal tract include adenoid, trabecular, and papillary structures, and the tumor cells are small and regular. Eosinophilic granules can be seen in the cytoplasm. Most tumor cell nuclei are round and of similar size, and mitotic events are rare. Rectal adenocarcinoma and RNETs are not always easy to distinguish, and accurate diagnosis depends on the detection of tumor markers, such as CgA and Syn, by IHC. In our study, 89.4% and 39.4% of tumors were positive for Syn and CgA, respectively.
Surgical resection is the main treatment option for RNETs, although the postoperative long-term prognosis is different between different grades and stages. The European Society for Medical Oncology guidelines state that surgery is the first treatment choice for gastrointestinal NETs[21]. Five-year survival rates of patients with RNETs can reach 80%-100%[19,22,23]. In our study, the 5-year survival rate was 96.2%, which is consistent with previous studies. The pathological grade of RNETs was based on the cell proliferation index (Ki-67 IHC staining) and the number of mitotic events per 10 HPFs, and the stage was based on infiltrative depth and tumor size. We did not observe any significant differences in survival between patients with stage I disease receiving local therapy and those who underwent radical surgery. Therefore, for patients with stage I RNETs, we recommend less invasive local surgery for treatment. For patients with stage II RNETs, due to the invasive depth of the tumor, there is a potential risk of lymph node metastasis. Therefore, we suggest curative resection. For patients with stages III and IV disease, due to the existence of obvious lymph node metastasis and distant organ metastasis, radical surgery might be the best choice.
Early NETs could obtain good benefit through endoscopic resection[24]. Another study performed in Chinese patients found that endoscopic submucosal resection and surgical treatment achieved satisfactory results and good prognosis in patients with RNETs. However, other reports indicate that mucosal stripping has a higher rate of complete resection than mucosal resection in early RNETs. The selection of operation mainly depends on the size of the tumor. Endoscopic resection can be performed in mucosal or submucosal layer NETs when the maximum diameter is ≤ 1 cm, and transanal resection, low rectal anterior resection, and mesorectal excision should be performed in patients with metastasis to the broad base or myometrial invasion or in patients with lymph node metastasis when the maximum tumor diameter is > 2 cm[25].
The prognosis of NETs is associated with many factors. The single factor analysis in our study showed that the prognosis of RNETs was correlated with tumor size, tumor stage, lymph node and vascular metastasis, and the degree of radical resection. A multivariate analysis showed that tumor size was an independent prognostic factor for RNETs. Zhang et al[26] found that the prognosis of colorectal NETs was closely related to WHO classification. They indicated that metastasis and the overall survival rate were statistically different in differently graded groups. Shields’s study found that tumor size was an independent risk factor affecting lymph node metastasis of colorectal NETs and was closely related to the prognosis of colorectal NETs. Another Chinese study reached a similar conclusion, but it also concluded that the focal depth of invasion and lymphatic invasion were important prognostic factors for colorectal NETs. Consistent with their conclusion, for the 12 patients who received endoscopic partial resection with positive basement in our study, metastasis occurred in 3 patients, and 2 patients died from the disease.
In conclusion, different grades and stages of RNETs have obviously different prognoses. The main treatment option is surgical excision. Determining the proper surgical methods is based on the size of the primary tumor. Endoscopic therapy can be used at early stages, and the operative method for late stage RNETs is similar to other malignant tumors. There are many factors influencing the long-term prognosis of RNETs. Early detection and radical surgery are still the best choices for the treatment of RNETs.
Epidemiology statistics show that an estimated 8000 people every year in the United States are diagnosed with NETs occurring in the gastrointestinal tract, including the stomach, intestine, appendix, colon, and rectum. The pathological changes and clinical symptoms of NETs are not specific, and therefore they are frequently misdiagnosed.
The aim of this study was to investigate the clinical symptoms, pathological characteristics, treatment, and prognosis of rectal NETs (RNETs).
To analyze the clinical and pathological data of 132 RNET cases at our hospital.
All RNETs were graded according to Ki-67 positivity and mitotic events. The tumors were staged as clinical stages I, II, III, and IV according to infiltrative depth and tumor size. COX proportional hazard model was used to assess the main risk factors for survival.
These 132 RNETs included 83 cases of G1, 21 cases of G2, and 28 cases of G3 (neuroendocrine carcinoma) disease. Immunohistochemical staining showed that 89.4% of RNETs were positive for synaptophysin and 39.4% positive for chromogranin A. There were 19, 85, 23, and 5 cases of clinical stages I, II, III, and IV, respectively. The median patient age was 52.96 years. The diameter of tumor, depth of invasion, and pathological grade were the main reference factors for the treatment of RNETs. The survival rates at 6, 12, 36, and 60 mo after operation were 98.5%, 94.6%, 90.2%, and 85.6%, respectively. Gender, tumor size, tumor grade, lymph node or distant organ metastasis, and radical resection were the main factors associated with prognosis of RNETs. Multivariate analysis showed that tumor size and grade were independent prognostic factors.
Different grades and stages of RNETs have obviously different prognoses. The main treatment option is surgical excision. Determining the proper surgical methods is based on the size of the primary tumor. Early detection and radical surgery are still the best choices for the treatment of RNETs. Gender, tumor size, tumor grade, lymph node or distant organ metastasis, and radical resection of RNETs are the main indices to evaluate prognosis.
| 1. | Salyers WJ, Vega KJ, Munoz JC, Trotman BW, Tanev SS. Neuroendocrine tumors of the gastrointestinal tract: Case reports and literature review. World J Gastrointest Oncol. 2014;6:301-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 2. | Maschmeyer G, Mügge LO, Kämpfe D, Kreibich U, Wilhelm S, Aßmann M, Schwarz M, Kahl C, Köhler S, Grobe N, Niederwieser D. A retrospective review of diagnosis and treatment modalities of neuroendocrine tumors (excluding primary lung cancer) in 10 oncological institutions of the East German Study Group of Hematology and Oncology (OSHO), 2010-2012. J Cancer Res Clin Oncol. 2015;141:1639-1644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Li ZS, Li Q. [The latest 2010 WHO classification of tumors of digestive system]. Zhonghua Bing Li Xue Za Zhi. 2011;40:351-354. [PubMed] |
| 4. | Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1877] [Article Influence: 81.6] [Reference Citation Analysis (1)] |
| 5. | Landry CS, Brock G, Scoggins CR, McMasters KM, Martin RC 2nd. A proposed staging system for rectal carcinoid tumors based on an analysis of 4701 patients. Surgery. 2008;144:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Oshitani N, Hamasaki N, Sawa Y, Hara J, Nakamura S, Matsumoto T, Kitano A, Arakawa T. Endoscopic resection of small rectal carcinoid tumours using an aspiration method with a transparent overcap. J Int Med Res. 2000;28:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Gustafsson BI, Kidd M, Modlin IM. Neuroendocrine tumors of the diffuse neuroendocrine system. Curr Opin Oncol. 2008;20:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Fendrich V, Bartsch DK. Surgical treatment of gastrointestinal neuroendocrine tumors. Langenbecks Arch Surg. 2011;396:299-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Rindi G, Klöppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, Erikssson B, Falchetti A, Falconi M, Komminoth P, Körner M, Lopes JM, McNicol AM, Nilsson O, Perren A, Scarpa A, Scoazec JY, Wiedenmann B; all other Frascati Consensus Conference participants; European Neuroendocrine Tumor Society (ENETS). TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449:395-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1196] [Cited by in RCA: 1095] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 10. | Shen C, Yin Y, Chen H, Tang S, Yin X, Zhou Z, Zhang B, Chen Z. Neuroendocrine tumors of colon and rectum: validation of clinical and prognostic values of the World Health Organization 2010 grading classifications and European Neuroendocrine Tumor Society staging systems. Oncotarget. 2017;8:22123-22134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Ikeda K, Kojima M, Saito N, Sakuyama N, Koushi K, Watanabe T, Sugihara K, Akimoto T, Ito M, Ochiai A. Current status of the histopathological assessment, diagnosis, and reporting of colorectal neuroendocrine tumors: A Web survey from the Japanese Society for Cancer of Colon and Rectum. Pathol Int. 2016;66:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Aytac E, Ozdemir Y, Ozuner G. Long term outcomes of neuroendocrine carcinomas (high-grade neuroendocrine tumors) of the colon, rectum, and anal canal. J Visc Surg. 2014;151:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Chagpar R, Chiang YJ, Xing Y, Cormier JN, Feig BW, Rashid A, Chang GJ, You YN. Neuroendocrine tumors of the colon and rectum: prognostic relevance and comparative performance of current staging systems. Ann Surg Oncol. 2013;20:1170-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Shafqat H, Ali S, Salhab M, Olszewski AJ. Survival of patients with neuroendocrine carcinoma of the colon and rectum: a population-based analysis. Dis Colon Rectum. 2015;58:294-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 15. | Chen CC, Lai YL, Jiang JK, Chu CH, Huang IP, Chen WS, Cheng AY, Yang SH. The evolving practice of hybrid natural orifice transluminal endoscopic surgery (NOTES) for rectal cancer. Surg Endosc. 2015;29:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Hu HK, Ke NW, Li A, Du XJ, Guo Q, Hu WM. Clinical characteristics and prognostic factors of gastroenteropancreatic neuroendocrine tumors: a single center experience in China. Hepatogastroenterology. 2015;62:178-183. [PubMed] |
| 17. | Wang AY, Ahmad NA. Rectal carcinoids. Curr Opin Gastroenterol. 2006;22:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Malik L, Chua YJ, Butt NS, Yip D. Single institutional series of neuroendocrine tumors managed in the Australian Capital Territory. Asia Pac J Clin Oncol. 2016;12:e133-e140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Magni E, Botteri E, Ravenda PS, Cassatella MC, Bertani E, Chiappa A, Luca F, Zorzino L, Bianchi PP, Adamoli L, Sandri MT, Zampino MG. Detection of circulating tumor cells in patients with locally advanced rectal cancer undergoing neoadjuvant therapy followed by curative surgery. Int J Colorectal Dis. 2014;29:1053-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Norton JA, Kivlen M, Li M, Schneider D, Chuter T, Jensen RT. Morbidity and mortality of aggressive resection in patients with advanced neuroendocrine tumors. Arch Surg. 2003;138:859-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 152] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Oberg K, Akerström G, Rindi G, Jelic S; ESMO Guidelines Working Group. Neuroendocrine gastroenteropancreatic tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v223-v227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Cusati D, Zhang L, Harmsen WS, Hu A, Farnell MB, Nagorney DM, Donohue JH, Que FG, Reid-Lombardo KM, Kendrick ML. Metastatic nonfunctioning pancreatic neuroendocrine carcinoma to liver: surgical treatment and outcomes. J Am Coll Surg. 2012;215:117-24; discussion 124-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Bergestuen DS, Aabakken L, Holm K, Vatn M, Thiis-Evensen E. Small intestinal neuroendocrine tumors: prognostic factors and survival. Scand J Gastroenterol. 2009;44:1084-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Jung HJ, Hong SJ, Han JP, Kim HS, Jeong GA, Cho GS, Kim HK, Ko BM, Lee MS. Long-term outcome of endoscopic and surgical resection for foregut neuroendocrine tumors. J Dig Dis. 2015;16:595-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Anthony LB, Strosberg JR, Klimstra DS, Maples WJ, O'Dorisio TM, Warner RR, Wiseman GA, Benson AB, Pommier RF; North American Neuroendocrine Tumor Society (NANETS). The NANETS consensus guidelines for the diagnosis and management of gastrointestinal neuroendocrine tumors (nets): well-differentiated nets of the distal colon and rectum. Pancreas. 2010;39:767-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 216] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 26. | Zhang XH, Lu XL, Wu N, Liu B, Wang FY, Zhang RS, Zhou XJ. [Clinicopathological features of colorectal neuroendocrine neoplasms and prognostic significance of WHO staging system]. Zhonghua Bing Li Xue Za Zhi. 2013;42:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Dhaliwal A, Hosseini M, Yeo SG S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Wang LL