Published online Jul 15, 2020. doi: 10.4251/wjgo.v12.i7.719
Peer-review started: March 25, 2020
First decision: April 26, 2020
Revised: May 3, 2020
Accepted: May 27, 2020
Article in press: May 27, 2020
Published online: July 15, 2020
Processing time: 111 Days and 22.8 Hours
Overexpression of SQSTM1 (sequestosome 1, P62) and nuclear factor-κB (NF-κB) plays an important role in the invasion and metastasis of a variety of malignant tumors.
To explore the expression of P62 and NF-κB in pancreatic cancer and their relationship with clinicopathological features.
The expression levels of P62 and NF-κB were analyzed by immunohistochemistry with a tissue chip containing 40 cases of human pancreatic carcinoma. Then we analyzed the correlation among P62 expression, phospho-P65 expression, and clinicopathological features of pancreatic carcinoma samples.
P62 expression was mainly observed in the cytoplasm of pancreatic carcinoma cells. Phosphorylated P65 (phospho-P65) was mainly expressed in the nucleus and cytoplasm of pancreatic carcinoma cells. There was a significant difference in P62 expression among T stages. And a significant difference in phosphor-P65 expression among pathology types was noted. In the cases with strongly positive P62 expression, significant differences were found in age. And there were significant differences in T stage and tumor-node-metastasis stage in the cases with strongly positive phosphor-P65 expression.
In pancreatic carcinoma, P62 expression is significantly correlated with T stage. It may be a valuable malignant indicator for human pancreatic carcinoma.
Core Tip: SQSTM1 (sequestosome 1, P62) and nuclear factor-κB play an important role in the invasion and metastasis of a variety of malignant tumors. We discovered that P62 expression can be used as a valuable indicator for the malignancy of human pancreatic carcinoma.
- Citation: Zhang ZY, Guo S, Zhao R, Ji ZP, Zhuang ZN. Clinical significance of SQSTM1/P62 and nuclear factor-κB expression in pancreatic carcinoma. World J Gastrointest Oncol 2020; 12(7): 719-731
- URL: https://www.wjgnet.com/1948-5204/full/v12/i7/719.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i7.719
Pancreatic cancer is one of the most common types of malignant digestive system cancer[1], and it has the fourth highest death rate of among all the deaths caused by cancer in the United States in the last decade[2]. Almost 90% of cases of pancreatic cancer can be detected with the mutation activation of the KRAS gene[3]. Ras mutated tumors have been shown to express a highly active nuclear factor-κB (NF-κB) signaling pathway[4]. And a previous study has shown that NF-κB activation plays an important role in the development of pancreatic cancer[4].
SQSTM1 (sequestosome 1, P62), a ubiquitin binding protein encoded by sqstm1, is involved in protein degradation in the ubiquitin proteasome system and autophagy lysosome system[5], and it is also one component of protein complexes in cells. Previous research has shown that P62 participates in the formation of cellular polyubiquitinated protein aggregates[6]. P62 has two sides in biological function. After the p62 gene was knocked out, the mice showed metabolic syndrome, including obesity and fat accumulation in the liver, decreased glucose tolerance and insulin resistance, and elevated plasma triglyceride and cholesterol[7]. A study showed that under normal physiological conditions, ERK1 signal was inhibited by P62, which prevented the over-proliferation and differentiation of adipocytes[8]. However, overexpression of P62 was found in small cell lung cancer, breast cancer, liver cancer, and other malignant tumor cells[9]. As a scaffold and adaptor protein in signal transduction pathways, P62 participates in the regulation of multiple signal transduction pathways, including the Ras/Raf/mitogen-activated protein kinase and NF-κB pathways, which can enhance the proliferation, migration, and invasion of tumor cells. The over-accumulation of P62 and the imbalance of NF-κB signal make mice more likely to produce tumors[10]. It has been confirmed that KRAS mutation can induce the overexpression of P62, which promotes the growth of pancreatic ductal adenocarcinoma[4].
It has been found that[11,12] NF-κB in pancreatic cancer with KRAS gene mutation is in a persistent activation state, which is closely related to the high expression of P62[13]. The elevation of AP-1 caused by P62 can lead to the expression of NF-κB (phosphor-P65) in pancreatic cancer cells. Our study has shown that the inhibition of P62 expression can effectively reduce the activity of NF-κB, and the rate of tumor growth in KRAS and p53 mutant mouse models[14]. P62 expression was also found decreased when NF-κB activity was inhibited. It is thus proved that there is a circular relationship between P62 and NF-κB, which leads to persistent activation of the NF-κB pathway[4].
This study aimed to explore the expression of P62 and NF-κB in pancreatic cancer and their relationship between with clinicopathological features, which will provide a theoretical basis for the prevention of pancreatic cancer and postponement of its development. Their correlations with clinicopathological feature were evaluated to determine whether P62 expression levels could be used to predict the prognosis in patients with pancreatic cancer.
We used a human tissue chip including 49 samples with information on age, sex, TNM stage, clinical stage, and pathological grade, purchased from Xi'an Besta Biotechnology Co., China. The samples were resected and pathologically confirmed, with 40 samples of pancreatic carcinoma tissues, 8 samples of normal pancreatic tissues as a negative control, and 1 sample of pheochromocytoma of the adrenal gland as a positive control.
Of the pancreatic carcinoma cases, 24 were male and 16 were female, whose age range was from 31 to 78 years, with a median age of 56.3 ± 11.7 years. The samples were divided into subgroups according to age (< 60 years and ≥ 60 years), pathological type (duct adenocarcinoma, adenocarcinoma, squamous cell carcinoma, and acinic cell carcinoma), T stage (T0, T1, T2, T3, and T4), N stage (N0, N1, N2, and N3), M stage (M0 and M1), and TNM classification (I, II, III, and IV). Hematoxylin-eosin-stained slides of all surgical specimens and corresponding adequate paraffin-embedded tissue sections were retrieved from the department’s archive. The Ethics Committee of Qilu Hospital, Shandong University, approved the study. The pathological tumor-node-metastasis (pTNM) classification was based on the TNM classification of the Union for International Cancer Control (2009).
Typical tumor tissue chips were present in all selected blocks. Informed consent for the use of the specimens was obtained from all patients. Phospho-P65 antibody (1:100, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and P62 (1:200, Wuhan Boshide, Inc., China) were used for phospho-P65 and P62 immunohistochemical staining, respectively.
The target protein was detected by the Elivison two-step method of immuno-histochemistry. All the tissues were fixed with 10% formalin, paraffin-embedded, cut into 4 μm serial sections, and baked for 60 min at 60°C. Then, the slices of tissues were dewaxed with xylene, absolute ethanol, 95% ethanol in water, 85% ethanol in water, and 70% ethanol in water. The cells were washed in PBS three times. A high-temperature plastic staining tray was filled with a beaker of antigen retrieval buffer for antigen retrieval. The enzyme was inactivated and the antigen was added to the reaction. Goat serum (50-100 µL) was added to the blocked tissues. For the primary antibody and secondary antibody reaction, 50 µL of universal IgG antibody-Fab-HRP polymer was added and incubated for 30 min. The tissues were washed in PBS three times. The glass slides were then stained, dehydrated, and sealed. After staining, the tumor tissue was examined in a standard manner using a light microscope (Olympus Corp, Tokyo, Japan).
Phospho-P65 was mainly expressed in the nucleus and cytoplasm of pancreatic carcinoma cells. P62 expression was mainly observed in the cytoplasm of pancreatic carcinoma cells. We assessed phospho-P65 and P62 staining. In brief, the scoring was as follows: (1) 0, < 10% of cells stained; (2) +, 10% to 25% of cells stained; (3) ++, 25% to 50% of cells stained; and (4) +++, > 50% of cells stained. For all cases, slides with a score ≥ ++ were considered positive.
All the data of the pancreatic carcinoma tissues were statistically analyzed using SPSS 22.0 software. The χ2 test was used to examine the relationships between the expression levels of phosphor-P65 and P62 and the clinicopathological features of the pancreatic carcinoma samples. Spearman rank correlation was applied to evaluate the relationship between phosphor-P65 and P62 expression levels. A two-sided P value of 0.05 was considered statistically significant.
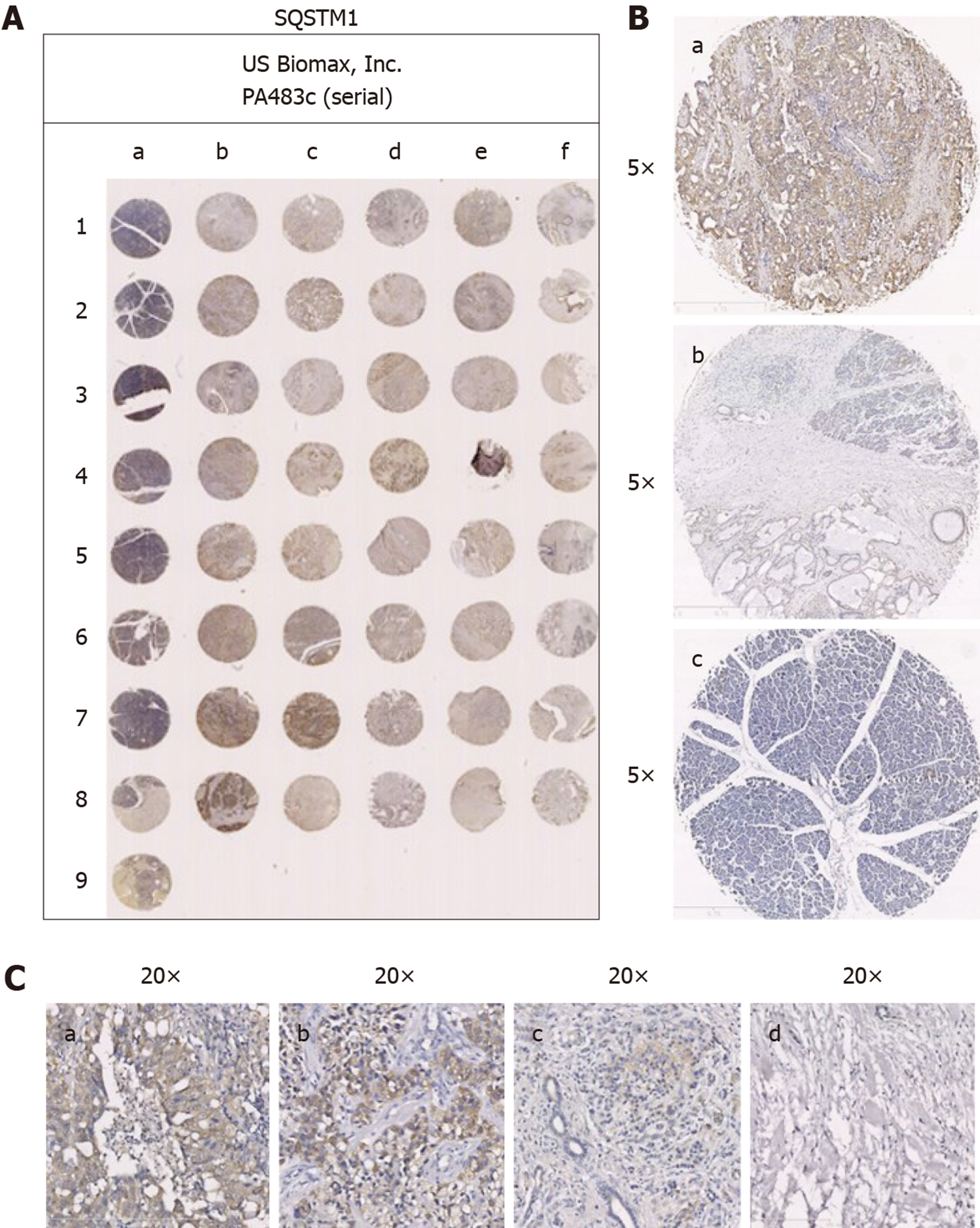
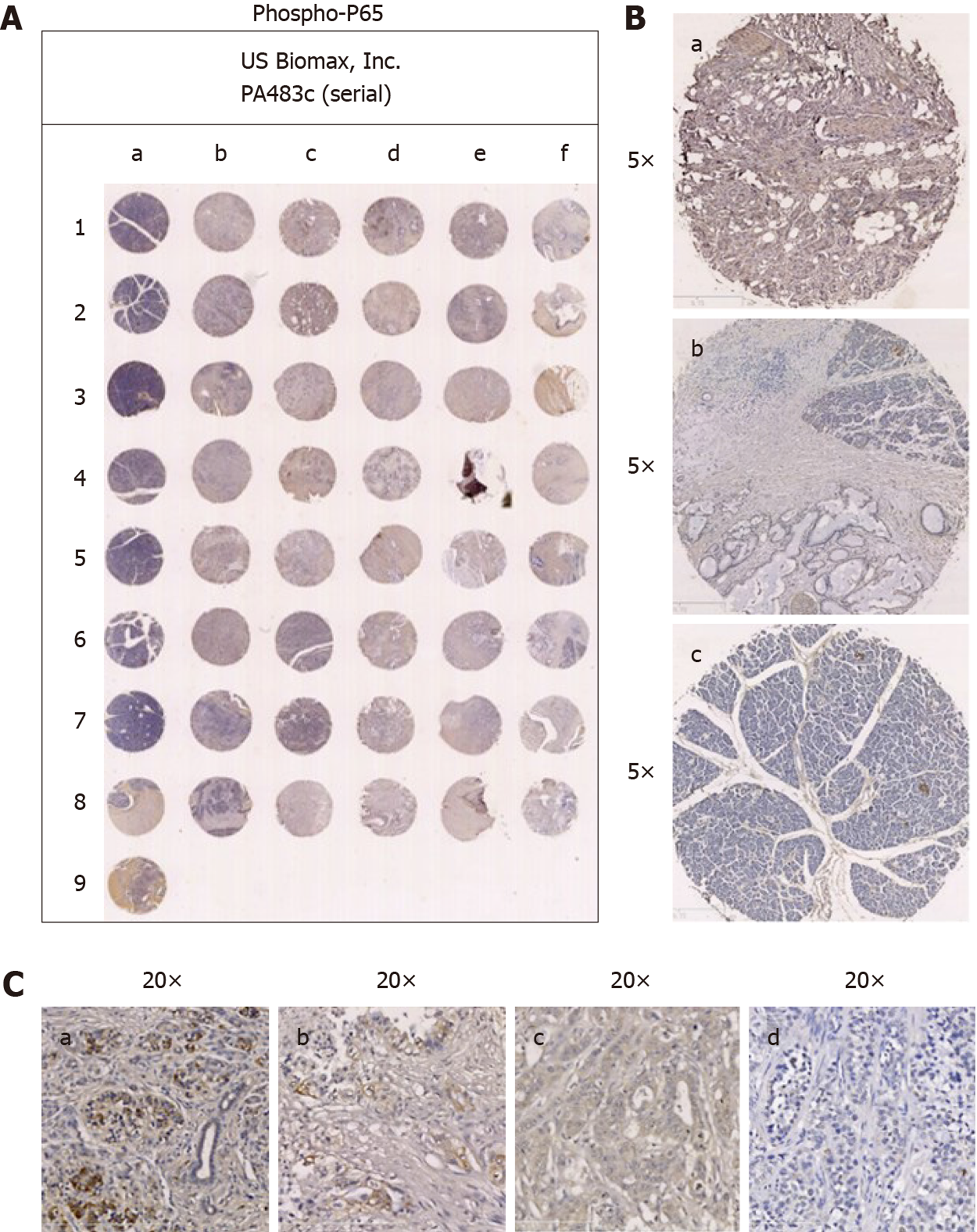
The tissue chips were analyzed by immunohistochemistry. P62 was mainly expressed in the cytoplasm of pancreatic carcinoma cells (Figure 1A), and phospho-P65 was mainly expressed in the nucleus and cytoplasm of pancreatic carcinoma cells (Figure 2A).
In the 40 pancreatic carcinoma specimens, significant positive P62 expression was seen in 22 cases (Figure 1A; 55%), and significant positive phospho-P65 expression was seen in 16 (Figure 2A; 40%). In the 29 duct adenocarcinoma specimens (72.5%), positive expression of phospho-P65 was seen in 27 cases (93.1%), and positive expression of P62 was seen in 26 (89.7%). In the 9 adenocarcinoma specimens (22.5%), positive phospho-P65 expression was seen in 8 cases (88.9%), and positive P62 expression was seen in 9 (100%). In the 1 squamous cell carcinoma specimen (2.5%), positive phospho-P65 expression was not seen, and positive P62 expression was seen. In the 1 acinic cell carcinoma specimen, positive P62 expression was not seen, and positive P62 expression was seen. In pancreatic carcinomas, P62 expression was mainly in pancreatic carcinoma cells compared with the paraneoplastic edge (Figure 1B, b), while normal tissues were negative for P62 expression (Figure 1B, c). Phospho-P65 expression was more obvious in pancreatic carcinoma cells than in the paraneoplastic edge (Figure 2B, b) and was negative in normal tissues (Figure 2B, c).
In the 40 pancreatic carcinoma specimens, we analyzed the relationship between phosphor-P65 or P62 expression and the clinical characteristics of the pancreatic carcinoma patients. A significant difference in phosphor-P65 expression among pathology types (P = 0.002) was found, while there were no statistically significant differences for sex, age, T stage, N stage, M stage, or TNM stage. There was a significant difference in P62 expression among T stages (P = 0.001), while there were no statistically significant differences for sex, age, pathological type, N stage, M stage, or TNM stage. Table 1 shows the details of the results.
| Clinicopathological feature | n | Phosph-P65 | P value1 | P62 | P value1 | ||||
| Positive | Negative | Positive | Negative | ||||||
| Gender | 40 | 35 | 5 | 1.000 | 37 | 3 | 0.141 | ||
| Male | 24 | 21 | 3 | 21 | 3 | ||||
| Female | 16 | 14 | 2 | 16 | 0 | ||||
| Age (yr) | 0.329 | 0.806 | |||||||
| < 60 | 24 | 22 | 2 | 22 | 2 | ||||
| ≥ 60 | 16 | 13 | 3 | 15 | 1 | ||||
| Pathology type | 0.002 | 0.746 | |||||||
| Duct adenocarcinoma | 29 | 27 | 2 | 26 | 3 | ||||
| Adenocarcinoma | 9 | 8 | 1 | 9 | 0 | ||||
| Squamous cell carcinoma | 1 | 0 | 1 | 1 | 0 | ||||
| Acinic cell carcinoma | 1 | 0 | 1 | 1 | 0 | ||||
| T stage | 0.885 | 0.001 | |||||||
| T0 | 0 | 0 | 0 | 0 | 0 | ||||
| T1 | 1 | 1 | 0 | 0 | 1 | ||||
| T2 | 10 | 9 | 1 | 10 | 0 | ||||
| T3 | 29 | 25 | 4 | 27 | 2 | ||||
| T4 | 0 | 0 | 0 | 0 | 0 | ||||
| N stage | 0.496 | 0.608 | |||||||
| N0 | 37 | 32 | 5 | 34 | 3 | ||||
| N1 | 3 | 3 | 0 | 3 | 0 | ||||
| N2 | 0 | 0 | 0 | 0 | 0 | ||||
| N3 | 0 | 0 | 0 | 0 | 0 | ||||
| M stage | 0.702 | 0.773 | |||||||
| M0 | 39 | 34 | 5 | 36 | 3 | ||||
| M1 | 1 | 1 | 0 | 1 | 0 | ||||
| TNM stage | 0.934 | 0.939 | |||||||
| I | 11 | 10 | 1 | 10 | 1 | ||||
| II | 28 | 25 | 3 | 26 | 2 | ||||
| III | 0 | 0 | 0 | 0 | 0 | ||||
| IV | 1 | 1 | 0 | 1 | 0 | ||||
We next analyzed the relationships between the positive expression of phospho-P65 and clinicopathological features in pancreatic carcinoma (n = 35). A significant difference of strong positive phosphor-P65 expression among T stages (P = 0.027) and TNM stages (P = 0.006) were found, while there were no statistically significant differences for sex, age, pathological type, N stage, or M stage. Table 2 shows the details of the results. We also analyzed the relationship between the positive expression of P62 and clinicopathological features of pancreatic carcinoma (n = 37). A significant difference in strong positive P62 expression was found for age (P = 0.036), while no significant differences were found for sex, pathological type, T stage N stage, M stage, and TNM stage. Table 3 shows the details of the results.
| Clinicopathological feature | n | Phosph-P65 | P value1 | |
| +++ | ++ | |||
| Gender | 35 | 16 | 19 | 0.678 |
| Male | 21 | 9 | 12 | |
| Female | 14 | 7 | 7 | |
| Age (yr) | 0.508 | |||
| < 60 | 22 | 11 | 11 | |
| ≥ 60 | 13 | 5 | 8 | |
| Pathology type | 0.181 | |||
| Duct adenocarcinoma | 27 | 14 | 13 | |
| Adenocarcinoma | 8 | 2 | 6 | |
| T stage | 0.027 | |||
| T0 | 0 | 0 | 0 | |
| T1 | 1 | 0 | 1 | |
| T2 | 9 | 1 | 8 | |
| T3 | 25 | 15 | 10 | |
| T4 | 0 | 0 | 0 | |
| N stage | 0.446 | |||
| N0 | 32 | 14 | 18 | |
| N1 | 3 | 2 | 1 | |
| N2 | 0 | 0 | 0 | |
| N3 | 0 | 0 | 0 | |
| M stage | 0.352 | |||
| M0 | 34 | 16 | 18 | |
| M1 | 1 | 0 | 1 | |
| TNM stage | 0.006 | |||
| I | 11 | 1 | 10 | |
| II | 23 | 15 | 8 | |
| III | 0 | 0 | 0 | |
| IV | 1 | 0 | 1 | |
| Clinicopathologic feature | n | P62 | P value1 | |
| +++ | ++ | |||
| Gender | 37 | 22 | 15 | 0.093 |
| Male | 21 | 10 | 11 | |
| Female | 16 | 12 | 4 | |
| Age (yr) | 0.036 | |||
| < 60 | 22 | 10 | 12 | |
| ≥ 60 | 15 | 12 | 3 | |
| Pathology type | 0.310 | |||
| Duct adenocarcinoma | 26 | 13 | 13 | |
| Adenocarcinoma | 9 | 7 | 2 | |
| Squamous cell carcinoma | 1 | 1 | 0 | |
| Acinic cell carcinoma | 1 | 1 | 0 | |
| T stage | 0.967 | |||
| T0 | 0 | 0 | 0 | |
| T1 | 0 | 0 | 0 | |
| T2 | 10 | 6 | 4 | |
| T3 | 27 | 16 | 11 | |
| T4 | 0 | 0 | 0 | |
| N stage | 0.791 | |||
| N0 | 34 | 20 | 14 | |
| N1 | 3 | 2 | 1 | |
| N2 | 0 | 0 | 0 | |
| N3 | 0 | 0 | 0 | |
| M stage | 0.220 | |||
| M0 | 36 | 22 | 14 | |
| M1 | 1 | 0 | 1 | |
| TNM stage | 0.469 | |||
| I | 10 | 6 | 4 | |
| II | 26 | 16 | 10 | |
| III | 0 | 0 | 0 | |
| IV | 1 | 0 | 1 | |
Next, we evaluated the relationship between phospho-P65 and P62 expression in pancreatic carcinoma. In the 40 samples, the positive rate of both phospho-P65 and P62 was 82.5% in pancreatic carcinoma. The negative rate of both phospho-P65 and P62 was 2.5% in pancreatic carcinoma. A total of 94.3% of phospho-P65-positive tissues showed positive P62 expression in pancreatic carcinoma. In addition, 89.2% of P62-positive tissues showed positive phospho-P65 expression in pancreatic carcinoma. The bivariate correlation between phospho-P65 and P62 analyzed by Spearman’s correlation was negative (r = 0.237, P = 0.069; Table 4).
| P62 | Phospho-P65 | Total | |
| - | + | ||
| - | 1 | 2 | 3 |
| + | 4 | 33 | 37 |
| Total | 5 | 35 | 40 |
According to the significant differences in phosphor-P65 expression for the pathological type of pancreatic carcinoma specimens, we continued to analyze the relations between the pathological type and other clinicopathological features (Table 5). In the 35 cases of phosphor-P65 positive pancreatic carcinoma specimens, pathological type had no statistically significant difference. The levels of phosphor-P65 expression in the cases diagnosed as the pathological type of duct adenocarcinoma or adenocarcinoma were stable in the patients with different clinical characteristics of sex, age, or T stage. Then, we analyzed the relations of P62 with T stage and other clinicopathological features. The P62 expression levels in cases in T1-T3 stage were stable in the patients with different clinical characteristics of sex, age, or pathological type (Table 6).
Pancreatic cancer is a kind of digestive system carcinoma with high malignancy[15]. Most of these patients are diagnosed in the late stage because of the painless and hidden growth of pancreatic masses, which leads to the low rate of surgical resection and the suboptimal effect of chemotherapy. The prognosis of these patients is poor, with a rising mortality rate and a less than 8% 5-year survival rate. According to the data published by the American Cancer Association in 2018, pancreatic cancer ranked fourth in cancer-related deaths in the United States[16].
The activity of the NF-kB pathway could play a role in the development of Kras-mutant pancreatic carcinoma, according to a previous study[17]. In this study, NF-kB was mainly expressed in the nucleus and cytoplasm of pancreatic carcinoma cells. A significantly different border was found between pancreatic carcinoma tissues and paraneoplastic tissues. In pancreatic carcinoma specimens, 16 specimens were seen to have strong positive expression of phosphor-P65 (≥ 50% of positive cells). The mutant Ras induces P62 expression through AP-1 to activate IKK2/b and NF-kB[18]. A previous study showed that the expression of P62 was induced by NF-kB activation during IL-1a stimulation of mouse and human PDAC cell lines[4]. This suggests the existence of an autoregulatory loop whereby NF-kB regulates P62 expression.
P62, as a scaffold and aptamer protein in signal transduction pathways, participates in the regulation of multiple signal transduction pathways, including the Ras/Raf/mitogen-activated protein kinase and NF-κB pathways[19]. P62 plays a key role in the regulation of mTOR activity and autophagy and can enhance the proliferation, migration, and invasion of tumor cells[20]. A previous study found that the expression of P62 in small cell lung cancer, breast cancer, liver cancer, and other kinds of malignant tumor cells is much higher than that in normal cells[21]. Another study showed that NF-κB signaling can be inhibited by knocking out the p62 gene to prevent the growth of lung cancer induced by reactive oxygen species[18], while the activation of KRAS can promote the growth of pancreatic ductal adenocarcinoma[4]. Silencing the p62 gene in lung adenocarcinoma cells with p62 overexpression induces the formation of autophagy with multiple layers, leading to tumor cell death[9]. The overexpression of P62 can enhance the resistance of tumor cells to oxidative damage and anticancer drugs[22]. Mutations in P62 can reduce the interaction between Keap1 and Nrf2 and have been detected in lung cancer, head and neck cancer, and gallbladder cancer[23]. Nrf2 activation is also involved in tumorigenesis and tumor growth[24]. In this study, P62 was observed to be positively expressed in different pathological types of pancreatic carcinoma, including duct adenocarcinoma, adenocarcinoma, squamous cell carcinoma, and acinic cell carcinoma. In the 40 pancreatic carcinoma specimens, 37 had positive P62 staining, while 35 had positive phosphor-P65 staining.
To investigate the clinical significance and relationship between P62 expression and clinicopathological features of human pancreatic carcinoma, we analyzed the immunohistochemistry data and clinicopathological features of 40 pancreatic carcinoma specimens. Positive P62 expression was more abundant in pancreatic carcinoma specimens with T2-T3 stage than in those with other stages, and this difference was significant. Furthermore, the relationship between the positive expression of P62 and clinicopathological features in pancreatic carcinoma was analyzed. Patients aged more than 60 years were found to have more strongly positive P62 expression than those aged less than 60 years, and there was a significant difference.
Overexpression of P62 was observed in mouse and human pancreatic ductal adenocarcinoma cell lines according to the autoregulatory loop between NF-kB and P62[21]. This feedback relationship was also detected in the specimens of pancreatic carcinoma patients. In duct adenocarcinoma specimens, 89.7% (26/29) had positive expression of P62, while 93.1% (27/29) had positive expression of phospho-P65. In adenocarcinoma specimens, the positive rate was 100% for P62 and 88.9% (8/9) for phospho-P65. In 1 case of squamous cell carcinoma and 1 case of acinic cell carcinoma, P62 expression was positive, but phospho-P65 expression was negative. We also evaluated the relationship between phospho-P65 and P62 expression in pancreatic carcinoma. In the 40 samples, the positive rate of both phospho-P65 and P62 was 82.5% in pancreatic carcinoma. A total of 94.3% of phospho-P65-positive tissues showed positive P62 expression in pancreatic carcinoma. In addition, 89.2% of P62-positive tissues showed positive phospho-P65 expression in pancreatic carcinoma. We used Spearman analysis to evaluate the relationship between NF-kB and P62 expression levels in pancreatic carcinoma patients, and the bivariate correlation between them was a negative relationship (P = 0.069). This result suggested that there was an autoregulatory loop between NF-kB and p62 and no expression differences between P62 and phosphor-P65. P62 protein might be a useful marker to confirm the diagnosis of pancreatic carcinoma.
We continued to analyze the clinical features associated with positive phospho-P65 or P62 expression. In the 35 cases of phosphor-P65 positive expression, there was no significant difference in pathological type. The levels of phosphor-P65 expression in the cases diagnosed as the pathological type of duct adenocarcinoma or adenocarcinoma were stable in the patients with different clinical characteristics of sex, age, and T stage. We also analyzed the relations of P62 with T stage and other clinicopathological features. The levels of P62 expression in cases in T1-T3 stage were stable in patients with different clinical characteristics of sex, age, and pathological type. As the number of samples with pancreatic carcinoma might limit the predictability of this correlation for prognostic analysis, we will continue to collect the samples for this research.
In summary, our study indicates that P62 expression can be used as a valuable malignant indicator for human pancreatic carcinoma. Therefore, NF-kB and P62 may be new targets for future carcinoma therapy.
Almost 90% cases of pancreatic cancer can be detected with the mutation activation of KRAS gene. Ras mutated tumors have been shown to express a highly active nuclear factor-κB (NF-κB) signaling pathway. NF-κB in pancreatic cancer with KRAS gene mutation was in a persistent activation state, which was closely related to the high expression of P62.
To detect the expression level of P62 in pancreatic cancer and find the relationship among P62, P65, and clinicopathological features, which will provide a theoretical basis for the prevention of pancreatic cancer and postponement of its development.
This study aimed to investigate the clinical characteristics of patients with pancreatic carcinomas and evaluate the relationship among P62, P65, and clinicopathological features.
Typical tumor tissue chips were used to detect the expression of phospho-P65 and P62 by immunohistochemical staining. SPSS 22.0 software was used to analyze the relationship between the expression of phosphor-P65 and P62 and the clinico-pathological features of pancreatic carcinoma samples.
P62 was mainly expressed in the cytoplasm of pancreatic carcinoma cells, and phospho-P65 was mainly expressed in the nucleus and cytoplasm of pancreatic carcinoma cells. Aprroximately 94.3% of phospho-P65 positive tissues showed positive P62 expression in pancreatic carcinomas. And 89.2% of P62 positive tissues showed positive phospho-P65 expression in pancreatic carcinomas. There was a significant difference in P62 expression among different T stages of pancreatic carcinoma.
P62 could be used as a valuable malignant indicator for human pancreatic carcinomas.
The correlation between clinicopathological features and P62 expression could be used to predict the prognosis in patients with pancreatic cancer. NF-kB and P62 may be new targets for future carcinoma therapy.
| 1. | Kidiyoor A, Schettini J, Besmer DM, Rego SL, Nath S, Curry JM, Roy LD, Dréau D, Mukherjee P. Pancreatic Cancer Cells Isolated from Muc1-Null Tumors Favor the Generation of a Mature Less Suppressive MDSC Population. Front Immunol. 2014;5:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 2. | Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969-2972. [PubMed] |
| 3. | Dajee M, Lazarov M, Zhang JY, Cai T, Green CL, Russell AJ, Marinkovich MP, Tao S, Lin Q, Kubo Y, Khavari PA. NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature. 2003;421:639-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 439] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 4. | Ling J, Kang Y, Zhao R, Xia Q, Lee DF, Chang Z, Li J, Peng B, Fleming JB, Wang H, Liu J, Lemischka IR, Hung MC, Chiao PJ. KrasG12D-induced IKK2/β/NF-κB activation by IL-1α and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:105-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 429] [Article Influence: 30.6] [Reference Citation Analysis (13)] |
| 5. | Su H, Wang X. p62 Stages an interplay between the ubiquitin-proteasome system and autophagy in the heart of defense against proteotoxic stress. Trends Cardiovasc Med. 2011;21:224-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Shin J. P62 and the sequestosome, a novel mechanism for protein metabolism. Arch Pharm Res. 1998;21:629-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 114] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Bitto A, Lerner CA, Nacarelli T, Crowe E, Torres C, Sell C. P62/SQSTM1 at the interface of aging, autophagy, and disease. Age (Dordr). 2014;36:9626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 8. | Rodriguez A, Durán A, Selloum M, Champy MF, Diez-Guerra FJ, Flores JM, Serrano M, Auwerx J, Diaz-Meco MT, Moscat J. Mature-onset obesity and insulin resistance in mice deficient in the signaling adapter p62. Cell Metab. 2006;3:211-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 252] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 9. | Nihira K, Miki Y, Ono K, Suzuki T, Sasano H. An inhibition of p62/SQSTM1 caused autophagic cell death of several human carcinoma cells. Cancer Sci. 2014;105:568-575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, Dipaola RS, Karantza-Wadsworth V, White E. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062-1075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1442] [Cited by in RCA: 1439] [Article Influence: 84.6] [Reference Citation Analysis (0)] |
| 11. | Carbone C, Melisi D. NF-κB as a target for pancreatic cancer therapy. Expert Opin Ther Targets. 2012;16 Suppl 2:S1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 12. | Chang Z, Ju H, Ling J, Zhuang Z, Li Z, Wang H, Fleming JB, Freeman JW, Yu D, Huang P, Chiao PJ. Cooperativity of oncogenic K-ras and downregulated p16/INK4A in human pancreatic tumorigenesis. PLoS One. 2014;9:e101452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Melisi D, Niu J, Chang Z, Xia Q, Peng B, Ishiyama S, Evans DB, Chiao PJ. Secreted interleukin-1alpha induces a metastatic phenotype in pancreatic cancer by sustaining a constitutive activation of nuclear factor-kappaB. Mol Cancer Res. 2009;7:624-633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Niu J, Li Z, Peng B, Chiao PJ. Identification of an autoregulatory feedback pathway involving interleukin-1alpha in induction of constitutive NF-kappaB activation in pancreatic cancer cells. J Biol Chem. 2004;279:16452-16462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Strobel O, Hinz U, Gluth A, Hank T, Hackert T, Bergmann F, Werner J, Büchler MW. Pancreatic adenocarcinoma: number of positive nodes allows to distinguish several N categories. Ann Surg. 2015;261:961-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 16. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15625] [Article Influence: 2232.1] [Reference Citation Analysis (11)] |
| 17. | Melisi D, Chiao PJ. NF-kappa B as a target for cancer therapy. Expert Opin Ther Targets. 2007;11:133-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Duran A, Linares JF, Galvez AS, Wikenheiser K, Flores JM, Diaz-Meco MT, Moscat J. The signaling adaptor p62 is an important NF-kappaB mediator in tumorigenesis. Cancer Cell. 2008;13:343-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 469] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 19. | Dror R, Lederman M, Umezawa K, Barak V, Pe'er J, Chowers I. Characterizing the involvement of the nuclear factor-kappa B (NF kappa B) transcription factor in uveal melanoma. Invest Ophthalmol Vis Sci. 2010;51:1811-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Lamark T, Svenning S, Johansen T. Regulation of selective autophagy: the p62/SQSTM1 paradigm. Essays Biochem. 2017;61:609-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 564] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 21. | Arbour KC, Jordan E, Kim HR, Dienstag J, Yu HA, Sanchez-Vega F, Lito P, Berger M, Solit DB, Hellmann M, Kris MG, Rudin CM, Ni A, Arcila M, Ladanyi M, Riely GJ. Effects of Co-occurring Genomic Alterations on Outcomes in Patients with KRAS-Mutant Non-Small Cell Lung Cancer. Clin Cancer Res. 2018;24:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 380] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 22. | Padmanabhan B, Tong KI, Ohta T, Nakamura Y, Scharlock M, Ohtsuji M, Kang MI, Kobayashi A, Yokoyama S, Yamamoto M. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol Cell. 2006;21:689-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 600] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 23. | Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E, Brock MV, Biswal S. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3:e420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 860] [Cited by in RCA: 885] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 24. | Shibata T, Kokubu A, Gotoh M, Ojima H, Ohta T, Yamamoto M, Hirohashi S. Genetic alteration of Keap1 confers constitutive Nrf2 activation and resistance to chemotherapy in gallbladder cancer. Gastroenterology. 2008;135:1358-1368, 1368.e1-1368.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 399] [Article Influence: 22.2] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Campanale M, Kramer JR, Sogabe I S-Editor: Wang JL L-Editor: Wang TQ E-Editor: Liu JH