Published online Jun 15, 2020. doi: 10.4251/wjgo.v12.i6.687
Peer-review started: January 23, 2020
First decision: March 24, 2020
Revised: April 9, 2020
Accepted: April 28, 2020
Article in press: April 28, 2020
Published online: June 15, 2020
Processing time: 144 Days and 8 Hours
The optimal time interval between neoadjuvant chemoradiotherapy (nCRT) and esophagectomy in esophageal cancer has not been defined.
To evaluate whether a prolonged time interval between the end of nCRT and surgery has an effect on survival outcome in esophageal cancer patients.
We searched PubMed, Embase, Web of Science, the Cochrane Library, Wanfang and China National Knowledge Infrastructure databases for relevant articles published before November 16, 2019, to identify potential studies that evaluated the prognostic role of different time intervals between nCRT and surgery in esophageal cancer. The hazard ratios and 95% confidence intervals (95%CI) were merged to estimate the correlation between the time intervals and survival outcomes in esophageal cancer, esophageal squamous cell carcinoma and adenocarcinoma using fixed- and random-effect models.
This meta-analysis included 12621 patients from 16 studies. The results demonstrated that esophageal cancer patients with a prolonged time interval between the end of nCRT and surgery had significantly worse overall survival (OS) [hazard ratio (HR): 1.107, 95%CI: 1.014-1.208, P = 0.023] than those with a shorter time interval. Subgroup analysis showed that poor OS with a prolonged interval was observed based on both the sample size and HRs. There was also significant association between a prolonged time interval and decreased OS in Asian, but not Caucasian patients. In addition, a longer wait time indicated worse OS (HR: 1.385, 95%CI: 1.186-1.616, P < 0.001) in patients with adenocarcinoma.
A prolonged time interval from the completion of nCRT to surgery is associated with a significant decrease in OS. Thus, esophagectomy should be performed within 7-8 wk after nCRT.
Core tip: Esophageal cancer is one of the most common malignant tumors worldwide. Neoadjuvant chemoradiotherapy (nCRT) is increasingly used as the standard treatment for most esophageal cancer patients. However, the optimal time interval for esophagectomy after nCRT in patients with esophageal cancer has not been defined. Therefore, we conducted a meta-analysis on 12621 patients from 16 studies to evaluate whether a prolonged time interval from the end of nCRT to surgery has an effect on survival outcome in esophageal cancer patients.
- Citation: Shang QX, Yang YS, Gu YM, Zeng XX, Zhang HL, Hu WP, Wang WP, Chen LQ, Yuan Y. Timing of surgery after neoadjuvant chemoradiotherapy affects oncologic outcomes in patients with esophageal cancer. World J Gastrointest Oncol 2020; 12(6): 687-698
- URL: https://www.wjgnet.com/1948-5204/full/v12/i6/687.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i6.687
Esophageal cancer is the sixth leading cause of cancer-related death worldwide[1]. In China, esophageal cancer led to 375000 deaths annually[2]. High-level evidence suggests that neoadjuvant chemoradiotherapy (nCRT) plus surgery compared with surgery alone improves survival in patients with local advanced esophageal squamous cell carcinoma (SCC) and adenocarcinoma (AC), and pathological complete response (pCR) after nCRT may have a potential impact on survival outcome[3,4]. However, there are many unclear factors which influence outcome in esophageal cancer patients.
The optimal time interval for esophagectomy after nCRT in patients with esophageal cancer has not been defined. According to current clinical practice, in most centers, patients usually undergo esophagectomy within 6 to 8 wk after completion of nCRT when they have fully recovered[5]. In rectal cancer patients, evidence[6] suggests that a longer waiting interval (more than 6-8 wk) significantly increases the rate of pCR without a detrimental outcome. Similarly, other studies[7,8] have revealed that a prolonged interval between nCRT (> 8 wk) and esophagectomy is associated with a higher pCR, which may improve survival in esophageal cancer patients. However, Ranney et al[9] and others[10,11] have indicated that the prognostic role of the time interval in esophageal cancer is still controversial. For these reasons, it is necessary to perform a meta-analysis to systematically and comprehensively investigate the impact of different intervals on survival outcome. In the present study, a pooled analysis of relevant studies was undertaken to evaluate whether a prolonged time interval from the end of nCRT to surgery has an effect on survival outcome in esophageal cancer patients.
We performed a systematic literature review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses. We searched PubMed, Embase, Web of Science, the Cochrane Library, Wanfang and China National Knowledge Infrastructure databases for relevant studies published before November 16, 2019. We identified articles using Medical Subject Heading and Test-word search strategy. Keywords included “esophageal neoplasms”, “neoadjuvant therapy”, “esophagectomy”, and “time interval”. In addition, the references listed in the articles were also checked.
The included studies satisfied the following criteria: (1) Comparisons were performed between longer time intervals and shorter time intervals from the completion of nCRT to surgery in esophageal cancer patients; (2) Survival-related outcomes were reported, such as overall survival (OS), progression-free survival (PFS) and disease-free survival; (3) Studies included human subjects; and (4) Articles were published in any language. The exclusion criteria were as follows: (1) Article types such as abstracts, letters, review articles, case reports and unpublished studies; (2) Studies with insufficient data to evaluate hazard ratio (HR) and 95%CI; and (3) For studies already reported or containing duplicate data, we included only the latest studies.
Two reviewers (Yi-Min Gu, Qi-Xin Shang) evaluated all potential eligible studies. Then, another two investigators (Han-Lu Zhang, Xiang-Yu Zhang) completed the full-text review independently. If disagreement occurred, a third investigator (Yu-Shang Yang) joined the discussion until a consensus was reached. The following information was extracted from the selected articles: First author, study year, study region, study design, ethnicity, sample size, age, nCRT regimen, cut-off value, outcome, follow-up, clinical stage, histological type and HR with 95%CI. The time interval was defined as the period of time from the completion of nCRT to surgery. When several time interval groups were included in the study, the subgroup events were combined at the cut-off value of 7-8 wk in order to compare the longer interval with the shorter time interval. Some studies have suggested an interval of 7-8 wk for esophageal cancer patients (Table 1); and a similar classification was used in a previously published meta-analysis of esophageal cancer[34]. OS was selected as the primary end point, while PFS and disease-free survival were secondary end points. The included studies quality was rated according to the Newcastle-Ottawa Scale (NOS) by two independent investigators (Yi-Min Gu, Wei-Peng Hu). Studies with NOS scores of 6 or higher were considered to be of high quality.
| Ref. | Year | Study region | Ethnicity | Study design | No. (M/F) | Age (yr) | NCRT regimen | Cut-off (d) | Outcome | Follow-up (mo) | Clinical stage | Histology | HR type | NOS score |
| Ruol et al[17] | 2010 | Italy | Caucasian | Prospective | 129 (99/30) | 60.8 | DDP+5FU ci/DDP+PTX/OXA+5FU ci+CF RT 45-50.4 Gy | 46 | OS | 60 | I-IV1 | SCC | U | 8 |
| Kim et al[18] | 2012 | United States | Caucasian | Prospective | 266 (235/31) | SI, 57; LI, 60 | Platinum-based RT 45Gy | 56 | OS/DFS | 99 | II-IVa1 | AC and SCC | U | 8 |
| Chiu et al[10] | 2013 | China | Asian | Retrospective | 276 (268/8) | SI, 56.8; LI, 53.5 | 5FU ci+DDP RT 30 Gy | 56 | OS | 60 | II-IV1 | SCC | U | 7 |
| Shapiro et al[7] | 2014 | United States | Caucasian | Retrospective | 325 (253/72) | 60 | PTX+CBP RT 41.4 Gy | 45 | OS/DFS | 60 | cT1-42, N0-11 | AC and SCC | U/M | 8 |
| Tessier et al[19] | 2014 | France | Caucasian | Prospective | 257 (227/30) | NR | 5FU ci+DDP RT 45 Gy | 49 | OS | 135 | I-III1 | AC and SCC | U | 8 |
| Shaikh et al[20] | 2015 | United States | Caucasian | Retrospective | 88 (62/26) | 61 | 5FU-based/PTX-based RT 45-60 Gy | 50 | OS | 87.7 | I-IV2 | AC and SCC | U | 7 |
| Wang et al[21] | 2015 | China | Asian | Prospective | 665 (636/29) | 53 | NA | 59 | OS | 60 | I-III2 | SCC | U | 7 |
| Haisley et al[5] | 2016 | United States | Caucasian | Prospective | 234 (191/43) | 64 | PTX+CBP/5FU ci+DDP NA | 56 | OS | 152 | I-IV2 | AC and SCC | U | 8 |
| Kathiravetpillai et al[22] | 2016 | Netherlands | Caucasian | Prospective | 190 (169/21) | NR | PTX+CBP/PTX+CBP+5FU RT41.4 Gy | 56 | OS/DFS | 60 | cT1-32, N0-32 | AC | U | 7 |
| Lee et al[11] | 2016 | United States | Caucasian | Retrospective | 5393 (4533/860) | 62 | NA RT 39.6-64.8 Gy | 64 | OS | 96 | I-IV2 | AC and SCC | M | 8 |
| Ranney et al[9] | 2017 | United States | Caucasian | Retrospective | 2444 (2193/251) | 61 | NA RT 40.4-50.4 Gy | 56 | OS | 60 | II-III2 | AC | M | 8 |
| Tsang et al[23] | 2017 | China | Asian | Prospective | 107(91/16) | 63 | 5FU ci+DDP RT 40 Gy | 64 | OS | 60 | I-III2 | SCC | U | 7 |
| Franko et al[14] | 2018 | United States | Caucasian | Retrospective | 1244 (810/434) | 60.5 | NA RT 45 Gy | 49 | OS | 75 | NR | SCC | U | 6 |
| Furukawa et al[24] | 2018 | Japan | Asian | Retrospective | 134 (116/18) | NR | 5FU+DTX/5FU+DDP/NDP RT 40 Gy | 56 | OS/DFS | 60 | I-IV2 | SCC | U | 7 |
| Singla et al[25] | 2018 | United States | Caucasian | Prospective | 226 (210/16) | 61 | DDP+Irinotecan/CBP+PTX/OXA+CAPE/5-FU+DDP RT 50.4 Gy | 49 | OS/PFS | 110 | I-IV2 | AC and SCC | U | 6 |
| Klevebro et al[8] | 2019 | Sweden | Caucasian | Prospective | 643 (536/107) | SI, 64; LI, 65 | DDP+5FU RT 40 Gy | 49 | OS | 60 | I-IVa2 | AC and SCC | U | 7 |
HRs) and 95%CIs were extracted from each article and combined to estimate the prognostic value. HR > 1 indicated a worse oncologic outcome in esophageal cancer patients with a longer time interval between nCRT therapy and surgery. If the study did not provide HRs and 95%CIs directly but a Kaplan-Meier curve instead, Engauge-Digitizer version 12 (http://markummitchell.github.io/engauge-digitizer/) was used to derive estimates from survival curves according to the method proposed by Parmar et al[12]. Cochran’s Q test and Higgins I-squared statistic were used to assess the heterogeneity of the included studies. Pooled estimates of HR and 95%CI were calculated initially with a fixed-effect model (Mantel–Haenszel method). If significant heterogeneity existed (Q-test, P < 0.10 or I2 > 50% was defined as statistically significant heterogeneity), a certified analysis using the random-effect model (DerSimonian–Laird method) was performed. Begg’s funnel plot and Egger's linear regression test were carried out to detect publication bias. All P-values were two-sided and significant publication bias was defined as P < 0.05. Multivariate models were chosen for a more accurate estimate of the effect of time interval on survival outcomes when both univariate and multivariate Cox regression analyses were performed. Subgroup analyses were performed on the basis of variables including histology, study design, ethnicity, sample size, and HR type. All statistical analyses were performed with Stata/SE 12.0 software (Stata Corp LLC, version 12.0 4905 Lakeway Drive College Station, TX, United States).
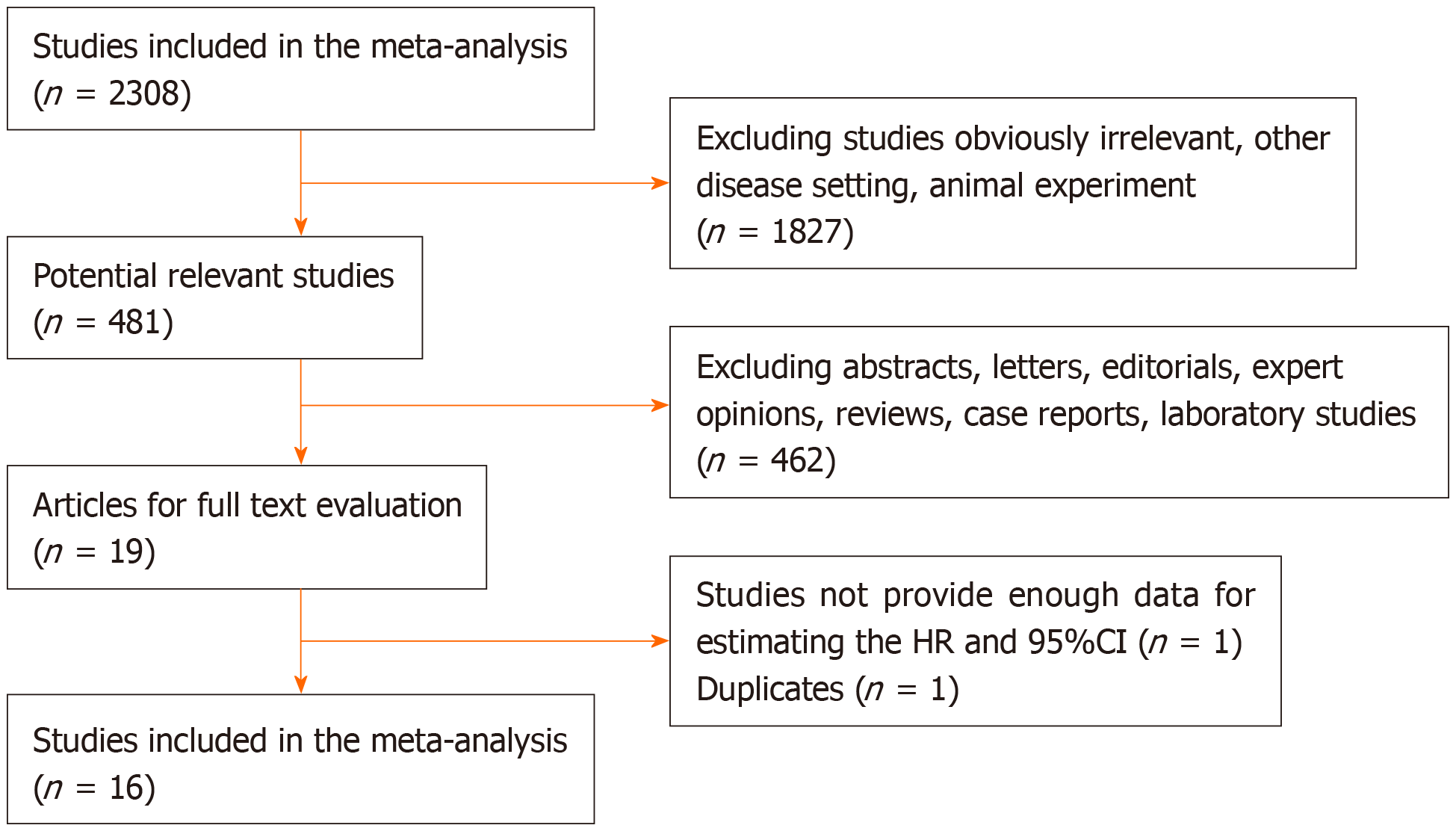
We screened 2308 eligible studies and identified 19 studies including two relevant articles from the same study by Franko et al[13,14] and two studies[15,16] without sufficient data to calculate HR and 95%CI. Sixteen studies were finally selected for the meta-analysis to determine whether the time interval from completion of nCRT to surgery has an effect on survival outcome[5,7-11,14,17-25]. Of these studies, HRs and 95%CIs were directly provided in three studies, while the other 13 studies all provided Kaplan-Meier curves; thus, we were able to obtain estimated HRs and 95%CIs indirectly. Moreover, in 13 studies, HRs were evaluated by univariate analysis and in three by multivariate analysis. The included articles were assessed to be of high quality, with a median quality score of 7.3 (range, 6-8). The identification of relevant studies is summarized in the flowchart (Figure 1).
The included studies were carried out in seven countries (United States, China, France, Italy, Netherlands, Japan, and Sweden) and published between 2010 and 2019. Of these studies, seven were retrospective and nine were prospective. The participants were Caucasian in 12 studies and were Asian in four studies. The median number of patients in each study was 789 (range, 88-5393), with a total of 12 621 patients, consisting of 7522 patients with a shorter time interval, and 5099 patients with a longer time interval between nCRT and surgery. The sample size in five studies was < 200 patients and was ≥ 200 patients in 11 studies. In addition, 1029, 219 and 189 patients underwent esophagectomy via Ivor-Lewis, Mckeown and transhiatal approaches, respectively, while six studies[5,8,11,20-22] did not provide the method of esophagectomy. The cut-off values in each study were not consistent and ranged from 45 d to 64 d. Six articles applied a cut-off value of 8 wk and five studies used 7 wk. Seven studies included whole stages, and three studies included advanced stage. Additional comprehensive characteristics of the relevant studies are shown in Table 1.
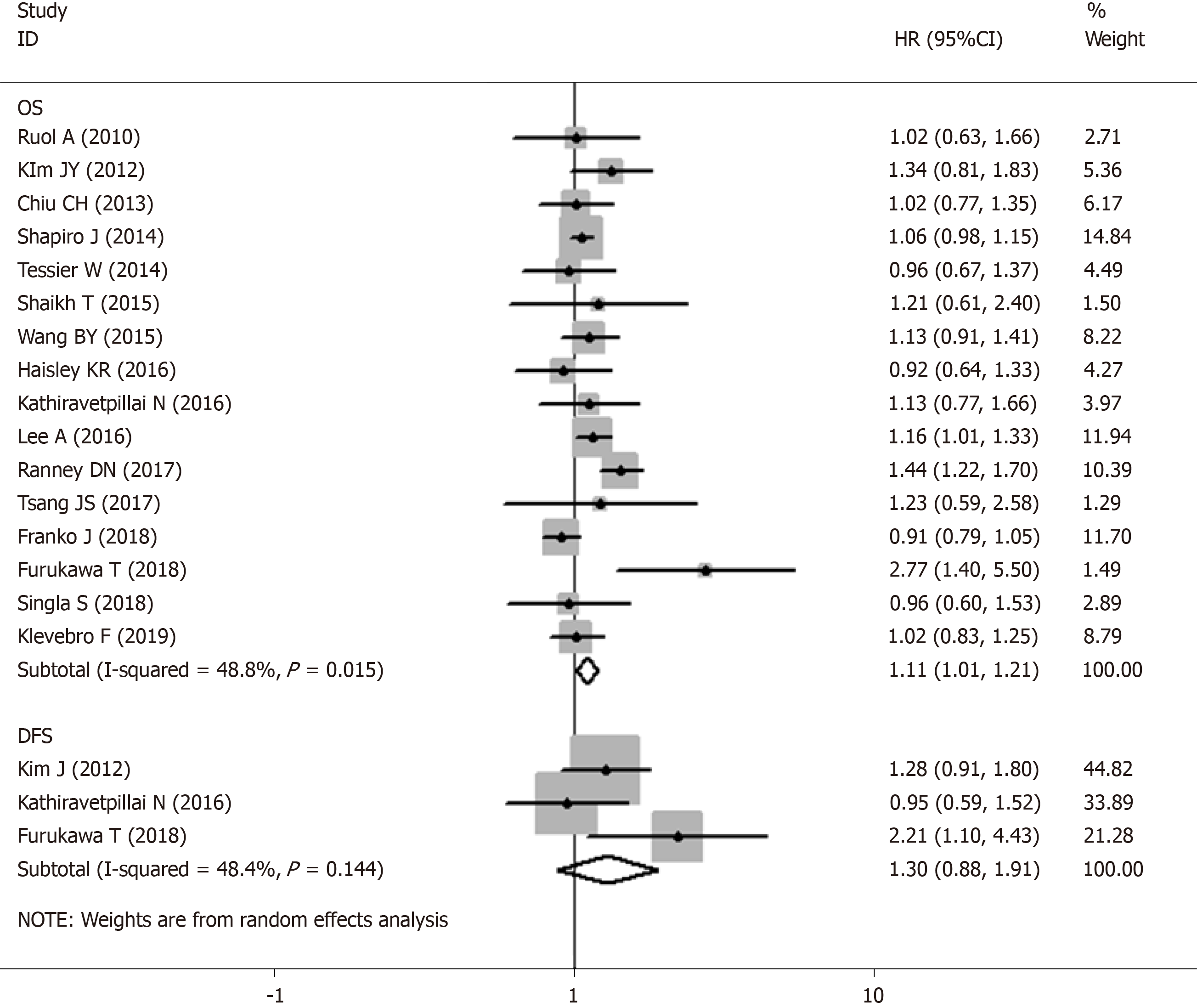
Sixteen studies were included in the meta-analysis on the effect of the time interval between nCRT and surgery on the OS of esophageal cancer patients. Notable heterogeneity was detected among the studies (I2 = 48.8%, P = 0.015; Figure 2); thus, a random-effect model was used. The results of our meta-analysis showed that esophageal cancer patients with a shorter wait time correlated with prolonged OS compared with an extended wait time, with a poor HR of 1.107 (95%CI: 1.014-1.208, P = 0.023; Table 2). Three studies which included 590 patients demonstrated that a longer time interval was related to shorter PFS (HR: 1.263, 95%CI: 0.976-1.633, P = 0.075; Table 2) without obvious heterogeneity (I2 = 48.4%, Ph = 0.144; Figure 2).
| Analysis | n | Ref. | Random-effects model | Fixed-effects model | Heterogeneity | |||
| HR (95%CI) | P value | HR (95%CI) | P value | I2 | Ph | |||
| Esophageal cancer OS | 16 | [5,7-11,13,16-24] | 1.107 (1.014-1.208) | 0.023 | 1.089 (1.036-1.145) | 0.001a | 48.80% | 0.015 |
| DFS | 3 | [17,21,23] | 1.300 (0.883-1.913) | 0.184 | 1.263 (0.976-1.633) | 0.075 | 48.40% | 0.144 |
| Subgroup 1: Study design | 16 | [5,7-11,13,16-24] | 1.107 (1.014-1.208) | 0.196 | 1.089 (1.036-1.145) | 0.196 | 0.00 | 0.865 |
| Prospective | 9 | [5,8,16-18,20-22,24] | 1.073 (0.964-1.194) | 0.064 | 1.073 (0.964-1.194) | 0.002a | 76.30% | 0.000 |
| Retrospective | 7 | [7,9-11,13,19,23] | 1.150 (0.992-1.332) | 0.023a | 1.094 (1.034-1.158) | 0.001a | 48.80% | 0.015 |
| Subgroup 2: Samplesize | 16 | [5,7-11,13,16-24] | 1.107 (1.014-1.208) | 0.023a | 1.089 (1.036-1.145) | 0.001a | 48.80% | 0.015 |
| < 200 | 5 | [16,19,21-23] | 1.294 (0.946-1.771) | 0.107 | 1.254 (0.985-1.597) | 0.067 | 34.70% | 0.190 |
| ≥ 200 | 11 | [5,7-11,13,17,18,20,24] | 1.089 (0.995-1.190) | 0.063 | 1.082 (1.028-1.139) | 0.002a | 54.20% | 0.016 |
| Subgroup 3: Ethnicity | 16 | [5,7-11,13,16-24] | 1.107 (1.014-1.208) | 0.064 | 1.107 (1.014-1.208) | 0.064 | 48.80% | 0.015 |
| Caucasian | 12 | [5,7-9,11,13,16-19,21,24] | 1.091 (0.995-1.197) | 0.150 | 1.091 (0.995-1.197) | 0.150 | 49.30% | 0.027 |
| Asian | 4 | [10,20,22,23] | 1.251 (0.922-1.696) | 0.023a | 1.251 (0.922-1.696) | 0.023a | 57.60% | 0.069 |
| Subgroup 4: HR type | 16 | [5,7-11,13,16-24] | 1.107 (1.014-1.208) | 0.023a | 1.089 (1.036-1.145) | 0.001a | 48.80% | 0.015 |
| Univariate | 13 | [5,8,10,13,16-24] | 1.053 (0.952-1.166) | 0.314 | 1.029 (0.949-1.115) | 0.492 | 23.20% | 0.209 |
| Multivariate | 3 | [7,9,11] | 1.194 (1.010-1.411) | 0.037a | 1.130 (1.059-1.204) | 0.000a | 81.00% | 0.005 |
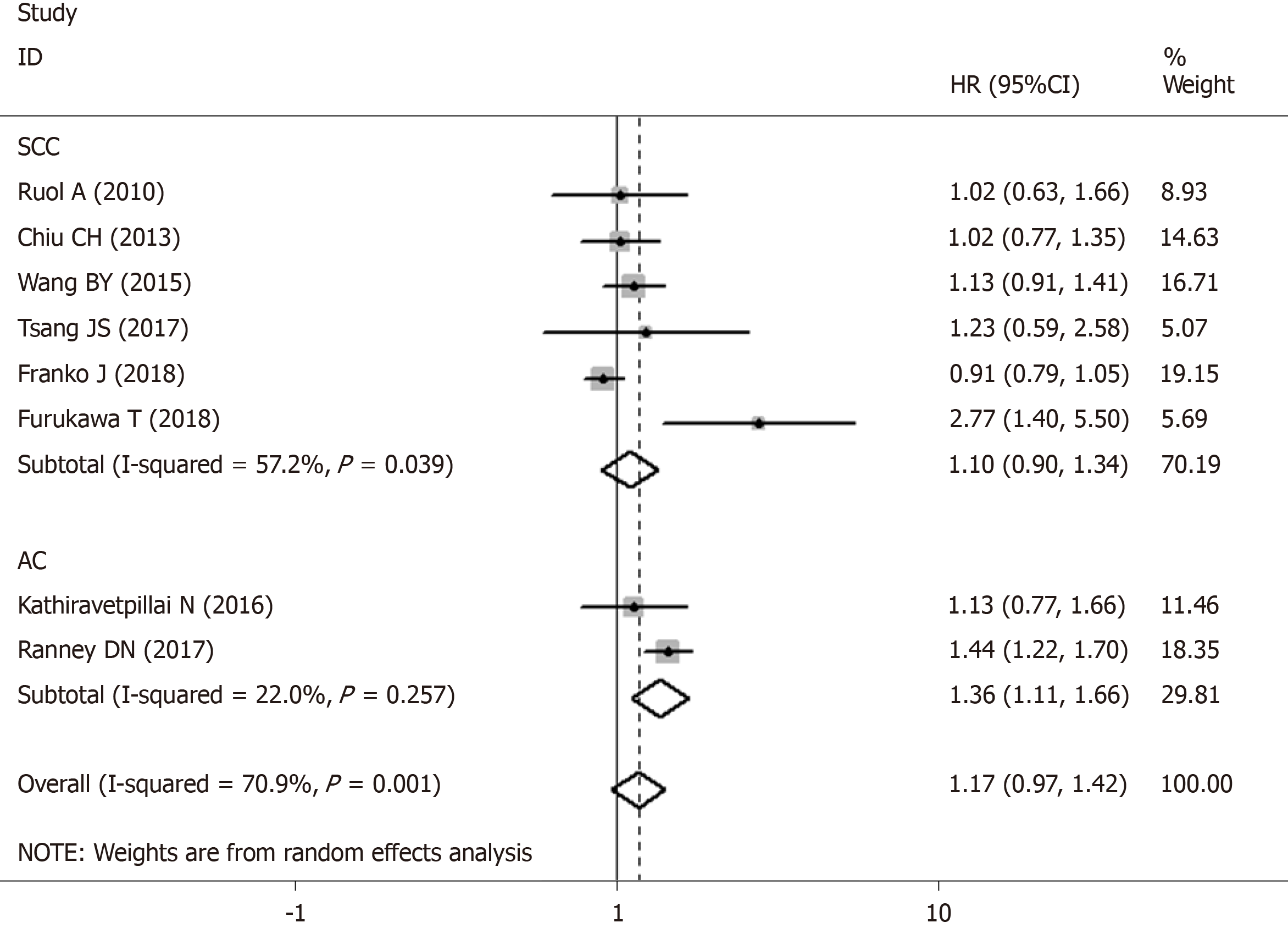
| Squamous cell carcinoma OS | 6 | [10,13,16,20,22,23] | 1.096 (0.896-1.341) | 0.371 | 1.009 (0.908-1.120) | 0.874 | 57.20% | 0.039 |
| Adenocarcinoma OS | 2 | [9,21] | 1.360 (1.111-1.664) | 0.003a | 1.385 (1.186-1.616) | 0.000a | 22.00% | 0.257 |
Two studies with 2634 patients reported data on a longer time interval and OS in AC patients. Pooled data from the two studies demonstrated that a prolonged time interval was significantly associated with worse OS with a HR estimate of 1.385 (95%CI: 1.186-1.616, P < 0.001; Table 2) without apparent heterogeneity (I2 = 22.00%, Ph = 0.257; Figure 3).
Meta-analysis of six studies revealed that SCC patients with a longer time interval had poor OS (HR: 1.096, 95%CI: 0.896-1.341, P = 0.371; Table 2) with significant heterogeneity (I2 = 57.2%, Ph = 0.039; Figure 3).
Subgroup analyses were conducted to investigate potential sources of heterogeneity across studies and to evaluate the consistency of the conclusions among different subpopulations of patients. Subgroup analyses based on study design, demonstrated that the merged HR was 1.073 (95%CI: 0.964-1.194, P = 0.064) for prospective cohort studies and 1.094 (95%CI: 1.034-1.158, P = 0.001) for retrospective analyses (Table 2).
Based on classifications by sample size, the merged HR was 1.254 (95%CI: 0.985-1.579, P = 0.067) for a sample size < 200 and 1.089 (95%CI: 0.995-1.190, P = 0.063) for a sample size ≥ 200. Stratification by ethnicity, revealed a combined HR of 1.091 (95%CI: 0.995-1.197, P = 0.150) in Caucasian populations and 1.251 (95%CI: 0.922-1.696, P = 0.023) in Asian cases. In addition, subgroup analysis was performed by univariate analysis (HR: 1.029, 95%CI: 0.949-1.115, P = 0.492) and multivariate analysis (HR: 1.194, 95 %CI: 1.010-1.411, P = 0.037). The results demonstrated that sample size (< 200 and ≥ 200) and statistical analysis approach (univariate and multivariate analyses) were both potential causes of significant heterogeneity (Table 2).
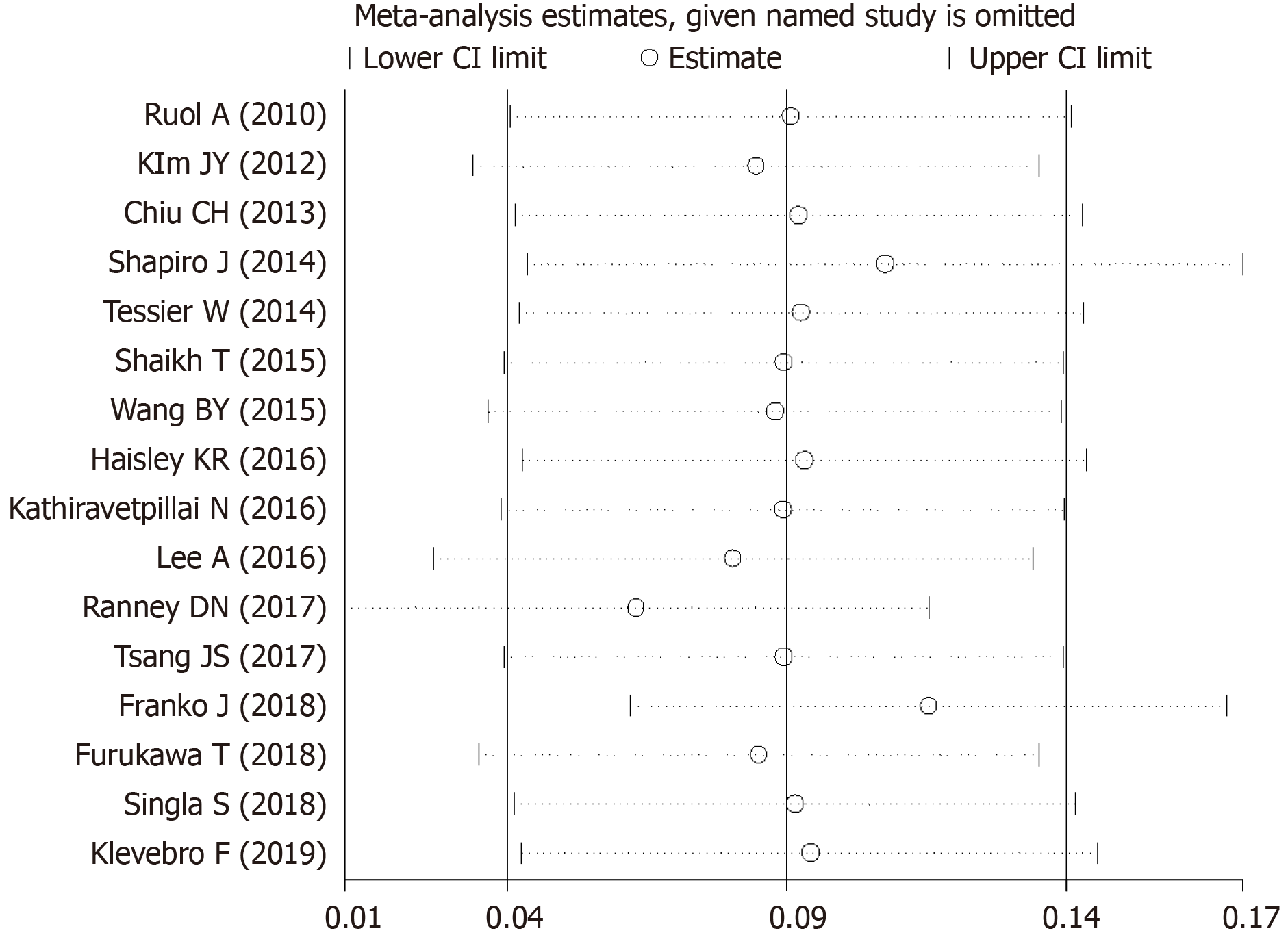
Due to the conspicuous heterogeneity among the studies, a sensitivity analysis was performed. We found that the combined results were still stable after the exclusion of any single study (Figure 4).
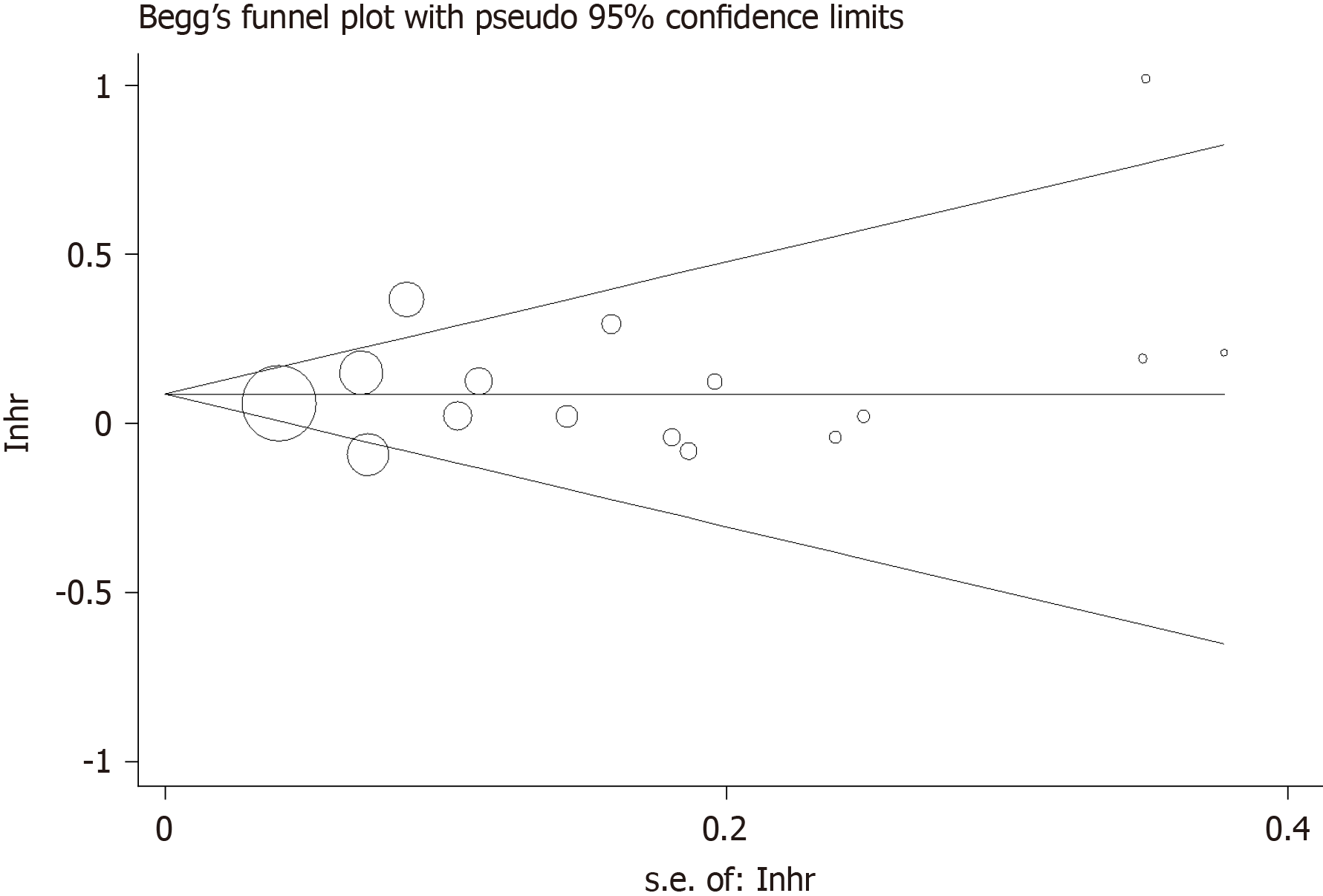
Investigations using Begg’s funnel plot (Figure 5) and Egger’s linear regression test did not indicate publication bias in the meta-analyses on the association between time to surgery and OS (Pr >|z| = 0.344 for Begg’s test and P >|t|= 0.432 for Egger’s test).
The role of preoperative chemoradiotherapy in improving survival among patients with potentially curable esophageal cancer is recognized in many randomized controlled studies[3,4]. However, esophagectomy cannot be performed immediately, as patients need to recover from the side effects of chemoradiotherapy, and an appropriate interval can induce the maximal radiotherapy response[26]. A strong relationship between the time interval and survival outcome has been reported for pancreatic tumor[27], rectal cancer[28], and non-small cell lung cancer[29].
Similarly, several studies have shown an association between time interval and survival in esophageal cancer patients. Shapiro et al[7] found that a prolonged interval after nCRT increased the pCR rate and may improve survival. However, the findings in some current studies do not support those of previous research. Ranney et al[9] found that OS was worse in the long-interval subgroup (HR: 1.44, 95%CI: 1.22-1.71, P < 0.001). This result is consistent with that of Chiu et al[10] who revealed that survival outcome did not improve following a long-term wait. In contrast, subgroup analysis showed that later resection may be hazardous, especially in patients who had a good response to nCRT.
This meta-analysis included 12621 esophageal cancer patients from 16 cohort studies, and demonstrated that patients with a longer time interval between nCRT and esophagectomy had significantly worse OS (HR: 1.107, 95%CI: 1.014-1.208, P = 0.023; Figure 2) than those with a shorter time interval. Subgroup analysis showed that OS with a prolonged interval was poor based on both the sample size and the HRs. There was also a significant association between a prolonged interval and worse OS in Asian, but not Caucasian patients. In addition, we found that a prolonged interval indicated worse OS (HR: 1.385, 95%CI: 1.186-1.616, P < 0.001; Table 2) in patients with AC. In contrast, a prolonged interval resulted in shorter OS without statistical significance (HR: 1.096, 95%CI: 0.896-1.341, P > 0.05; Table 2) in SCC patients. Taking all of these findings into consideration, worse OS was noted in the longer time interval group. There are several possible explanations for this result. One possible explanation may be the disproportionate number of medically complex patients between the two groups, which could have decreased the OS[5]. Patients need to optimize medical comorbidities during see-and-wait follow-up. As a result, disease-specific survival may be closer to the real evaluation rather than OS. Another possible explanation is that the longer wait time was not due to preference or chance in the patients, but due to their poor physical condition after nCRT, which may have put them at an inherent disadvantage in terms of survival.
Some studies have shown that radiation-induced fibrosis may also make surgical dissection technically demanding with delayed surgery leading to higher complication rates[2]. In contrast, Haisley et al[5] found no effect on mortality and no increase in complications in the longer time interval group. Another source of uncertainty is cancer stage; however, no significant difference in initial clinical stage was observed between the longer time interval and shorter time interval groups[14,23]. In addition, due to the heterogeneity of genotype and phenotype in esophageal cancer as well as constitutive resistance to individual cytotoxic drugs[30], chemotherapy is rarely beneficial in all patients, and some researchers have reported that the pCR rate following preoperative chemoradiation for esophageal cancer could reach 20%-35%[31]. Further well-designed and large-scale studies are needed to determine whether the time interval from the end of nCRT to surgery has an effect on survival outcome and to assess whether disease-specific survival differs by type of pathological response.
The strength of our study is that this is the first meta-analysis to investigate whether the time interval between nCRT and surgery affects survival outcome using pooled HRs. The total sample size in the 16 included studies was 12 621 patients with a survival of five or more years. Moreover, the larger number of included studies ensured the inclusion of subgroup analyses. To date, three similar meta-analyses have been published. The earliest meta-analysis by Lin et al[32] included only five eligible studies and found that a longer time interval did not impact the five-year OS and pCR rates. The next meta-analysis was performed by Tie et al[33], but did not reveal if a prolonged time interval had a significant impact on the five-year OS and pCR rates. Moreover, this study included conference abstracts, which may have introduced potential bias. The third study by Qin et al[34] found that a prolonged interval between nCRT and surgery was significantly correlated with higher pCR and surgical mortality rate in esophageal cancer patients. Their study included only nine articles containing 5830 patients with a five-year survival, which were less than half of the sample size in our study. In addition, they did not investigate the association between time interval and survival outcomes.
Several limitations in our studies should be carefully addressed. The most important limitation was the fact that most of the studies included were retrospective. An additional uncontrolled factor is that heterogeneity was a potential factor that may have affected interpretation of the results. The source of heterogeneity in this study could be age, nCRT regimen, cut-off value, and ypTNM stage.
In conclusion, despite these limitations, this meta-analysis confirmed that a prolonged time interval between the completion of nCRT and surgery is related to decreased OS of esophageal cancer patients. It is suggested that esophagectomy should be performed within 7-8 wk after nCRT in view of OS, especially in patients with good recovery and response to nCRT. As some potential biases were hardly adjusted, our results still require further confirmation.
The optimal time interval for esophagectomy after neoadjuvant chemoradiotherapy (nCRT) in patients with esophageal cancer has not been defined.
Some studies have revealed that a prolonged interval (> 8 wk) between nCRT and esophagectomy is associated with a higher pathological complete response, which may improve survival in esophageal cancer patients. However, others have indicated that the prognostic role of the time interval in esophageal cancer is still controversial. For these reasons, it is necessary to perform a meta-analysis to systematically and comprehensively investigate the impact of different intervals on survival outcome in these patients.
To evaluate whether a prolonged time interval between the end of nCRT and surgery has an effect on survival outcome through meta-analysis.
The research methods meta-analysis that were adopted to realize the objectives.
The results demonstrated that esophageal cancer patients with a prolonged time interval between the end of nCRT and surgery had significantly worse overall survival (OS) (HR: 1.107, 95%CI: 1.014-1.208, P = 0.023) than those with a shorter time interval. Subgroup analysis showed that poor OS with a prolonged interval was observed based on both the sample size and HRs. There was also significant association between a prolonged time interval and decreased OS in Asian, but not Caucasian patients. In addition, a longer waiting time resulted in worse OS (HR: 1.385, 95%CI: 1.186-1.616, P < 0.001) in patients with adenocarcinoma.
This meta-analysis confirmed that a prolonged time interval between the completion of nCRT and surgery is related to decreased OS of esophageal cancer patients. It is suggested that esophagectomy should be performed within 7-8 wk after nCRT in view of OS, especially in patients with good recovery and response to nCRT.
Several limitations in this analysis should be carefully addressed. The most important limitation was the fact that most of the studies included were retrospective. An additional uncontrolled factor is that heterogeneity was a potential factor that may have affected interpretation of the results. The source of heterogeneity in this study could be age, nCRT regimen, cut-off value, and ypTNM stage. As some potential biases were hardly adjusted, further well-designed and large-scale studies are needed to determine whether the time interval from the end of nCRT to surgery has an effect on survival outcome and to assess whether disease-specific survival differs by type of pathological response.
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21462] [Article Influence: 1951.1] [Reference Citation Analysis (6)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13323] [Article Influence: 1332.3] [Reference Citation Analysis (4)] |
| 3. | van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, Verheul HM, Spillenaar Bilgen EJ, van Dekken H, van der Sangen MJ, Rozema T, Biermann K, Beukema JC, Piet AH, van Rij CM, Reinders JG, Tilanus HW, van der Gaast A; CROSS Group. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3288] [Cited by in RCA: 4253] [Article Influence: 303.8] [Reference Citation Analysis (3)] |
| 4. | Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, Mao W, Xiang J, Han Y, Chen Z, Yang H, Wang J, Pang Q, Zheng X, Yang H, Li T, Lordick F, D'Journo XB, Cerfolio RJ, Korst RJ, Novoa NM, Swanson SJ, Brunelli A, Ismail M, Fernando HC, Zhang X, Li Q, Wang G, Chen B, Mao T, Kong M, Guo X, Lin T, Liu M, Fu J; AME Thoracic Surgery Collaborative Group. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol. 2018;36:2796-2803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 768] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 5. | Haisley KR, Laird AE, Nabavizadeh N, Gatter KM, Holland JM, Vaccaro GM, Thomas CR Jr, Schipper PH, Hunter JG, Dolan JP. Association of Intervals Between Neoadjuvant Chemoradiation and Surgical Resection With Pathologic Complete Response and Survival in Patients With Esophageal Cancer. JAMA Surg. 2016;151:e162743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Petrelli F, Sgroi G, Sarti E, Barni S. Increasing the Interval Between Neoadjuvant Chemoradiotherapy and Surgery in Rectal Cancer: A Meta-analysis of Published Studies. Ann Surg. 2016;263:458-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 190] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 7. | Shapiro J, van Hagen P, Lingsma HF, Wijnhoven BP, Biermann K, ten Kate FJ, Steyerberg EW, van der Gaast A, van Lanschot JJ; CROSS Study Group. Prolonged time to surgery after neoadjuvant chemoradiotherapy increases histopathological response without affecting survival in patients with esophageal or junctional cancer. Ann Surg. 2014;260:807-813; discussion 813-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Klevebro F, Nilsson K, Lindblad M, Ekman S, Johansson J, Lundell L, Ndegwa N, Hedberg J, Nilsson M. Association between time interval from neoadjuvant chemoradiotherapy to surgery and complete histological tumor response in esophageal and gastroesophageal junction cancer: a national cohort study. Dis Esophagus. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Ranney DN, Mulvihill MS, Yerokun BA, Fitch Z, Sun Z, Yang CF, D'Amico TA, Hartwig MG. Surgical resection after neoadjuvant chemoradiation for oesophageal adenocarcinoma: what is the optimal timing? Eur J Cardiothorac Surg. 2017;52:543-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Chiu CH, Chao YK, Chang HK, Tseng CK, Chan SC, Liu YH, Chen WH. Interval between neoadjuvant chemoradiotherapy and surgery for esophageal squamous cell carcinoma: does delayed surgery impact outcome? Ann Surg Oncol. 2013;20:4245-4251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Lee A, Wong AT, Schwartz D, Weiner JP, Osborn VW, Schreiber D. Is There a Benefit to Prolonging the Interval Between Neoadjuvant Chemoradiation and Esophagectomy in Esophageal Cancer? Ann Thorac Surg. 2016;102:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815-2834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 13. | Franko J, Voynov G, Goldman CD. Esophagectomy Timing After Neoadjuvant Therapy for Distal Esophageal Adenocarcinoma. Ann Thorac Surg. 2016;101:1123-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Franko J, McAvoy S. Timing of esophagectomy after neoadjuvant chemoradiation treatment in squamous cell carcinoma. Surgery. 2018;164:455-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Müller AK, Lenschow C, Palmes D, Senninger N, Hummel R, Lindner K. [Timing of esophagectomy in multimodal therapy of esophageal cancer: Impact of time interval between neoadjuvant therapy and surgery on outcome and response]. Chirurg. 2015;86:874-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | van der Werf LR, Dikken JL, van der Willik EM, van Berge Henegouwen MI, Nieuwenhuijzen GAP, Wijnhoven BPL; Dutch Upper Gastrointestinal Cancer Audit (DUCA) group. Time interval between neoadjuvant chemoradiotherapy and surgery for oesophageal or junctional cancer: A nationwide study. Eur J Cancer. 2018;91:76-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Ruol A, Rizzetto C, Castoro C, Cagol M, Alfieri R, Zanchettin G, Cavallin F, Michieletto S, Da Dalt G, Sileni VC, Corti L, Mantoan S, Zaninotto G, Ancona E. Interval between neoadjuvant chemoradiotherapy and surgery for squamous cell carcinoma of the thoracic esophagus: does delayed surgery have an impact on outcome? Ann Surg. 2010;252:788-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Kim JY, Correa AM, Vaporciyan AA, Roth JA, Mehran RJ, Walsh GL, Rice DC, Ajani JA, Maru DM, Bhutani MS, Welsh J, Marom EM, Swisher SG, Hofstetter WL. Does the timing of esophagectomy after chemoradiation affect outcome? Ann Thorac Surg. 2012;93:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Tessier W, Gronnier C, Messager M, Hec F, Mirabel X, Robb WB, Piessen G, Mariette C. Does timing of surgical procedure after neoadjuvant chemoradiation affect outcomes in esophageal cancer? Ann Thorac Surg. 2014;97:1181-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Shaikh T, Ruth K, Scott WJ, Burtness BA, Cohen SJ, Konski AA, Cooper HS, Astsaturov I, Meyer JE. Increased time from neoadjuvant chemoradiation to surgery is associated with higher pathologic complete response rates in esophageal cancer. Ann Thorac Surg. 2015;99:270-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Wang BY, Chen HS, Hsu PK, Shih CS, Liu CY, Liu CC, Wu SC. Clinical impact of the interval between chemoradiotherapy and esophagectomy in esophageal squamous cell carcinoma patients. Ann Thorac Surg. 2015;99:947-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Kathiravetpillai N, Koëter M, van der Sangen MJ, Creemers GJ, Luyer MD, Rutten HJ, Nieuwenhuijzen GA. Delaying surgery after neoadjuvant chemoradiotherapy does not significantly influence postoperative morbidity or oncological outcome in patients with oesophageal adenocarcinoma. Eur J Surg Oncol. 2016;42:1183-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Tsang JS, Tong DKH, Lam KO, Law BTT, Wong IYH, Chan DKK, Chan FSY, Kwong D, Law S. Appropriate timing for surgery after neoadjuvant chemoradiation for esophageal cancer. Dis Esophagus. 2017;30:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Furukawa T, Hamai Y, Hihara J, Emi M, Yamakita I, Ibuki Y, Kurokawa T, Okada M. Impact of Interval Between Neoadjuvant Chemoradiation and Surgery Upon Morbidity and Survival of Patients with Squamous Cell Carcinoma of Thoracic Esophagus. Anticancer Res. 2018;38:5239-5245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Singla S, Gabriel E, Alnaji R, Du W, Attwood K, Nava H, Hochwald SN, Kukar M. Complete pathologic response is independent of the timing of esophagectomy following neoadjuvant chemoradiation for esophageal cancer. J Gastrointest Oncol. 2018;9:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Huang J, Barbera L, Brouwers M, Browman G, Mackillop WJ. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J Clin Oncol. 2003;21:555-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 429] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 27. | Chen KT, Devarajan K, Milestone BN, Cooper HS, Denlinger C, Cohen SJ, Meyer JE, Hoffman JP. Neoadjuvant chemoradiation and duration of chemotherapy before surgical resection for pancreatic cancer: does time interval between radiotherapy and surgery matter? Ann Surg Oncol. 2014;21:662-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Lefevre JH, Mineur L, Kotti S, Rullier E, Rouanet P, de Chaisemartin C, Meunier B, Mehrdad J, Cotte E, Desrame J, Karoui M, Benoist S, Kirzin S, Berger A, Panis Y, Piessen G, Saudemont A, Prudhomme M, Peschaud F, Dubois A, Loriau J, Tuech JJ, Meurette G, Lupinacci R, Goasgen N, Parc Y, Simon T, Tiret E. Effect of Interval (7 or 11 weeks) Between Neoadjuvant Radiochemotherapy and Surgery on Complete Pathologic Response in Rectal Cancer: A Multicenter, Randomized, Controlled Trial (GRECCAR-6). J Clin Oncol. 2016;34:3773-3780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 346] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 29. | Gao SJ, Corso CD, Wang EH, Blasberg JD, Detterbeck FC, Boffa DJ, Decker RH, Kim AW. Timing of Surgery after Neoadjuvant Chemoradiation in Locally Advanced Non-Small Cell Lung Cancer. J Thorac Oncol. 2017;12:314-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 30. | Ling ZQ, Qi CJ, Lu XX, Qian LJ, Gu LH, Zheng ZG, Zhao Q, Wang S, Fang XH, Yang ZX, Yin J, Mao WM. Heterogeneity of chemosensitivity in esophageal cancer using ATP-tumor chemosensitivity assay. Acta Pharmacol Sin. 2012;33:401-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Berger AC, Farma J, Scott WJ, Freedman G, Weiner L, Cheng JD, Wang H, Goldberg M. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005;23:4330-4337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 410] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 32. | Lin G, Han SY, Xu YP, Mao WM. Increasing the interval between neoadjuvant chemoradiotherapy and surgery in esophageal cancer: a meta-analysis of published studies. Dis Esophagus. 2016;29:1107-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Tie H, He F, Shen J, Zhang B, Ye M, Chen B, Wu Q. Prolonged interval between neoadjuvant chemoradiotherapy and esophagectomy does not benefit the outcome in esophageal cancer: a systematic review and meta-analysis. Dis Esophagus. 2018;31:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Qin Q, Xu H, Liu J, Zhang C, Xu L, Di X, Zhang X, Sun X. Does timing of esophagectomy following neoadjuvant chemoradiation affect outcomes? A meta-analysis. Int J Surg. 2018;59:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Okamoto H, Ono T, Petrucciani N S-Editor: Wang J L-Editor: MedE-Ma JY E-Editor: Qi LL