Published online Apr 15, 2020. doi: 10.4251/wjgo.v12.i4.483
Peer-review started: December 21, 2019
First decision: January 19, 2020
Revised: February 5, 2020
Accepted: March 22, 2020
Article in press: March 22, 2020
Published online: April 15, 2020
Processing time: 116 Days and 3.8 Hours
In early gastric cancer (GC), tumor markers are increased in the blood. The levels of these markers have been used as important indexes for GC screening, early diagnosis and prognostic evaluation. However, specific tumor markers have not yet been discovered. Diagnosis based on a single tumor marker has limited significance. The detection rate of GC is still very low.
To improve the diagnostic value of blood markers for GC.
We used a multiparameter joint analysis of 77 indexes of malignant GC and gastric polyp (GP), 64 indexes of GC and healthy controls (Ctrls).
By analyzing the data, there are 27 indexes in the final Ctrls vs GC with P values < 0.01, the area under the curve (AUC) of albumin is the largest in Ctrls vs GC, and the AUC was 0.907. 30 indexes in GP vs GC have P values < 0.01. Among them, the D-dimer showed an AUC of 0.729. The 27 indexes in Ctrls vs GC and 30 indexes in GP vs GC were used for binary logistic regression, discriminant analysis, classification tree analysis and artificial neural network analysis model. For the ability to distinguish between Ctrls vs GC, GP vs GC, artificial neural networks had better diagnostic value when compared with classification tree, binary logistic regression, and discriminant analysis. When compared Ctrl and GC, the overall prediction accuracy was 92.9%, and the AUC was 0.992 (0.980, 1.000). When compared GP and GC, the overall prediction accuracy was 77.9%, and the AUC was 0.969 (0.948, 0.990).
The diagnostic effect of multi-parameter joint artificial neural networks analysis is significantly better than the single-index test diagnosis, and it may provide an assistant method for the detection of GC.
Core tip: In this study, we aimed to improve the diagnostic value of blood markers for gastric cancer. By comparing the binary logistic regression, discriminant analysis, classification tree and artificial neural network analysis, finally, artificial neural networks had better diagnostic value. When compared healthy control and gastric cancer, gastric polyp and gastric cancer, the area under the curve was 0.992 (0.980, 1.000) and 0.969 (0.948, 0.990), respectively. Based on artificial neural network and serum index, a novel diagnostic model for gastric cancer may be provided for clinical practice.
- Citation: Zhang ZG, Xu L, Zhang PJ, Han L. Evaluation of the value of multiparameter combined analysis of serum markers in the early diagnosis of gastric cancer. World J Gastrointest Oncol 2020; 12(4): 483-491
- URL: https://www.wjgnet.com/1948-5204/full/v12/i4/483.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i4.483
According to estimates by the World Health Organization, nearly 7 million people worldwide die from cancer every year, and this number is increasing every year. Gastric cancer (GC) is a common malignant tumor that endangers human health. GC ranks second in cancer-related deaths. In China, GC is one of the most malignant tumors with high morbidity and mortality[1]. GC deaths account for approximately 25% to 30% of all cancer-type deaths[2]. The pathogenesis of GC involves physical aging, eating habits and psychological factors[3-5]. The development and progression of GC is a multistage process involving multiple changes at the gene and molecular levels. In the early stage of GC, there are precancerous lesions, most of which remain unchanged, and a small part of which develop into cancer. The Correa cascade is the most common pattern of GC[6]. In current clinical practice, the main treatment for GC is surgical treatment. The 5-year survival rate is very low[7]; however, if GC is detected early, then the 5-year survival rate can be as high as 90%[8]. In developed countries, such as Japan, where the early diagnosis of GC reached 50%, the five-year survival rate reached 90%[9]. The early diagnosis and treatment of GC are extremely important for patients with GC.
Currently, many methods for diagnosing GC are used in scientific research and clinical practice[10]. Among these methods, plasma biomarker detection is an important detection method. The most commonly used tumor markers for early GC detection include carcinoembryonic antigen (CEA), carbohydrate antigens (CA): CA19-9, CA72-4, CA125, CA24-2, CA50, and pepsinogen and alpha-fetoprotein (AFP)[11]. However, these tumor biomarkers are poorly specific and sensitive, and thus far, they have not been used alone for the diagnosis of GC[11,12]. In early GC, tumor markers, such as CEA and CA-724, are increased in the blood. The levels of these markers have been used as important indexes for GC screening, early diagnosis and prognostic evaluation[13]. However, specific tumor markers have not yet been discovered. Diagnosis based on a single tumor marker has limited significance[14]. The detection rate of GC is still very low.
In this study, to distinguish between healthy controls (Ctrls) vs GC, gastric polyp (GP) and GC, we analyzed the routine blood detection indexes of GC diagnosis by using binary logistic regression, discriminant analysis, classification tree and artificial neural network. We aimed to use multiparameter joint analysis to improve diagnostic sensitivity and specificity and provide a new potential method for the early diagnosis of GC in clinical practice.
The serum samples of the patients involved in this study were obtained from the blood samples of patients admitted to the Beijing Daxing District People’s Hospital from April 2016 to April 2019 and confirmed by imaging and pathology. Sample collection and data screening were approved by the Ethics Committee of Beijing Daxing District People’s Hospital.
The inclusion criteria of the disease group were complete clinical and pathological data of the patient, with clear imaging and pathological diagnosis, and no radiotherapy, chemotherapy or other immunotherapy before surgery. The exclusion criteria for the disease group were patients with major diseases associated with the study, combined with other types of tumors, or individuals that had received radiotherapy, chemotherapy, or other immunotherapy before surgery. As shown in Table 1, this study included 144 GP and 253 GC patients. A total of 370 healthy controls were examined for tumor markers and imaging examinations. There were no diseases associated with this study, and both tumor markers and imaging examinations were qualified.
| Test variables | Gastric cancer | Benign | Normal |
| Sex | |||
| Male | 188 (74.31) | 69 (47.92) | 232 (62.70) |
| Female | 65 (25.69) | 75 (52.08) | 138 (37.30) |
| Age (yr) | |||
| < 40 | 10 (3.95) | 16 (11.11) | 45 (12.16) |
| 40-60 | 93 (36.76) | 74 (51.39) | 272 (73.51) |
| ≥ 60 | 150 (59.29) | 54 (37.50) | 53 (14.33) |
| T | |||
| 1 | 5 (1.98) | ||
| 1a | 9 (3.56) | ||
| 1b | 15 (5.93) | ||
| 2 | 45 (17.79) | ||
| 3 | 77 (30.43) | ||
| 4 | 2 (0.79) | ||
| 4a | 17 (6.72) | ||
| 4b | 1 (0.40) | ||
| is | 6 (2.37) | ||
| N | |||
| 0 | 93 (36.76) | ||
| 1 | 24 (9.49) | ||
| 1a | 4 (1.58) | ||
| 1b | 4 (1.58) | ||
| 2 | 12 (4.74) | ||
| 2a | 5 (1.98) | ||
| 2b | 20 (7.91) | ||
| 3a | 9 (3.56) | ||
| 3b | 6 (2.37) | ||
| M | |||
| 0 | 175 (69.17) | ||
| 1 | 43 (17.00) | ||
| TNM | |||
| 0 | 6 (2.37) | ||
| I | 57 (22.53) | ||
| II | 50 (19.76) | ||
| III | 67 (26.48) | ||
| IV | 43 (17.00) | ||
| Unknown | 30 (11.86) | ||
All subjects involved in the study provided early morning fasting peripheral blood samples. EDTA was used as an anticoagulant, and after centrifugation at 3500 r/min for 7 min, the patient serum was collected in a new Eppendorf tube. The serum was then dispensed into 3 tubes and labeled and immediately stored in a -80°C. During the collection process, it is necessary to pay attention to the removal of serum samples of hemolysis or lipemia and avoid repeated freezing and thawing during the test. When testing, directly remove the thawed test samples.
Using SPSS 22.0 statistical software, 77 indexes of GC and GP, 64 indexes of GC and Ctrls were analyzed. The serum levels of each index of GC and GP, Ctrls of GC were compared by an independent samples t test[15]. The diagnostic value was evaluated by the area under curve (AUC) of the receiver operating characteristic (ROC), and the cutoff value was determined by the Youden index. The combination of indexes was analyzed by statistical methods, such as binary logistic regression analysis, discriminant analysis, classification tree and artificial neural network[16-21]. P < 0.01 was considered statistically significant.
There were significant differences in 40 indexes between Ctrls vs GC, and 24 indexes had no significant difference; 39 indexes of GP vs GC were significantly different, and 38 indexes had no significant difference. The ROC were generated for 40 indexes with significant differences in Ctrls vs GC and 39 indexes with significant differences between GP vs GC. Among these indexes, the largest AUC in Ctrls vs GC was and ALB, with values of 0.907. When the ALB cutoff value was 42.05, the sensitivity and specificity were 93.0% and 79.1%, respectively. In GP vs GC, the largest AUC was for D-dimer. The AUC value was 0.729. When the D-dimer cutoff value was 0.435, the sensitivity and specificity were 55.3% and 81.2%, respectively.
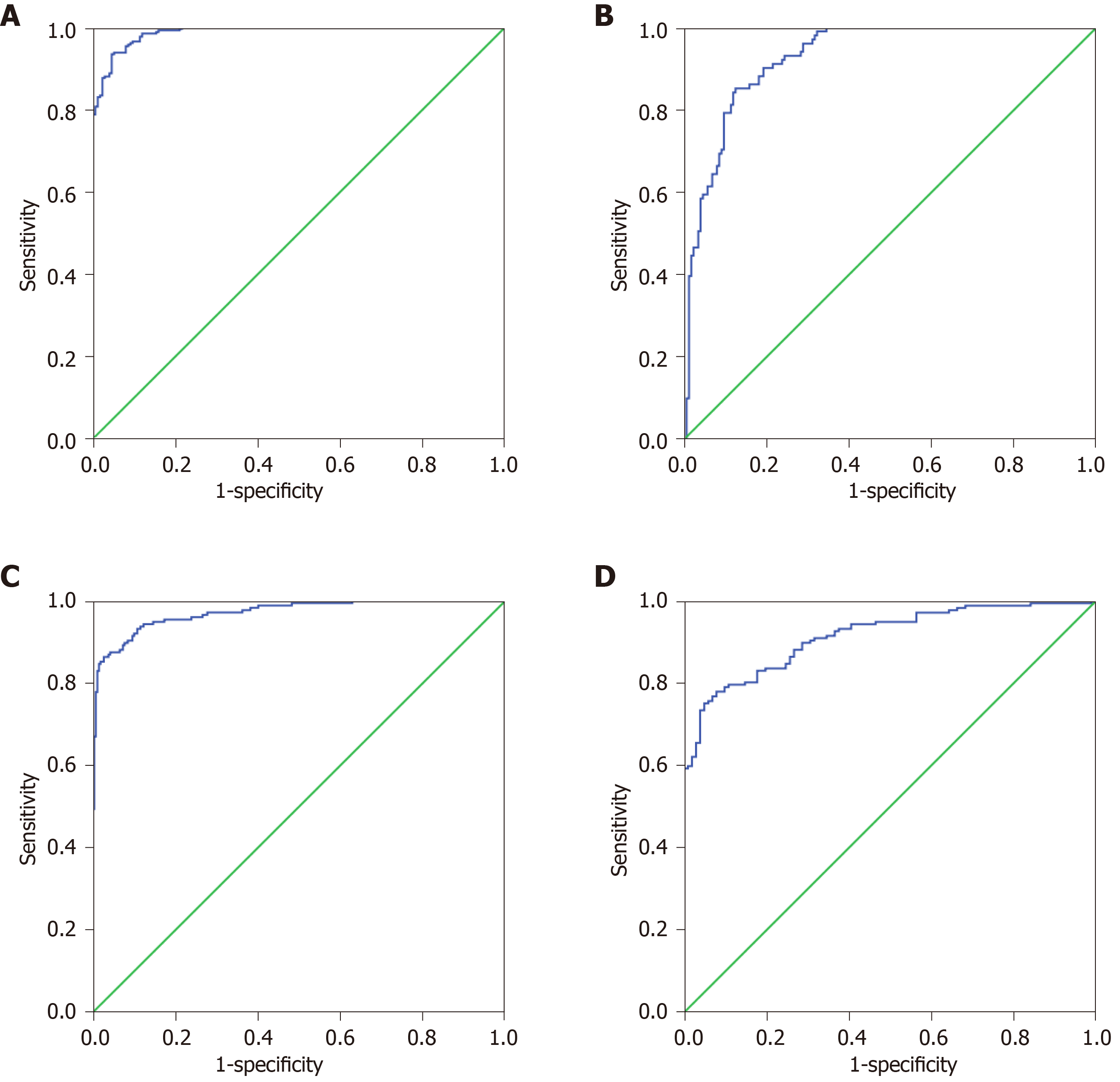
The 27 indexes in Ctrls vs GC and 30 indexes in GP vs GC were used to establish a binary logistic regression analysis model (70% of the data). As shown in Figure 1A, the AUC for Ctrls vs GC was 0.989 (0.982, 0.995). When the cutoff value was 0.675, the sensitivity and specificity were 93.4% and 95.5%, respectively. As shown in Figure 1B, the AUC of GP vs GC was 0.929 (0.901, 0.958), when the cutoff value was 0.477, the sensitivity and specificity were 85.1% and 87.6%, respectively. Binary logistic regression analysis is significantly better than the distinction between Ctrls vs GC for distinguishing GP vs GC. As shown in Figure 1C, the AUC of Ctrls vs GC was 0.971 (0.957, 0.985), and when the cutoff value was 0.470, the sensitivity and specificity were 86.4% and 97.3%, respectively. As shown in Figure 1D, the GP vs GC AUC was 0.914 (0.882, 0.946), and when the cutoff value was 0.462, the sensitivity and specificity were 78.0% and 92.1%, respectively. Discriminant analysis is significantly better than the distinction between Ctrls vs GC for distinguishing GP vs GC.
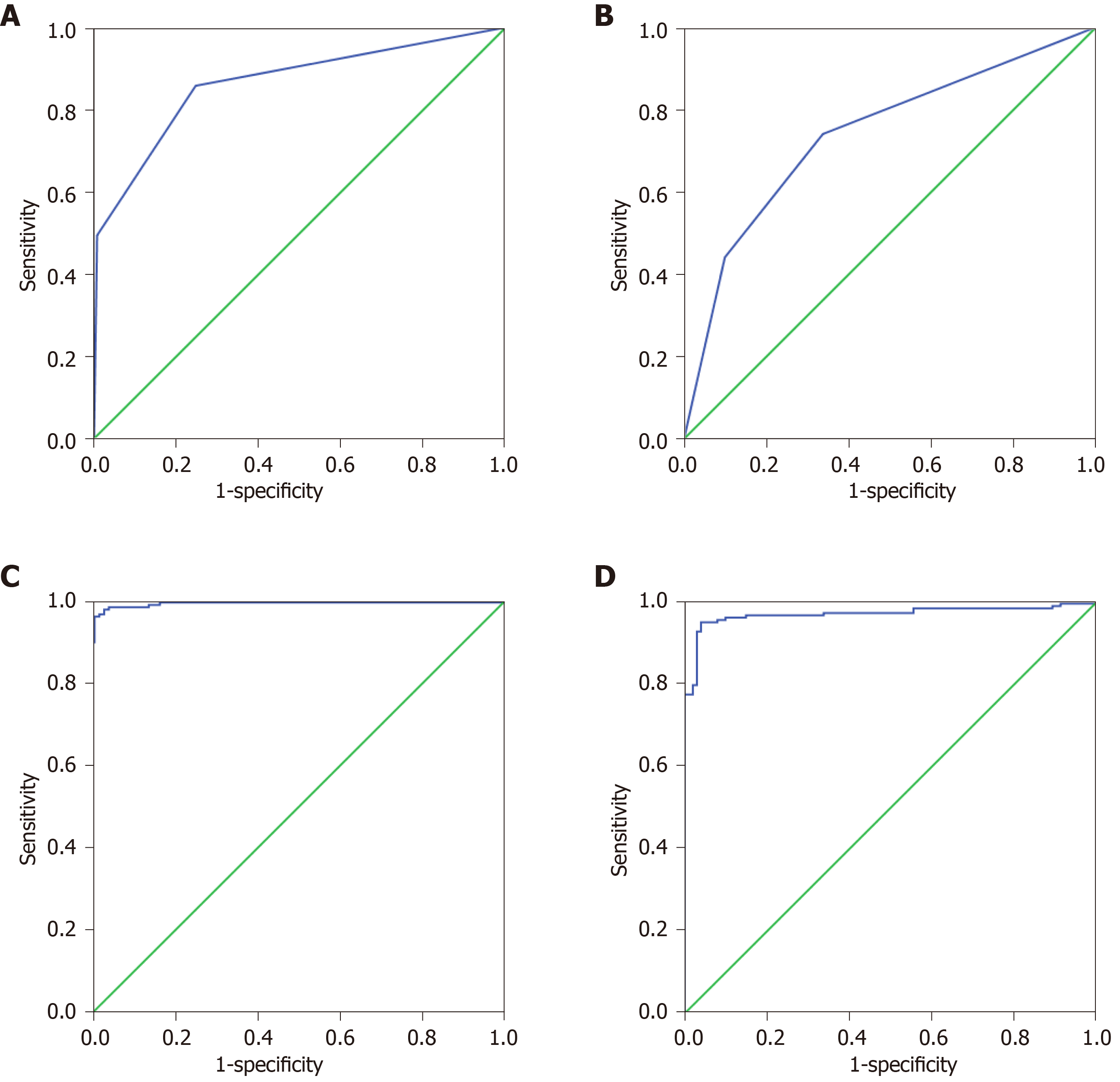
The 27 indexes in Ctrls vs GC and 30 indexes in GP vs GC were used to establish a classification tree analysis model. As shown in Figure 2A, the AUC of Ctrls vs GC was 0.863 (0.826, 0.900), and when the cutoff value was 0.520, the sensitivity and specificity were 74.0% and 76.3%, respectively. The prediction accuracy rate of the Ctrls was 100%, the prediction accuracy rate of the mGC was 48.2%, and the overall prediction accuracy rate was 76.8%. As shown in Figure 2B, the AUC of GP vs GC was 0.739 (0.680, 0.799), and when the cutoff value was 0.290, the sensitivity and specificity were 85.8% and 75.3%, respectively. The predictive accuracy rate of the GP was 62.1%, the correct rate of the GC was 67.8%, and the overall prediction accuracy rate was 65.9%. As shown in Figure 2C, the AUC of Ctrls vs GC was 0.992 (0.980, 1.000). When the cutoff value is 0.837, the sensitivity and specificity were 96.0% and 99.6%, respectively; the prediction accuracy rate of the Ctrls was 97.5%, the prediction accuracy rate of the GC was 84.8%, and the overall prediction accuracy rate was 92.9%. As shown in Figure 2D, the AUC of bGC vs mGC was 0.969 (0.948, 0.990); when the cutoff value was 0.970, the sensitivity and specificity were 94.9% and 96.0%, respectively. The predictive accuracy rate of GP was 71.0%, the predictive accuracy rate of GC was 82.6%, and the overall prediction accuracy rate was 77.9%.
Through saliency analysis and ROC curve analysis, there were 27 indexes in the final Ctrls vs GC with a P value of < 0.01 and 30 indexes in the GP vs GC with a P value of < 0.01. Among these indexes, the maximum AUC of Ctrls vs GC is ALB, and the AUC values were 0.907. The maximum AUC of GP vs GC is D-dimer, and the AUC was 0.729. Pre-ALB levels had been demonstrated to correlate with the outcomes of surgical patients[22,23]. It was usually used to assess the nutritional status. Lots of studies demonstrated that the poor postoperative nutritional status of GC may be related to worse prognosis[24,25]. In our study, we found that it was related to the development of GC. D-dimer is a widely used biomarker for evaluating the ability of coagulation and fibrinolysis, and involved in the progression of cancers[26]. Plasma D-dimer levels was significantly increased in GC patients with distant metastases, and it may be a promising biomarker of detection of GC[27]. In addition, high plasma D-dimer level may also predict poor prognosis in gynecological tumor[28].
With the rapid development of molecular technology, kinds of molecular detection methods had been explored[29-33]. Many statistical methods currently used in the multi-index joint detection analysis of cancer[15,21,34-36], such as binary logistic regression, discriminant analysis, classification tree and artificial neural network, have achieved good results[16-20]. For example, the artificial neural network model was applied in lung cancer-assisted diagnosis, and the effects of back-propagation neural network and Fisher discriminant model on lung cancer screening were compared by the joint detection of four biomarkers. The results showed that the back-propagation neural network predicts lung cancer model better than the Fisher discriminant analysis, which can provide excellent and intelligent diagnostic tools for lung cancer[37]. Li et al[38] used binary logistic regression analysis to analyze various cytokines in serum for the early detection of GC. Feng et al[39] used the ANN model established by six serum tumor markers to distinguish lung cancer, to identify not only benign lung diseases and normal people but also three common gastrointestinal cancers. These results showed that the artificial neural network model may be an excellent intelligent system to distinguish lung cancer[39]. Su et al[40] applied a classification decision tree model to distinguish between GC and healthy controls. This model is able to distinguish between GC patients and healthy volunteers. The sensitivity in the training set is 95.6%, and the specificity is 92.0%. In the blinded group, this model was able to distinguish GC samples from other samples with a specificity of 88.0%, a sensitivity of 85.3%, and an accuracy of 86.4%. By measuring serum CEA and CA19-9 together, these values were higher than those obtained in the parallel analysis. Therefore, a decision tree analysis demonstrating a serum proteomics model is likely to be used for the diagnosis of GC[40].
For distinguishing Ctrls vs GC, binary logistic regression, discriminant analysis, classification tree analysis and artificial neural network were significantly better than GP vs GC. Binary logistic regression, discriminant analysis and artificial neural network analysis of the ROC curve AUC and the maximum cutoff value corresponding to the sensitivity and specificity were greater than the AUC maximum single index. Therefore, the diagnostic effect of multiparameter joint analysis is significantly better than that of the single-index test. Through the comparison of these four methods, we have the ability to distinguish Ctrls vs mGC, bGC vs mGC, artificial neural network > binary logistic regression > discriminant analysis > classification tree. However, the results may be effected because of the relatively little sample size and lack of independent validation of the model which was built in our study. We propose that the artificial neural network analysis method has good prospects for the multi-index joint detection of tumors, and further research in this area should be carried out in the future.
Tumor markers are increased in the blood in early gastric cancer (GC). The levels of these markers have been used as important indexes for GC screening, early diagnosis and prognostic evaluation.
Specific tumor markers have not yet been discovered. Diagnosis based on a single tumor marker has limited significance. The detection rate of GC is still very low.
In this study, we aimed to improve the diagnostic value of blood markers for GC.
In this study, to distinguish between healthy controls (Ctrls) vs GC, gastric polyp (GP) and GC, we analyzed the routine blood detection indexes of GC diagnosis by using binary logistic regression, discriminant analysis, classification tree and artificial neural network.
By analyzing the data, there are 27 indexes in the final Ctrls vs GC with P values < 0.01, the area under the curve (AUC) of albumin is the largest in Ctrls vs GC, and the AUC was 0.907. For 30 indexes in GP vs GC have P values < 0.01. Among them, the D-dimer showed an AUC of 0.729. The 27 indexes in Ctrls vs GC and 30 indexes in GP vs GC were used for binary logistic regression, discriminant analysis, classification tree analysis and artificial neural network analysis model. The overall prediction accuracy was 92.9%, and the AUC was 0.992 (0.980, 1.000).
The diagnostic effect of multi-parameter joint artificial neural networks analysis is significantly better than the single-index test diagnosis, and it may provide an assistant method for the detection of GC.
We propose that the artificial neural network analysis method has good prospects for the multi-index joint detection of tumors, and further research in this area should be carried out in the future.
| 1. | Ning FL, Zhang CD, Wang P, Shao S, Dai DQ. Endoscopic resection versus radical gastrectomy for early gastric cancer in Asia: A meta-analysis. Int J Surg. 2017;48:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Zheng Q, Chen C, Guan H, Kang W, Yu C. Prognostic role of microRNAs in human gastrointestinal cancer: A systematic review and meta-analysis. Oncotarget. 2017;8:46611-46623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | Kalisperati P, Spanou E, Pateras IS, Korkolopoulou P, Varvarigou A, Karavokyros I, Gorgoulis VG, Vlachoyiannopoulos PG, Sougioultzis S. Inflammation, DNA Damage, Helicobacter pylori and Gastric Tumorigenesis. Front Genet. 2017;8:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 4. | Maleki SS, Röcken C. Chromosomal Instability in Gastric Cancer Biology. Neoplasia. 2017;19:412-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Sunakawa Y, Lenz HJ. Molecular classification of gastric adenocarcinoma: translating new insights from the cancer genome atlas research network. Curr Treat Options Oncol. 2015;16:17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Futawatari N, Fukuyama T, Yamamura R, Shida A, Takahashi Y, Nishi Y, Ichiki Y, Kobayashi N, Yamazaki H, Watanabe M. Early gastric cancer frequently has high expression of KK-LC-1, a cancer-testis antigen. World J Gastroenterol. 2017;23:8200-8206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Ahn S, Park DY. Practical Points in Gastric Pathology. Arch Pathol Lab Med. 2016;140:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Beeharry MK, Liu WT, Yan M, Zhu ZG. New blood markers detection technology: A leap in the diagnosis of gastric cancer. World J Gastroenterol. 2016;22:1202-1212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Pasechnikov V, Chukov S, Fedorov E, Kikuste I, Leja M. Gastric cancer: prevention, screening and early diagnosis. World J Gastroenterol. 2014;20:13842-13862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 247] [Cited by in RCA: 308] [Article Influence: 25.7] [Reference Citation Analysis (2)] |
| 10. | Uedo N, Yao K. Endoluminal Diagnosis of Early Gastric Cancer and Its Precursors: Bridging the Gap Between Endoscopy and Pathology. Adv Exp Med Biol. 2016;908:293-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Tsai MM, Wang CS, Tsai CY, Huang HW, Chi HC, Lin YH, Lu PH, Lin KH. Potential Diagnostic, Prognostic and Therapeutic Targets of MicroRNAs in Human Gastric Cancer. Int J Mol Sci. 2016;17:945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 12. | Tong W, Ye F, He L, Cui L, Cui M, Hu Y, Li W, Jiang J, Zhang DY, Suo J. Serum biomarker panels for diagnosis of gastric cancer. Onco Targets Ther. 2016;9:2455-2463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Feng F, Tian Y, Xu G, Liu Z, Liu S, Zheng G, Guo M, Lian X, Fan D, Zhang H. Diagnostic and prognostic value of CEA, CA19-9, AFP and CA125 for early gastric cancer. BMC Cancer. 2017;17:737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 285] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 14. | Zhang Q, Qu H, Sun G, Li Z, Ma S, Shi Z, Zhao E, Zhang H, He Q. Early postoperative tumor marker responses provide a robust prognostic indicator for N3 stage gastric cancer. Medicine (Baltimore). 2017;96:e7560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Xu W, Zhao Y, Nian S, Feng L, Bai X, Luo X, Luo F. Differential analysis of disease risk assessment using binary logistic regression with different analysis strategies. J Int Med Res. 2018;46:3656-3664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Bicciato S. Artificial neural network technologies to identify biomarkers for therapeutic intervention. Curr Opin Mol Ther. 2004;6:616-623. [PubMed] |
| 17. | Berger RP, Beers SR, Richichi R, Wiesman D, Adelson PD. Serum biomarker concentrations and outcome after pediatric traumatic brain injury. J Neurotrauma. 2007;24:1793-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 134] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Lin KC, Wu HP, Huang CY, Lin CY, Chang CF. Discriminant analysis of serum inflammatory biomarkers which differentiate pediatric appendicitis from other acute abdominal diseases. Acta Paediatr Taiwan. 2007;48:125-130. [PubMed] |
| 19. | Navaglia F, Fogar P, Basso D, Greco E, Padoan A, Tonidandel L, Fadi E, Zambon CF, Bozzato D, Moz S, Seraglia R, Pedrazzoli S, Plebani M. Pancreatic cancer biomarkers discovery by surface-enhanced laser desorption and ionization time-of-flight mass spectrometry. Clin Chem Lab Med. 2009;47:713-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Liu Z, Lin S, Tan MT. Sparse support vector machines with Lp penalty for biomarker identification. IEEE/ACM Trans Comput Biol Bioinform. 2010;7:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Zhang P, Zou M, Wen X, Gu F, Li J, Liu G, Dong J, Deng X, Gao J, Li X, Jia X, Dong Z, Chen L, Wang Y, Tian Y. Development of serum parameters panels for the early detection of pancreatic cancer. Int J Cancer. 2014;134:2646-2655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Saito H, Kono Y, Murakami Y, Shishido Y, Kuroda H, Matsunaga T, Fukumoto Y, Osaki T, Ashida K, Fujiwara Y. Prognostic Significance of the Preoperative Ratio of C-Reactive Protein to Albumin and Neutrophil-Lymphocyte Ratio in Gastric Cancer Patients. World J Surg. 2018;42:1819-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 23. | Saito H, Kono Y, Murakami Y, Shishido Y, Kuroda H, Matsunaga T, Fukumoto Y, Osaki T, Ashida K, Fujiwara Y. Postoperative Serum Albumin is a Potential Prognostic Factor for Older Patients with Gastric Cancer. Yonago Acta Med. 2018;61:72-78. [PubMed] |
| 24. | Jin Y, Yong C, Ren K, Li D, Yuan H. Effects of Post-Surgical Parenteral Nutrition on Patients with Gastric Cancer. Cell Physiol Biochem. 2018;49:1320-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Wu M, Pan Y, Jia Z, Wang Y, Yang N, Mu J, Zhou T, Guo Y, Jiang J, Cao X. Preoperative Plasma Fibrinogen and Serum Albumin Score Is an Independent Prognostic Factor for Resectable Stage II-III Gastric Cancer. Dis Markers. 2019;2019:9060845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Dai H, Zhou H, Sun Y, Xu Z, Wang S, Feng T, Zhang P. D-dimer as a potential clinical marker for predicting metastasis and progression in cancer. Biomed Rep. 2018;9:453-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Repetto O, De Re V. Coagulation and fibrinolysis in gastric cancer. Ann N Y Acad Sci. 2017;1404:27-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (1)] |
| 28. | Xu L, He F, Wang H, Gao B, Wu H, Zhao S. A high plasma D-dimer level predicts poor prognosis in gynecological tumors in East Asia area: a systematic review and meta-analysis. Oncotarget. 2017;8:51551-51558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Gao W, Long L, Tian X, Xu F, Liu J, Singh PK, Botella JR, Song C. Genome Editing in Cotton with the CRISPR/Cas9 System. Front Plant Sci. 2017;8:1364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 30. | Guo J, Li K, Jin L, Xu R, Miao K, Yang F, Qi C, Zhang L, Botella JR, Wang R, Miao Y. A simple and cost-effective method for screening of CRISPR/Cas9-induced homozygous/biallelic mutants. Plant Methods. 2018;14:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Lei KJ, Lin YM, An GY. miR156 modulates rhizosphere acidification in response to phosphate limitation in Arabidopsis. J Plant Res. 2016;129:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Sun Q, Qiao J, Zhang S, He S, Shi Y, Yuan Y, Zhang X, Cai Y. Changes in DNA methylation assessed by genomic bisulfite sequencing suggest a role for DNA methylation in cotton fruiting branch development. PeerJ. 2018;6:e4945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Yu J, Zhang Y, Liu J, Wang L, Liu P, Yin Z, Guo S, Ma J, Lu Z, Wang T, She Y, Miao Y, Ma L, Chen S, Li Y, Dai S. Proteomic discovery of H2O2 response in roots and functional characterization of PutGLP gene from alkaligrass. Planta. 2018;248:1079-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Liu MM, Wen L, Liu YJ, Cai Q, Li LT, Cai YM. Application of data mining methods to improve screening for the risk of early gastric cancer. BMC Med Inform Decis Mak. 2018;18:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | Mohammadzadeh F, Noorkojuri H, Pourhoseingholi MA, Saadat S, Baghestani AR. Predicting the probability of mortality of gastric cancer patients using decision tree. Ir J Med Sci. 2015;184:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Zhang Y, Liu Y, Zhang J, Wu X, Ji X, Fu T, Li Z, Wu Q, Bu Z, Ji J. Construction and external validation of a nomogram that predicts lymph node metastasis in early gastric cancer patients using preoperative parameters. Chin J Cancer Res. 2018;30:623-632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Duan X, Yang Y, Tan S, Wang S, Feng X, Cui L, Feng F, Yu S, Wang W, Wu Y. Application of artificial neural network model combined with four biomarkers in auxiliary diagnosis of lung cancer. Med Biol Eng Comput. 2017;55:1239-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Li J, Xu L, Run ZC, Feng W, Liu W, Zhang PJ, Li Z. Multiple cytokine profiling in serum for early detection of gastric cancer. World J Gastroenterol. 2018;24:2269-2278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Feng F, Wu Y, Wu Y, Nie G, Ni R. The effect of artificial neural network model combined with six tumor markers in auxiliary diagnosis of lung cancer. J Med Syst. 2012;36:2973-2980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 40. | Su Y, Shen J, Qian H, Ma H, Ji J, Ma H, Ma L, Zhang W, Meng L, Li Z, Wu J, Jin G, Zhang J, Shou C. Diagnosis of gastric cancer using decision tree classification of mass spectral data. Cancer Sci. 2007;98:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Avalos-Gonzalez J, Chivu-Economescu M, Ryan EM, Schmidt J S-Editor: Wang JL L-Editor: A E-Editor: Xing YX