Published online Mar 15, 2020. doi: 10.4251/wjgo.v12.i3.358
Peer-review started: November 2, 2019
First decision: November 22, 2019
Revised: December 31, 2019
Accepted: January 14, 2020
Article in press: January 14, 2020
Published online: March 15, 2020
Processing time: 131 Days and 4.7 Hours
Multi-phase computed tomography (CT) or magnetic resonance imaging (MRI) has been the standard of care for hepatocellular carcinoma (HCC) diagnosis for years.
We report a case series of four patients in whom positron emission tomography-computed tomography (PET-CT) scan complemented the conventional CT/MRI scans in evaluating treatment response. In these four cases the conventional multi-phase CT and MRI failed to identify residual HCC disease post-treatment, while PET-CT complemented and aided in treatment response evaluation. In each case, the addition of PET-CT identified and located residual HCC disease, allowed retreatment, and altered medical management.
This case series suggests that PET-CT should perhaps play a role in the HCC management algorithm, in addition to the conventional contrast-enhanced multi-phase scans.
Core tip: This is a case series of four hepatocellular carcinoma patients who had undergone locoregional therapies. The conventional multi-phase computed tomography and magnetic resonance imaging scans failed to identify residual hepatocellular carcinoma disease post-treatment, while positron emission tomography-computed tomography scan complemented in treatment response evaluation by identifying and locating residual disease, allowing retreatment.
- Citation: Cheng JT, Tan NE, Volk ML. Utility of positron emission tomography-computed tomography scan in detecting residual hepatocellular carcinoma post treatment: Series of case reports. World J Gastrointest Oncol 2020; 12(3): 358-364
- URL: https://www.wjgnet.com/1948-5204/full/v12/i3/358.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i3.358
Hepatocellular carcinoma (HCC) is a well-known complication of chronic liver disease and cirrhosis. It has remained as one of the leading causes of death worldwide[1], responsible for nearly 746000 deaths in 2012[1]. It is the second most common cause of death from cancer globally[1,2]. The incidence of HCC in the United States has been rising in the past four decades[3-5]. Multi-phase computed tomography (CT) or magnetic resonance imaging (MRI) has been the standard of care for HCC diagnosis for years[6]. HCC lesions are known to display arterial enhancement and delayed washout on multi-phase CT or MRI[7]. These contrast-enhanced multi-phase cross-sectional imaging modalities have also been utilized for follow-up on known cases of HCC, especially in determining the response to treatment[8]. Positron emission tomography (PET) scan has been considered unreliable as an imaging modality for HCC diagnosis and for treatment response follow-up due to its lack of sensitivity[9,10]. Many HCC tumors do not show up on PET scan[11]. This case series intends to describe cases in which PET scan complemented the conventional multi-phase CT or MRI in evaluating treatment response.
(1) Case 1: A 62-year-old male with known hepatitis C cirrhosis self-referred to our liver center for further management; (2) Case 2: A 69-year-old male with cryptogenic cirrhosis was referred to our liver center with a 3.3 cm liver lesion in segment 6/7 that appeared to be hypodense without enhancement on a multi-phase CT scan; (3) Case 3: A 62-year-old male with compensated cirrhosis secondary to chronic hepatitis C was referred to our center with HCC tumors based on outside MRI; and (4) Case 4: A 75-year-old female with chronic hepatitis C and compensated cirrhosis was referred to our center due to two HCC tumors, 8.4 cm and 1.2 cm based on multi-phase MRI.
(1) Case 1: The patient was discovered to have HCC upon routine surveillance multi-phase CT, with original tumor burden of 4.2 cm in segment 3 cm and 2.6 cm in segment 5/6. He then received multiple trans-arterial chemo-embolization (TACE) treatments to both lobes of the liver; (2) Case 2: Our multi-disciplinary liver tumor board subsequently reviewed the outside CT scan and confirmed the findings of a non-enhancing hypodense liver lesion. Alpha fetoprotein (AFP) was less than 10 ng/mL. The tumor board recommended a biopsy, which revealed poorly differentiated HCC, based on histological characteristics and immunohistochemical staining. The patient underwent TACE; (3) Case 3: The patient was subsequently treated for HCC tumors (2.1 cm in segment 8, and 1.8 cm and 1.2 cm in segment 6) with two TACE treatments and one microwave ablation (MWA). He was also listed for liver transplant, and a PET/CT scan was done to rule out lung metastasis. He had had tuberculosis, successfully treated many years ago; a chest CT had shown a cavitary lesion within some infiltrate in the right upper lung; and (4) Case 4: The patient’s AFP remained normal in the single digit (ng/mL) at baseline. She subsequently underwent TACE and proton treatments as recommended by our tumor board. The patient underwent multi-phase MRI for monitoring treatment response every three to four months subsequently, and was deemed in complete response for more than two years after the second treatment with proton. She also underwent hepatitis C treatment successfully and achieved sustained virologic response with negative viral titer more than two years from the end of treatment.
(1) Case 1: Negative for diabetes or cardiac disease; (2) Case 2: Diabetes mellitus type 2, atrial fibrillation, skin cancer, esophageal varices; (3) Case 3: Tuberculosis; and (4) Case 4: Diabetes mellitus type 2, and atrial fibrillation.
(1) Non-contributory (Case 1, 3, 4); and (2) He was exposed to agent orange in the early 1970s (in his 20s) (Case 2).
Anicteric; abdomen soft, non-distended, and non-tender to palpation; liver not palpable; no asterixis (Case 1, 2, 3, 4).
(1) Case 1: AFP rose to 7344 ng/mL approximately 16 mo after presentation; it raised concerns of extrahepatic metastasis, though recent bone scan and chest CT were both negative; (2) Case 2: The patient’s AFP remained low throughout his course, 9.4 ng/mL at the time of the PET/CT scan; his total bilirubin was mildly elevated 2.3 mg/dL while his albumin remained normal, 3.8 g/dL; international normalized ratio (INR) was 1.2; (3) Case 3: The patient’s AFP remained normal throughout his course, 3.8 ng/mL at the time of the PET/CT scan; his albumin remained normal, 3.8 g/dL, while his total bilirubin was slightly elevated 1.5 mg/dL; INR remained normal 1.1; and (4) Case 4: AFP started to increase about 31 mo after presentation, to 24.7 ng/mL, and later to 75 ng/mL in month 36. Total bilirubin had remained normal 0.3 mg/dL, and so had albumin 3.7 g/dL.
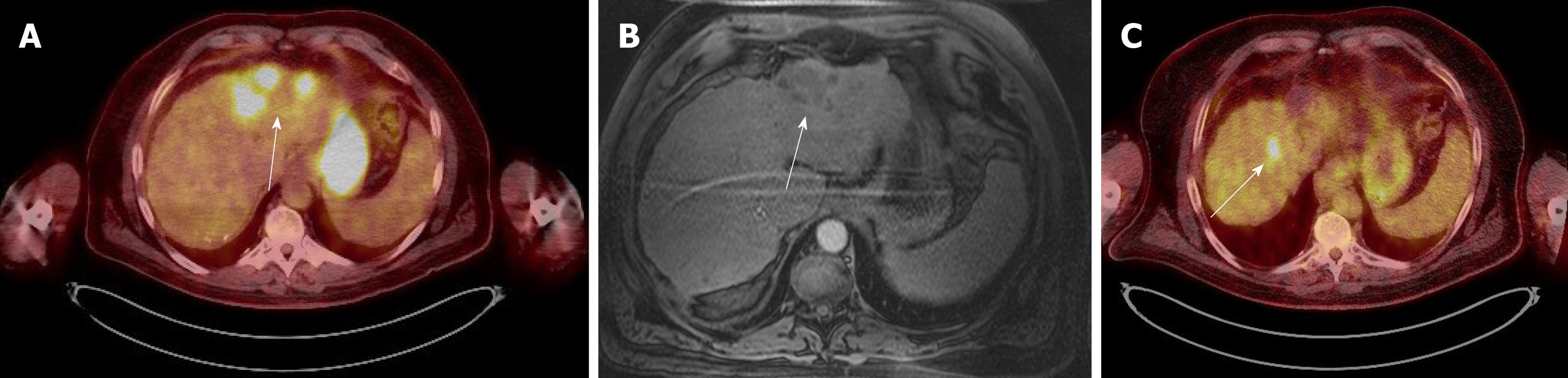
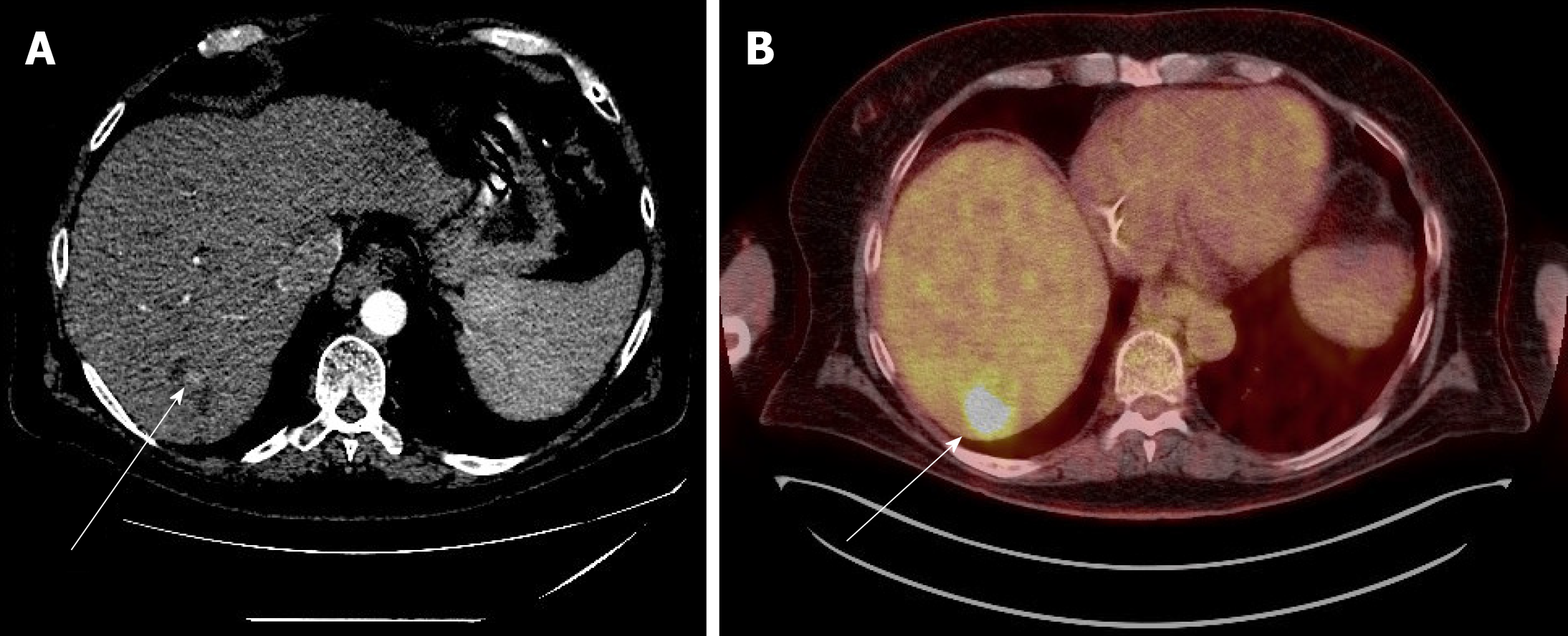
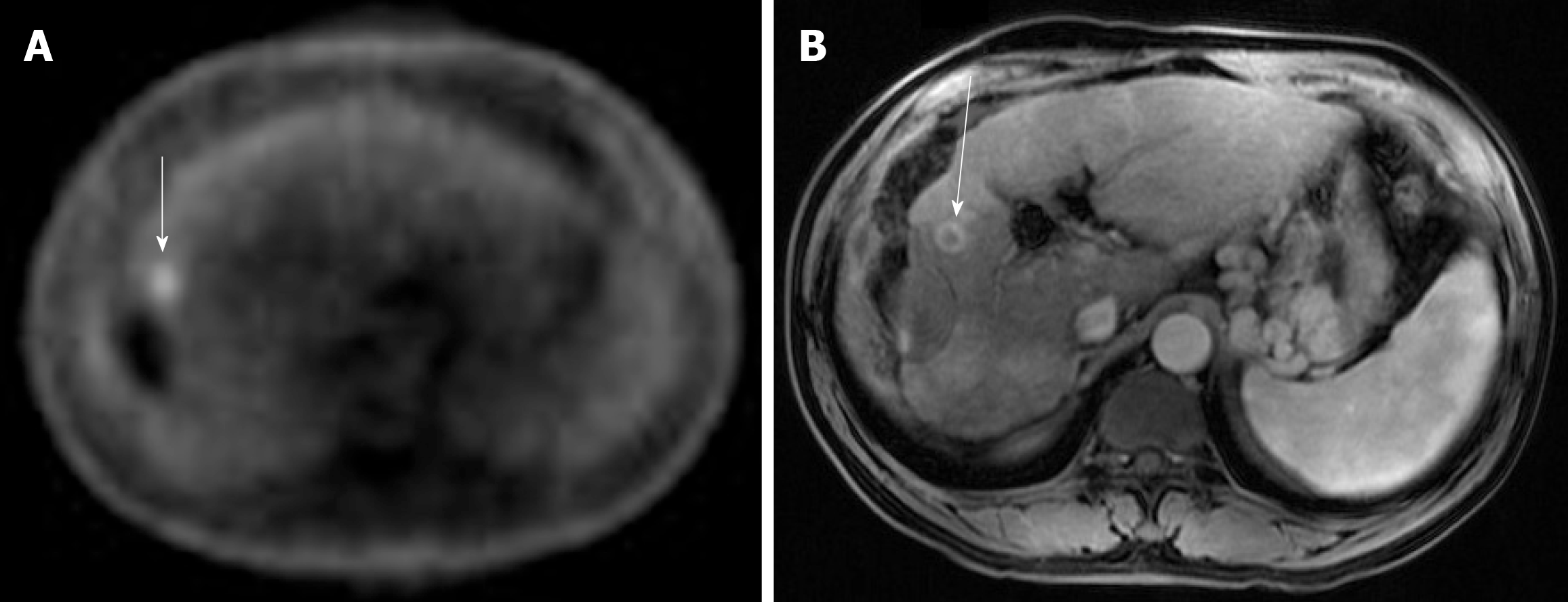
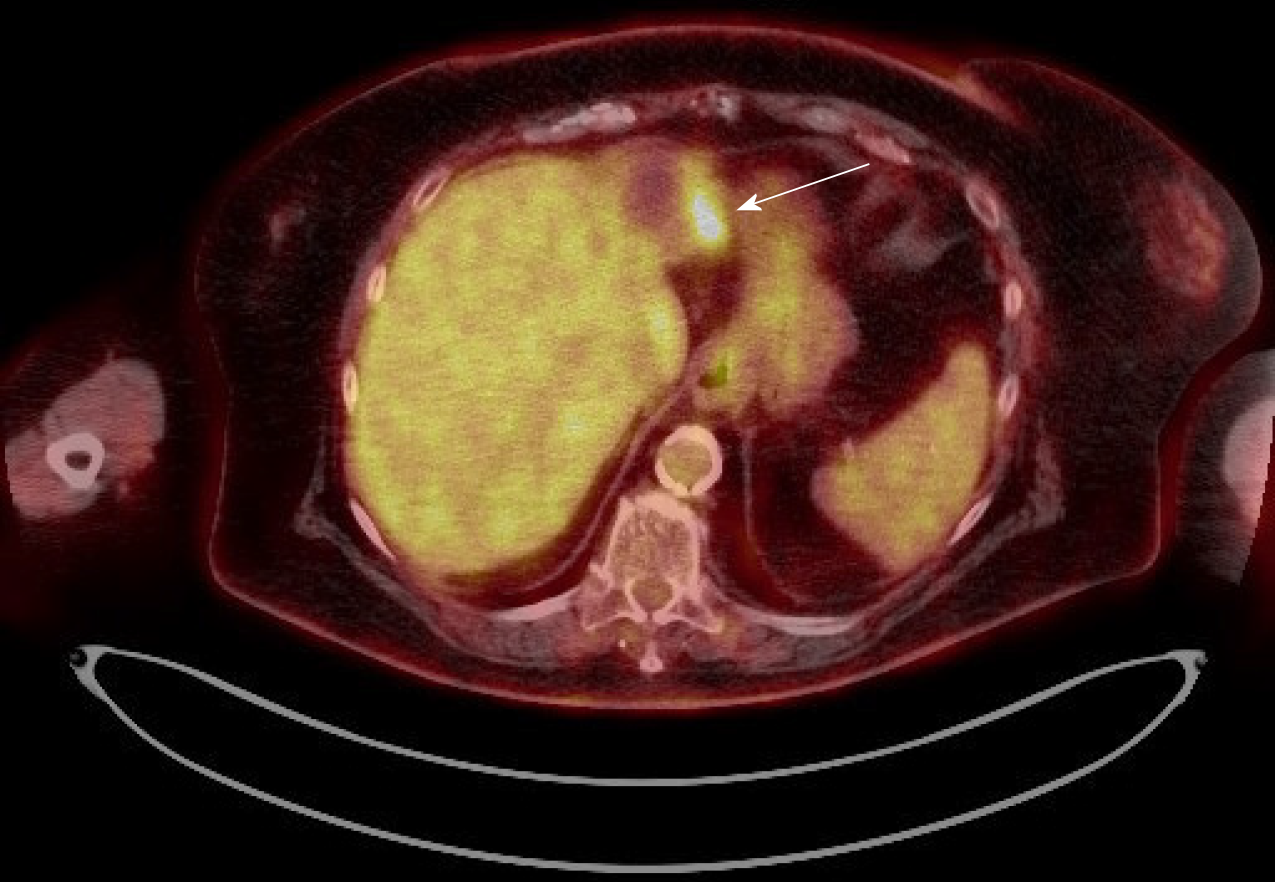
Case 1: A PET-CT scan was done to search for metastasis, and it revealed three foci of increased fludeoxyglucose (FDG) activity within the treated area of segment 3 (Figure 1A), while showing no FDG activity in the treated area of segment 5/6; no metastasis was identified. A repeat multi-phase MRI was done concurrently, and it failed to reveal any arterial enhancement in the liver (Figure 1B); (2) Case 2: The one-month post-TACE multi-phase CT scan was again inconclusive, showing a 4.2 cm hypodense lesion (Figure 2A) similar to the pre-TACE CT scan. A PET-CT scan was performed, revealing an FDG avid uptake of 3.5 cm × 3.2 cm in measurement at the same area of the liver (Figure 2B) previously biopsied and treated, consistent with residual HCC; (3) Case 3: The PET/CT scan 16 wk post MWA incidentally showed a small site of localized metabolic activity corresponding to a low-density lesion adjacent to a larger right hepatic lobe mass which demonstrated absent metabolic activity, consistent with residual HCC in the treated segment-6 lesion (Figure 3A); no FDG activity in the lungs or elsewhere. A multi-phase MRI a few weeks prior had revealed focal bleed at the periphery of the treatment zone post MWA without evidence of any viable tumor (Figure 3B); and (4) Case: A multi-phase MRI in month 36 was negative for any arterial enhancement, but concurrently a PET-CT scan in month 36 revealed positive FDG uptake at the periphery of the treated lesion in segment 2/3 (Figure 4).
Recurrent HCC post locoregional therapies (Case 1, 2, 3, 4).
The patient then underwent more TACE treatments. Both multi-phase MRI scans and PET-CT scans were utilized to monitor treatment response. PET-CT scans subsequently showed residual disease in the left lobe. Treatment modality was changed to proton after the fourth TACE to the segment-3 HCC, approximately 29 mo after presentation.
The patient underwent and completed a course of proton treatment consisted of 15 fractions.
The patient underwent third TACE approximately five months after the MWA.
The patient opted out of recommended laparoscopic ablation, citing her advanced age and the invasiveness of the proposed procedure. She later elected to start nivolumab infusion.
The patient’s AFP responded from 3841 ng/mL before the proton treatment to 7 ng/mL after proton. He remained in complete response based on both multi-phase MRI and PET-CT scans every three months until approximately 41 mo after presentation when a new focus of FDG uptake was seen in the dome; a concurrent multi-phase MRI again failed to reveal any arterial enhancement. The dome lesion was treated with proton. Both PET-CT and multi-phase MRI three months post-proton showed the dome lesion well treated, but there was a recurrent HCC focus with arterial enhancement and washout, as well as FDG uptake (Figure 1C), at the previously treated area in segment 6. The patient received proton treatment to segment 6 approximately 48 mo after presentation. The PET-CT scans aided in detecting HCC for this patient and allowed appropriate treatments to prolong his survival. He was followed at our center for a total of 52 mo.
The patient was followed up at our center for a total 9 mo. After the proton therapy, he decided to follow up with another institution closer to his residence.
Both multi-phase MRI and PET-CT scans one-month post-TACE showed no residual HCC in the liver. The patient was followed up at our center for a total of 37 mo.
The patient has tolerated nivolumab infusion well for 14 mo, currently on 2 mg/kg every 2 wk. She has been followed at our center for a total of 58 mo.
We have described a series of four cases in which the conventional multi-phase CT and MRI failed to identify residual HCC disease post-treatment, while the FDG PET-CT scan aided in evaluating treatment response (Table 1). In all these cases, FDG PET-CT scans detected residual HCC tumors in treatment zone status post locoregional therapy while the contrast-enhanced multiphase scans could not, and these allowed for timely treatment and meaningful survival.
| Case | Indication for PET-CT | How PET-CT changed management |
| Case 1 | To rule out metastatic HCC while AFP in the 7000s; chest CT and bone scan had been negative | The PET-CT scans successfully detected residual HCC in the treated areas in both lobes and allowed for appropriate treatments to prolong his survival by at least 36 mo; multiple multi-phase MRI scans failed to do so. Subsequently PET-CT scan subsequently detected a new HCC lesion when MRI did not |
| Case 2 | To evaluate treatment response in a biopsy-proven hepatocellular carcinoma mixed with poorly differentiated carcinoma, which had had atypical characteristics on multi-phase CTs (MRI was contraindicated due to his pacemaker) | The PET-CT scan successfully revealed residual carcinoma and allowed for further treatment in prolonging survival |
| Case 3 | To rule out metastatic HCC disease to the lungs in which anatomy had been distorted due to prior Tb infection | The PET-CT scan successfully detected residual HCC while a multi-phase MRI failed to do so. The PET-CT scan subsequently detected a metastatic focus to the bone and averted liver transplant |
| Case 4 | To aid in the investigation of rising AFP in a treated HCC patient when multi-phase MRI scans had been negative | The PET-CT scan identified a recurrent HCC focus in the periphery of a previously treated HCC tumor location |
Cirrhosis occasionally could alter the vasculature and distort the manifestation of arterial enhancement and delayed washout in HCC tumors via multi-phase CT or MRI, thereby decreasing the sensitivity and specificity of these contrast-enhanced imaging modalities[6,12], not to mention when these HCC tumors have been treated with locoregional therapies or even adjuvant systemic therapy (Case 1). In our case series PET-CT scans appeared very useful when the AFP was elevated and the contrast-enhanced scans did not reveal any pathognomonic findings in treated tumors. The utility and strengths of PET-CT scans are likely underestimated since it is not part of the standard of care in screening for and monitoring HCC, even in the latest United States guidelines[13,14]; it is certainly not part of our institution’s protocol yet. There have been several studies describing the efficacy of combining the traditional 18F-FDG isotope with another isotope, 11C-acetate, in the utility of PET-CT scan in the detection of HCC[15-19]. This dual-tracer approach appears to be quite promising in complementing multi-phase CT or MRI scans, as well as FDG PET-CT scan.
PET-CT scans can be very helpful in select HCC cases for monitoring of treatment response, especially when contrast-enhanced multi-phase scans fail to identify pathognomonic findings of residual HCC tumors. A prospective study comparing the addition of dual-tracer PET-CT scan to the conventional multi-phase CT or MRI, vs multi-phase CT or MRI alone in detecting HCC tumors, is needed to improve the evaluation of treatment response in this disease.
| 1. | World Health Organization. Cancer Fact Sheets; 2018. Available from: https://gco.iarc.fr/today/fact-sheets-cancers. |
| 2. | Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47 Suppl:S2-S6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 894] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 3. | El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2221] [Cited by in RCA: 2135] [Article Influence: 79.1] [Reference Citation Analysis (1)] |
| 4. | El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:817-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 686] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 5. | Shaw JJ, Shah SA. Rising incidence and demographics of hepatocellular carcinoma in the USA: what does it mean? Expert Rev Gastroenterol Hepatol. 2011;5:365-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Sangiovanni A, Manini MA, Iavarone M, Romeo R, Forzenigo LV, Fraquelli M, Massironi S, Della Corte C, Ronchi G, Rumi MG, Biondetti P, Colombo M. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut. 2010;59:638-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 312] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 7. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6629] [Article Influence: 441.9] [Reference Citation Analysis (1)] |
| 8. | Hennedige T, Venkatesh SK. Imaging of hepatocellular carcinoma: diagnosis, staging and treatment monitoring. Cancer Imaging. 2013;12:530-547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 9. | Khan MA, Combs CS, Brunt EM, Lowe VJ, Wolverson MK, Solomon H, Collins BT, Di Bisceglie AM. Positron emission tomography scanning in the evaluation of hepatocellular carcinoma. J Hepatol. 2000;32:792-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 295] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 10. | Wolfort RM, Papillion PW, Turnage RH, Lillien DL, Ramaswamy MR, Zibari GB. Role of FDG-PET in the evaluation and staging of hepatocellular carcinoma with comparison of tumor size, AFP level, and histologic grade. Int Surg. 2010;95:67-75. [PubMed] |
| 11. | Teefey SA, Hildeboldt CC, Dehdashti F, Siegel BA, Peters MG, Heiken JP, Brown JJ, McFarland EG, Middleton WD, Balfe DM, Ritter JH. Detection of primary hepatic malignancy in liver transplant candidates: prospective comparison of CT, MR imaging, US, and PET. Radiology. 2003;226:533-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 134] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Yu NC, Chaudhari V, Raman SS, Lassman C, Tong MJ, Busuttil RW, Lu DS. CT and MRI improve detection of hepatocellular carcinoma, compared with ultrasound alone, in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 13. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3144] [Article Influence: 393.0] [Reference Citation Analysis (3)] |
| 14. | Roberts LR, Sirlin CB, Zaiem F, Almasri J, Prokop LJ, Heimbach JK, Murad MH, Mohammed K. Imaging for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. Hepatology. 2018;67:401-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 362] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 15. | Ho CL, Yu SC, Yeung DW. 11C-acetate PET imaging in hepatocellular carcinoma and other liver masses. J Nucl Med. 2003;44:213-221. [PubMed] |
| 16. | Ho CL, Chen S, Yeung DW, Cheng TK. Dual-tracer PET/CT imaging in evaluation of metastatic hepatocellular carcinoma. J Nucl Med. 2007;48:902-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 155] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 17. | Park JW, Kim JH, Kim SK, Kang KW, Park KW, Choi JI, Lee WJ, Kim CM, Nam BH. A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma. J Nucl Med. 2008;49:1912-1921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 219] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 18. | Hwang KH, Choi DJ, Lee SY, Lee MK, Choe W. Evaluation of patients with hepatocellular carcinomas using [(11) C] acetate and [(18) F] FDG PET/CT: A preliminary study. Appl Radiat Isot. 2009;67:1195-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Cheung TT, Ho CL, Lo CM, Chen S, Chan SC, Chok KS, Fung JY, Yan Chan AC, Sharr W, Yau T, Poon RT, Fan ST. 11C-acetate and 18F-FDG PET/CT for clinical staging and selection of patients with hepatocellular carcinoma for liver transplantation on the basis of Milan criteria: surgeon's perspective. J Nucl Med. 2013;54:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bramhall SR, Corrales FJ, Guo JS, Niu ZS, Sun XT, Tajiri K S-Editor: Zhang L L-Editor: A E-Editor: Qi LL