Published online Mar 15, 2020. doi: 10.4251/wjgo.v12.i3.289
Peer-review started: September 6, 2019
First decision: October 14, 2019
Revised: November 18, 2019
Accepted: January 6, 2020
Article in press: January 6, 2020
Published online: March 15, 2020
Processing time: 187 Days and 20.3 Hours
The single nucleotide polymorphisms of interleukin-21 (IL-21) gene were confirmed to be related to various diseases, but no studies have examined the possible role of IL-21 single nucleotide polymorphisms (SNPs) (rs907715, rs2221903, and rs12508721) in gastric precancerous lesions.
To explore the associations between SNPs of IL-21 gene (rs907715, rs2221903, and rs12508721) and gastric precancerous lesions in a Chinese population.
Three SNPs of IL-21 were genotyped using polymerase chain reaction–ligase detection reaction in 588 cases and 290 healthy controls from May 2013 to December 2016 in northwestern China. Gastric precancerous lesions were confirmed by endoscopic examination and categorized as non-atrophic gastritis, atrophic gastritis, and intestinal metaplasia. Descriptive statistic and logistic regression were used for data analyses.
IL-21 rs907715 genotype CC and C frequencies were higher in in patients with gastric precancerous lesions than in the controls (OR = 1.59, 95%CI: 1.06-2.38, P = 0.013; OR = 1.28, 95%CI: 1.01-2.22, P = 0.044, respectively) after adjusting for confounding factors. For SNP rs907715 in intestinal metaplasia patients, significant differences between cases and controls were observed in the frequencies of genotype CC and C (OR = 1.92, 95%CI: 1.24-2.98, P = 0.004; OR = 1.53, 95%CI: 1.04-2.24, P = 0.028, respectively); for non-atrophic gastritis and atrophic gastritis patients, the CC and C genotypes showed no significant association with risk in all models. No association between either rs2221903 or rs12508721 and gastric precancerous lesions was found in the present study. In the haplotype analysis, the TC haplotype (rs907715 and rs12508721) and TT haplotype (rs2221903 and rs907715) were more frequent in the case group than control group (P < 0.05).
Our findings indicate that SNP rs907715 of IL-21 gene is associated with gastric precancerous lesions. The TC haplotype (rs907715 and rs12508721) and TT haplotype (rs2221903 and rs907715) increased the risk of gastric precancerous lesions. If confirmed, these findings will shed light on the etiology of precancerous lesions.
Core tip: This study investigated the associations between single nucleotide polymorphisms of interleukin-21 (IL-21) gene (rs907715, rs2221903 and rs12508721) and gastric precancerous lesions in a Chinese population. The results showed an association between IL-21 rs907715 polymorphism and gastric precancerous lesions. IL-21 rs907715 genotype CC and C frequencies were higher in patients with gastric precancerous lesions than in the controls. Single nucleotide polymorphism rs907715 increased in CC and C genotypes were associated with intestinal metaplasia patients when examined separately. These findings may help clarify the etiology of gastric cancer.
- Citation: Wang XQ, Li Y, Terry PD, Kou WJ, Zhang Y, Hui ZZ, Ren XH, Wang MX. Association between interleukin-21 gene rs907715 polymorphism and gastric precancerous lesions in a Chinese population. World J Gastrointest Oncol 2020; 12(3): 289-300
- URL: https://www.wjgnet.com/1948-5204/full/v12/i3/289.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i3.289
Gastric cancer (GC) is the fifth most common malignancy worldwide, and ranks second in incidence and mortality among all malignancies in China[1,2]. GC is considered to be a multistep progression from non-atrophic gastritis (NAG), atrophic gastritis (AG), intestinal metaplasia (IM), dysplasia, to gastric adenocarcinoma[3]. The worldwide prevalences of AG and IM were 33% and 25% respectively[4]. As the specific recognizable stages of the precancerous cascade[5], gastric precancerous lesions can increase the risk of GC[6,7], so clarifying the etiology of gastric precancerous lesions is of great significance in preventing the development of GC[8]. Multiple factors contribute to the occurrence and development of gastric precancerous lesions, including environmental factors, such as Helicobacter pylori infection[9,10], high salt intake[11,12], alcohol consumption[12], and smoking status[13]. Some studies have explored genetic risk factors for precancerous lesions such as interleukin (IL)-1, IL-8, IL-10, and IL-22[14-18], but less attention has been given to IL-21.
IL-21 is an immune modulatory cytokine produced mainly by activated CD4+T cells and natural killer (NK) cells, and has multiple effects on innate and adaptive immune responses[19]. The activity of IL-21 is mediated via binding to a compound receptor consisting of IL-21R and γ chain[20,21], and the biological functions of IL-21 include promoting T-cell proliferation, stimulating B-cell differentiation, and enhancing NK-cell activation[22,23]. IL-21 plays important roles in inflammatory, antiviral, and antitumor responses[24]. Single nucleotide polymorphisms (SNPs) of the IL-21 gene can change the expression level of mRNA, resulting in a change in protein expression or autoantibody production[25]. SNPs of IL-21 have been associated with various diseases of the immune system including systemic lupus erythematosus[26], Graves’ disease[27], rheumatoid arthritis[28], and hepatitis B virus (HBV) infection[29]. Several SNPs of IL-21 (rs907715, rs2221903 and rs12508721) have also been associated with the susceptibility to cancer[30-33]. For example, SNPs rs907715 and rs2221903 reduce the susceptibility to non-small cell lung cancer[30], and SNP rs12508721 is related to thyroid cancer[31], breast cancer[32] and HBV-related hepatocellular carcinoma[33]. Previous studies have found that IL-21 may be associated with the risk of gastric precancerous lesions[34,35]. However, no studies have examined the possible role of IL-21 SNPs (rs907715, rs2221903 and rs12508721) in gastric precancerous lesions. Therefore, the present study explored associations between SNPs of IL-21 (rs907715, rs2221903 and rs12508721) and risk of gastric precancerous lesions in a northwestern Chinese population.
This study was conducted from May 2014 to December 2016 in hospitals from three cities (Yulin, North; Xi'an, Middle; Hanzhong, South) in Shaanxi Province, China (Figure 1). Men and women with gastrointestinal symptoms requiring upper endoscopy examination were screened for study eligibility. Individuals diagnosed with GC were excluded, while a total of 1674 subjects who had undergone upper gastrointestinal endoscopy, completed pathological and 24-hour urine testing were included. The medical records of all subjects were reviewed retrospectively.
Of the eligible and willing subjects, 588 with NAG, AG, or IM (cases) and 290 without any diagnosis of gastric diseases or H. pylori infection (controls) were enrolled. This study was performed in accordance with the Declaration of Helsinki of the World Medical Association and was approved by the Institutional Review Board of Xi’an Jiaotong University Health Science Center. Informed consent was obtained from all subjects.
Demographic information was obtained from subjects’ medical records including age, gender, smoking status, drinking status, height, and weight. For smoking status and drinking status, subjects were dichotomized as “yes” or “no”. Body mass index was calculated as weight in kilograms divided by height in meters squared.
Daily salt intake was determined by 24-hour urine sodium excretion and was dichotomized as “high salt” and “non-high salt” according to the median of the controls (representing the general population). Subjects were asked to excrete and discard their first urine at 7 a.m. and to collect all urine over the following 24-hours, including the next day first urine at 7 a.m. Total volumes of the collection were measured. Urinary sodium levels were measured by the ion selective electrode method using by Olympus AU 680 autoanalyser.
In this study, NAG, AG, and IM were diagnosed by endoscopic findings and based on updated Sydney system criteria[36] and Atrophy Club criteria[37]. The serum H. pylori IgG antibody test was performed by an enzyme-linked immunosorbent assay on the same day of endoscopy. IgG values ≥ 10 U/mL were considered as “H. pylori infection” and < 10 U/mL as negative results[38].
Three SNPs (rs907715, rs2221903, and rs12508721) of IL-21 were genotyped in cases and controls. Genomic DNA was extracted from 5 mL peripheral blood samples using the Blood DNA Kit (Tiangen, Beijing, China), and stored at -80°C until subsequent assay. SNPs were genotyped using the polymerase chain reaction (PCR)–ligase detection reaction method using assay-on-demand probes and primers: C_8949748_10 for rs907715, C_16167441_10 for rs2221903, C_1597500_10 for rs12508721. The forward and reverse primers are shown in Table 1. All primers were designed using the Primer3 program (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_http://www.cgi). The reaction was performed in a total volume of 20 µL, containing genomic DNA (1 µL), buffer (2 µL), MgCl2 (0.6 µL), dNTPs (2 µL), Taq polymerase (0.2 µL), 2 µL of each primer, and 12.2 µL ddH2O. PCR conditions were as follows: denaturation at 95 °C for 2 min, 94 °C for 30 s; annealing at 56 °C for 90 s; extension at 40 cycles of 65 °C for 30 s, and a final extension at 65 °C for 10 min. Following amplification, PCR products were submitted for DNA sequencing.
| Primer | Type | Primer sequences, 5′→3′ |
| rs907715 | F | 5′-ATAGATGAGGAAAGTGAGATC-3′ |
| R | 5′- CTTTGCTTATTTGATATATTCC-3′ | |
| rs2221903 | F | 5′-GGACCACATATTGCCAG ACAC-3′ |
| R | 5′-GACACTGACGCCCATATTGAT-3′ | |
| rs12508721 | F | 5′-ATGGGACTAAAGT CAAGGTG-3′ |
| R | 5′-AGATGGCTTCTAGAGTCTGG-3′ |
Trizol was used for extraction of mRNA from six intestinal epithelium tissues according to the manufacturer’s instructions. Quantification of mRNA of rs907715 was performed using BioEasy SYBR Green Real Time PCR Kit in a 20 μL reaction volume, containing SYBR Green Master Mix (10 μL), PCR Forward Primer (0.8 μL), cDNA (2 μL), ROX (0.4 μL) and nuclease-free water (6 μL). Extension was performed under the following conditions: Initial denaturation at 95 °C for 5 min, followed by 40 cycles at 95 °C for 5 s and 60 °C for 34 s. All reactions were performed in duplicate. Using the 2−ΔΔCt method[39] to calculate the relative mRNA expression levels.
Descriptive statistics were used to describe demographic characteristics of all subjects in our study. Genotype frequencies of three SNPs (rs907715, rs2221903, and rs12508721) were obtained by statistical description, and Hardy-Weinberg equilibrium was analyzed using the chi-squared goodness of fit test. Logistic regression models were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) of gastric precancerous lesions for genotype, controlling for demographic and lifestyle factors (age, gender, body mass index, drinking status, smoking status, daily salt intake and region). Distribution normality of IL-21 mRNA expression was assessed using the Kolmogorov–Smirnov test, differences among three genotypes were measured using an independent sample Student’s t-test. All analyses were performed with SPSS 22.0 software (IBM, Chicago, IL, United States). A two-tailed P < 0.05 was considered statistically significant.
A total of 1674 subjects were included in the study, aged 26 to 88 with a mean age of 49.8 (SD = 11.4). The incidences of NAG, AG, and IM were 6.9%, 6.3%. and 21.9%, respectively (Table 2). The NAG incidence in the south region was higher than that in other two regions, while AG and IM incidences in middle region were higher than those in other two regions.
| Characteristic | North | Middle | South | Total | ||||
| n = 742 | 44.3 (%) | n = 488 | 29.2 (%) | n = 444 | 26.5 (%) | n = 1674 | 100 (%) | |
| Gender | ||||||||
| Female | 464 | 62.5 | 128 | 26.2 | 136 | 30.6 | 728 | 43.5 |
| Male | 278 | 37.5 | 360 | 73.8 | 308 | 69.4 | 946 | 56.5 |
| BMI | 742 | 20.0 ± 3.2 | 488 | 20.6 ± 2.8 | 444 | 20.3 ± 2.2 | 1674 | 20.6 ± 2.0 |
| Age in yr | 742 | 52.9 ± 15.1 | 488 | 46.3 ± 12.8 | 444 | 47.5 ± 15.0 | 1674 | 49.8 ± 11.4 |
| < 40 | 110 | 36.2 ± 5.4 | 174 | 36.9 ± 5.2 | 76 | 36.7 ± 3.9 | 360 | 35.8 ± 2.9 |
| 40-49 | 220 | 45.1 ± 4.2 | 202 | 44.7 ± 4.5 | 288 | 44.8 ± 2.1 | 710 | 45.5 ± 2.0 |
| 50-59 | 150 | 54.6 ± 6.1 | 28 | 51.8 ± 1.9 | 8 | 54.4 ± 2.5 | 186 | 54.2 ± 2.0 |
| ≥ 60 | 262 | 66.1 ± 5.0 | 84 | 68.3 ± 11.1 | 72 | 77.0 ± 10.4 | 418 | 68.0 ± 8.4 |
| Lifestyle | ||||||||
| High salt | 230 | 31.0 | 112 | 23.0 | 56 | 12.6 | 398 | 23.8 |
| Smoking | 314 | 42.3 | 142 | 29.1 | 318 | 71.6 | 774 | 46.2 |
| Drinking | 286 | 38.5 | 116 | 23.8 | 184 | 41.4 | 586 | 35.0 |
| Clinical diagnosis | ||||||||
| H. pylori infection | 512 | 69.0 | 296 | 60.7 | 360 | 81.1 | 1168 | 69.8 |
| NAG | ||||||||
| None | 700 | 94.3 | 470 | 96.3 | 388 | 87.4 | 1558 | 93.1 |
| Mild | 23 | 3.1 | 10 | 2.1 | 35 | 7.9 | 68 | 4.1 |
| Moderate | 16 | 2.2 | 6 | 1.2 | 17 | 3.8 | 39 | 2.3 |
| Severe | 3 | 0.4 | 2 | 0.4 | 4 | 0.9 | 9 | 0.5 |
| AG | ||||||||
| None | 722 | 97.3 | 438 | 89.8 | 408 | 91.9 | 1568 | 93.7 |
| Mild | 13 | 1.8 | 33 | 6.8 | 23 | 5.1 | 69 | 4.1 |
| Moderate | 6 | 0.8 | 14 | 2.8 | 10 | 2.3 | 30 | 1.8 |
| Severe | 1 | 0.1 | 3 | 0.6 | 3 | 0.7 | 7 | 0.4 |
| IM | ||||||||
| None | 612 | 82.5 | 328 | 67.2 | 368 | 82.9 | 1308 | 78.1 |
| Mild | 87 | 11.7 | 104 | 21.3 | 48 | 10.9 | 239 | 14.3 |
| Moderate | 34 | 4.6 | 45 | 9.2 | 23 | 5.1 | 102 | 6.1 |
| Severe | 9 | 1.2 | 11 | 2.3 | 5 | 1.1 | 25 | 1.5 |
High salt intake was associated with an increased risk of NAG (OR = 2.58, 95%CI: 1.21-4.88, P = 0.011) (Table 3); H. pylori infection was correlated with decreased risk of AG (OR = 0.39, 95% 95%CI: 0.65-0.99, P = 0.041); and smoking was related to increased risk of NAG (OR = 2.15, 95%CI: 1.19-4.44, P = 0.015) and IM (OR = 1.97, 95%CI: 1.40-2.58, P = 0.005). Compared with the south region, subjects in the middle region had a lower risk of NAG (OR = 0.33, 95%CI: 0.28-0.51, P = 0.009) and a higher risk of IM (OR = 2.95, 95%CI: 1.45-4.33, P = 0.007); subjects in the north region had a lower risk of AG (OR = 0.33, 95%CI: 0.21-0.83, P = 0.010).
| Risk factors | NAG | AG | IM | ||||
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | ||
| Age | 1.09 (0.99-1.03) | 0.315 | 0.90 (0.88-1.01) | 0.412 | 1.01 (0.99-1.02) | 0.313 | |
| BMI | 1.01 (0.93-1.11) | 0.421 | 0.89 (0.78-1.32) | 0.587 | 1.11 (0.98-1.24) | 0.134 | |
| Gender | Female | - | - | - | |||
| Male | 1.52 (0.79-2.14) | 0.156 | 0.91 (0.42-1.83) | 0.792 | 1.30 (0.88-1.91) | 0.199 | |
| High salt | No | - | - | - | |||
| Yes | 2.58 (1.21-4.88) | 0.011 | 0.59 (0.24-1.77) | 0.315 | 1.01 (0.55-1.41) | 0.699 | |
| H. pylori infection | No | - | - | - | |||
| Yes | 0.59 (0.37-1.08) | 0.141 | 0.39 (0.65-0.99) | 0.041 | 0.90 (0.77-1.31) | 0.555 | |
| Smoking | No | - | - | - | |||
| Yes | 2.15 (1.19-4.44) | 0.015 | 1.11 (0.77-2.10) | 0.515 | 1.97 (1.40-2.58) | 0.005 | |
| Drinking | No | - | - | - | |||
| Yes | 1.00 (0.99-1.39) | 0.057 | 0.731 (0.49-1.19) | 0.161 | 0.95 (0.89-1.33) | 0.668 | |
| Region | South | - | - | - | |||
| Middle | 0.33 (0.28-0.51) | 0.009 | 0.71 (0.46-1.99) | 0.669 | 2.95 (1.45-4.33) | 0.007 | |
| North | 0.55 (0.40-1.01) | 0.053 | 0.33 (0.21-0.83) | 0.010 | 1.41 (0.89-2.01) | 0.313 | |
The genotype distributions of each group were consistent with Hardy-Weinberg equilibrium (P > 0.05) (Table 4). In univariate analyses, differences in the distribution frequency of rs907715 genotypes CC and C between cases and controls were statistically significant (OR = 1.77, 95%CI: 1.19-2.63, P = 0.005; OR = 1.43, 95%CI: 1.02-2.01, P = 0.039, respectively). Results were similar after adjusting for confounding factors (OR = 1.59, 95%CI: 1.06-2.38, P = 0.013; OR = 1.28, 95%CI: 1.01-2.22, P = 0.044, respectively). Results were also similar for IM when examined separately (OR = 1.92, 95%CI: 1.24-2.98, P = 0.004; OR = 1.53, 95%CI: 1.04-2.24, P = 0.028, respectively). The distribution frequencies of genotype CC and C were not statistically different between cases and controls in all models.
| SNPs set | Geno-type | Control (n = 290, %) | NAG (n = 116, %) | AG (n = 106, %) | IM (n = 366, %) | OR1 | P1 value | OR2 | P2 value | OR3 | P3 value | OR4 | P4 value | OR5 | P5 value |
| rs907715 | TT | 70 (24.1) | 25 (21.6) | 19 (17.9) | 63 (17.2) | - | - | - | - | - | |||||
| CT | 143 (49.3) | 53 (45.7) | 50 (47.2) | 170 (46.5) | 1.04 (0.60-1.81) | 0.896 | 1.29 (0.71-2.35) | 0.408 | 1.32 (0.88-1.98) | 0.179 | 1.25 (0.87-1.80) | 0.230 | 1.13 (0.62-1.74) | 0.439 | |
| CC | 77 (26.6) | 38 (32.7) | 37 (34.9) | 133 (36.3) | 1.38 (0.76-2.52) | 0.290 | 1.77 (0.93-3.36) | 0.079 | 1.92 (1.24-2.98) | 0.004 | 1.77 (1.19-2.63) | 0.005 | 1.59 (1.06-2.38) | 0.013 | |
| C | 220 (75.9) | 91 (78.4) | 87 (82.1) | 303 (82.8) | 1.16 (0.69-1.94) | 0.578 | 1.46 (0.83-2.56) | 0.190 | 1.53 (1.04-2.24) | 0.028 | 1.43 (1.02-2.01) | 0.039 | 1.28 (1.01-2.22) | 0.044 | |
| rs12508721 | TT | 39 (13.4) | 14 (12.1) | 18 (17.0) | 54 (14.8) | - | - | - | - | - | |||||
| CT | 151 (52.1) | 57 (49.1) | 55 (51.9) | 178 (48.6) | 1.05 (0.53-2.08) | 0.885 | 0.79 (0.42-1.50) | 0.467 | 0.85 (0.54-1.36) | 0.498 | 0.87 (0.57-1.33) | 0.525 | 0.82 (0.55-1.28) | 0.618 | |
| CC | 100 (34.5) | 45 (38.8) | 33 (31.1) | 134 (36.6) | 1.25 (0.62-2.54) | 0.529 | 0.72 (0.36-1.42) | 0.335 | 0.97 (0.60-1.57) | 0.895 | 0.96 (0.62-1.50) | 0.863 | 0.88 (0.59-1.33) | 0.879 | |
| C | 251 (86.6) | 102 (87.9) | 88 (83.0) | 312 (85.2) | 1.13 (0.59-2.17) | 0.709 | 0.76 (0.41-1.40) | 0.375 | 0.90 (0.58-1.40) | 0.634 | 0.91 (0.60-1.36) | 0.639 | 0.83 (0.49-1.19) | 0.801 | |
| rs2221903 | TT | 231 (79.7) | 90 (77.6) | 83 (78.3) | 271 (74.0) | - | - | - | - | - | |||||
| CT | 52 (17.9) | 24 (20.7) | 21 (19.8) | 80 (21.9) | 1.19 (0.69-2.04) | 0.539 | 1.12 (0.64-1.98) | 0.685 | 1.31 (0.89-1.94) | 0.173 | 1.25 (0.87-1.79) | 0.223 | 1.08 (0.53-1.55) | 0.459 | |
| CC | 7 (2.4) | 2 (1.7) | 2 (1.9) | 15 (4.1) | 0.73 (0.15-3.60) | 0.701 | 0.80 (0.16-3.91) | 0.777 | 1.83 (0.73-4.56) | 0.190 | 1.41 (0.59-3.41) | 0.441 | 1.19 (0.41-2.87) | 0.503 | |
| C | 59 (20.3) | 26 (22.4) | 23 (21.7) | 95 (26.0) | 1.13 (0.67-1.91) | 0.643 | 1.09 (0.63-1.87) | 0.769 | 1.37 (0.95-1.99) | 0.092 | 1.27 (0.90-1.79) | 0.171 | 1.08 (0.81-1.48) | 0.311 |
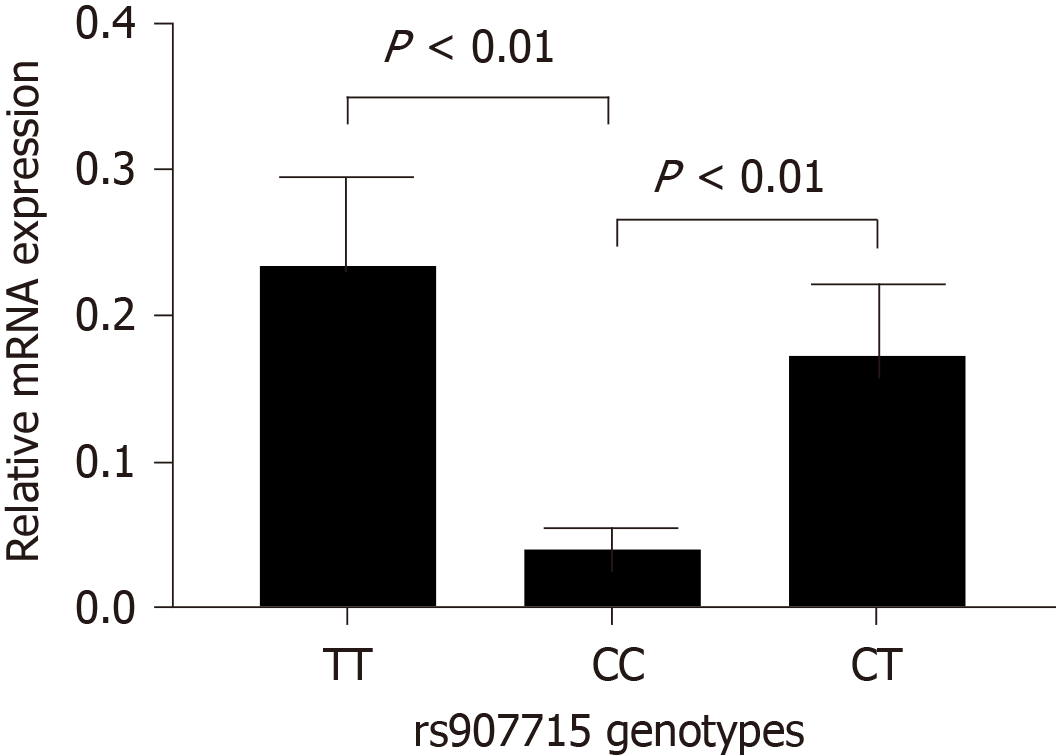
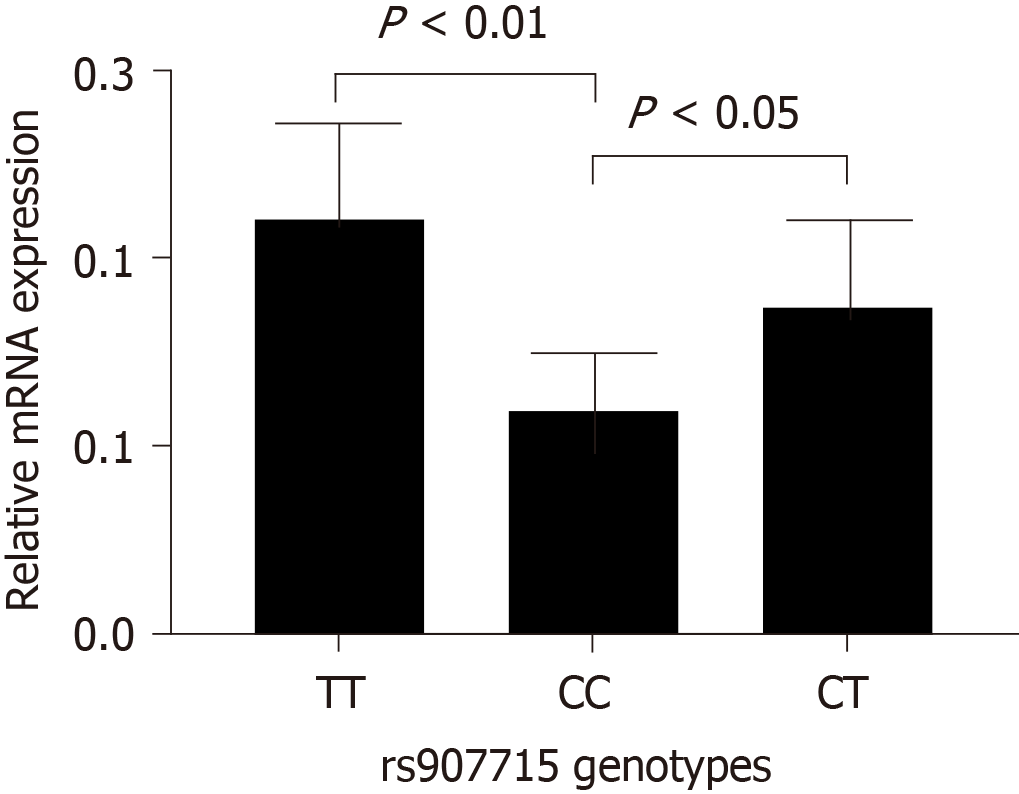
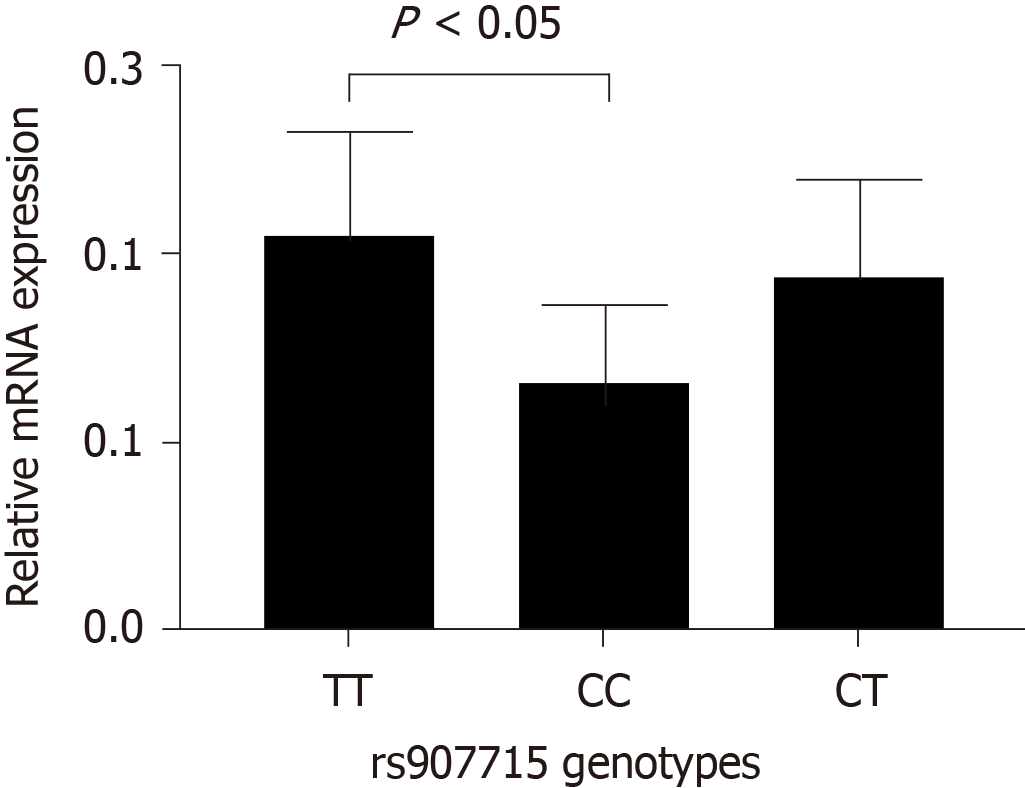
Analyses of rs907715 mRNA expression in intestinal epithelium tissue from six subjects with IM showed significant differences between CC genotype and TT genotype (P < 0.001), CC genotype and CT genotype (P < 0.01) (Figure 2). Similarly, rs907715 mRNA expression levels in six NAG tissues and six atrophic gastritis tissues were conducted. For NAG tissues, the expression level of rs907715 CC genotype was significantly different from that among the rs907715 CT genotype (P < 0.05) and TT genotype (P < 0.01) (Figure 3); for atrophic gastritis tissues, the expression level showed significant difference between rs907715 CC genotype and TT genotype (P < 0.05) (Figure 4).
The results showed that the TC haplotype (rs907715 and rs12508721) was significantly associated with AG and IM (OR = 3.91, P = 0.003; OR = 2.02, P = 0.004, respectively), and it appeared to be a risk haplotype; the TT haplotype (rs2221903 and rs907715) was significantly associated with IM (OR = 1.44, P = 0.023) and it appeared to be a risk haplotype (Table 5).
| SNPs | Haplo-type | Control (n = 290, %) | NAG (n = 116, %) | AG (n = 106, %) | IM (n = 366, %) | χ2-1 | P1 value | OR1 | χ2-2 | P2 value | OR2 | χ2-3 | P3 value | OR3 |
| rs907715| rs12508721 | CC | 0.496 | 0.543 | 0.575 | 0.571 | 0.713 | 0.397 | 1.20 (0.78-1.86) | 1.936 | 0.164 | 1.37 (0.88-2.15) | 3.612 | 0.057 | 1.35 (1.00-1.84) |
| CT | 0.066 | 0.060 | 0.057 | 0.071 | 0.037 | 0.847 | 1.09 (0.45-2.67) | 0.104 | 0.747 | 1.17 (0.45-3.01) | 0.077 | 0.781 | 0.92 (0.50-1.69) | |
| TC | 0.163 | 0.138 | 0.047 | 0.087 | 0.368 | 0.544 | 1.21 (0.66-2.23) | 8.984 | 0.003 | 3.91 (1.51-10.11) | 8.509 | 0.004 | 2.02 (1.25-3.26) | |
| TT | 0.275 | 0.259 | 0.321 | 0.271 | 0.125 | 0.724 | 1.09 (0.67-1.78) | 0.763 | 0.382 | 0.81 (0.50-1.31) | 0.024 | 0.878 | 1.03 (0.73-1.45) | |
| rs2221903| rs907715 | CC | 0.128 | 0129 | 0.123 | 0.158 | 0.002 | 0.963 | 0.99 (0.52-1.87) | 0.017 | 0.896 | 1.05 (0.53-2.06) | 1.264 | 0.264 | 0.78 (0.50-1.21) |
| CT | 0.006 | 0.026 | 0.019 | 0.014 | 2.450 | 0.118 | 0.26 (0.04-1.59) | 1.113 | 0.292 | 0.36 (0.05-2.60) | 0.701 | 0.404 | 0.50 (0.10-2.60) | |
| TC | 0.434 | 0.474 | 0.509 | 0.484 | 0.527 | 0.468 | 0.85 (0.55-1.31) | 1.759 | 0.185 | 0.74 (0.47-1.16) | 1.571 | 0.210 | 0.82 (0.60-1.12) | |
| TT | 0.432 | 0.371 | 0.349 | 0.344 | 1.244 | 0.265 | 1.29 (0.83-2.00) | 2.158 | 0.142 | 1.41 (0.89-2.24) | 5.157 | 0.023 | 1.44 (1.05-1.98) |
Our results suggested that rs907715 genotypes CC and C confer increased susceptibility to IM and total gastric precancerous lesions, whereas no association was found for rs2221903 or rs12508721. Because we are not aware of any previous study that directly addressed these associations, our findings should be interpreted cautiously.
Regarding rs907715, Liu et al[30], for example, reported that genotype AA and A allele of rs907715 were associated with the decreased susceptibility to non-small cell lung cancer. Xiao et al[31] revealed that the G allele of rs907715 increased the susceptibility to Graves’ disease. Moreover, a case-control study found that serum IL-21 levels in HBV patients with rs907715 genotype AA were lower than those in patients with genotype AG/GG; this genotype was independently related to sustained virological response[40]. A meta-analysis showed that the genotype distribution of IL-21 rs907715 was significantly different between systemic lupus erythematosus patients and healthy controls in all genetic models[26]. All of these findings suggest that rs907715 of IL-21 may to some extent exert effects on antitumor, antiviral and/or inflammatory processes. However, other studies have shown no association between rs907715 and thyroid cancer[31] and breast cancer[32]. Hence, the associations we observed in our study should be addressed in future studies.
The IL-21 gene is located on human chromosome 4q26-27 and plays an important role in anti-tumor immunopathology[41]. Previous studies have found that IL-21 is overexpressed in H. pylori-infected gastric mucosa[42], and is correlated with the occurrence and development of gastritis[34,35]. Moreover, studies have found that the concentration of IL-21 is increased in both tissue and serum of GC patients[43,44]. Thus, IL-21 may play a role in the development and progression of GC and gastritis-related diseases. Previous evidence has shown that SNP rs907715 is associated with increased IL-21 transcription and expression[40,45]. The rs907715, locating in the third intron of IL-21 gene, may be a surrogate marker for mutations with functional consequences[25]. SNPs including rs907715 may be in linkage disequilibrium with a variant correlated with mRNA translation, thereafter may lead to the change of protein expression[27]. Therefore, SNP rs907715 of IL-21 gene may alter the mRNA expression levels and regulate the function of IL-21. This suggested that rs907715 may be related to the risk of gastric precancerous lesions by influencing the activities of IL-21.
The data regarding TC haplotype frequency (rs907715 and rs12508721) in AG and IM patients compared to controls showed that this haplotype may be a risk for gastric precancerous lesions. Similarly, the TT haplotype frequency (rs2221903 and rs907715) in IM patient compared to controls showed that this haplotype may be a risk for IM. This result suggested that the two haplotypes, according to the IL-21 polymorphisms, might be the important genetic factors for susceptibility to gastric precancerous lesions.
The present study selected subjects in three cities from north to south in Shaanxi province, which enhanced the power of population representation and made our results more credible. However, this study also has several limitations. First, all cases and controls were selected from participants experiencing upper gastrointestinal symptoms, which may cause a potential selection bias and increase the positive results of the study. Second, controls were screened from subjects with non-H. pylori infection and non-precancerous lesions to represent the general population, this led to a mismatched number of cases and controls, which may weaken the testing effectiveness. Third, this study was a case-control study and unable to draw a causal relationship.
In conclusion, our study found that SNP rs907715 was associated with gastric precancerous lesions, and the TC haplotype (rs907715 and rs12508721) and TT haplotype (rs2221903 and rs907715) increased the risk of gastric precancerous lesions, which may help clarify the etiology of GC. Further studies are required to elucidate the roles of rs907715 in development of gastric precancerous lesions at a molecular level, which may provide new targets for therapeutic interventions.
Previous studies have found that interleukin-21 (IL-21) may be associated with the risk of gastric precancerous lesions, and single nucleotide (SNPs) of the IL-21 gene are associated with various diseases or cancer. Clarifying the possible role of IL-21 SNPs (rs907715, rs2221903 and rs12508721) in gastric precancerous lesions is of great significance in preventing the development of gastric cancer.
However, no studies have examined the possible role of IL-21 SNPs (rs907715, rs2221903 and rs12508721) in gastric precancerous lesions.
Therefore, the present study explored the associations between SNPs of IL-21 (rs907715, rs2221903 and rs12508721) and risk of gastric precancerous lesions in a north western Chinese population, which may help clarify the etiology of gastric cancer and provide new targets for therapeutic interventions.
Gastric precancerous lesions were confirmed by endoscopic examination and categorized as non-atrophic gastritis, atrophic gastritis, and intestinal metaplasia. Three SNPs of IL-21 (rs907715, rs2221903 and rs12508721) were genotyped using polymerase chain reaction–ligase detection reaction in 588 cases and 290 healthy controls. Descriptive statistic and logistic regression were used for data analyses.
We found an association between IL-21 rs907715 polymorphism and gastric precancerous lesions. IL-21 rs907715 genotype CC and C frequencies in patients with gastric precancerous lesions were higher than in controls. SNP rs907715 increased in CC and C genotypes were associated with intestinal metaplasia patients when examined separately. However, the exact role of rs907715 in development of gastric precancerous lesions at a molecular level remains to be studied.
In conclusion, our findings indicate that SNP rs907715 of IL-21 gene is associated with gastric precancerous lesions.
If confirmed by other studies, the results of our study suggest that IL-21 rs907715 polymorphisms may shed light on the etiology of precancerous lesions.
| 1. | American Cancer Society. Global Cancer Facts & Figures 4th Edition. Atlanta: American Cancer Society 2018; 33-34 Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/global-cancer-facts-and-figures/global-cancer-facts-and-figures-4th-edition.pdf. |
| 2. | Chen W, Zheng R, Zhang S, Zeng H, Xia C, Zuo T, Yang Z, Zou X, He J. Cancer incidence and mortality in China, 2013. Cancer Lett. 2017;401:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 352] [Article Influence: 39.1] [Reference Citation Analysis (1)] |
| 3. | Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735-6740. [PubMed] |
| 4. | Marques-Silva L, Areia M, Elvas L, Dinis-Ribeiro M. Prevalence of gastric precancerous conditions: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2014;26:378-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis. 2012;13:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 578] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 6. | Lage J, Uedo N, Dinis-Ribeiro M, Yao K. Surveillance of patients with gastric precancerous conditions. Best Pract Res Clin Gastroenterol. 2016;30:913-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Zhang L, Liu Y, You P, Feng G. Occurrence of gastric cancer in patients with atrophic gastritis during long-term follow-up. Scand J Gastroenterol. 2018;53:843-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Dinis-Ribeiro M, Areia M, de Vries AC, Marcos-Pinto R, Monteiro-Soares M, O'Connor A, Pereira C, Pimentel-Nunes P, Correia R, Ensari A, Dumonceau JM, Machado JC, Macedo G, Malfertheiner P, Matysiak-Budnik T, Megraud F, Miki K, O'Morain C, Peek RM, Ponchon T, Ristimaki A, Rembacken B, Carneiro F, Kuipers EJ; European Society of Gastrointestinal Endoscopy; European Helicobacter Study Group; European Society of Pathology; Sociedade Portuguesa de Endoscopia Digestiva. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy. 2012;44:74-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 503] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 9. | Watari J, Chen N, Amenta PS, Fukui H, Oshima T, Tomita T, Miwa H, Lim KJ, Das KM. Helicobacter pylori associated chronic gastritis, clinical syndromes, precancerous lesions, and pathogenesis of gastric cancer development. World J Gastroenterol. 2014;20:5461-5473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 202] [Cited by in RCA: 211] [Article Influence: 17.6] [Reference Citation Analysis (6)] |
| 10. | Shimizu T, Chiba T, Marusawa H. Helicobacter pylori-Mediated Genetic Instability and Gastric Carcinogenesis. Curr Top Microbiol Immunol. 2017;400:305-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Song JH, Kim YS, Heo NJ, Lim JH, Yang SY, Chung GE, Kim JS. High Salt Intake Is Associated with Atrophic Gastritis with Intestinal Metaplasia. Cancer Epidemiol Biomarkers Prev. 2017;26:1133-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Thapa S, Fischbach LA, Delongchamp R, Faramawi MF, Orloff M. Association between Dietary Salt Intake and Progression in the Gastric Precancerous Process. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Ferro A, Morais S, Rota M, Pelucchi C, Bertuccio P, Bonzi R, Galeone C, Zhang ZF, Matsuo K, Ito H, Hu J, Johnson KC, Yu GP, Palli D, Ferraroni M, Muscat J, Malekzadeh R, Ye W, Song H, Zaridze D, Maximovitch D, Aragonés N, Castaño-Vinyals G, Vioque J, Navarrete-Muñoz EM, Pakseresht M, Pourfarzi F, Wolk A, Orsini N, Bellavia A, Håkansson N, Mu L, Pastorino R, Kurtz RC, Derakhshan MH, Lagiou A, Lagiou P, Boffetta P, Boccia S, Negri E, La Vecchia C, Peleteiro B, Lunet N. Tobacco smoking and gastric cancer: meta-analyses of published data versus pooled analyses of individual participant data (StoP Project). Eur J Cancer Prev. 2018;27:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Chiurillo MA. Role of gene polymorphisms in gastric cancer and its precursor lesions: current knowledge and perspectives in Latin American countries. World J Gastroenterol. 2014;20:4503-4515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Cai M, Dai S, Chen W, Xia C, Lu L, Dai S, Qi J, Wang M, Wang M, Zhou L, Lei F, Zuo T, Zeng H, Zhao X. Environmental factors, seven GWAS-identified susceptibility loci, and risk of gastric cancer and its precursors in a Chinese population. Cancer Med. 2017;6:708-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Liu S, Liu JW, Sun LP, Gong YH, Xu Q, Jing JJ, Yuan Y. Association of IL10 gene promoter polymorphisms with risks of gastric cancer and atrophic gastritis. J Int Med Res. 2018;46:5155-5166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Negovan A, Iancu M, Fülöp E, Bănescu C. Helicobacter pylori and cytokine gene variants as predictors of premalignant gastric lesions. World J Gastroenterol. 2019;25:4105-4124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Hnatyszyn A, Wielgus K, Kaczmarek-Rys M, Skrzypczak-Zielinska M, Szalata M, Mikolajczyk-Stecyna J, Stanczyk J, Dziuba I, Mikstacki A, Slomski R. Interleukin-1 gene polymorphisms in chronic gastritis patients infected with Helicobacter pylori as risk factors of gastric cancer development. Arch Immunol Ther Exp (Warsz). 2013;61:503-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Long D, Chen Y, Wu H, Zhao M, Lu Q. Clinical significance and immunobiology of IL-21 in autoimmunity. J Autoimmun. 2019;99:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 152] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 20. | Habib T, Senadheera S, Weinberg K, Kaushansky K. The common gamma chain (gamma c) is a required signaling component of the IL-21 receptor and supports IL-21-induced cell proliferation via JAK3. Biochemistry. 2002;41:8725-8731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 165] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 553] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 22. | Al-Chami E, Tormo A, Khodayarian F, Rafei M. Therapeutic utility of the newly discovered properties of interleukin-21. Cytokine. 2016;82:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Davis ID, Skak K, Smyth MJ, Kristjansen PE, Miller DM, Sivakumar PV. Interleukin-21 signaling: functions in cancer and autoimmunity. Clin Cancer Res. 2007;13:6926-6932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Spolski R, Leonard WJ. Interleukin-21: a double-edged sword with therapeutic potential. Nat Rev Drug Discov. 2014;13:379-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 441] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 25. | Liu J, Cen H, Ni J, Zhang M, Li P, Yang XK, Leng RX, Pan HF, Ye DQ. Association of IL-21 polymorphisms (rs907715, rs2221903) with susceptibility to multiple autoimmune diseases: a meta-analysis. Autoimmunity. 2015;48:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 26. | Qi JH, Qi J, Xiang LN, Nie G. Association between IL-21 polymorphism and systemic lupus erythematosus: a meta-analysis. Genet Mol Res. 2015;14:9595-9603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Zeng H, Yan H, Zhang Z, Fang W, Ding R, Huang L, Chen M, Zhang J. Association between IL-21 gene rs907715 polymorphisms and Graves' disease in a Southern Chinese population. Exp Ther Med. 2014;8:213-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Malinowski D, Paradowska-Gorycka A, Safranow K, Pawlik A. Interleukin-21 gene polymorphism rs2221903 is associated with disease activity in patients with rheumatoid arthritis. Arch Med Sci. 2017;13:1142-1147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Feng L, Li B, Li B. Meta-analysis of correlation between rs907715, rs2221903 and rs12508721 polymorphisms in IL-21 and susceptibility to Hepatitis B. Minerva Med. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Liu L, Shi F, Li S, Liu X, Wei L, Zhang J, Ju X, Yu J. IL-21 polymorphisms rs907715 and rs2221903 are associated with decreased non-small cell lung cancer susceptibility. Int J Clin Exp Med. 2015;8:19460-19465. [PubMed] |
| 31. | Xiao M, Hu S, Tang J, Zhang L, Jiang H. Interleukin (IL)-21 promoter polymorphism increases the risk of thyroid cancer in Chinese population. Gene. 2014;537:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | You Y, Deng J, Zheng J, Hu M, Li N, Wu H, Li W, Lu J, Zhou Y. IL-21 gene polymorphism is associated with the prognosis of breast cancer in Chinese populations. Breast Cancer Res Treat. 2013;137:893-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Zhang QX, Li SL, Yao YQ, Li TJ. Association between interleukin-21 gene polymorphisms (rs12508721) and HBV-related hepatocellular carcinoma. Int J Immunogenet. 2016;43:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Bagheri N, Azadegan-Dehkordi F, Shirzad M, Zamanzad B, Rahimian G, Taghikhani A, Rafieian-Kopaei M, Shirzad H. Mucosal interleukin-21 mRNA expression level is high in patients with Helicobacter pylori and is associated with the severity of gastritis. Cent Eur J Immunol. 2015;40:61-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Nishiura H, Iwamoto S, Kido M, Aoki N, Maruoka R, Ikeda A, Chiba T, Watanabe N. Interleukin-21 and tumor necrosis factor-α are critical for the development of autoimmune gastritis in mice. J Gastroenterol Hepatol. 2013;28:982-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3624] [Article Influence: 120.8] [Reference Citation Analysis (6)] |
| 37. | Rugge M, Correa P, Dixon MF, Fiocca R, Hattori T, Lechago J, Leandro G, Price AB, Sipponen P, Solcia E, Watanabe H, Genta RM. Gastric mucosal atrophy: interobserver consistency using new criteria for classification and grading. Aliment Pharmacol Ther. 2002;16:1249-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 269] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 38. | Wang XQ, Yan H, Terry PD, Wang JS, Cheng L, Wu WA, Hu SK. Interaction between dietary factors and Helicobacter pylori infection in noncardia gastric cancer: a population-based case-control study in China. J Am Coll Nutr. 2012;31:375-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149116] [Cited by in RCA: 139206] [Article Influence: 5568.2] [Reference Citation Analysis (3)] |
| 40. | Wang X, Xu ZQ, Fu JJ, Cheng LW, Li Y, Li L, Pan XC. Role of interleukin-21 and interleukin-21 receptor polymorphisms in the treatment of HBeAg-positive chronic hepatitis B patients with peginterferon. Medicine (Baltimore). 2018;97:e10891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 41. | Yao JY, Chao K, Li MR, Wu YQ, Zhong BH. Interleukin-21 gene polymorphisms and chronic hepatitis B infection in a Chinese population. World J Gastroenterol. 2015;21:4232-4239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Caruso R, Fina D, Peluso I, Fantini MC, Tosti C, Del Vecchio Blanco G, Paoluzi OA, Caprioli F, Andrei F, Stolfi C, Romano M, Ricci V, MacDonald TT, Pallone F, Monteleone G. IL-21 is highly produced in Helicobacter pylori-infected gastric mucosa and promotes gelatinases synthesis. J Immunol. 2007;178:5957-5965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Su Z, Sun Y, Zhu H, Liu Y, Lin X, Shen H, Chen J, Xu W, Xu H. Th17 cell expansion in gastric cancer may contribute to cancer development and metastasis. Immunol Res. 2014;58:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Meng X, Yu X, Dong Q, Xu X, Li J, Xu Q, Ma J, Zhou C. Distribution of circulating follicular helper T cells and expression of interleukin-21 and chemokine C-X-C ligand 13 in gastric cancer. Oncol Lett. 2018;16:3917-3922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | Wang H, Wang M, Feng Z, Chen L, Gao L, Li Q, Zhang L, Ma J. Functional interleukin-21 polymorphism is a protective factor of diffuse large B-cell lymphoma. DNA Cell Biol. 2014;33:775-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited Manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kaabachi W, Raffaniello R S-Editor: Wang YQ L-Editor: Filipodia E-Editor: Qi LL