Published online Feb 15, 2020. doi: 10.4251/wjgo.v12.i2.228
Peer-review started: October 8, 2019
First decision: November 11, 2019
Revised: December 19, 2019
Accepted: January 6, 2020
Article in press: January 6, 2020
Published online: February 15, 2020
Processing time: 130 Days and 0.6 Hours
Liver metastases secondary to breast cancer are associated with unfavourable prognosis. Radioembolization with ytrrium-90 is an emerging option for management of liver metastases of breast cancer when other systemic therapies have failed to achieve disease control. However, unlike the case of other liver tumours (colorectal/melanoma metastases/cholangiocarcinoma), its role in the management of breast liver metastases is yet to be elucidated.
The aims of this systematic review were to (1) assess the effect of radioembolization with yttrium-90 on tumour response; and (2) to estimate patient survival post radioembolization.
The review was performed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses. A systematic literature search was performed using the PubMed and EMBASE databases from January 2007 to December 2018. The initial search yielded 265 reports which were potentially suitable for inclusion in this review. Studies published in English reporting at least one outcome of interest were considered to be suitable for inclusion. Conference abstracts; case reports, animal studies and reports not published in English were excluded from this review. Data was retrieved from each individual report on the name of primary author, year of publication, patient demographics, type of microspheres used, radiation dose delivered to tumour, duration of follow-up, disease control rate (%), tumour response, and overall patient survival.
The final number of studies which met the inclusion criteria was 12 involving 452 patients. There were no randomized controlled trials identified after the literature search. The age of the patients included in this review ranged from 52 to 61 years. The duration of the follow up period post-radioembolization ranged from 6 to 15.7 mo. The total number of patients with breast metastases not confined to the liver was 236 (52.2%). Cumulative analysis revealed that radioembolization with yttrium-90 conferred tumour control rate in 81% of patients. Overall survival post-radioembolization ranged from 3.6 to 20.9 mo with an estimated mean survival of 11.3 mo.
Radioembolization with ytrrium-90 appears to confer control of tumour growth rate in most patients, however its effect on patient survival need to be elucidated further. Furthermore, quality evidence in the form of randomized trials is needed in order to assess the effect of radioembolization in more depth.
Core tip: This is the first systematic review on the subject of liver radioembolization with yttrium-90 for breast metastases. Our paper reports cumulative findings of the 12 studies included on two important outcomes that of tumour response to embolization and patient survival. The paper summarises the current evidence available in the field and also makes recommendations for future areas of research in clinical practice.
- Citation: Feretis M, Solodkyy A. Yttrium-90 radioembolization for unresectable hepatic metastases of breast cancer: A systematic review. World J Gastrointest Oncol 2020; 12(2): 228-236
- URL: https://www.wjgnet.com/1948-5204/full/v12/i2/228.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i2.228
Breast cancer (BC) is the most common cancer in women and is associated with a life-time risk of incidence of 10%-15%[1,2]. However, the presence of BRCA1 or BRCA2 genes increases the life-time risk to 50%[3]. Breast cancer metastases will develop in up to 50% of patients with bone (85%), liver (50%), and lungs (20%) being the commonest sites[4,5]. The average 5-year survival rate for patients with breast cancer is 90% but if the cancer has spread to a distant part of the body, the 5-year survival rate drops dramatically to 27%[6]. Median survival for patients with liver metastases is generally very poor ranging from 4-21 mo[7,8].
The treatment options available for patients with liver metastases are limited. Palliative systemic chemotherapy is the commonest approach to metastatic breast cancer aiming to prolong survival. Resection of liver metastases in breast cancer has not been widely adopted perhaps due to the presence of multi-segmental liver disease at the time of diagnosis[9].
Transarterial radioembolization with yttrium-90 (TARE) microspheres offers an alternative radiotherapy option in the management of primary and secondary intrahepatic tumours[10]. Yttrium-90 microspheres are injected into the hepatic artery feeding the tumour and emit radiation at a local level. The advantage of TARE, in contrast to non-selective radiotherapy, is the ability to deliver high dose radiation to the tumour with minimal collateral damage to the normal liver parenchyma[11]. Liver radioembolization with yttrium-90 has been previously used to manage unresectable intrahepatic cholangiocarcinoma, colorectal and melanoma liver metastases[12-14]. The role of TARE in the management pathway of breast liver metastases is yet to be elucidated.
The purpose of this review was to systematically review the literature on the role of TARE in the management of breast liver metastases and summarise all evidence available on treatment response and patient survival. The primary outcomes of interest of this study were (1) to assess tumour response to TARE; and (2) to estimate overall patient survival following TARE as reported in the literature.
The Preferred Reporting Item for Systematic Reviews and Meta-analyses statements were followed to conduct this systematic review[15].
Published English-language manuscripts were considered for review and inclusion in this study. A systematic literature search was performed in PubMed and EMBASE databases from January 2007 to December 2018. The following search terms were used in order to identify the relevant bibliography: “yttrium” or “yttrium-90” or “Y90” or “radio-embolization” and “breast”. All full text studies, and abstracts identified were screened independently by the two authors in order to identify those concerning transarterial radio-embolization with yttrium-90 (TARE) of breast liver metastases. The PubMed function” related studies” was used to broaden the search and the reference list of all potentially relevant studies was analysed.
Studies published in the English-language reporting at least one of the primary outcomes of interest were included in this review. Conference abstracts; case reports, animal studies and reports not published in English were excluded from this review. The final decision regarding study eligibility for inclusion in this review was reached by mutual agreement between the two authors.
Data of interest from each study were extracted using standardised data collection database. The following information was extracted from each study: Name of primary author, year of publication, patient demographics, duration of follow-up, disease control rate (%), tumour response, type of spheres used and overall patient survival. Data was extracted by each of the two authors independently for data validation purposes.
Descriptive statistics (absolute frequencies, percentages and mean or median values) were used to report study and patient data. Due to the high heterogeneity among studies and the lack of randomized controlled trials, performing a meta-analysis was not deemed to be appropriate.
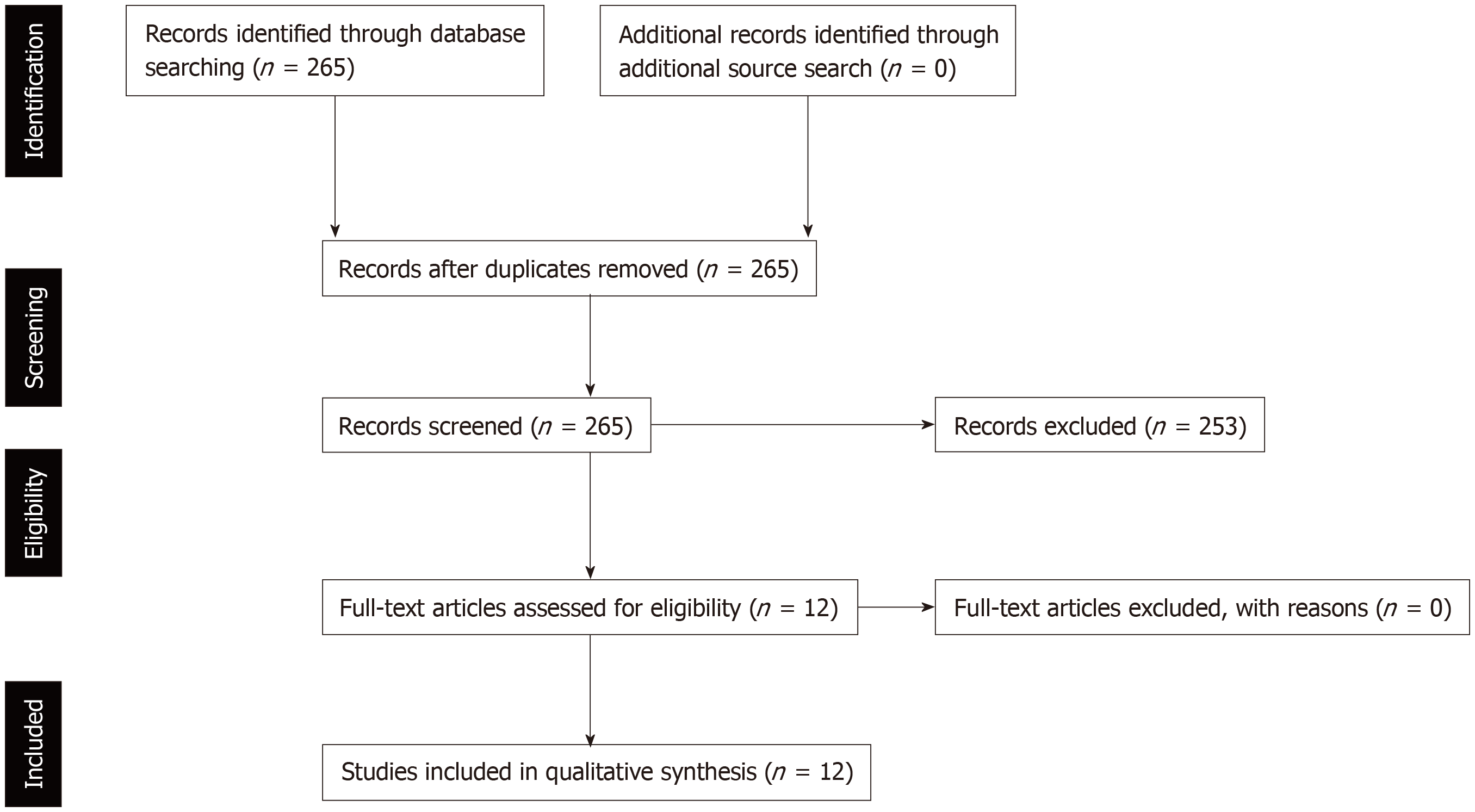
The literature search initially yielded 265 reports from January 2007 to December 2018. After screening the titles and abstracts of the reports identified a total number of 12 cohort studies were included in this systematic review (Figure 1). There were no randomized trials identified after the literature search.
The total number of patients originating from the 12 studies included was 452. Patient baseline demographic characteristics from the reports included in this review are summarised in Table 1. The age of the patients included in this review ranged from 52 to 61 years[16-27]. Data on the number of patients with extra-hepatic disease present at the time of radioembolization was available in 9/12 studies[17-19,21-25,27]. The total number of patients with breast metastases not confined to the liver was 236 (52.2%). The type of microspheres used to deliver the radioembolization to the hepatic metastases was clearly identifiable in 10 studies[16-19,21-24,26,27]. Currently there are two types of commercially available yttrium-microspheres. Resin microspheres (SIR-spheres, SIRTex Medical Limited, Sydney, Australia) were used in six studies whereas glass microspheres (TheraSphere, MDS, Nordion Inc., Ottawa, Canada) were used in 2 studies. In 2 of the studies included patients received treatment by a combination of resin and glass microspheres[26,27]. Data on the dose of yttrium-90 delivered to the patients was extractable from 9 studies[16,17,19,21-23,25-27]. The radiation dose delivered to the hepatic metastases varied from 0.8-2.1GBq (Table 1).
| Ref. | Number of patients with breast cancer | Mean age (yr) | Number of patients with extrahepatic disease | Type of microsphere used | Activity infused (GBq) |
| Bangash et al[16], 2007 | 27 | 52 | N/A | Glass | 2.05 |
| Coldwell et al[17], 2007 | 44 | 58 | 29 (66%) | Resin | 2.11 |
| Stuart et al[18], 2008 | 7 | N/A | 1 (14%) | Resin | N/A |
| Jakobs et al[19], 2008 | 30 | 58 | 17 (57%) | Resin | 1.9 |
| Cianni et al[20], 2010 | 32 | N/A | N/A | N/A | N/A |
| Haug et al[21], 2012 | 58 | 58 | 38 (65%) | Resin | 1.8 |
| Saxena et al[22], 2014 | 40 | 54.4 | 24 (60%) | Resin | 1.67 |
| Gordon et al[23], 2014 | 75 | 53.7 | 58 (77%) | Glass | 1.52 |
| Bagni et al[24], 2015 | 17 | 59.2 | 10 (59%) | Resin | N/A |
| Fendler et al[25], 2016 | 81 | 611 | 54 (67%) | N/A | 1.61 |
| Pieper et al[26], 2016 | 44 | 56.1 | N/A | Resin = 56, Glass = 13 | 1.35 |
| Chang et al[27], 2018 | 30 | 55* | 5 (17%) | Resin = 46, Glass = 3 | 0.81 |
The duration of the follow up period post-radioembolization was reported clearly in 4 studies (range 6-15.7 mo)[17,19,21,22]. Data on tumour/disease response to radioembolization and patient survival is summarised in Table 2. Data on tumour response to Ytrrium-90 treatment was retrievable from 11 studies included in this review[16,17,19-27]. Tumour response to radioembolization, defined as tumour appearance on follow-up versus baseline imaging, was described in all 12 studies included [data available on 357/452 subjects, (81%)]. Tumour response was evaluated using the Response Evaluation Criteria in Solid Tumours (RECIST, n = 7 studies), the modified Response Evaluation Criteria in Solid Tumours (mRECIST, n = 1 study) or the World Health Organization (WHO) classification method (n = 2 studies)[28-30]. Two further studies did not provide information on the criteria used to assess response to treatment[18,25]. In summary, according to the WHO/RECIST criteria, patients are sub-categorized in four groups when comparing post treatment imaging with baseline imaging for up to two target lesions: (1) Complete response (CR) if all lesions disappear; (2) Partial response (PR) if the sum of the longest diameters decreases at least 30%; (3) Stable disease (SD) if neither partial response or progressive disease is present; and (4) Progressive disease (PD) if the sum of the longest diameters increases by at least 20%[28-30]. Following radioembolization, disease control rate, calculated as the sum of CR + PR + SD, was achieved in 282 patients (77%, Table 2). Post-radioembolization imaging revealed CR in 30 subjects (8.2%, data available from 5 studies); PR in 113 subjects (30.8%, data available from 9 studies); SD in 94 subjects (26%, data available from 8 studies) and PD in 49 subjects (13.4%, data available from 10 studies). Patient survival post- radioembolization was reported in 9 studies. Overall survival post-radioembolization ranged from 3.6 to 20.9 mo with an estimated mean survival of 11.3 mo.
| Ref. | Evaluable patients | Assessment criteria | Follow up (mo) | Tumour response rate (%) | Cases of CR/PR/SD/PD | Overall survival (mo) |
| Bangash et al[16], 2007 | 23 | WHO | N/A | 21/23 (91%) | CR = 9 (39%); PR = 12 (52%); SD = 2 (9%); PD = 0 | N/A |
| Coldwell et al[17], 2007 | 36 | WHO | 14 | 34/36 (94.4%) | CR = 0; PR = 17 (47.2%); SD = 17 (47%); PD=2 (6%) | > 14 for those with CR/PR, 3.6 for those with SD/PD |
| Stuart et al[18], 2008 | 7 | N/A | N/A | N/A | N/A | 20.91 |
| Jakobs et al[19], 2008 | 23 | RECIST | 15.7 | 22/23 (97.2%) | CR = 0; PR = 14 (61%); SD = 8 (35%); PD = 1 (4%) | 9.6 |
| Cianni et al[20], 2010 | 32 | RECIST | N/A | 32/32 (100%) | CR = 14 (44%); PR = 11 (34%); SD = 7 (22%); PD = 0 | N/A |
| Haug et al[21], 2012 | 43 | RECIST | 6 | 38/43 (88%) | CR = 0; PR = 11 (26%); SD = 27 (62%); PD = 5 (12%) | 10.8 |
| Saxena et al[22], 2014 | 38 | RECIST | 11.21 | 27/38 (71%) | CR = 2 (5%); PR = 10 (26%); SD = 15 (39%); PD = 11 (29%) | 13.6 |
| Gordon et al[23], 2014 | 25 | RECIST | N/A | 21/25 (84%) | CR = 3 (12%); PR/SD = 18 (72%); PD = 4 (16%) | 6.61 |
| Bagni et al[24], 2015 | 17 | RECIST | N/A | 17/17 (100%) | CR = 2 (12%); PR = 15 (88%); SD = 0; PD = 0 | 13.5 |
| Fendler et al[25], 2016 | 56 | N/A | N/A | 29/56 (52%) | N/A | 81 |
| Pieper et al[26], 2016 | 38 | RECIST | N/A | 27/38 (71%) | CR = 0; PR = 11 (29%); SD = 16 (42%); PD = 11 (29%) | 6 |
| Chang et al[27], 2018 | 29 | mRECIST | N/A | 14/29 (48%) | CR = 0; PR = 12 (40%); SD = 2 (0.6%); PD = 15 (50%) | 12.9 |
In this report the relevant medical literature was systematically reviewed and the results of 12 studies are summarised. The primary outcomes of this review were survival and radiological response to radioembolization with Yttrium-90 microspheres for inoperable breast liver metastases. In summary, data from the studies included has demonstrated that radioembolization of breast cancer liver metastases with yttrium-90 confers a disease control rate of 81% with an estimated mean survival of 11.3 mo.
The development of liver metastases from breast cancer is associated with poor prognosis. Hepatic resection is a potential treatment option for patients, but unfortunately in the vast majority of cases the disease is unresectable at the time of diagnosis of liver metastases[31]. Other liver-directed therapies have been previously attempted for liver-only disease with the primary aim of palliating and prolonging survival. These treatments include radiofrequency and microwave ablation[32,33], transarterial chemoembolization[34] and stereotactic body radiotherapy[35]. Despite employing these treatment modalities, the reported median survival in patients with liver metastases remains poor ranging from 5-12 mo[8,36].
TARE with Yttrium-90 is an increasingly popular treatment choice in patients with unresectable liver involvement. It is a combination of embolization and radiotherapy techniques. During the procedure radioactive microspheres are injected via peripheral access into hepatic artery and due to their small size of 15-40 uM lodged into arteriolar level of liver vascular system. A high radiation dose can be delivered to the tumour itself saving healthy liver cells in comparison to external radiation technique. A previous structured review concluded that TARE for inoperable breast liver metastases, is well tolerated by patients especially when compared to the side effects associated with systemic chemotherapy[37]. The overall survival data retrieved from the studies included in this present review varied from 6 to 20.9 mo[17-19,21-27]. Although data from the studies included should be interpreted with caution due to the heterogeneity of the methodology in the reports included, the overall impression is that radioembolization is a promising option considering that over 50% of the total number patients included in this review had metastases beyond the liver at the time of TARE. Furthermore, survival data from one of the studies included, demonstrate that patients who have a complete or partial response to embolization treatment have a survival over 12 months compared to 3.6 months in those patients who failed to respond[17]. As an extension of the above one may speculate that radioembolization instead of being a monotherapy could have a synergistic role to systemic chemotherapy as it has been previously the case in colorectal liver metastases. In the context of colorectal liver metastasis, a previous randomized controlled trial demonstrated that the addition of radioembolization with ytrrium-90 to 5-fluorouracil treatment led to a significantly prolonged progression free survival and a non-statistically significant prolonged overall survival[13].
The response at a tumour level in the case of breast liver metastases to radioembolization has been a matter of debate in the literature. First of all the fact that, unlike the case of colorectal or uveal melanoma metastases which are often confined to the liver, breast cancer patients often have more extensive disease spread making radioembolization a modality less likely to succeed. However, it has been previously suggested that breast liver metastases are hypervascular compared to colorectal liver metastases which are described as hypovascular[38,39]. Therefore, the ratio between the number of spheres arriving at the level of the tumour versus the number of spheres arriving to healthy liver may be higher in the case of breast liver metastases making radioembolization an appropriate treatment modality for breast metastases. Data on tumour response to radioembolization could be retrieved from ten of the studies included in this review. However, interpretation of the data is limited by the use of different criteria (WHO vs RECIST) in the studies included[28,29]. Disease control rates varied from 48%-100% with an estimated mean response to TARE of 81%. The 2 studies[16,17] which used the WHO criteria to assess response to TARE reported disease response over 90%, whereas the rest of the studies reported disease control rates of 48%-100% based on the RECIST/mRECIST criteria[19-24,26,27]. The heterogeneity in the criteria used to assess tumour response rates, the inconsistency in the type of microspheres used and the different timings that post-TARE radiological surveillance was perfromed along with the retrospective nature of the studies identified, make it difficult to draw safe conclusions on the efficacy of TARE in disease control and necessitate the need for more quality evidence to be produced. Nevertheless, the results appear to be encouraging with an estimated mean disease control rate of over 80%. A recent systematic review on the role of TARE in disease control rate in cases of unresectable liver metastases secondary to melanoma reported a median control rate of 73.6%[14]. The findings of this review were promising and highlight the need for more quality evidence to explore the role of TARE either as a monotherapy or synergistically with systemic therapies in the future.
There are some limitations in the findings reported by this systematic review. First of all the absence of randomized controlled trials and the retrospective nature of the reports included carries the risk of selection bias. Furthermore, there is heterogeneity between the studies included and no standardised reporting system on the control-rate of the disease post-radioembolization. Differences between studies included were the type of spheres used to deliver the treatment locally, the variable radiation dose, variable presence of extrahepatic disease, previous chemotherapy and the length of follow-up.
This review, despite its limitations, highlights the potentially beneficial role of radio-embolization with yttrium microspheres in cases with inoperable liver metastases secondary to breast cancer. However, future randomized trials are need comparing systemic chemotherapy, local radiation and transarterial chemoembolization in order to identify the most suitable treatment modality for patients with inoperable hepatic metastases secondary to breast cancer. Standardization of the method that radioembolization is delivered by and the reporting systems used would be highly desirable.
Breast cancer liver metastases are associated with dismal prognosis. Previous reports in the literature on liver metastases secondary to melanoma or colorectal origin have shown promising results with the use of transarterial embolization. The aim of this review was to consolidate the evidence available in the literature on the use of transarterial embolization for management of breast liver metastases.
The aim of this review was to consolidate the evidence currently available on transarterial embolization for breast liver metastases in a systematic fashion. This relatively new technique is not widely available and its role in the management pathway of breast metastases has not been clearly described before. Patients with breast liver metastases have poor prognosis despite advances in chemotherapy and therefore transarterial embolization could be of benefit for those patients with advanced disease.
The main outcomes of interest were tumour response and patient survival following radioembolization with ytrrium-90 spheres.
A systematic literature search was performed in PubMed and EMBASE databases from January 2007 to December 2018. The following search terms were used in order to identify the relevant studies of interest: “yttrium” or “yttrium-90” or “Y90” or “radio-embolization” and “breast”.
The final number of studies which met the inclusion criteria was 12 involving 452 patients. There were no randomized controlled trials identified after the literature search. The age of the patients included in this review was ranged from 52-61 years. The duration of the follow up period post-radioembolization ranged from 6 to 15.7 mo. The total number of patients with breast metastases not confined to the liver was 236 (52.2%). Cumulative analysis revealed that radioembolization with yttrium-90 conferred tumour control rate in 81% of patients. Overall survival post-radioembolization ranged from 3.6 to 20.9 mo with an estimated mean survival of 11.3 mo.
Radioembolization with ytrrium-90 appears to confer control of tumour growth rate in most patients. The effect on patient survival need to be elucidated further. The findings reported in this review are limited by the absence of randomized trials on the subject and the heterogeneity in the methodology of the studies included. It is therefore highly desirable for more quality evidence to be produced in order to assess mor accurately the role of radioembolization with yttrium-90.
The findings of this review highlight the need for more quality evidence to be produced in the form of randomized controlled trials. Standardisation of types of spheres used, timing of imaging modalities and criteria used in order to assess the effect of radioembolization is required. Furthermore, the potentially synergistic role of radioembolization for patients on palliative chemotherapy should be evaluated as it may confer a significant impact on survival.
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9983] [Article Influence: 907.5] [Reference Citation Analysis (15)] |
| 2. | Eichbaum MH, Kaltwasser M, Bruckner T, de Rossi TM, Schneeweiss A, Sohn C. Prognostic factors for patients with liver metastases from breast cancer. Breast Cancer Res Treat. 2006;96:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg A, Pasini B, Radice P, Manoukian S, Eccles DM, Tang N, Olah E, Anton-Culver H, Warner E, Lubinski J, Gronwald J, Gorski B, Tulinius H, Thorlacius S, Eerola H, Nevanlinna H, Syrjäkoski K, Kallioniemi OP, Thompson D, Evans C, Peto J, Lalloo F, Evans DG, Easton DF. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2571] [Cited by in RCA: 2601] [Article Influence: 113.1] [Reference Citation Analysis (0)] |
| 4. | Hoe AL, Royle GT, Taylor I. Breast liver metastases--incidence, diagnosis and outcome. J R Soc Med. 1991;84:714-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 85] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Zinser JW, Hortobagyi GN, Buzdar AU, Smith TL, Fraschini G. Clinical course of breast cancer patients with liver metastases. J Clin Oncol. 1987;5:773-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 114] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Cancer. Net Editorial Board. Breast Cancer: Statistics 2019. [Accessed September 2019] Available from: https://www.cancer.net/cancer-types/breast-cancer/statistics. |
| 7. | O'Reilly SM, Richards MA, Rubens RD. Liver metastases from breast cancer: The relationship between clinical, biochemical and pathological features and survival. Eur J Cancer. 1990;26:574-577. [RCA] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Insa A, Lluch A, Prosper F, Marugan I, Martinez-Agullo A, Garcia-Conde J. Prognostic factors predicting survival from first recurrence in patients with metastatic breast cancer: analysis of 439 patients. Breast Cancer Res Treat. 1999;56:67-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 130] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Chua TC, Saxena A, Liauw W, Chu F, Morris DL. Hepatic resection for metastatic breast cancer: a systematic review. Eur J Cancer. 2011;47:2282-2290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. J Vasc Interv Radiol. 2006;17:1251-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 512] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 11. | Riaz A, Kulik LM, Mulcahy MF, Lewandowski RJ, Salem R. Yttrium-90 radioembolization in the management of liver malignancies. Semin Oncol. 2010;37:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Al-Adra DP, Gill RS, Axford SJ, Shi X, Kneteman N, Liau SS. Treatment of unresectable intrahepatic cholangiocarcinoma with yttrium-90 radioembolization: a systematic review and pooled analysis. Eur J Surg Oncol. 2015;41:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 13. | Hendlisz A, Van den Eynde M, Peeters M, Maleux G, Lambert B, Vannoote J, De Keukeleire K, Verslype C, Defreyne L, Van Cutsem E, Delatte P, Delaunoit T, Personeni N, Paesmans M, Van Laethem JL, Flamen P. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol. 2010;28:3687-3694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 277] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 14. | Jia Z, Jiang G, Zhu C, Wang K, Li S, Qin X. A systematic review of yttrium-90 radioembolization for unresectable liver metastases of melanoma. Eur J Radiol. 2017;92:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18665] [Cited by in RCA: 18060] [Article Influence: 1062.4] [Reference Citation Analysis (1)] |
| 16. | Bangash AK, Atassi B, Kaklamani V, Rhee TK, Yu M, Lewandowski RJ, Sato KT, Ryu RK, Gates VL, Newman S, Mandal R, Gradishar W, Omary RA, Salem R. 90Y radioembolization of metastatic breast cancer to the liver: toxicity, imaging response, survival. J Vasc Interv Radiol. 2007;18:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Coldwell DM, Kennedy AS, Nutting CW. Use of yttrium-90 microspheres in the treatment of unresectable hepatic metastases from breast cancer. Int J Radiat Oncol Biol Phys. 2007;69:800-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Stuart JE, Tan B, Myerson RJ, Garcia-Ramirez J, Goddu SM, Pilgram TK, Brown DB. Salvage radioembolization of liver-dominant metastases with a resin-based microsphere: initial outcomes. J Vasc Interv Radiol. 2008;19:1427-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Jakobs TF, Hoffmann RT, Fischer T, Stemmler HJ, Tatsch K, La Fougere C, Murthy R, Reiser MF, Helmberger TK. Radioembolization in patients with hepatic metastases from breast cancer. J Vasc Interv Radiol. 2008;19:683-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Cianni R, Urigo C, Notarianni E, Saltarelli A, D'Agostini A, Iozzino M, Dornbusch T, Cortesi E. Radioembolisation using yttrium 90 (Y-90) in patients affected by unresectable hepatic metastases. Radiol Med. 2010;115:619-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Haug AR, Tiega Donfack BP, Trumm C, Zech CJ, Michl M, Laubender RP, Uebleis C, Bartenstein P, Heinemann V, Hacker M. 18F-FDG PET/CT predicts survival after radioembolization of hepatic metastases from breast cancer. J Nucl Med. 2012;53:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Saxena A, Kapoor J, Meteling B, Morris DL, Bester L. Yttrium-90 radioembolization for unresectable, chemoresistant breast cancer liver metastases: a large single-center experience of 40 patients. Ann Surg Oncol. 2014;21:1296-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Gordon AC, Gradishar WJ, Kaklamani VG, Thuluvath AJ, Ryu RK, Sato KT, Gates VL, Salem R, Lewandowski RJ. Yttrium-90 radioembolization stops progression of targeted breast cancer liver metastases after failed chemotherapy. J Vasc Interv Radiol. 2014;25:1523-1532, 1532.e1-1532.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Bagni O, Filippi L, Pelle G, Cianni R, Schillaci O. Total Lesion Glycolysis and Sequential (90)Y-Selective Internal Radiation Therapy in Breast Cancer Liver Metastases: Preliminary Results. Cancer Biother Radiopharm. 2015;30:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Fendler WP, Lechner H, Todica A, Paprottka KJ, Paprottka PM, Jakobs TF, Michl M, Bartenstein P, Lehner S, Haug AR. Safety, Efficacy, and Prognostic Factors After Radioembolization of Hepatic Metastases from Breast Cancer: A Large Single-Center Experience in 81 Patients. J Nucl Med. 2016;57:517-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Pieper CC, Meyer C, Wilhelm KE, Block W, Nadal J, Ahmadzadehfar H, Willinek WA, Schild HH. Yttrium-90 Radioembolization of Advanced, Unresectable Breast Cancer Liver Metastases-A Single-Center Experience. J Vasc Interv Radiol. 2016;27:1305-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Chang J, Charalel R, Noda C, Ramaswamy R, Kim SK, Darcy M, Foltz G, Akinwande O. Liver-dominant Breast Cancer Metastasis: A Comparative Outcomes Study of Chemoembolization Versus Radioembolization. Anticancer Res. 2018;38:3063-3068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12751] [Cited by in RCA: 13141] [Article Influence: 505.4] [Reference Citation Analysis (8)] |
| 29. | Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207-214. [RCA] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 30. | van Persijn van Meerten EL, Gelderblom H, Bloem JL. RECIST revised: implications for the radiologist. A review article on the modified RECIST guideline. Eur Radiol. 2010;20:1456-1467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Selzner M, Morse MA, Vredenburgh JJ, Meyers WC, Clavien PA. Liver metastases from breast cancer: long-term survival after curative resection. Surgery. 2000;127:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 152] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | Covey AM, Sofocleous CT. Radiofrequency ablation as a treatment strategy for liver metastases from breast cancer. Semin Intervent Radiol. 2008;25:406-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Lawes D, Chopada A, Gillams A, Lees W, Taylor I. Radiofrequency ablation (RFA) as a cytoreductive strategy for hepatic metastasis from breast cancer. Ann R Coll Surg Engl. 2006;88:639-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Wang M, Zhang J, Ji S, Shao G, Zhao K, Wang Z, Wu A. Transarterial chemoembolisation for breast cancer with liver metastasis: A systematic review. Breast. 2017;36:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Goodman KA, Wiegner EA, Maturen KE, Zhang Z, Mo Q, Yang G, Gibbs IC, Fisher GA, Koong AC. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys. 2010;78:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 220] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 36. | Schneebaum S, Walker MJ, Young D, Farrar WB, Minton JP. The regional treatment of liver metastases from breast cancer. J Surg Oncol. 1994;55:26-31; discussion 32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 67] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Smits ML, Prince JF, Rosenbaum CE, van den Hoven AF, Nijsen JF, Zonnenberg BA, Seinstra BA, Lam MG, van den Bosch MA. Intra-arterial radioembolization of breast cancer liver metastases: a structured review. Eur J Pharmacol. 2013;709:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Namasivayam S, Martin DR, Saini S. Imaging of liver metastases: MRI. Cancer Imaging. 2007;7:2-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 39. | Namasivayam S, Salman K, Mittal PK, Martin D, Small WC. Hypervascular hepatic focal lesions: spectrum of imaging features. Curr Probl Diagn Radiol. 2007;36:107-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Koukourakis GV S-Editor: Zhang L L-Editor: A E-Editor: Qi LL