Published online Dec 15, 2020. doi: 10.4251/wjgo.v12.i12.1443
Peer-review started: August 5, 2020
First decision: September 24, 2020
Revised: September 28, 2020
Accepted: October 20, 2020
Article in press: October 20, 2020
Published online: December 15, 2020
Processing time: 127 Days and 4.2 Hours
The number of dissected lymph nodes (LNs) in rectal cancer after neoadjuvant therapy has a controversial effect on the prognosis.
To investigate the prognostic impact of the number of LN dissected in rectal cancer patients after neoadjuvant therapy.
We performed a systematic review and searched PubMed, Embase (Ovid), MEDLINE (Ovid), Web of Science, and Cochrane Library from January 1, 2000 until January 1, 2020. Two reviewers examined all the publications independently and extracted the relevant data. Articles were eligible for inclusion if they compared the number of LNs in rectal cancer specimens resected after neoadjuvant treatment (LNs ≥ 12 vs LNs < 12). The primary endpoints were the overall survival (OS) and disease-free survival (DFS).
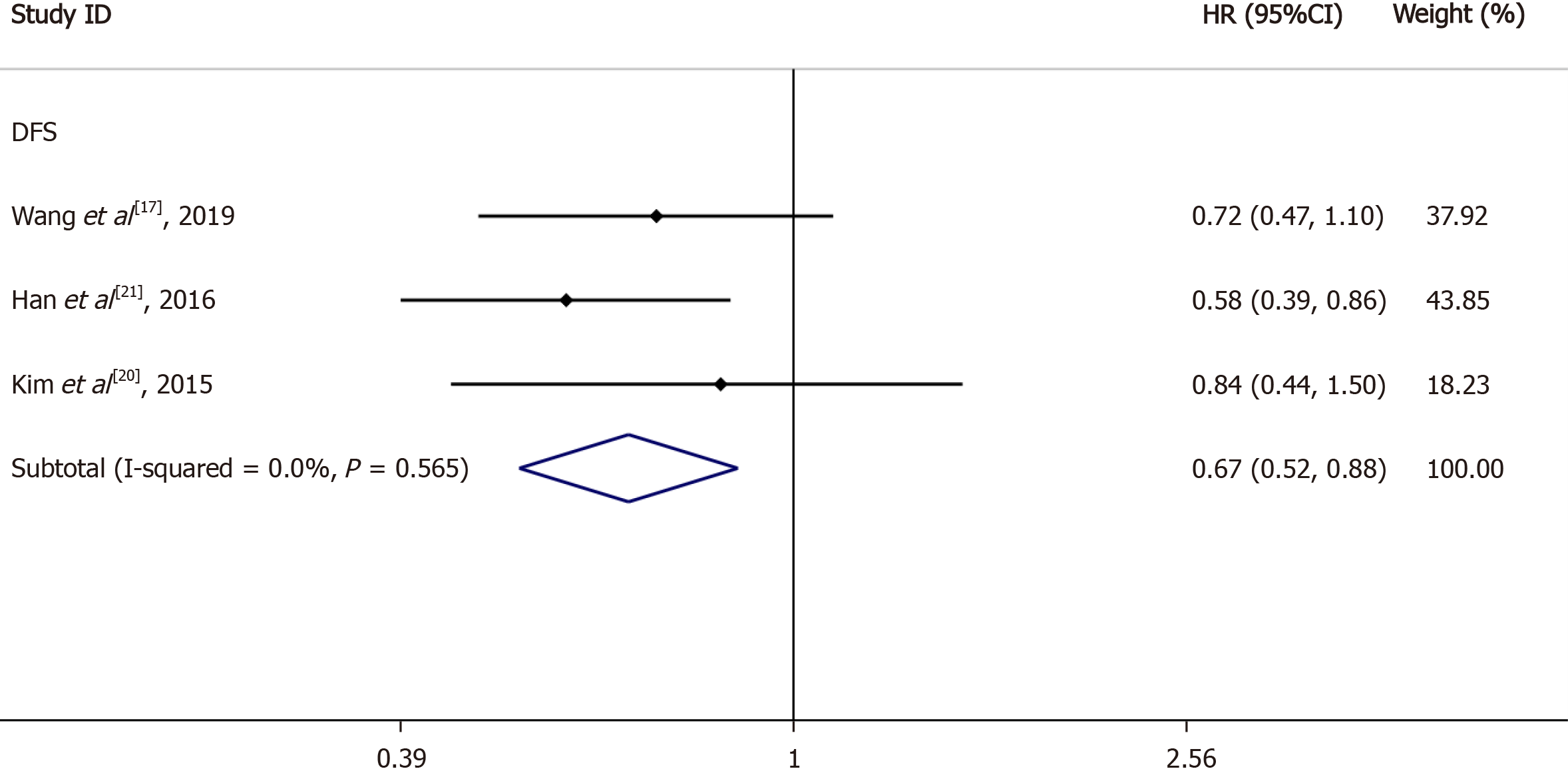
Nine articles were included in the meta-analyses. Statistical analysis revealed a statistically significant difference in OS [hazard ratio (HR) = 0.76, 95% confidence interval (CI): 0.66-0.88, I2 = 12.2%, P = 0.336], DFS (HR = 0.76, 95%CI: 0.63-0.92, I2 = 68.4%, P = 0.013), and distant recurrence (DR) (HR = 0.63, 95%CI: 0.48-0.93, I2 = 30.5%, P = 0.237) between the LNs ≥ 12 and LNs < 12 groups, but local recurrence (HR = 0.67, 95%CI: 0.38-1.16, I2 = 0%, P = 0.348) showed no statistical difference. Moreover, subgroup analysis of LN negative patients revealed a statistically significant difference in DFS (HR = 0.67, 95%CI: 0.52-0.88, I2 = 0%, P = 0.565) between the LNs ≥ 12 and LNs < 12 groups.
Although neoadjuvant therapy reduces LN production in rectal cancer, our data indicate that dissecting at least 12 LNs after neoadjuvant therapy may improve the patients’ OS, DFS, and DR.
Core Tip: After neoadjuvant treatment of rectal cancer, the lymph node (LN) output is significantly reduced. There is no consensus on the relationship between the number of LNs resected and the prognosis of rectal cancer after neoadjuvant treatment. This is the first meta-analysis to compare the impact of the number of LNs on the prognosis of rectal cancer after neoadjuvant treatment. We studied the effects of resection of at least 12 LNs and less than 12 LNs after neoadjuvant treatment of rectal cancer on overall survival, disease-free survival, distant recurrence, and local recurrence.
- Citation: Tan L, Liu ZL, Ma Z, He Z, Tang LH, Liu YL, Xiao JW. Prognostic impact of at least 12 lymph nodes after neoadjuvant therapy in rectal cancer: A meta-analysis. World J Gastrointest Oncol 2020; 12(12): 1443-1455
- URL: https://www.wjgnet.com/1948-5204/full/v12/i12/1443.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i12.1443
Colorectal cancer is ranked third and second among global cancer morbidity and mortality, respectively. The incidence rate of colorectal cancer is third in men and fourth in women[1]. In 2018, 43030 new cases of rectal cancer were diagnosed in the United States[2].
According to both the International Union Against Cancer (UICC) and American Joint Commission on Cancer (AJCC), a minimum of 12 lymph nodes (LNs) should be obtained from surgical specimens to stage a colorectal cancer[3,4]. Today, the standard of care for patients with locally advanced rectal cancer is neoadjuvant chemoradiation therapy (CRT), followed by total mesorectal excision (TME)[5,6]. However, many studies have reported a significant decrease in the number of LNs retrieved from patients with rectal cancer who have received preoperative chemoradiation[7-12]. For example, a meta-analysis reported an average reduction of 3.9 LNs in neoadjuvant CRT compared with no neoadjuvant CRT[12]. Therefore, it remains controversial whether 12 or more resected LNs should be recommended by the UICC or AJCC after many neoadjuvant treatments for rectal cancer.
In the past 20 years, neoadjuvant therapy has been widely applied in rectal cancer. An increasing number of scholars have focused on the influence of the number of resected LNs after neoadjuvant therapy on the prognosis of rectal cancer. Presently, resecting more than 12 LNs or fewer than 12 LNs after neoadjuvant treatment for rectal cancer is controversial for prognosis.
Given the prognostic impact of the number of LNs, we performed a first series of meta-analyses to compare the prognostic impact of surgical resection of greater than 12 vs fewer than 12 LNs in patients with rectal cancer after neoadjuvant treatment.
For this systematic review, we adhered to the Meta-analysis of Observational Studies guidelines[13] and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement[14]. A systematic search was performed based on the following databases: PubMed, Embase (Ovid), MEDLINE (Ovid), Web of Science, and Cochrane Library from January 1, 2000 to January 1, 2020. We used “rectal cancer”, “neoadjuvant therapy”, “preoperative radiotherapy”, “preoperative chemotherapy”, “lymph nodes” and corresponding free words to search the literature in the above databases. Regardless of the type of study, the articles were eligible for inclusion if they compared the number of LNs in rectal cancer specimens resected after neoadjuvant treatment (LNs ≥ 12 vs LNs < 12).
First, all the identified titles and abstracts were examined by two independent reviewers (Tan L and Liu ZL). Next, the same two reviewers independently examined the full text of potentially relevant articles. In the event of disagreement, a third reviewer (Ma Z) was consulted and the relevant articles were discussed until a consensus was reached.
The following relevant information was extracted from all the included publications: First author, year of publication, country, number of patients, age, tumor grade, neoadjuvant therapy, surgery, years of follow-up, and outcome type. The main outcomes were the overall survival (OS) and disease-free survival (DFS) differences between the LNs ≥ 12 and LNs < 12 groups in patients after neoadjuvant therapy. The secondary outcomes were the distant recurrence (DR) and local recurrence (LR) differences between the LNs ≥ 12 and LNs < 12 groups in patients after neoadjuvant therapy. Therefore, if available, the following data were extracted: Hazard ratios (HRs), 95% confidence intervals (CIs), and P values of OS, DFS, DR, and LR. When the literature did not report HRs, only OS and DFS Kaplan-Meier curves and Engauge Digitizer (version 10.8) were used to determine the survival rate at the corresponding time points on the curve, followed by the HR calculation table[15]. We took the countdown if the HR reported in the literature was LNs < 12 vs LNs ≥ 12. All the data were independently extracted by two authors (Tan L and Liu ZL) and compared for consistency.
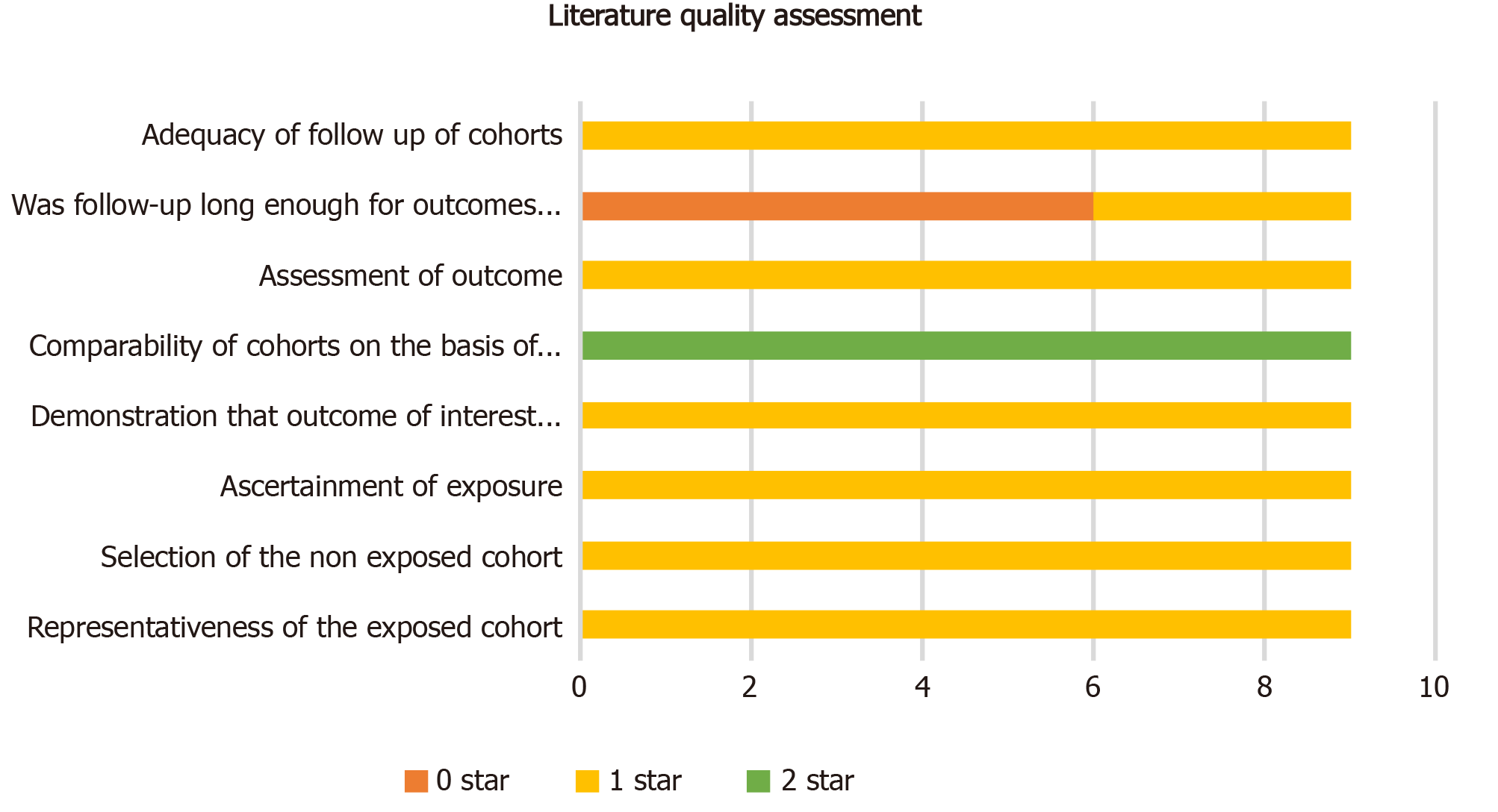
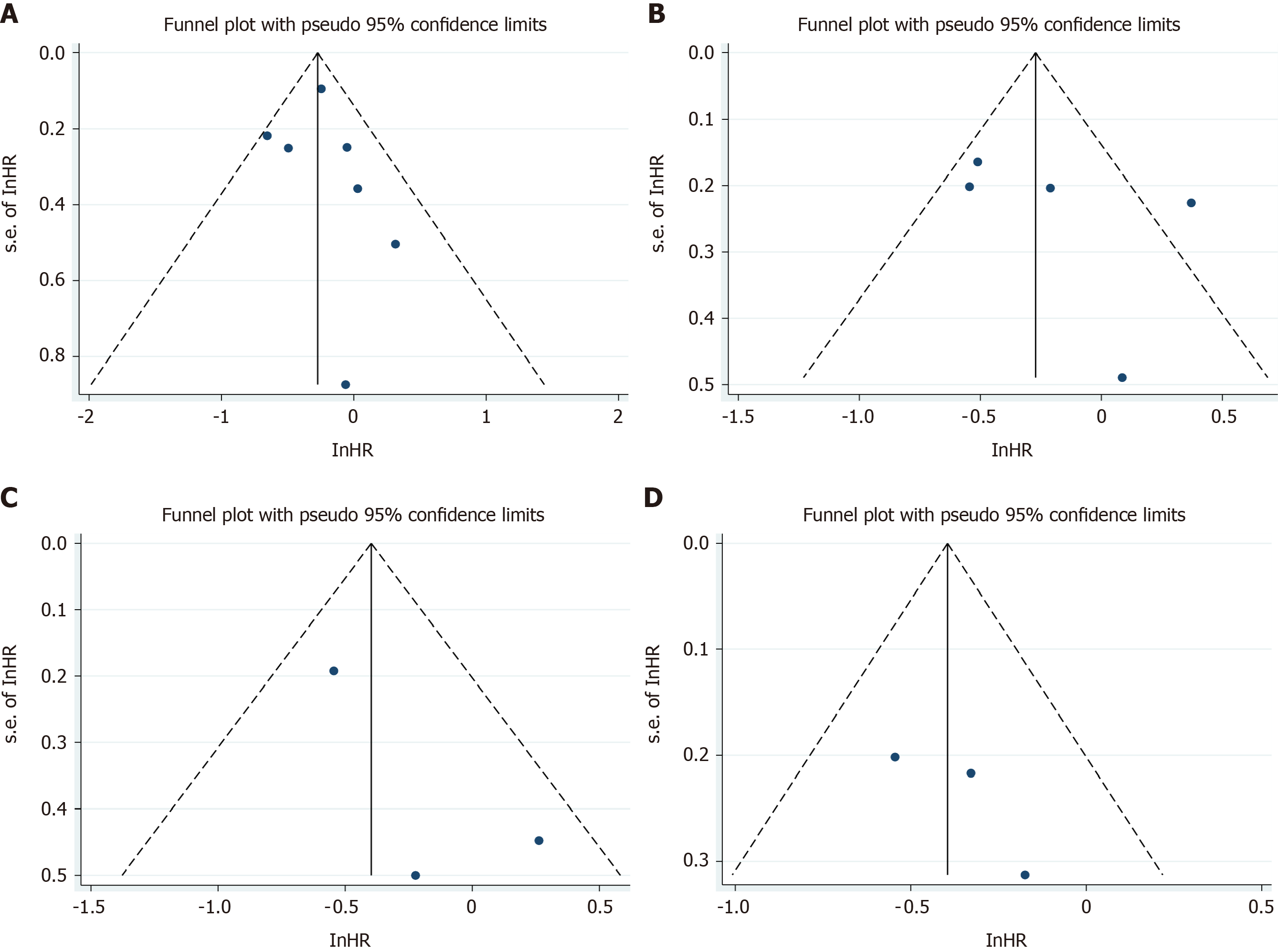
The quality of the included studies was assessed using the Newcastle-Ottawa Scale (NOS), with a maximum of nine points per study. Publication bias was assessed by visual inspection of the symmetry of the funnel plot. Since we considered that DFS heterogeneity was derived from patients with positive LNs, we performed subgroup analysis of the DFS of LN negative patients based on LNs ≥ 12 vs LNs < 12.
We used the Stata (version 15.3) Meta package for meta-analysis[16]. Binary outcome data are reported as HRs with 95%CIs using the Mantel–Haenszel method. Weighted mean differences were calculated for the effect size of continuous variables. Heterogeneity was assessed using I2 statistics, with values above 50% considered considerable heterogeneity. An a priori decision to use the random-effects model was made to account for the assumed considerable heterogeneity between the studies.
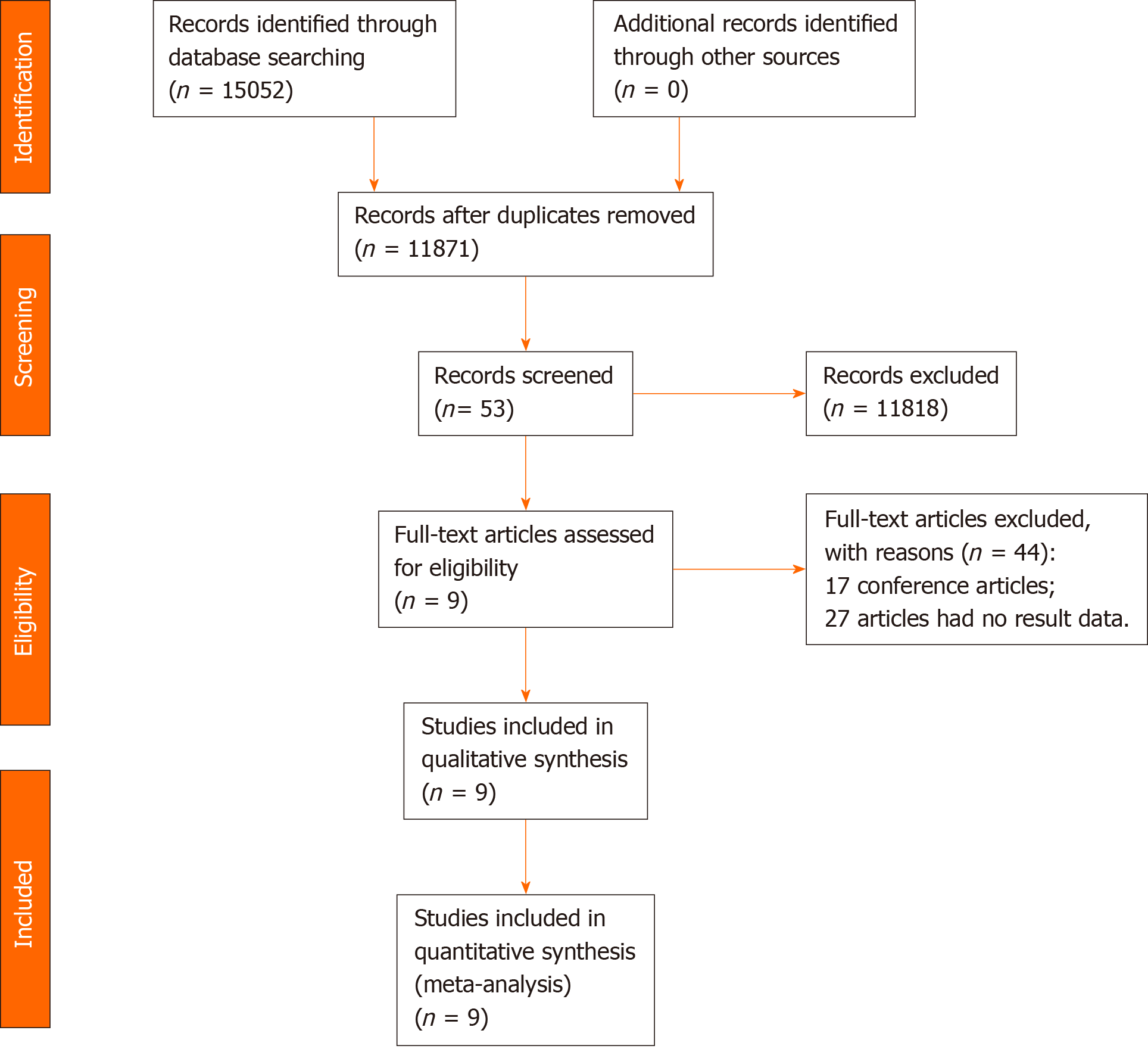
After removing duplicates, our computer-aided search yielded 11871 publications from PubMed, Medline (Ovid), Embase (Ovid), Web of Science, and Cochrane Library (Figure 1). In total, nine publications and 4494 patients with rectal cancer were eligible for inclusion. Table 1 indicates the characteristics of the included studies. Seven[17-23] of the nine studies (3840 patients) reported the main endpoint of OS; five[17,19-21,24] of the nine studies (1811 patients) reported the endpoint of DFS; three[17,23,25] of nine studies (953 patients) reported the endpoint of DR; two[17,25] of the nine studies (716 patients) reported the endpoint of LR. The NOS scores of the nine studies ranged from eight to nine (Figure 2). The literature collected was considered to be qualified.
| Ref. | Country | n | Age (yr) | Stage | Treatment | Surgery | Year of follow-up | Outcome |
| Wang et al[17], 2019 | China | 495 | < 50: 160; > 50: 335 | I-IV | Neoadjuvant treatment (RT 45-55 Gy + capecitabine) | AR, APR, Hartmann | No report | OS, DFS, LR, DR |
| Lykke et al[18], 2015 | Denmark | 2123 | 60-75 | I-IV | Neoadjuvant treatment | TME | No report | OS |
| de Campos-Lobato et al[23], 2013 | United States | 237 | 57 (49-66) | II-III | Neoadjuvant treatment | LAR, APR | 55 (36-77) mo | OS, DR, LR |
| Kim et al[20], 2015 | South Korea | 302 | 39-73 | I-IV | Neoadjuvant treatment (IV 5-FU leucovorin or oral 5-FU based) | LAR, APR, CAA | 57 mo | OS, DFS |
| Doll et al[22], 2009 | Germany | 102 | 18-75 | I-IV | Neoadjuvant diochemotherapy (RT 45 Gy + 5-FU) | (L)AR, Miles | No reports | OS |
| La Torre et al[19], 2013 | Italy | 123 | 67.9 (27-91) | I-IV | Neoadjuvant diochemotherapy (RT 45 Gy + 5-FU) | LAR, APR | 50 (9–120) mo | OS, DFS |
| Kim et al[24], 2015 | South Korea | 433 | 62 ± 11.1 | I-IV | Perioperative chemoradiation (45.0–50.4 Gy + 5-FU and leucovorin) | TME | 41.2 mo | DFS |
| Han et al[21], 2016 | South Korea | 458 | 60 (22-99) | I-III | Neoadjuvant treatment (RT 45–50.4 Gy + 5-FU) | TME | 52 mo | OS, DFS |
| Klos et al[25], 2010 | United States | 221 | 53 ± 13 | - | neoadjuvant treatment (RT 45.0–50.4 Gy + 5-FU) | TME | 36 (21.6-63.6) mo | LR, DR |
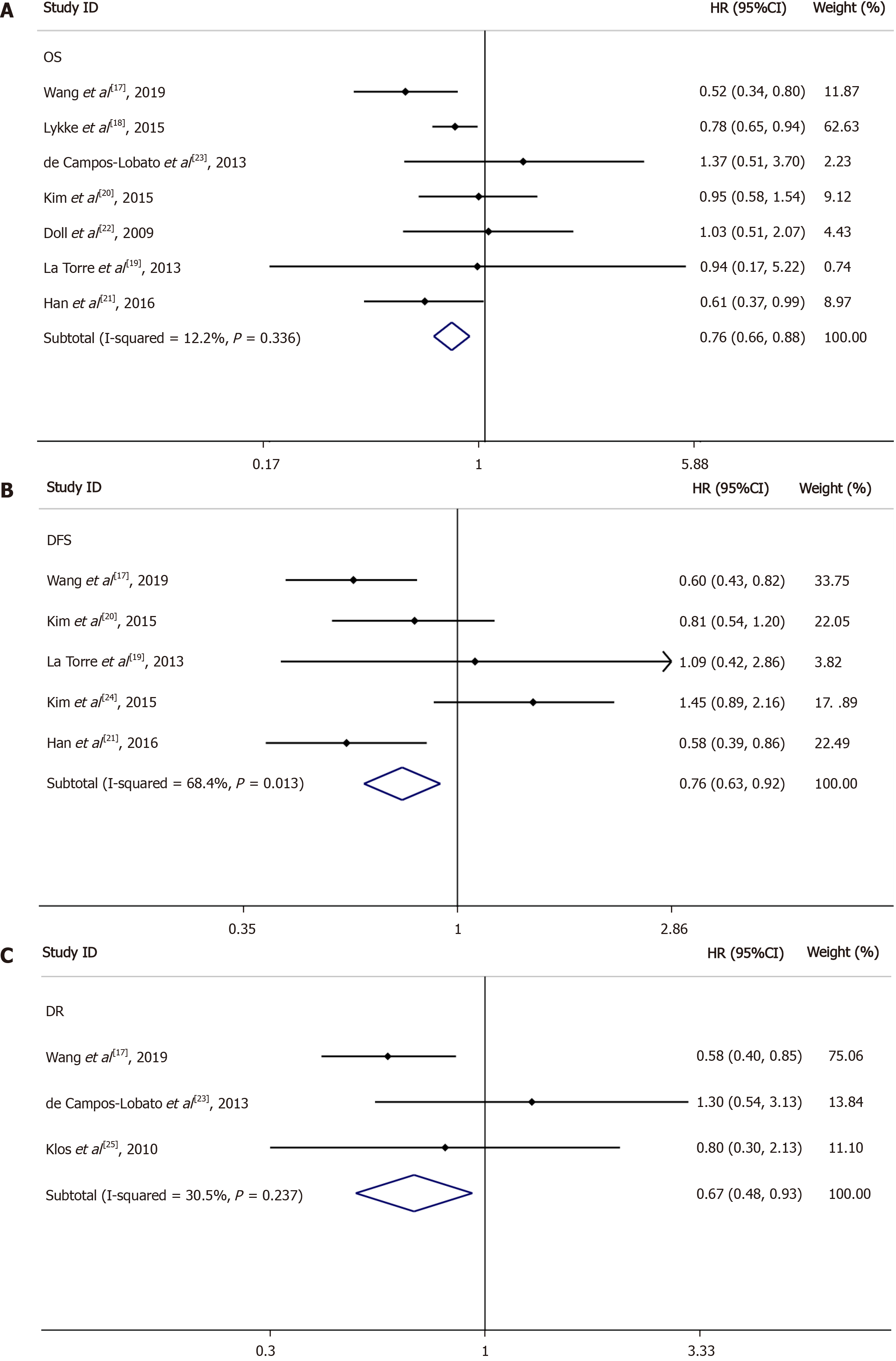
OS for LNs ≥ 12 vs LNs < 12: Seven of the nine included studies reported OS data based on at least 12 LNs vs fewer than 12 LNs; the HRs and 95%CIs of these studies and the summary HRs are shown in Figure 3A. The total summary estimated HR was 0.76 (95%CI: 0.66-0.88, P = 0.336). Heterogeneity tests showed that the trials did not have heterogeneity (I2 = 12.2%, P = 0.336).
DFS for LNs ≥ 12 vs LNs < 12: Among the nine studies collected, five reported DFS data based on at least 12 LNs and fewer than 12 LNs; the 95%CIs and HRs for each study and the summary HRs are shown in Figure 3B. The total summary estimated HR was 0.76 (95%CI: 0.63-0.92, P = 0.013). Heterogeneity tests showed that the trials had significant heterogeneity (I2 = 68.4%, P = 0.013).
DR for LNs ≥ 12 vs LNs < 12: Three of the nine included studies reported DR data based on LN ≥ 12 vs LN < 12; the 95%CIs and HRs for each study and the summary HRs are shown in Figure 3C. The total summary estimated HR was 0.67 (95%CI: 0.48-0.93, P = 0.237). Heterogeneity tests showed that the trials had no significant heterogeneity (I2 = 30.5%, P = 0.237).
LR for LNs ≥ 12 vs LNs < 12: Two of the nine included studies reported LR data based on LN ≥ 12 vs LN < 12. The total summary estimated HR was 0.67 (95%CI: 0.38-1.16, P = 0.348), with no statistical significance.
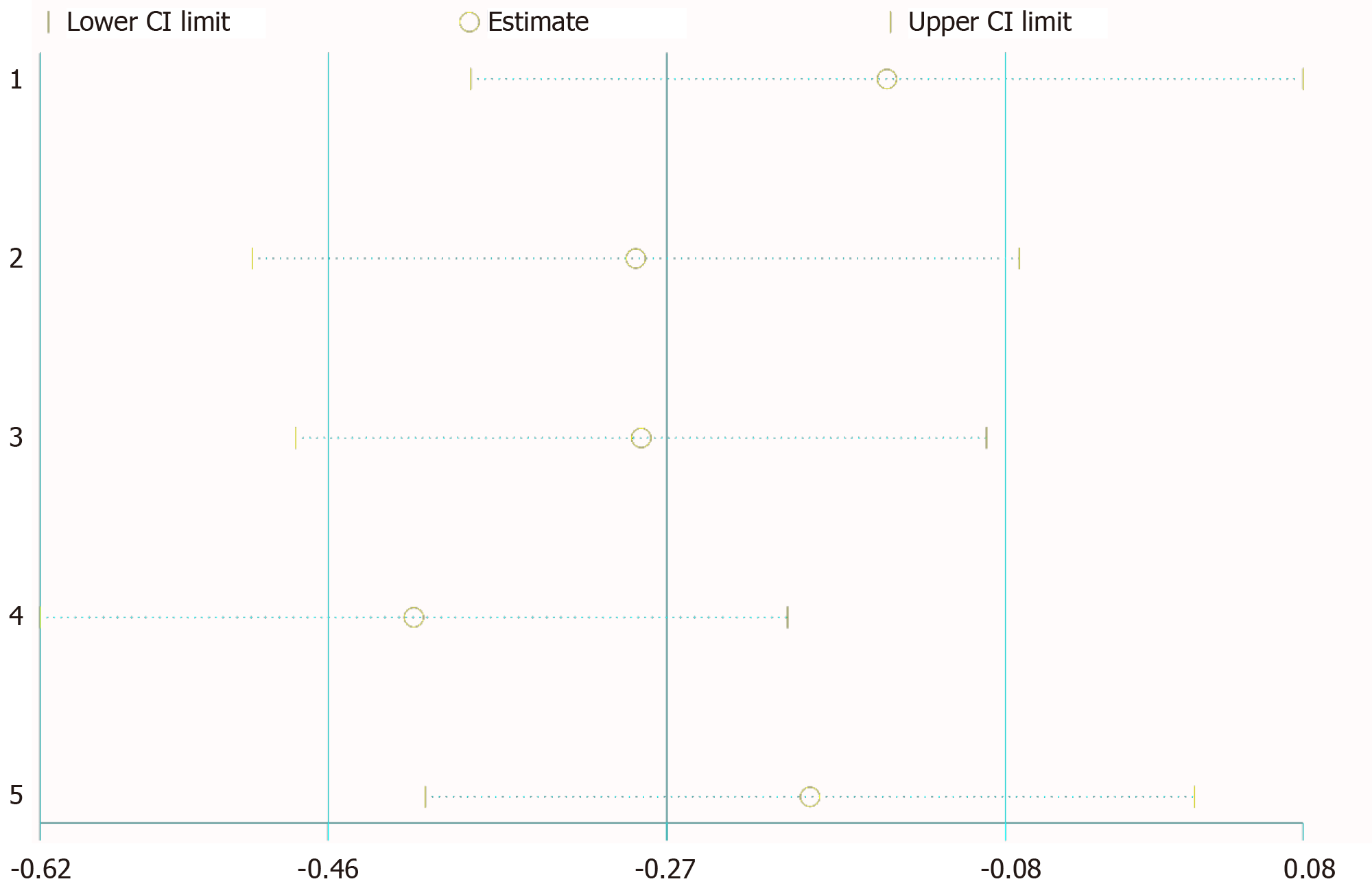
Sensitivity analysis showed that any deletion of a set of data had no effect on the results (Figure 4). We considered heterogeneity in patients with LN positivity. So, we conducted subgroup analysis of LN negative patients; the HRs and 95%CIs for each study and the summary HRs are shown in Figure 5. The total summary estimated HR was 0.67 (95%CI: 0.52-0.88), and no heterogeneity was found.
Publication bias was assessed by visual examination of the symmetry of the funnel plot. Our funnel plot showed no publication bias (Figure 6).
The AJCC and College of American Pathologists recommend examination of a minimum of 12 LNs to stage rectal cancer accurately. Sampling of 12 LNs may not be achievable in patients who have received neoadjuvant chemoradiation therapy[4,26]. Therefore, it remains controversial whether 12 LNs can be used as an accurate staging index for rectal cancer patients who have received preoperative neoadjuvant chemoradiation therapy. The mean number of LNs retrieved from rectal cancers treated with neoadjuvant therapy is significantly lower than that from rectal cancers treated by surgery alone[8,9]. The number of LNs needed to stage neoadjuvant-treated cases accurately is unknown. In patients receiving neoadjuvant radiotherapy and chemotherapy, the number of LN dissections needs to reach 12. Is this requirement suitable as a risk factor to evaluate the prognosis of patients with rectal tumors? Is it possible that the number of evaluated LN dissections is fewer than 12 because neoadjuvant radiotherapy and chemotherapy will lead to a decrease in the number of LN dissections? To confirm whether the number of LN dissections to judge the prognosis of rectal cancer patients who received neoadjuvant radiotherapy and chemotherapy is still applicable to 12, we performed this meta-analysis study.
The main finding of the present study is that, among patients with rectal cancer, dissecting at least 12 LNs after neoadjuvant treatment improved OS and DR compared with dissecting fewer than 12 LNs. In this study, we confirmed that at least 12 LNs should be dissected in rectal cancer patients after neoadjuvant radiotherapy and chemotherapy to evaluate the prognosis of patients well, and the number of LNs dissectedwas closely related to the improvement in the survival rate of rectal cancer patients. Additionally, for subgroup analysis, the DFS of LN negative patients with greater than 12 LNs resected was better than that of patients with fewer than 12 LNs dissected. These data suggest that surgical resection of at least 12 LNs after neoadjuvant treatment of rectal cancer improves prognosis.
Most scholars agree that the LN yield affects the prognosis of rectal cancer. Presently, they have studied the effect of resection of 12 LNs on the prognosis. At the same time, many scholars have studied the effect of different numbers of LNs resected on the prognosis of patients with neoadjuvant therapy for rectal cancer. We summarize the literature on the effect of different LN numbers on the prognosis in the last 10 years (Table 2). Yeo et al[27] showed that at least 8.5 LNs removed from rectal cancer surgery after neoadjuvant therapy could significantly improve the 5-year OS. La Torre et al[19], Tsai et al[28], and Han et al[21] found that at least 6, 7, and 8 LNs resected after neoadjuvant treatment could improve the prognosis. Pitto et al[29] found that at least 10 to 20 LNs resected after neoadjuvant radiotherapy improved the 5-year OS compared with fewer than 9 and more than 20. The above studies indicated that the small number of LNs dissected after neoadjuvant therapy is not a sign of a good tumor response to neoadjuvant therapy, and a relatively large number of LNs is still needed to be dissected to ensure a good prognosis.
| Ref. | n | Treatment | Number of LNs compared | OS (HR or percent) | DFS (HR or percent) |
| Yeo et al[27], 2020 | 94 | Neoadjuvant CRT (RT 45 Gy + capecitabine) | LNs ≥ 8.5 vs LNs < 8.5 | HR: 0.31 (95%CI: 0.15-0.64, P < 0.001) | - |
| LNs ≥ 16.5 vs LNs < 16.5 | - | HR: 0.46 (95%CI: 0.17-1.27, P = 0.13) | |||
| La Torre et al[19], 2013 | 123 | Neoadjuvant CRT (RT 45 Gy + 5-FU) | LNs ≥ 6 vs LNs < 6 | 5-yr OS: 84% vs 75% (P = 0.03) | 5-yr DFS: 83% vs 75% (P = 0.03) |
| Tsai et al[28], 2011 | 372 | Neoadjuvant CRT (RT 45 Gy + 5-FU and/or capecitabine) | LNs > 7 vs LNs ≤ 7 | 5-yr OS: 86.9% vs 81% (P = 0.067) | - |
| Han et al[21], 2016 | 458 | Neoadjuvant CRT (RT 45–50.4 Gy + 5-FU) | LNs ≥ 8 vs LNs < 8 | HR: 0.5 (95%CI: 0.2-0.9, P = 0.002) | HR: 0.6 (95%CI: 0.4-1.1, P = 0.042) |
| Pitto et al[29], 2020 | 104 | Neoadjuvant RT (RT 45 Gy + capecitabine) | LNs: 10-20 vs LNs ≤ 9 and ≥ 20 | - | HR: 0.313 (95%CI: 0.1-0.99, P = 0.049) |
The prognostic impact of resecting more than 12 LNs and fewer than 12 LNs after neoadjuvant treatment for rectal cancer is controversial. For example, Dev et al[30] found that resecting fewer than 12 LNs in rectal cancer patients undergoing neoadjuvant radiotherapy should be considered a better prognostic factor, but Wang et al[31] and Lykke et al[18] believed that resecting at least 12 LNs is an independent and favorable prognostic factor for rectal cancer after neoadjuvant therapy. Moreover, Khan et al[32] and La Torre et al[19] believed that at least 12 LNs dissected after neoadjuvant treatment of rectal cancer do not affect the prognosis. Our meta-analysis combining the available data showed that resection of at least 12 LNs after neoadjuvant therapy improves the prognosis.
The LN harvest is influenced by several factors, including the patient’s anatomic and pathologic workup, surgical dissection technique, and use of methylene blue and neoadjuvant treatment[12,33-36]. Pathological techniques are considered a factor that affects the LN yield due to improper specimen analysis and processing. Factors associated with patients, such as advanced age and obesity, are associated with lower LN yields[9,34,37,38]. Standard TME should be performed to help achieve optimal tumor resection. The injection of methylene blue solution into the inferior mesenteric artery is an effective and simple way to increase the LN harvest in the histopathological examination of the TME of rectal specimens[39], especially those receiving neoadjuvant therapy[40-42]. Presently, the use of neoadjuvant radiotherapy and chemotherapy is the standard treatment for rectal cancer in many European countries, leading to fewer LN tests[43]. If 12 LNs are considered the number needed for the accurate staging of stage II tumors, only 20% of cases treated with neoadjuvant therapy had adequate LN sampling[9]. To date, the number of dissected LNs needed to stage neoadjuvant-treated cases accurately is unknown. Additionally, the clinical significance of this information is unknown in the neoadjuvant setting because postoperative therapy is indicated in all patients who receive preoperative therapy regardless of the surgical pathology results. Therefore, technical measures are needed to improve the postoperative LN detection rate in patients with rectal cancer after neoadjuvant radiotherapy and chemotherapy. For example, standard TME in combination with the injection of methylene blue into the inferior mesenteric artery can be used to increase LN yield after neoadjuvant therapy. At the same time, the application of nano-carbon lymphatic tracer technology can also effectively improve the detection rate of postoperative LNs in patients with rectal cancer.
In recent decades, the therapeutic effect of rectal cancer has made great progress with the development of laparoscopic technology and medical devices. Murphy et al[44] found that the 5-year relative survival of rectal cancer improved significantly from 1992-1996 to 2010-2014. The emergence of neoadjuvant therapy, especially neoadjuvant radiotherapy and chemotherapy, significantly reduced the local recurrence rate and tumor staging of patients[45,46]. Neoadjuvant radiotherapy and chemotherapy have been regarded as the standard treatments for locally advanced rectal cancer, and the side effects of neoadjuvant radiotherapy and chemotherapy cannot be ignored, such as chronic sexual dysfunction[47] and diarrhea[48,49]. Some patients with high-risk diseases may need more intensive treatment, while others may have severe side effects due to the use of current protocols[50]. The criteria for the inclusion of patients with rectal cancer to undergo neoadjuvant radiotherapy and chemotherapy need to be further optimized, and multidisciplinary team discussion is warranted to determine whether a patient should receive neoadjuvant therapy for rectal cancer.
This meta-analysis was mostly limited by its inclusion of cohort study data only; no randomized controlled study was included. Cohort studies are prone to introduce bias, and two of these studies did have OS results. The HR data in four studies could not be extracted directly and were calculated from Kaplan-Meier curves, a calculation process that may cause errors. Additionally, this study only analyzed the prognosis of patients in the LNs ≥ 12 and LNs < 12 groups after neoadjuvant therapy. Insufficient data existed to analyze the effect of other LN numbers on the prognosis. Differences in surgical treatment reported in the literature, as well as different surgical procedures, may influence the LN yield, which may lead to bias.
Although neoadjuvant therapy reduces the production of LNs in rectal cancer, our data indicate that dissecting at least 12 LNs after neoadjuvant therapy may improve the patients’ OS, DFS, and DR.
Neoadjuvant therapy significantly reduces the number of yielded lymph nodes (LNs) for rectal cancer, and the number of dissected LNs in rectal cancer after neoadjuvant therapy has a controversial effect on the prognosis.
Studies have shown that the number of LNs after rectal cancer is significantly reduced after neoadjuvant therapy. Some scholars have found that less than 12 LNs in rectal cancer patients receiving neoadjuvant radiotherapy should be considered as a better prognostic factor. However, others believe that dissecting at least 12 LNs is an independent and favorable prognostic factors for rectal cancer after neoadjuvant therapy. Therefore, it is necessary to conduct a meta-analysis to systematically and comprehensively study the influence of the number of LNs retrieved after neoadjuvant treatment on the survival outcome of patients with rectal cancer.
To evaluate the effect of LN production in rectal cancer after neoadjuvant treatment on survival through meta-analysis.
The meta-analysis methods were adopted to realize the objectives.
Nine articles were included in the meta-analyses. Statistical analysis revealed a statistically significant difference in overall survival (OS) [hazard ratio (HR) = 0.76, 95% confidence interval (CI) = 0.66-0.88, I2 = 12.2%, P = 0.336], disease-free survival (DFS) (HR = 0.76, 95%CI: 0.63-0.92, I2 = 68.4%, P = 0.013), and distant recurrence (DR) (HR = 0.63, 95%CI: 0.48-0.93, I2 = 30.5%, P = 0.237) between the LNs ≥ 12 and LNs < 12 groups, but local recurrence (HR = 0.67, 95%CI: 0.38-1.16, I2 = 0%, P = 0.348) showed no statistical difference. Moreover, subgroup analysis of LN negative patients revealed a statistically significant difference in DFS (HR = 0.67, 0.95%CI: 0.52-0.88, I2 = 0%, P = 0.565) between the LNs ≥ 12 and LNs < 12 groups.
This meta-analysis confirmed that dissecting at least 12 LNs after neoadjuvant therapy may improve the patients’ OS, DFS, and DR.
Some limitations in this analysis should be handled carefully. The most important limitation is that the included studies are all retrospective. Because some potential deviations are difficult to adjust, further careful design and large-scale randomized controlled trial experiments are needed to determine the effect of the number of anatomical LNs on the prognosis of rectal cancer after neoadjuvant treatment. In addition, because neoadjuvant therapy reduces LN yield, further research is needed on the impact of different LN numbers on prognosis, such as 6 LNs, 7 LNs, and 8 LNs.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56683] [Article Influence: 7085.4] [Reference Citation Analysis (135)] |
| 2. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11573] [Cited by in RCA: 13302] [Article Influence: 1662.8] [Reference Citation Analysis (4)] |
| 3. | Greene FL. The American Joint Committee on Cancer: updating the strategies in cancer staging. Bull Am Coll Surg. 2002;87:13-15. [PubMed] |
| 4. | Sobin LH. TNM classification: clarification of number of regional lymph nodes for pN0. Br J Cancer. 2001;85:780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Gollins S, Moran B, Adams R, Cunningham C, Bach S, Myint AS, Renehan A, Karandikar S, Goh V, Prezzi D, Langman G, Ahmedzai S, Geh I. Association of Coloproctology of Great Britain & Ireland (ACPGBI): Guidelines for the Management of Cancer of the Colon, Rectum and Anus (2017) - Multidisciplinary Management. Colorectal Dis. 2017;19 Suppl 1:37-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, Arnold D; ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 311] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 7. | Sermier A, Gervaz P, Egger JF, Dao M, Allal AS, Bonet M, Morel P. Lymph node retrieval in abdominoperineal surgical specimen is radiation time-dependent. World J Surg Oncol. 2006;4:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Wichmann MW, Müller C, Meyer G, Strauss T, Hornung HM, Lau-Werner U, Angele MK, Schildberg FW. Effect of preoperative radiochemotherapy on lymph node retrieval after resection of rectal cancer. Arch Surg. 2002;137:206-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 155] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Baxter NN, Morris AM, Rothenberger DA, Tepper JE. Impact of preoperative radiation for rectal cancer on subsequent lymph node evaluation: a population-based analysis. Int J Radiat Oncol Biol Phys. 2005;61:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 159] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Nagtegaal ID, van de Velde CJ, van der Worp E, Kapiteijn E, Quirke P, van Krieken JH; Cooperative Clinical Investigators of the Dutch Colorectal Cancer Group. Macroscopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control. J Clin Oncol. 2002;20:1729-1734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 688] [Article Influence: 28.7] [Reference Citation Analysis (9)] |
| 11. | Rullier A, Laurent C, Capdepont M, Vendrely V, Belleannée G, Bioulac-Sage P, Rullier E. Lymph nodes after preoperative chemoradiotherapy for rectal carcinoma: number, status, and impact on survival. Am J Surg Pathol. 2008;32:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 147] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 12. | Mechera R, Schuster T, Rosenberg R, Speich B. Lymph node yield after rectal resection in patients treated with neoadjuvant radiation for rectal cancer: A systematic review and meta-analysis. Eur J Cancer. 2017;72:84-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14425] [Cited by in RCA: 17252] [Article Influence: 663.5] [Reference Citation Analysis (0)] |
| 14. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18665] [Cited by in RCA: 18040] [Article Influence: 1061.2] [Reference Citation Analysis (1)] |
| 15. | Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4738] [Cited by in RCA: 5106] [Article Influence: 268.7] [Reference Citation Analysis (1)] |
| 16. | Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1058] [Cited by in RCA: 1684] [Article Influence: 140.3] [Reference Citation Analysis (0)] |
| 17. | Wang Y, Zhou M, Yang J, Sun X, Zou W, Zhang Z, Zhang J, Shen L, Yang L, Zhang Z. Increased lymph node yield indicates improved survival in locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. Cancer Med. 2019;8:4615-4625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Lykke J, Jess P, Roikjaer O; Danish Colorectal Cancer Group. Increased Lymph Node Yield Is Associated With Improved Survival in Rectal Cancer Irrespective of Neoadjuvant Treatment: Results From a National Cohort Study. Dis Colon Rectum. 2015;58:823-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | La Torre M, Mazzuca F, Ferri M, Mari FS, Botticelli A, Pilozzi E, Lorenzon L, Osti MF, Marchetti P, Enrici RM, Ziparo V. The importance of lymph node retrieval and lymph node ratio following preoperative chemoradiation of rectal cancer. Colorectal Dis. 2013;15:e382-e388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Kim WR, Han YD, Cho MS, Hur H, Min BS, Lee KY, Kim NK. Oncologic Impact of Fewer Than 12 Lymph Nodes in Patients Who Underwent Neoadjuvant Chemoradiation Followed by Total Mesorectal Excision for Locally Advanced Rectal Cancer. Medicine. 94:e1133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Han J, Noh GT, Yeo SA, Cheong C, Cho MS, Hur H, Min BS, Lee KY, Kim NK. The number of retrieved lymph nodes needed for accurate staging differs based on the presence of preoperative chemoradiation for rectal cancer. Medicine. 95:e4891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Doll D, Gertler R, Maak M, Friederichs J, Becker K, Geinitz H, Kriner M, Nekarda H, Siewert JR, Rosenberg R. Reduced lymph node yield in rectal carcinoma specimen after neoadjuvant radiochemotherapy has no prognostic relevance. World J Surg. 2009;33:340-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | de Campos-Lobato LF, Stocchi L, de Sousa JB, Buta M, Lavery IC, Fazio VW, Dietz DW, Kalady MF. Less than 12 nodes in the surgical specimen after total mesorectal excision following neoadjuvant chemoradiation: it means more than you think! Ann Surg Oncol. 2013;20:3398-3406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Kim HJ, Jo JS, Lee SY, Kim CH, Kim YJ, Kim HR. Low Lymph Node Retrieval After Preoperative Chemoradiation for Rectal Cancer is Associated with Improved Prognosis in Patients with a Good Tumor Response. Ann Surg Oncol. 2015;22:2075-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Klos CL, Shellito PC, Rattner DW, Hodin RA, Cusack JC, Bordeianou L, Sylla P, Hong TS, Blaszkowsky L, Ryan DP, Lauwers GY, Chang Y, Berger DL. The effect of neoadjuvant chemoradiation therapy on the prognostic value of lymph nodes after rectal cancer surgery. Am J Surg. 2010;200:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Bostwick DG, Grignon DJ, Hammond ME, Amin MB, Cohen M, Crawford D, Gospadarowicz M, Kaplan RS, Miller DS, Montironi R, Pajak TF, Pollack A, Srigley JR, Yarbro JW. Prognostic factors in prostate cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:995-1000. [PubMed] |
| 27. | Yeo CS, Syn N, Liu H, Fong SS. A lower cut-off for lymph node harvest predicts for poorer overall survival after rectal surgery post neoadjuvant chemoradiotherapy. World J Surg Oncol. 2020;18:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Tsai CJ, Crane CH, Skibber JM, Rodriguez-Bigas MA, Chang GJ, Feig BW, Eng C, Krishnan S, Maru DM, Das P. Number of lymph nodes examined and prognosis among pathologically lymph node-negative patients after preoperative chemoradiation therapy for rectal adenocarcinoma. Cancer. 2011;117:3713-3722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Pitto F, Zoppoli G, Scabini S, Romairone E, Fiocca R, Ballestrero A, Sparavigna M, Malaspina L, Valle L, Grillo F, Mastracci L. Lymph node number, surface area and lymph node ratio are important prognostic indicators in neoadjuvant chemoradiotherapy treated rectal cancer. J Clin Pathol. 2020;73:162-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Dev K, Shivran N, Gurawalia J, Pandey A, Kumar S, Kurpad V, Nayak S, Arjunan R. Less Than 12 Lymph Nodes in The Surgical Specimen after Neo-Adjuvant Chemo-Radiotherapy in Rectal Cancer: Five Years Survival Analysis. Eur J Surg Oncol. 2020;46:e78. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Wang Y, Zhou M, Yang L, Zhang J, Deng W, Shen L, Yao Y, Liang L, Zhang Z. Prognostic value of lymph node yield in locally advanced rectal cancer with neoadjuvant chemoradiotherapy. J Clin Oncol. 2018;36:e15680. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 32. | Khan M, Hakeem A, Scott N, Botterill I. Significance of the lymph node count after neo-adjuvant treatment for rectal cancer. Gut. 2015;64:A542. [DOI] [Full Text] |
| 33. | Thorn CC, Woodcock NP, Scott N, Verbeke C, Scott SB, Ambrose NS. What factors affect lymph node yield in surgery for rectal cancer? Colorectal Dis. 2004;6:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Miller ED, Robb BW, Cummings OW, Johnstone PA. The effects of preoperative chemoradiotherapy on lymph node sampling in rectal cancer. Dis Colon Rectum. 2012;55:1002-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 35. | Mekenkamp LJ, van Krieken JH, Marijnen CA, van de Velde CJ, Nagtegaal ID; Pathology Review Committee and the Co-operative Clinical Investigators. Lymph node retrieval in rectal cancer is dependent on many factors--the role of the tumor, the patient, the surgeon, the radiotherapist, and the pathologist. Am J Surg Pathol. 2009;33:1547-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 36. | Liu J, Huang P, Zheng Z, Chen T, Wei H. Modified methylene blue injection improves lymph node harvest in rectal cancer. ANZ J Surg. 2017;87:247-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Shen SS, Haupt BX, Ro JY, Zhu J, Bailey HR, Schwartz MR. Number of lymph nodes examined and associated clinicopathologic factors in colorectal carcinoma. Arch Pathol Lab Med. 2009;133:781-786. [PubMed] |
| 38. | Görög D, Nagy P, Péter A, Perner F. Influence of obesity on lymph node recovery from rectal resection specimens. Pathol Oncol Res. 2003;9:180-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Klepšytė E, Samalavičius NE. Injection of methylene blue solution into the inferior mesenteric artery of resected rectal specimens for rectal cancer as a method for increasing the lymph node harvest. Tech Coloproctol. 2012;16:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Borowski DW, Banky B, Banerjee AK, Agarwal AK, Tabaqchali MA, Garg DK, Hobday C, Hegab M, Gill TS. Intra-arterial methylene blue injection into ex vivo colorectal cancer specimens improves lymph node staging accuracy: a randomized controlled trial. Colorectal Dis. 2014;16:681-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Münster M, Hanisch U, Tuffaha M, Kube R, Ptok H. Ex Vivo Intra-arterial Methylene Blue Injection in Rectal Cancer Specimens Increases the Lymph-Node Harvest, Especially After Preoperative Radiation. World J Surg. 2016;40:463-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Reima H, Saar H, Innos K, Soplepmann J. Methylene blue ex vivo staining of resected colorectal cancer specimens to enhance lymph node retrieval: a randomised controlled trial. Conference: 8th Congress of the Baltic Association of Surgeons; 2015; Estonia. Eesti Arst. 2015;94:77. |
| 43. | Marijnen CA, Nagtegaal ID, Klein Kranenbarg E, Hermans J, van de Velde CJ, Leer JW, van Krieken JH; Pathology Review Committee and the Cooperative Clinical Investigators. No downstaging after short-term preoperative radiotherapy in rectal cancer patients. J Clin Oncol. 2001;19:1976-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 249] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 44. | Murphy CC, Wallace K, Sandler RS, Baron JA. Racial Disparities in Incidence of Young-Onset Colorectal Cancer and Patient Survival. Gastroenterology. 2019;156:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 131] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 45. | Abraha I, Aristei C, Palumbo I, Lupattelli M, Trastulli S, Cirocchi R, De Florio R, Valentini V. Preoperative radiotherapy and curative surgery for the management of localised rectal carcinoma. Cochrane Database Syst Rev. 2018;10:CD002102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 46. | Pettersson D, Lörinc E, Holm T, Iversen H, Cedermark B, Glimelius B, Martling A. Tumour regression in the randomized Stockholm III Trial of radiotherapy regimens for rectal cancer. Br J Surg. 2015;102:972-978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 47. | Hendren SK, O'Connor BI, Liu M, Asano T, Cohen Z, Swallow CJ, Macrae HM, Gryfe R, McLeod RS. Prevalence of male and female sexual dysfunction is high following surgery for rectal cancer. Ann Surg. 2005;242:212-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 448] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 48. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R; German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4342] [Cited by in RCA: 4558] [Article Influence: 207.2] [Reference Citation Analysis (7)] |
| 49. | Ansari N, Solomon MJ, Fisher RJ, Mackay J, Burmeister B, Ackland S, Heriot A, Joseph D, McLachlan SA, McClure B, Ngan SY. Acute Adverse Events and Postoperative Complications in a Randomized Trial of Preoperative Short-course Radiotherapy Versus Long-course Chemoradiotherapy for T3 Adenocarcinoma of the Rectum: Trans-Tasman Radiation Oncology Group Trial (TROG 01.04). Ann Surg. 2017;265:882-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 50. | Rana N, Chakravarthy AB, Kachnic LA. Neoadjuvant Treatment for Locally Advanced Rectal Cancer: New Concepts in Clinical Trial Design. Curr Treat Options Oncol. 2017;18:13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Grundmann R, Uhlmann D S-Editor: Chen XF L-Editor: Wang TQ P-Editor: Li JH