Published online Nov 15, 2020. doi: 10.4251/wjgo.v12.i11.1336
Peer-review started: July 13, 2020
First decision: September 17, 2020
Revised: September 27, 2020
Accepted: October 15, 2020
Article in press: October 15, 2020
Published online: November 15, 2020
Processing time: 122 Days and 3.1 Hours
Colonoscopy is the accepted gold standard for the detection of colorectal cancer. However, colonoscopy is less effective in preventing colon cancer in the right side compared with the left side.
To investigate the feasibility of a novel type of retroflexion colonoscope, EC-3490Ti colonoscope, for detection of proximal colon lesions.
In this prospective trial, we recruited patients who underwent colonoscopy for screening or surveillance. When the endoscopists could not grasp the whole observation of the right-side colon mucosa in the forward view (FV), insertion and withdrawal were repeatedly performed in the FV group with the EC38-i10F colonoscope while retroflexion was performed in the retroflexed view (RV) group with the EC-3490Ti colonoscope. Adenoma detection rate, the total number of adenomas per positive participant, the success rate of retroflexion, and endoscope withdrawal time were recorded and compared.
The total adenoma detection rate (39.3% vs 37.7%, P = 0.646) did not show any significant difference between the two groups. However, the polyp detection rate (59.6% vs 51.0%, P = 0.002), adenoma detection rate in the right colon (21.6% vs 14.4%, P = 0.012), and the total number of adenomas per positive participant (2.1 vs 1.7, P = 0.011) reached statistical significance. Retroflexion was achieved in 91.7% of our cohort. Compared with the FV group, the withdrawal time was significantly prolonged in the RV group (586.1 ± 124.4 s vs 508.8 ± 129.6 s, P < 0.001). In contrast, the proportion of additional ancillary pressure decreased (27.4% vs 45.7%, P < 0.001), and the visual analog scale pain scores did not increase (2.7 ± 1.4 vs 2.8 ± 1.4, P = 0.377).
Retroflexion in the proximal colon could be performed successfully and safely with the EC-3490Ti colonoscope. This maneuver could detect more adenomas effectively.
Core Tip: The current prospective randomized trial assessed the feasibility and efficacy of retroflexion colonoscopy in the proximal colon. Unlike previous studies, retroflexion was performed when the mucosa could not be exposed completely in the forward view due to the folds and flexures, instead of second insertion and withdrawal. Retroflexion in the right colon could be performed successfully and safely with the EC-3490Ti colonoscope and detect more adenomas effectively.
- Citation: Li WK, Wang Y, Wang YD, Liu KL, Guo CM, Su H, Liu H, Wu J. Diagnostic value of novel retroflexion colonoscopy in the right colon: A randomized controlled trial. World J Gastrointest Oncol 2020; 12(11): 1336-1345
- URL: https://www.wjgnet.com/1948-5204/full/v12/i11/1336.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i11.1336
Colorectal cancer (CRC) is the third most commonly diagnosed malignancy and the second leading cause of cancer death worldwide[1]. According to the latest data of the Chinese National Cancer Center[2], the incidence and mortality of CRC both ranked fifth in China. Pathological and genetic evidence[3,4] suggests that > 90% of CRCs gradually develop from adenomas, during a period of 7-12 years. Screening and endoscopic polypectomy can reduce the mortality rate of CRC remarkably. Therefore, colonoscopy is currently considered the gold standard for CRC screening. However, interval cancers (ICs) still occur in patients who have undergone colonoscopy screening, due to a low detection rate and high miss rate for adenoma[5,6]. Furthermore, colonoscopy has unsatisfactory protection for the right-side colon because of the anatomical and morphological characteristics of proximal colon neoplasms compared with those on the left side[7,8]. Corley et al[9] found that with every percent increase in adenoma detection rate (ADR), the morbidity of ICs decreased by 3% within 10 years.
Various potential methods have been applied to improve ADR, especially in the proximal colon. Proper colonic cleaning, a high rate of cecal intubation, sufficient withdrawal time, and specialized training to recognize subtle polyps are required. Moreover, various new instruments have been used by endoscopists, including image-enhanced endoscopy, full spectrum endoscopy (FUSE), extra-wide-angle-view colonoscopy (EWAVE), and third eye retroscopy (TER). However, the evidence supporting the efficacy of these measures is not sufficient[10].
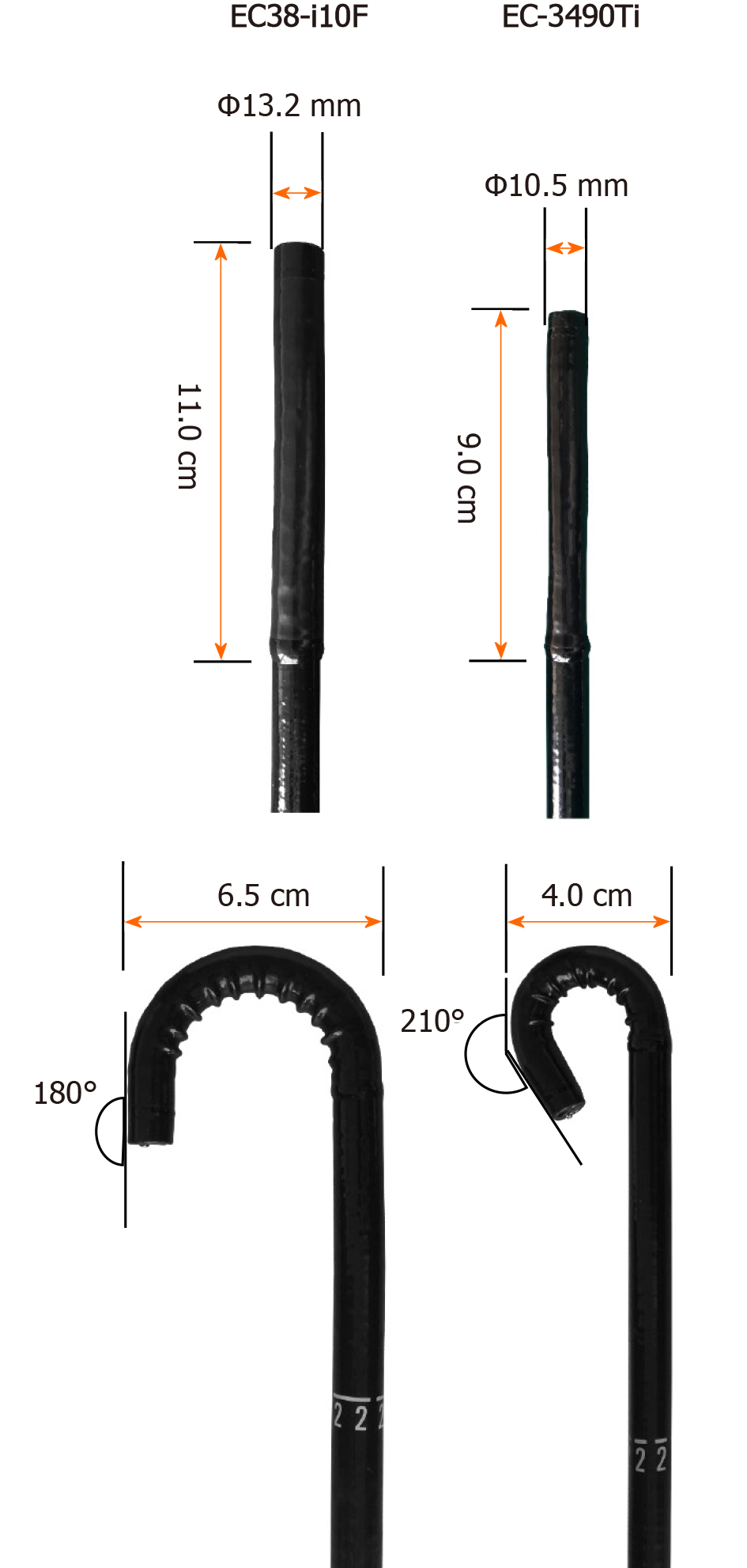
Retroflexion during withdrawal of a colonoscope refers to making a J-turn with the bending section of the colonoscope, primarily aiming to increase the diagnostic view in the rectum[11,12]. Retroflexion could improve the visualization of the back wall of the haustral folds and the inner curvatures of the colonic flexures, making it possible to detect more lesions in the right colon. Recently, PENTAX MEDICAL (Hoya, Tokyo, Japan) developed a novel type of retroflexion colonoscope, EC-3490Ti, with shorter bending section, wider retroflexion angle, and smaller retroflexion semidiameter (Figure 1), making retroflexion easier.
Therefore, we conducted a prospective randomized trial to assess the feasibility and efficiency of this new retroflexion colonoscope in the proximal colon.
This was a single-center prospective randomized trial conducted in the Endoscopy Center of Beijing Shijitan Hospital, Capital Medical University (Beijing, China). The working protocol was approved by the ethics committee of Beijing Shijitan Hospital, Capital Medical University, and informed consent was obtained from each participant.
We enrolled patients aged ≥ 18 years who underwent total colonoscopy for CRC screening or surveillance. The exclusion criteria included: (1) Familial adenomatous polyposis and hereditary non-polyposis colorectal cancer syndrome; (2) Inflammatory bowel disease; (3) Incomplete colonoscopy; (4) Inadequate bowel preparation [Boston bowel preparation scale (BBPS) < 6]; (5) Advanced CRC; and (6) Receiving anticoagulant medication.
The enrolled patients were randomly assigned in a 1:1 ratio to the retroflexed view (RV) group or forward view (FV) group. Randomization was carried out using a computer-generated random sequence. The allocation was placed in a sealed envelope and kept by an independent nurse who was not involved in this study. The endoscopists were blinded to the result of randomization until the start of the colonoscopy.
For bowel cleaning, all patients were advised to take a low-fiber diet for 3 d and were given 4 L of polyethylene glycol solution at split doses the day before colonoscopy. Endoscopists evaluated the quality of bowel preparation using the criteria of BBPS. Colonoscopy was performed without sedation. All procedures were performed by four experienced endoscopists, who had performed > 3000 colonoscopies.
The right colon was defined as the colon from the cecum to the splenic flexure, including the cecum, ascending colon, hepatic flexure, and transverse colon. In each group, a colonoscope was intubated in the cecum routinely. Afterwards, the colonoscope was withdrawn to search for adenomas and other lesions. When the endoscopists could not grasp the whole observation of the right-side colon mucosa in the FV due to haustral folds and hepatic flexures in the proximal colon, insertion and withdrawal were repeatedly performed using the EC38-i10F colonoscope in the FV group while retroflexion was performed in the RV group with the EC-3490Ti colonoscope. Retroflexion was accomplished by using a maneuver similar to that used for rectal retroflexion. Successful retroflexion was defined as the insertion tube being visible to the endoscopist. After withdrawal to the splenic flexure, the rest of the colon was examined in a conventional FV in the two groups. The location and size, estimated by open biopsy forceps, were recorded for all detected polyps. The visualized lesions were removed by biopsy, endoscopic mucosal resection, or endoscopic submucosal dissection and sent for histopathological diagnoses by an experienced pathologist.
The primary outcome parameter of this study was ADR, which was the proportion of patients with at least one adenoma detected. The secondary outcome measures included ADR for the right colon (R-ADR), polyp detection rate (PDR), total number of adenomas per positive participant (APP), total number of adenomas per colonoscopy (APC), success rate of retroflexion, withdrawal duration, and degree of pain assessed with the visual analog scale (VAS).
According to the data of our center, the ADR of EC38-i10F endoscopy was 31.6% in 2018. Based on the results of previous studies, we hypothesized that ADR could be increased by 10% with retroflexion examination of the right colon, compared with forward withdrawal alone. Power analysis indicated that a minimum of 361 participants were required in each group, assuming a 0.05 significance level and 0.8 power using two-sided equivalence to test each hypothesis.
IBM SPSS 23.0 (Armonk, NY, United States) was used for all statistical analyses. Continuous variables were compared using Student’s t test if normally distributed and the Mann–Whitney test if not normally distributed. Pearson’s c2 test or Fisher’s exact test was used to analyze categorical data and compare proportions. Univariate analysis and logistic regression analysis were carried out to evaluate predictors associated with unsuccessful retroflexion in the proximal colon. P < 0.05 was considered statistically significant. The statistical methods of this study were reviewed by Qing-Kun Song from Beijing Shijitan Hospital, Capital Medical University.
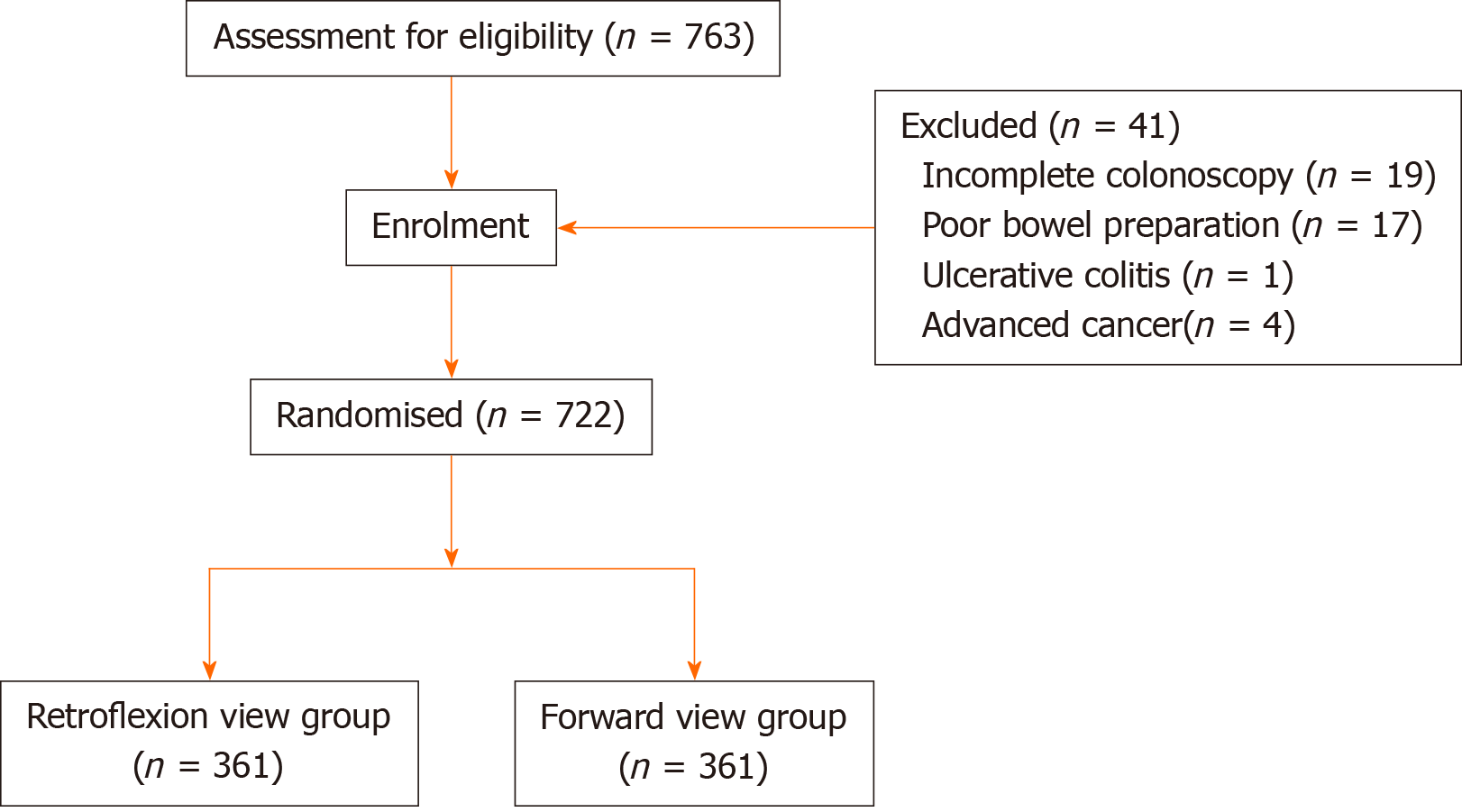
During the 6-mo period, 763 patients consented to participate in the study. Forty-one participants were excluded as a consequence of incomplete colonoscopy (n = 19), poor bowel preparation (n = 17), ulcerative colitis (n = 1), or advanced cancer (n = 4). Finally, 361 patients were enrolled into each arm (Figure 2). Baseline demographic characteristics are listed in Table 1. The median age was 55 years in the RV group and FV group. There were no significant differences between the two groups with respect to age, sex, body mass index (BMI), indication for colonoscopy, smoking history, family history of CRC, and bowel preparation (Table 1). No immediate or delayed complications occurred.
| RV (n = 361) | FV (n = 361) | P value | |
| Age (yr) | 55 (50-65) | 55 (45-63) | 0.052 |
| Sex, n (%) | |||
| Male | 183 (50.7) | 168 (46.5) | 0.264 |
| Female | 178 (49.3) | 193 (53.5) | |
| BMI (kg/m2) | 24.38 (22.31-25.95) | 24.22 (20.76-26.99) | 0.055 |
| Indication for colonoscopy, n (%) | |||
| Screening (no history of colon polyps) | 287 (79.5) | 304 (84.2) | 0.101 |
| Surveillance (past history of colon polyps) | 74 (20.5) | 57 (15.8) | |
| Current smokers, n (%) | 92 (25.5) | 90 (25.5) | 0.864 |
| Family history of colorectal cancer, n (%) | 111 (30.7) | 104 (28.8) | 0.569 |
| BBPS | 7 (7-8) | 7 (7-8) | 0.195 |
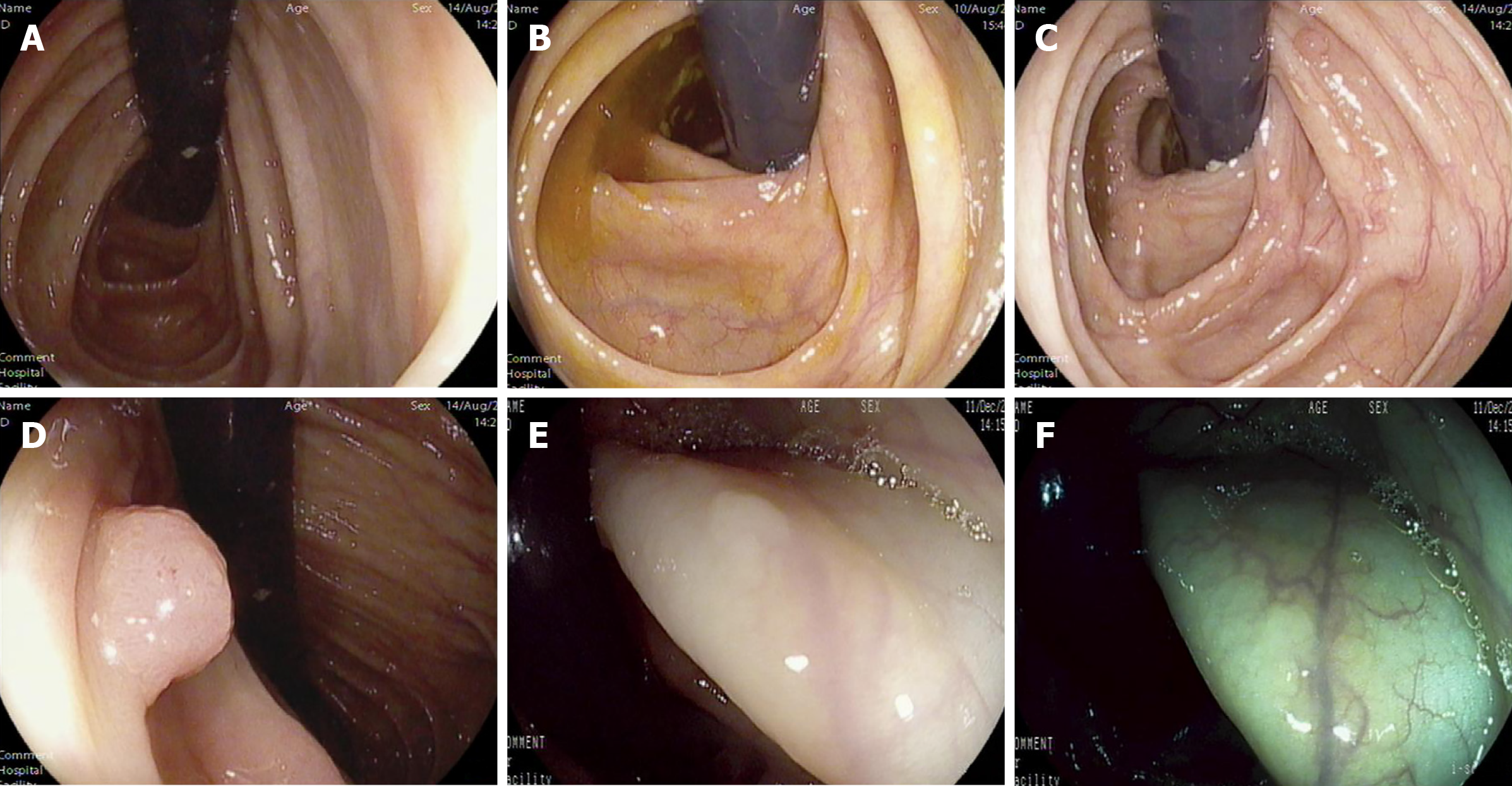
The total number of polyps detected in the RV group was 497, including an additional 75 found by retroflexion. Histopathological analysis confirmed 297 adenomas, including 63 assigned to retroflexed vision. A total of 417 polyps and 235 adenomas were identified in the FV group. In the RV group, 142 (39.3%) patients had at least one lesion compared with 136 (37.7%) in the FV group. ADR, the primary outcome measure, showed no significant differences between the two groups (P = 0.646). Among the secondary outcome parameters, there was also no significant difference in APC (0.8 vs 0.7, P = 0.280) between the RV and FV groups. However, the PDR (59.6% vs 51.0%, P = 0.002), R-ADR (21.6% vs 14.4%, P = 0.012), and APP (2.1 vs 1.7, P = 0.011) reached statistical significance between the two groups. Tables 2 and 3 show the details for the size and the location of lesions detected, respectively. Several pictures of detected lesions are shown in Figure 3.
| Polyps | Adenomas | |||
| RV | FV | RV | FV | |
| ≤ 5 mm | 295 | 314 | 126 | 133 |
| 6-10 mm | 143 | 81 | 113 | 80 |
| > 10 mm | 59 | 22 | 58 | 22 |
| Polyps | Adenomas | |||
| RV | FV | RV | FV | |
| Right colon | 244 | 141 | 145 | 90 |
| Left colon | 186 | 181 | 125 | 105 |
| Rectum | 67 | 95 | 27 | 40 |
To evaluate the applicability of the novel retroflexion colonoscopy, we calculated several related indicators. The colonoscope was inserted into the cecum in 351 (97.2%) of 361 cases. The scores of abdominal pain did not show a significant difference between the two groups during colonoscopy (RV 2.7 ± 1.4 vs FV 2.8 ± 1.4, P = 0.377). The duration of withdrawal in the RV group was significant longer than that of the FV group (586.1 ± 124.4 vs 508.8 ± 129.6 s, P < 0.001). Compared with conventional colonoscopy, the new approach required less ancillary pressure (27.4% vs 45.7%, P < 0.001).
Retroflexion in the right-side colon was achieved in 331 (91.7%) of 361 patients. On univariate analysis, age, sex, abdominal surgery, and excessive looping were significantly associated with failure to retroflex. Body height, weight, BMI, bowel cleanliness, and smoking history were not related to the failure. Logistic regression analysis showed that age > 60 years, female sex, previous abdominal surgery, and instrumental looping were powerful predictors (Table 4).
| Odds ratio (95%CI) | P value | |
| Age > 65 yr | 5.26 (2.17-12.8) | < 0.001 |
| Female sex | 2.92 (1.17-7.31) | 0.022 |
| Previous abdominal surgery | 5.83 (2.26-15.04) | < 0.001 |
| Instrumental looping | 3.49 (1.33-9.12) | 0.011 |
Colorectal adenomas are considered to be a precancerous state of CRC. Benefiting from colonoscopy screening and polypectomy among adults aged ≥ 50 years, the morbidity of CRC declined by 2%-3% annually in Western developed countries[13]. However, about 20% of adenomas were missed during colonoscopy, which weakened the protective effect on CRC, especially in the proximal colon[6,14].
Various colonoscopy techniques were attempted in several studies to improve adenoma detection. Compared with white-light imaging, narrow-band imaging could improve ADR and PDR significantly[15]. Nulsen et al[16] conducted a retrospective study, including 3998 participants, suggesting that overall ADR, ADR for the proximal colon, and ADR for advanced adenomas could be improved significantly after adopting FUSE. A multicenter randomized controlled trial conducted by Ikematsu et al[17] showed that blue-laser imaging significantly increased the mean number of adenomas per patient, rather than ADR.
Retroflexed inspection in the right-side colon has been proposed as a complementary maneuver following the routine forward withdrawal, which could potentially improve diagnostic visibility. However, the results of previous work have revealed controversial evidence for this maneuver. A prospective multicenter cohort study, conducted by Chandran et al[18] in 2014, showed that retroflexion following forward examination improved the ADR from 24.64% to 26.4%. There was no significant difference in ADR when the proximal colon was re-examined in the FV vs RV (46% vs 47%), as revealed in a high-quality randomized controlled trial[19] . A systematic review and meta-analysis[20], including eight studies (with a total of 3660 cases), suggested that second examination of the proximal colon in retroflexion detected an additional 16.9% of right-sided adenomas that would have been missed by traditional colonoscopy. Recently, a multicenter randomized controlled trial suggested that a second examination of the right colon after standard withdrawal was associated with improvement of ADR, but by which method did not matter [RV (9%) or FV (12%)][21].
Our study aimed to evaluate the utility of the EC-3490Ti colonoscope in the retroflexion of the proximal colon. According to previous studies, compared with FV, retroflexion could not improve the ADR obviously in the second withdrawal, which is not feasible in clinical practice. Concerning this weakness, we did not perform a second withdrawal operation. Unlike previous studies, retroflexion was performed when the mucosa could not be exposed completely in the FV due to the folds and flexures of the colon, instead of the second insertion and withdrawal.
In the present study, the calculated ADR in the RV group was slightly higher than that in the control group (39.3% vs 37.3%, P = 0.646). These results are in line with the previous study[19]. Proximal colon retroflexion failed to yield a higher ADR compared with that in the FV. We propose several explanations for this. First, visualization of the whole colon mucosa could not be achieved by retroflexion, as there were also some blind spots on the colon wall hidden by the insertion tube itself. During the examination, endoscopists attempted to compensate for this by rotating the insertion tube, but the results were still unsatisfactory. Second, adenomas located in the proximal colon were flatter or more depressed than those in the distal colon, which made it difficult to distinguish them from the normal mucosa. Third, it could be speculated that adenomas located in hidden positions on the proximal sides of folds and flexures of the colon were not the dominant mechanism resulting in unsuccessful detection during colonoscopy[22]. However, no other plausible mechanism has been put forward until now. Lastly, the ADR may not be comprehensive for the evaluation of colonoscopy detection, although it is targeted as one of the most critical indicators of colonoscopy quality control[9]. In our study, additional 65 adenomas in 65/361 (18.0%) patients were identified. Still, the total ADR was only improved from 38.8% to 39.3% with additional retroflexion, excluding the cases with at least one adenoma detected on the initial forward withdrawal or detected in the distal colon. In this regard, the number of adenomas detected, as crucial as the ADR, should be used as an indicator to assess the endoscopic operation. Hence, we added other indicators to evaluate the detection efficiency of the novel endoscopy, such as APP, APC, R-ADR, and PDR. PDR, R-ADR, and APP were significantly increased with retroflexion during withdrawal in the proximal colon, compared with the FV. Based on these results, retroflexion in the right colon during withdrawal could facilitate detecting more adenomas and polyps.
In the present study, retroflexion in the right-side colon was successfully performed in approximately 92% of our cohort, which was identical to prior studies[18-20]. The significant predictors of failed retroflexion were advanced age, female sex, previous abdominal surgery, and instrumental looping. The observation time of RV was approximately 1 min longer than that of FV. We believe that this operation is new for our endoscopists, and retroflexion should be performed gently, which should take several seconds. Compared with conventional colonoscopy, the novel endoscopy requires less ancillary pressure during insertion, which may be associated with its flexible manipulation due to the shorter bending section and wider retroflexion angle. Besides, the new endoscopy did not increase the abdominal pain in patients, and the cecum arrival rate reached 95% as recommended in the guidelines. No major RV-related adverse event was noted in our study. Additionally, retroflexion could also provide valuable information to assess and remove lesions that are difficult to access in the prograde view[23,24]. The experience of our team suggests that retroflexion in the proximal colon is a safe and useful maneuver, as a complement to forward inspection. However, one serious adverse event of perforation was reported in the meta-analysis mentioned earlier[20]. We recommend that the procedure be stopped if any resistance is felt while turning the bending section.
There were several limitations in our study. First, the operators were experienced endoscopists, and the learning curve for the maneuver was unclear. Second, this trial did not adopt a double-blind design. Although the results could not reject bias altogether, we consider that there may have been some bias because it was impossible for our colleagues to overlook lesions in the FV group deliberately. Finally, this study was conducted in our center alone, thus large-scale multicenter trials are needed to explore the utility of retroflexion in the right-side colon.
Retroflexion in the proximal colon can be performed successfully and safely with the EC-3490Ti colonoscope. This maneuver could increase the number of adenomas detected and improve ADR. As a result, we suggest that retroflexion should be adopted as a complementary maneuver during screening or surveillance colonoscopy in the right colon.
Colonoscopy is the most effective method in the screening and prevention of colorectal cancer (CRC), and it could reduce the mortality from CRC. However, colonoscopy is less effective in preventing CRC in the right-side compared with the left-side colon.
Failure to detect more preneoplastic lesions is regarded as one of the mechanisms in the development of interval CRC. Retroflexion in the proximal colon allows for better visualization of the folds and the hepatic flexure, which may increase adenoma detection rate (ADR).
The current study aimed to investigate the effectiveness and safety of the EC-3490Ti colonoscope in detecting adenomas in the proximal colon.
We enrolled patients who underwent colonoscopy for screening or surveillance for CRC. When the endoscopists could not grasp the whole observation of the colon mucosa in the forward view, retroflexion was performed in the retroflexion view group with the EC-3490Ti colonoscope, while insertion and withdrawal were repeatedly conducted with the EC38-i10F colonoscope. ADR, total number of adenomas per positive participant (APP), success rate of retroflexion, and withdrawal time were compared.
The success rate of proximal retroflexion was 91.7%. There were no complications with the maneuver. Polyp detection rate, ADR for the right colon, and APP were significantly increased with retroflexion during withdrawal in the proximal colon, compared with the forward view group.
Proximal retroflexion with the EC-3490Ti colonoscope in the right colon could be accomplished safely and effectively. Retroflexion in the proximal colon significantly increases the detection of adenomas compared with conventional colonoscopy.
Retroflexion should be adopted as a complementary procedure in the future for the improvement of CRC prevention.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56675] [Article Influence: 7084.4] [Reference Citation Analysis (135)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13326] [Article Influence: 1332.6] [Reference Citation Analysis (4)] |
| 3. | Leslie A, Carey FA, Pratt NR, Steele RJ. The colorectal adenoma-carcinoma sequence. Br J Surg. 2002;89:845-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 478] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 4. | Wu Z, Liu Z, Ge W, Shou J, You L, Pan H, Han W. Analysis of potential genes and pathways associated with the colorectal normal mucosa-adenoma-carcinoma sequence. Cancer Med. 2018;7:2555-2566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Dawwas MF. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:2539-2540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 933] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 7. | Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 623] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 8. | Rondagh EJ, Bouwens MW, Riedl RG, Winkens B, de Ridder R, Kaltenbach T, Soetikno RM, Masclee AA, Sanduleanu S. Endoscopic appearance of proximal colorectal neoplasms and potential implications for colonoscopy in cancer prevention. Gastrointest Endosc. 2012;75:1218-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, Zauber AG, de Boer J, Fireman BH, Schottinger JE, Quinn VP, Ghai NR, Levin TR, Quesenberry CP. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1668] [Article Influence: 139.0] [Reference Citation Analysis (1)] |
| 10. | ASGE Technology Committee, Konda V, Chauhan SS, Abu Dayyeh BK, Hwang JH, Komanduri S, Manfredi MA, Maple JT, Murad FM, Siddiqui UD, Banerjee S. Endoscopes and devices to improve colon polyp detection. Gastrointest Endosc. 2015;81:1122-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Grobe JL, Kozarek RA, Sanowski RA. Colonoscopic retroflexion in the evaluation of rectal disease. Am J Gastroenterol. 1982;77:856-858. [PubMed] |
| 12. | Rex DK, Vemulapalli KC. Retroflexion in colonoscopy: why? Gastroenterology. 2013;144:882-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11573] [Cited by in RCA: 13302] [Article Influence: 1662.8] [Reference Citation Analysis (4)] |
| 14. | Baxter NN, Warren JL, Barrett MJ, Stukel TA, Doria-Rose VP. Association between colonoscopy and colorectal cancer mortality in a US cohort according to site of cancer and colonoscopist specialty. J Clin Oncol. 2012;30:2664-2669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 277] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 15. | Vișovan II, Tanțău M, Pascu O, Ciobanu L, Tanțău A. The role of narrow band imaging in colorectal polyp detection. Bosn J Basic Med Sci. 2017;17:152-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Nulsen B, Ungaro RC, Davis N, Turvall E, Deutsch L, Lewis B. Changes in Adenoma Detection Rate With Implementation of Full-spectrum Endoscopy: A Report of 3998 Screening Colonoscopies. J Clin Gastroenterol. 2018;52:885-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Ikematsu H, Sakamoto T, Togashi K, Yoshida N, Hisabe T, Kiriyama S, Matsuda K, Hayashi Y, Matsuda T, Osera S, Kaneko K, Utano K, Naito Y, Ishihara H, Kato M, Yoshimura K, Ishikawa H, Yamamoto H, Saito Y. Detectability of colorectal neoplastic lesions using a novel endoscopic system with blue laser imaging: a multicenter randomized controlled trial. Gastrointest Endosc. 2017;86:386-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 18. | Chandran S, Parker F, Vaughan R, Mitchell B, Fanning S, Brown G, Yu J, Efthymiou M. Right-sided adenoma detection with retroflexion versus forward-view colonoscopy. Gastrointest Endosc. 2015;81:608-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Kushnir VM, Oh YS, Hollander T, Chen CH, Sayuk GS, Davidson N, Mullady D, Murad FM, Sharabash NM, Ruettgers E, Dassopoulos T, Easler JJ, Gyawali CP, Edmundowicz SA, Early DS. Impact of retroflexion vs. second forward view examination of the right colon on adenoma detection: a comparison study. Am J Gastroenterol. 2015;110:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 20. | Cohen J, Grunwald D, Grossberg LB, Sawhney MS. The Effect of Right Colon Retroflexion on Adenoma Detection: A Systematic Review and Meta-analysis. J Clin Gastroenterol. 2017;51:818-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Núñez Rodríguez MH, Díez Redondo P, Riu Pons F, Cimavilla M, Hernández L, Loza A, Pérez-Miranda M. Proximal retroflexion vs second forward view of the right colon during screening colonoscopy: A multicentre randomized controlled trial. United European Gastroenterol J. 2020;8:725-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Harrison M, Singh N, Rex DK. Impact of proximal colon retroflexion on adenoma miss rates. Am J Gastroenterol. 2004;99:519-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Pishvaian AC, Al-Kawas FH. Retroflexion in the colon: a useful and safe technique in the evaluation and resection of sessile polyps during colonoscopy. Am J Gastroenterol. 2006;101:1479-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Rex DK, Khashab M. Colonoscopic polypectomy in retroflexion. Gastrointest Endosc. 2006;63:144-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dubois A, Sheu B, Vollmers H S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Li JH