Published online Oct 15, 2020. doi: 10.4251/wjgo.v12.i10.1167
Peer-review started: April 13, 2020
First decision: April 26, 2020
Revised: May 24, 2020
Accepted: August 25, 2020
Article in press: August 25, 2020
Published online: October 15, 2020
Processing time: 184 Days and 3.8 Hours
Numerous studies have demonstrated that human epididymis protein 4 (HE4) is overexpressed in various malignant tissues including ovarian, endometrial, lung, breast, pancreatic, and gastric cancers. However, no study has examined the diagnostic impact of HE4 in patient with esophageal squamous cell carcinoma (ESCC) until now.
To analyze the value of four serum tumor markers for the diagnosis of ESCC, and examine the associations of serum levels of HE4 with ESCC patients’ clinicopathological characteristics.
The case group consisted of 80 ESCC patients, which were compared to a control group of 56 patients with benign esophageal disease. Serum levels of HE4, carcinoma embryonic antigen (CEA), alpha fetal protein, and carbohydrate antigen 19-9 (CA19-9) were detected by ELISA. The associations of serum HE4 levels with ESCC patients’ clinicopathological characteristics such as gender, tumor location, and pathological stage were also examined after operation.
The result of ELISA showed that serum HE4 level was significantly higher in the patients with ESCC than in the controls, and the staining intensity was inversely correlated with the pathological T and N stages. Serum HE4 levels had a sensitivity of 66.2% and specificity of 78.6% when the cutoff value was set at 3.9 ng/mL. Moreover, the combined HE4 and CA19-9 increased the sensitivity to 83.33%, and interestingly, the combination of HE4 with CEA led to the most powerful sensitivity of 87.5%. Furthermore, A positive correlation was observed between HE4 serum levels and pathological T and N stages (P = 0.0002 and 0.0017, respectively), but there was no correlation between HE4 serum levels and ESCC patient gender (P = 0.4395) or tumor location (P = 0.6777).
The results of this study suggest that detection of serum HE4 levels may be useful in auxiliary diagnosis and evaluation of the progression of ESCC.
Core Tip: Numerous studies have demonstrated that human epididymis protein 4 (HE4) is overexpressed in various malignant tissues including ovarian, endometrial, lung, breast, pancreatic, and gastric cancers. However, no study has examined the diagnostic impact of HE4 in patient with esophageal squamous cell carcinoma (ESCC) until now. In this study, we quantified serum HE4 levels via ELISA using a non-biased database consisting of 80 ESCC patients, which were compared to a control group of 56 patients with benign esophageal disease. It was found that serum HE4 was significantly higher in ESCC patients than in the control group, and the staining intensity was inversely correlated with the pathological T and N stages. It has been suggested that serum HE4 can have a potential role as an early diagnostic biomarker for ESCC.
- Citation: Liu SY, Ahsan Bilal M, Zhu JH, Li SM. Diagnostic value of serum human epididymis protein 4 in esophageal squamous cell carcinoma. World J Gastrointest Oncol 2020; 12(10): 1167-1176
- URL: https://www.wjgnet.com/1948-5204/full/v12/i10/1167.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i10.1167
Esophageal cancer (EC), considered a serious malignancy with respect to prognosis and mortality rate, ranks 7th in terms of incidence (572000 new cases) and 6th in mortality overall (509000 deaths) worldwide, and it was responsible for an estimated 1 in every 20 cancer deaths in 2018[1]. Also, as one of the most commonly diagnosed cancers among men in China, the estimated number of new cases of EC was 291238 in 2011, while the number of deaths was 218957 in the same year[2]; by 2015, just 4 years later, this number had increased to 477900 and 375000, respectively[3]. The two main types of EC are squamous cell carcinoma (ESCC) and adenocarcinoma, and in China, 90% of cases are ESCC[4]. Despite many advances in diagnosis and treatment, the 5-year survival rate for all ESCC patients ranges only from 15% to 20%[5]. The overall 5-year survival after surgical resection is also poor, and the reason for that is the relatively late stage of diagnosis and rapid clinical progression[6,7]. By the time of diagnosis, the cancer has usually already spread to multiple organs. Therefore, a better means of early diagnosis is very important for improving the prognosis of patients with ESCC.
Tumor markers used for diagnosis and follow-up of patients with ESCC include carcinoembryonic antigen (CEA), carbohydrate antigen 72-4 (CA72-4), CA19-9, alpha fetal protein (AFP), CA24-2, and squamous cell carcinoma antigen[8,9]. Moreover, they can be used for the monitoring of tumor recurrence or progression, and have been used extensively for this purpose in patient management. However, clinical use of these markers has been restricted because of the lack of sensitivity.
Human epididymis protein 4 (HE4), also called whey-acidic-protein four-disulfide core domain protein 2, a 25 kDa secreted glycoprotein, is specifically expressed in epididymis, lung, and trachea tissues[10]. HE4 carries a protease inhibitor activity through interactions with serine proteases, Prss35 and Prss23, which are implicated in kidney fibrosis in a mouse model[11]. Numerous studies have demonstrated that HE4 is overexpressed in various malignant tissues including ovarian, endometrial, lung, pancreatic, and gastric cancers[12-16]. The prognostic value of serum HE4 as a biomarker for ovarian and endometrial cancers has also been well recognized[17-19]. Moreover, the combination of serum HE4 with CA125 levels has shown an increased power for early detection of ovarian cancers[20]. Interestingly, serum HE4 could be a potential diagnostic marker for small cell lung cancer, non-small cell lung cancer, and breast, ovarian, and endometrial cancers[20-30]. Furthermore, recent studies have indicated that when overexpressed in ovarian and endometrial cancer cells, HE4 is able to promote cell proliferation, adhesion, and invasion, and may play an active role in tumor pathogenesis and progression[31,32]. These new findings may partially explain why HE4 is highly expressed in many types of human malignancies.
HE4 was found to be of little benefit in diagnosis, prognosis, and other clinical settings of several human cancers. But there is little research focusing on the diagnostic value of HE4 in ESCC. In the present study, we evaluated the associations between serum HE4 levels and clinicopathological parameters in a cohort of ESCC patients. The goal of the current study was to examine the relationship between HE4 and clinicopathological variables of ESCC patients, and to assess the value of serum HE4, either alone or in combination with additional serum markers, for the screening and/or diagnosis of ESCC.
The case group of this study included 80 patients with ESCC admitted to the Department of Thoracic Surgery, the Second Affiliated Hospital of Xi'an Jiaotong University from January 2017 and June 2019. All patients were verified to have ESCC by imaging examination, such as computed tomography and electronic gastroscopy, and the diagnosis was confirmed by tissue biopsy or postoperative pathological examination. Then, the histological diagnoses after surgical resection were confirmed by pathology according to the 8th edition of tumor-node-metastasis (TNM) staging system of the American Joint Committee on Cancer[33]. The patients ranged in age from 44 years to 79 years, with a mean age of 61.6 ± 15.3 years. Characteristics of the study cohort are summarized in Table 1.
| Group | n | HE4 (pmol/L) | CEA (ng/mL) | AFP (ng/mL) | CA19-9 (ng/mL) |
| Case group | 80 | 5.746 ± 3.360 | 4.274 ± 2.530 | 3.358 ± 1.559 | 8.450 ± 5.157 |
| Benign control group | 56 | 3.294 ± 1.738 | 2.471 ± 1.702 | 2.561 ± 1.587 | 5.271 ± 4.636 |
| P value | < 0.0001 | 0.0022 | 0.0042 | 0.0003 |
A benign control group included 56 patients with benign esophageal disease who visited our hospital during the same period. The group included 40 men and 16 women, aged 44-81 years old with a mean age of 63.8 ± 8.7 years.
Morning fasting peripheral blood samples were collected from patients of the two groups before any relevant treatment. The levels of HE4, CEA, AFP, and CA 19-9 were detected in serum samples by ELISA. The matched reagents (DHE400, R&D Systems, Inc., Minneapolis, MN, United States) were used according to protocols from the manufacturers. Measurement procedures were carried out following the manufacturers’ instructions and the values were read at the recommended wave length of 450 nm.
The correlations between HE4 expression status and clinicopathological characteristics of ESCC patients were analyzed using the χ2 test or Fisher’s exact test, if appropriate. The area under the receiver operating characteristic (ROC) curve (AUC) was used to compare the diagnostic efficiency of serum markers. P < 0.05 was considered statistically significant.
Serum levels of HE4, CEA, AFP, and CA 19-9 significantly differed among the case and benign control groups, respectively. Serum marker levels were significantly higher in the case group than in the benign control group (Table 1).
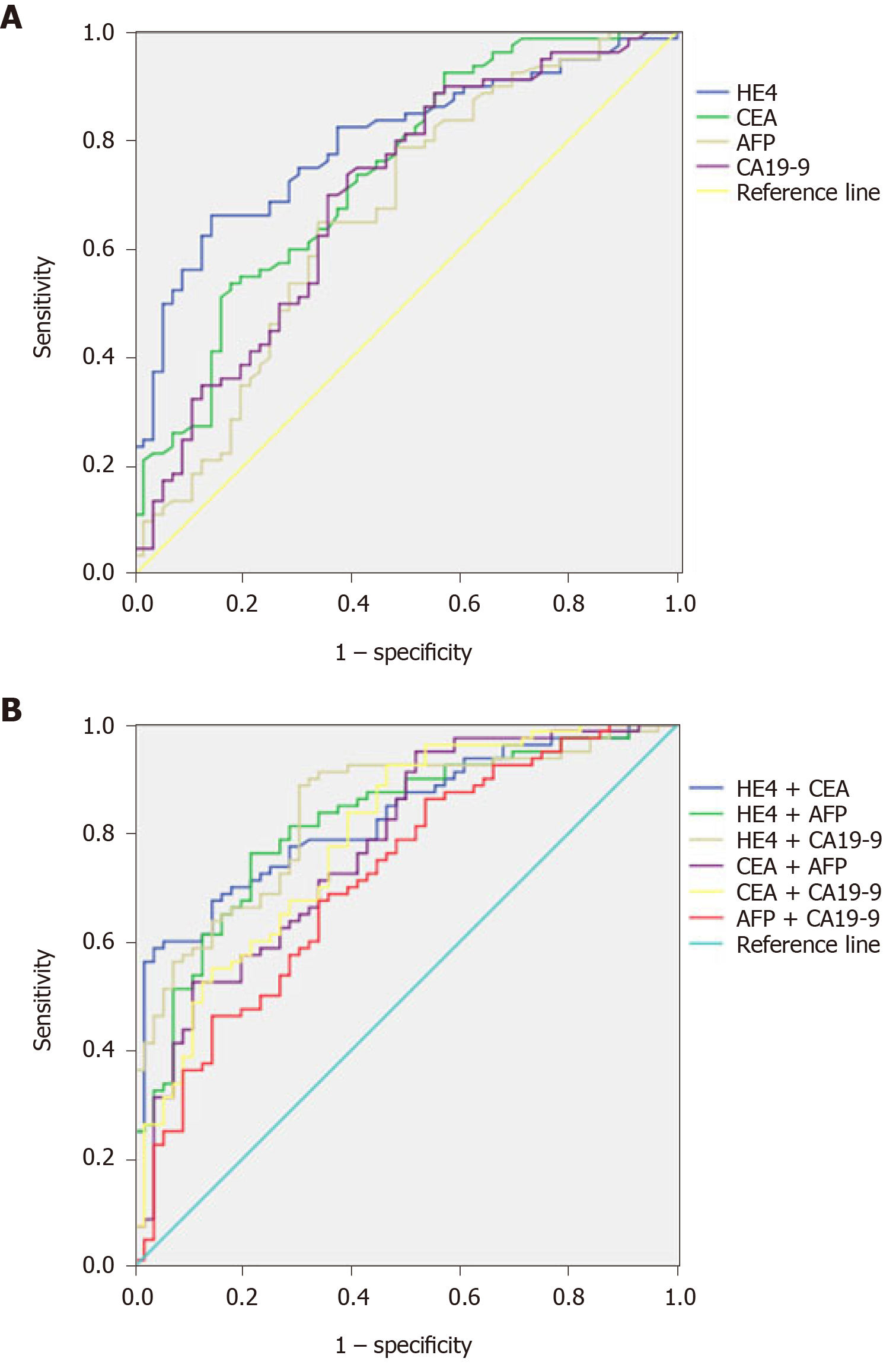
A ROC curve was plotted to verify the optimum cut-off point for HE4, which was 3.9 ng/mL (Figure 1A). According to ROC analysis, HE4 had a slightly higher specificity and sensitivity (66.2% and 85.7%) in the diagnosis of ESCC. The sensitivity and specificity of serum markers HE4, CEA (53.7% and 82.1%), AFP (65.0% and 86.0%), and CA19-9 (73.1% and 60.7%) differed with each other but not significantly in ESCC (Table 2).
| Variable | AUC | Standard error | Asymptotic significance | Asymptotic 95%CI |
| HE4 | 0.795 | 0.038 | 0.000 | 0.717-0.859 |
| CEA | 0.737 | 0.043 | 0.000 | 0.665-0.809 |
| AFP | 0.666 | 0.049 | 0.001 | 0.580-0.744 |
| CA19-9 | 0.697 | 0.047 | 0.000 | 0.612-0.773 |
To assess the diagnostic value of each serum marker for ESCC, we analyzed their AUCs. Serum markers HE4 (AUC = 0.795), CEA (AUC = 0.737), AFP (AUC = 0.666), and CA 19-9 (AUC = 0.697) showed the most promise as diagnostic markers for ESCC (Table 3; Figure 1A).
| Variable | HE4 | CEA | AFP | CA19-9 |
| Sensitivity | 66.2 | 53.7 | 65.0 | 73.1 |
| Specificity | 85.7 | 82.1 | 66.0 | 60.7 |
To assess the diagnostic value of combination of four serum markers for ESCC, we also analyzed the AUCs of their different combinations. Serum markers HE4 + CEA (AUC = 0.828), HE4 + AFP (AUC = 0.819), HE4 + CA19-9 (AUC = 0.839), CEA + AFP (AUC = 0.775), CEA + CA19-9 (AUC = 0.790), and AFP + CA19-9 (AUC = 0.717) showed the most promise as diagnostic markers for ESCC (Table 4; Figure 1B).
| Variable combination | AUC | Standard error | Asymptotic significance | Asymptotic 95%CI |
| HE4 + CEA | 0.828 | 0.034 | 0.000 | 0.761-0.895 |
| HE4 + AFP | 0.819 | 0.036 | 0.000 | 0.748-0.890 |
| HE4 + CA19-9 | 0.837 | 0.034 | 0.000 | 0.770-0.904 |
| CEA + AFP | 0.775 | 0.041 | 0.000 | 0.696-0.855 |
| CEA + CA19-9 | 0.790 | 0.039 | 0.000 | 0.713-0.867 |
| AFP + CA19-9 | 0.717 | 0.045 | 0.000 | 0.635-0.805 |
A total 80 ESCC patients underwent operation and were included in this study, and their clinical and demographic characteristics are presented in Table 5. A positive correlation was observed between HE4 serum levels and pathological T and N stages (P= 0.0002 and 0.0017, respectively), but there was no correlation between HE4 serum levels and ESCC tumor location (P = 0.6777).
| Total | Serum HE4 levels | P value | ||
| ≤ 3.9 ng/mL | > 3.9 ng/mL | |||
| All cases | 80 | 27 | 53 | |
| Gender | 0.4395 | |||
| Male | 56 (70.0) | 17 (63.0) | 39 (73.6) | |
| Female | 24 (30.0) | 10 (47.0) | 14 (26.4) | |
| Tumor location | 0.6777 | |||
| Upper | 16 (23.8) | 5 (18.5) | 11 (20.8) | |
| Middle | 45 (56.4) | 14 (51.9) | 31 (58.4) | |
| Lower | 19 (23.8) | 8 (29.6) | 11 (20.8) | |
| T grade | 0.0002 | |||
| T1-2 | 21 (26.3) | 14 (51.9) | 7 (13.2) | |
| T3-4 | 59 (73.7) | 13 (48.1) | 46 (86.8) | |
| N stage | 0.0017 | |||
| N0 | 26 (32.5) | 15 (55.6) | 11 (20.8) | |
| N1-2 | 54 (67.5) | 12 (44.4) | 42 (79.2) | |
HE4 belongs to one of the whey acidic protein four-disulfide core domain protein family and has features of trypsin inhibitors. Multiple studies have verified that HE4 expression is associated with several human malignant tumors, such as pulmonary carcinoma, oophoroma, endometrial carcinoma, and urinary bladder carcinoma[34]. However, the association between HE4 and ESCC remained unclear. Comparison of published data raised a possibility for increasing incidence of ESCC in recent years, and markers for early screening are the key to improving the poor prognosis of ESCC patients. Biomarkers in various body fluids, such as in serum, have been investigated. Relevant studies have verified that, as a cancer-related serological marker, HE4 can be highly expressed in serum and malignant hydrothorax of cancer patients. Besides, it is a useful index for auxiliary diagnosis of early disease. HE4 overexpression with serum levels > 150 pm is common in 78% of ovarian cancer patients as compared to breast (13%), endometrial (25%), gastrointestinal (16%), and lung (42%) tumors[35]. The sensitivity and specificity of HE4 serum expression in epithelial ovarian cancer patients are superior to those of CA125, which led to the United States Food and Drug Administration approval of HE4 as a tumor marker to monitor the efficacy and recurrence of ovarian tumors[36]. In the meantime, joint detection of HE4 and indexes such as CEA, CYFRA21-1, and NSE can significantly improve the efficiency of pulmonary carcinoma diagnosis[37].
This study indicated that serum levels of HE4, CEA, AFP, and CA 19-9 were significantly higher in patients with ESCC than in benign controls. The AUCs of those four serum markers indicated that they may be useful for auxiliary diagnosis of ESCC, with HE4 and CEA showing the highest promise as diagnostic markers. It is noteworthy that increased diagnostic value of HE4 has been achieved through combination with other biomarkers for, in this study, the serum levels of HE4 and previously investigated markers including CEA, AFP, and CA19-9 were all elevated in serum of ESCC patients. The lack of correlation between serum HE4 levels and CEA, AFP, or CA19-9 pointed to an independent diagnostic value of HE4. Indeed, AUC analysis revealed that combination of HE4 with CA19-9 led to a slight increase in the AUC (0.837) compared with HE4 (0.795) or CA19-9 alone (0.697), albeit the slight reduction in specificity. AUC analysis revealed that serum HE4 levels are of potential diagnostic value for ESCC. The relatively high sensitivity warrants further studies with larger sample size on the possible value of this combination as a screening tool for the auxiliary diagnosis and evaluation of ESCC.
Surgery is the main treatment especially for early ESCC, and a previous study indicated that the most important clinical strategy basis for treatment (such as surgery) of ESCC patients is TNM classification[38]. However, it is difficult to obtain complete and accurate TNM and other pathology data of patients preoperatively. For this reason, it may be important to find some other preoperative prognostic factors for performing the surgery and evaluating the outcome of ESCC patients. In addition, our data showed that, serum HE4 levels of T stage 3-4 ESCC patients were significantly higher than those of T stage 1-2 patients (P = 0.0002. Furthermore, according to N category, serum HE4 levels of N1-2 ESCC patients were significantly higher than those of N0 patients (P = 0.0017). AUC analysis of serum marker levels also revealed that HE4 had the highest diagnostic efficiency for ESCC, suggesting that it may be an auxiliary index for metastasis and may help evaluate tumor progression. Interestingly, HE4 serum levels appear to be especially higher in advanced stage ESCC, and appreciably, but not ideally, the levels of sensitivity and specificity were obtained in this study. These findings, together with its advantage of noninvasiveness, warrant further investigation on the efficacy of HE4 as a potential early diagnostic or screening tool for ESCC patients.
Collectively, our data for the first time implicates HE4 overexpression as a serum marker for ESCC and demonstrates that HE4 expression is a potential target for the diagnosis of ESCC. Further validation experiments in larger cohort of patients are required to determine the efficacy of ELISA-based HE4 serum assay as a non-invasive tool for the diagnosis and/or screening ESCC. Therefore, future studies should focus on the use of molecular biomarkers to predict patient survival and to select the ESCC patients who will benefit from early diagnosis and specific treatment, including adjuvant chemotherapy and even targeted therapy.
Esophageal squamous cell carcinoma (ESCC) is the predominant histological subtype of esophageal cancer in East Asian countries especially in China, where it accounts for more than 90% of total EC cases. Effective screening and early diagnosis are the key for improved management of ESCC patients. Tumor markers used for diagnosis and follow-up of patients with ESCC include carcinoembryonic antigen (CEA), carbohydrate antigen 72-4 (CA72-4), CA19-9, alpha fetal protein (AFP), CA24-2, and squamous cell carcinoma antigen. However, clinical use of these markers for diagnosis of ESCC has been restricted because of the lack of sensitivity.
The early diagnosis of ESCC remains a clinical challenge. Biomarkers predictive of early diagnosis and prognosis may help design more effective and even targeted therapies for ESCC patients. To date, overexpression of human epididymis protein 4 (HE4) has been demonstrated in a range of malignant neoplasms. The sensitivity and specificity of HE4 serum expression in epithelial ovarian cancer patients are superior to those of other serum biomarkers, which led to the United States Food and Drug Administration approval of HE4 as a biomarker for the detection of ovarian cancer in women presenting with an ovarian cyst or pelvic mass as part of the risk of ovarian malignancy algorithm. However, the diagnostic value of serum HE4 in ESCC patients remains unknown.
We have carried out this study to investigate the diagnostic value of HE4 in patients with ESCC. The relationship between clinical, demographic, and pathological characteristics and HE4 was also assessed.
Eighty patients diagnosed with ESCC who underwent surgical resection at our hospital between January 2017 and June 2019 were included in this study. They were compared to a control group of 56 patients with benign esophageal disease. Serum levels of HE4, CEA, AFP, and CA19-9 were detected using ELISA.
We found that serum HE4 level was higher in cases with ESCC than in the controls. Serum HE4 levels had a sensitivity of 66.2% and specificity of 78.6% when the cut-off value was set at 3.9 ng/mL. Moreover, the combined HE4 and CA19-9 increased the sensitivity to 83.33%, and interestingly, the combination of HE4 with CEA led to the most powerful sensitivity of 87.5%. Furthermore, a positive correlation was observed between HE4 serum levels and pathological T and N stages, but there was no correlation between HE4 serum levels and ESCC patients' gender and tumor location.
HE4 is significantly higher expressed in patients with ESCC, and the staining intensity is inversely correlated with the pathological stage. The results of this study suggest that detection of serum HE4 levels may be useful in auxiliary diagnosis and evaluation of the progression of ESCC.
Biomarkers predictive of patient prognosis may help design more effective and targeted therapies for ESCC. This study indicates that ESCC patients have abnormal expression of serum tumor markers such as HE4, which therefore may be useful for auxiliary diagnosis and evaluation of ESCC. Furthermore, it seems that detection of serum HE4 levels may potentially be useful in auxiliary early diagnosis and evaluation of the progression of ESCC patients. However, further studies are required to verify ESCC progression according to the serum HE4 level at the time of diagnosis, and even treatment can be planned accordingly.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56651] [Article Influence: 7081.4] [Reference Citation Analysis (134)] |
| 2. | Zeng H, Zheng R, Zhang S, Zuo T, Xia C, Zou X, Chen W. Esophageal cancer statistics in China, 2011: Estimates based on 177 cancer registries. Thorac Cancer. 2016;7:232-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 214] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 3. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13323] [Article Influence: 1332.3] [Reference Citation Analysis (4)] |
| 4. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21462] [Article Influence: 1951.1] [Reference Citation Analysis (6)] |
| 5. | Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1956] [Cited by in RCA: 2001] [Article Influence: 153.9] [Reference Citation Analysis (5)] |
| 6. | Feng JF, Chen QX. Prognostic significance of preoperative CA72-4 in patients with esophageal squamous cell carcinoma. Arch Iran Med. 2013;16:338-342. [PubMed] |
| 7. | Tachibana M, Kinugasa S, Hirahara N, Yoshimura H. Lymph node classification of esophageal squamous cell carcinoma and adenocarcinoma. Eur J Cardiothorac Surg. 2008;34:427-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Zhao H, Chen W, Wu J, Wang L, Mao W. Clinical significance of preoperative serum tumor markers in esophageal squamous cell carcinoma. J Cancer Res Ther. 2014;10 Suppl:C179-C185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Shimada Y, Watanabe G, Kawamura J, Soma T, Okabe M, Ito T, Inoue H, Kondo M, Mori Y, Tanaka E, Imamura M. Clinical significance of osteopontin in esophageal squamous cell carcinoma: comparison with common tumor markers. Oncology. 2005;68:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Drapkin R, von Horsten HH, Lin Y, Mok SC, Crum CP, Welch WR, Hecht JL. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res. 2005;65:2162-2169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 411] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 11. | LeBleu VS, Teng Y, O'Connell JT, Charytan D, Müller GA, Müller CA, Sugimoto H, Kalluri R. Identification of human epididymis protein-4 as a fibroblast-derived mediator of fibrosis. Nat Med. 2013;19:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 195] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 12. | Nozaki K, Ogawa M, Williams JA, Lafleur BJ, Ng V, Drapkin RI, Mills JC, Konieczny SF, Nomura S, Goldenring JR. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology. 2008;134:511-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 153] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Hough CD, Sherman-Baust CA, Pizer ES, Montz FJ, Im DD, Rosenshein NB, Cho KR, Riggins GJ, Morin PJ. Large-scale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res. 2000;60:6281-6287. [PubMed] |
| 14. | Huang T, Jiang SW, Qin L, Senkowski C, Lyle C, Terry K, Brower S, Chen H, Glasgow W, Wei Y, Li J. Expression and diagnostic value of HE4 in pancreatic adenocarcinoma. Int J Mol Sci. 2015;16:2956-2970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Angioli R, Capriglione S, Scaletta G, Aloisi A, Miranda A, De Cicco Nardone C, Terranova C, Plotti F. The role of HE4 in endometrial cancer recurrence: how to choose the optimal follow-up program. Tumour Biol. 2016;37:4973-4978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Benati M, Montagnana M, Danese E, Paviati E, Giudici S, Ruzzenente O, Franchi M, Lippi G. The clinical significance of DJ-1 and HE4 in patients with endometrial cancer. J Clin Lab Anal. 2018;32:e22223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Anastasi E, Marchei GG, Viggiani V, Gennarini G, Frati L, Reale MG. HE4: a new potential early biomarker for the recurrence of ovarian cancer. Tumour Biol. 2010;31:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 18. | Będkowska GE, Ławicki S, Gacuta E, Pawłowski P, Szmitkowski M. M-CSF in a new biomarker panel with HE4 and CA 125 in the diagnostics of epithelial ovarian cancer patients. J Ovarian Res. 2015;8:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Bie Y, Zhang Z. Diagnostic value of serum HE4 in endometrial cancer: a meta-analysis. World J Surg Oncol. 2014;12:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Urban N. Designing early detection programs for ovarian cancer. Ann Oncol. 2011;22 Suppl 8:viii6-viii18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Iwahori K, Suzuki H, Kishi Y, Fujii Y, Uehara R, Okamoto N, Kobayashi M, Hirashima T, Kawase I, Naka T. Serum HE4 as a diagnostic and prognostic marker for lung cancer. Tumour Biol. 2012;33:1141-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Wang X, Fan Y, Wang J, Wang H, Liu W. Evaluating the expression and diagnostic value of human epididymis protein 4 (HE4) in small cell lung cancer. Tumour Biol. 2014;35:6847-6853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Lamy PJ, Plassot C, Pujol JL. Serum HE4: An Independent Prognostic Factor in Non-Small Cell Lung Cancer. PLoS One. 2015;10:e0128836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Wojcik E, Tarapacz J, Rychlik U, Stasik Z, Sas-Korczynska B, Skotnicki P, Kulpa JK. Human Epididymis Protein 4 (HE4) in Patients with Small-Cell Lung Cancer. Clin Lab. 2016;62:1625-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Bian J, Sun X, Li B, Ming L. Clinical Significance of Serum HE4, CA125, CA724, and CA19-9 in Patients With Endometrial Cancer. Technol Cancer Res Treat. 2017;16:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 26. | Cymbaluk-Płoska A, Chudecka-Głaz A, Pius-Sadowska E, Sompolska-Rzechuła A, Machaliński B, Surowiec A, Menkiszak J. Clinical importance of serum HE4 and MMP2 levels in endometrial cancer patients. Onco Targets Ther. 2017;10:3169-3175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Li J, Chen H, Curcuru JR, Patel S, Johns TO, Patel D, Qian H, Jiang SW. Serum HE4 Level as a Biomarker to Predict the Recurrence of Gynecologic Cancers. Curr Drug Targets. 2017;18:1158-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Stiekema A, Lok C, Korse CM, van Driel WJ, van der Noort V, Kenter GG, Van de Vijver KK. Serum HE4 is correlated to prognostic factors and survival in patients with endometrial cancer. Virchows Arch. 2017;470:655-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 29. | Kamei M, Yamashita S, Tokuishi K, Hashioto T, Moroga T, Suehiro S, Ono K, Miyawaki M, Takeno S, Yamamoto S, Kawahara K. HE4 expression can be associated with lymph node metastases and disease-free survival in breast cancer. Anticancer Res. 2010;30:4779-4783. [PubMed] |
| 30. | Lu M, Ju S, Shen X, Wang X, Jing R, Yang C, Chu H, Cong H. Combined detection of plasma miR-127-3p and HE4 improves the diagnostic efficacy of breast cancer. Cancer Biomark. 2017;18:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Lu R, Sun X, Xiao R, Zhou L, Gao X, Guo L. Human epididymis protein 4 (HE4) plays a key role in ovarian cancer cell adhesion and motility. Biochem Biophys Res Commun. 2012;419:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Li J, Chen H, Mariani A, Chen D, Klatt E, Podratz K, Drapkin R, Broaddus R, Dowdy S, Jiang SW. HE4 (WFDC2) Promotes Tumor Growth in Endometrial Cancer Cell Lines. Int J Mol Sci. 2013;14:6026-6043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Inada M, Nishimura Y, Ishikawa K, Nakamatsu K, Wada Y, Uehara T, Fukuda K, Anami S, Doi H, Kanamori S. Comparing the 7th and 8th editions of the American Joint Committee on Cancer/Union for International Cancer Control TNM staging system for esophageal squamous cell carcinoma treated by definitive radiotherapy. Esophagus. 2019;16:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Macedo AC, da Rosa MI, Lumertz S, Medeiros LR. Accuracy of serum human epididymis protein 4 in ovarian cancer diagnosis: a systematic review and meta-analysis. Int J Gynecol Cancer. 2014;24:1222-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Moore RG, Brown AK, Miller MC, Skates S, Allard WJ, Verch T, Steinhoff M, Messerlian G, DiSilvestro P, Granai CO, Bast RC. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. 2008;108:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 485] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 36. | Moore RG, Hill EK, Horan T, Yano N, Kim K, MacLaughlan S, Lambert-Messerlian G, Tseng YD, Padbury JF, Miller MC, Lange TS, Singh RK. HE4 (WFDC2) gene overexpression promotes ovarian tumor growth. Sci Rep. 2014;4:3574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 37. | Tang QF, Zhou ZW, Ji HB, Pan WH, Sun MZ. Value of serum marker HE4 in pulmonary carcinoma diagnosis. Int J Clin Exp Med. 2015;8:19014-19021. [PubMed] |
| 38. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5537] [Cited by in RCA: 6607] [Article Influence: 412.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shimizu Y S-Editor: Wang JL L-Editor: Wang TQ P-Editor: Li JH