Published online Aug 15, 2019. doi: 10.4251/wjgo.v11.i8.634
Peer-review started: March 20, 2019
First decision: June 4, 2019
Revised: June 7, 2019
Accepted: June 20, 2019
Article in press: June 20, 2019
Published online: August 15, 2019
Processing time: 152 Days and 9.3 Hours
Triplet chemotherapy, with docetaxel-5FU-oxaliplatin FLOT regimen recently became the standard perioperative treatment for localized gastric cancer (GC). An adapted regimen called TeFOX was recently tested in metastatic setting and gave promising results.
To determine safety and efficacy of TeFOX perioperative regimen.
This monocentric retrospective study aims to test efficacy and safety of the perioperative TeFOX regimen given alone or in combination with trastuzumab in patients with localized GC. TeFOX consist in docetaxel (50 mg/m²) with oxaliplatin 85 mg/m² and and leucovorin (400 mg/m2) 5 FU bolus (400 mg/m2) on day 1, followed by continuous infusion of 5FU for 46 h (2400 mg/m2) every 2 wk.
Thirty-three consecutive patients were included in this retrospective study. Eighteen patients have a gastroesophageal junction cancer and 11 have a GC. Median follow-up of surviving patients was 32 mo. R0 resection was obtained in 30 (91) patients. Twelve patients (36) had a pathological complete response and 8 (24) patients a nearly complete pathological response. Median OS and PFS were not reached at data base lock. We have observed 6 metastatic relapses and 1 localized relapse. No relapse was observed in patients with pathological complete responses. The most common grade 3-4 adverse events were peripheral neuropathy (21) and asthenia (20).
TeFOX regimen could be safely administrated in perioperative treatment of localized GC. TeFOX and the FLOT regimen have comparable efficacy and safety profiles.
Core tip: Triplet chemotherapy with docetaxel-5FU-oxaliplatin FLOT regimen recently became the standard perioperative treatment for localized gastric cancer. An adapted regimen called TeFOX was recently tested in metastatic setting and gave promising results. We provide here evidence based on our experience of the safety and efficacy of this regimen in patients treated in neoadjuvant setting.
- Citation: Basso V, Orry D, Fraisse J, Vincent J, Hennequin A, Bengrine L, Ghiringhelli F. Safety and efficacy of a docetaxel-5FU-oxaliplatin regimen with or without trastuzumab in neoadjuvant treatment of localized gastric or gastroesophageal junction cancer: A retrospective study. World J Gastrointest Oncol 2019; 11(8): 634-641
- URL: https://www.wjgnet.com/1948-5204/full/v11/i8/634.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i8.634
Gastric cancer (GC) is a major problem of health worldwide. In 2018, 783000 patients died of a GC, making it the third leading cause of cancer death[1]. Upon metastatic disease, the prognosis is poor with less than 2 years of median survival, even with new chemotherapeutic approaches. Prognosis is better in localized tumours and surgery can cure about 90% of T1 N0 tumours[1]. For more advanced tumours, the prognosis remains poor. Standard of care involves a perioperative treatment. With such treatment, the 5-year relapse free survival reaches 30%-40%. For a long time, based on MAGIC trial and on FFCD 9703 phase 3 study, the standard treatment was association of fluoropyrimidin plus platin with or without epirubicin[2,3]. Such therapies improve 5-year relapse free survival of around 20% with surgery only and around 35% with perioperative chemotherapy.
Recently, a new combination of platin and taxane improved outcome in patients with metastatic disease. In addition, usage of trastuzumab demonstrated its efficacy in first line of metastatic GC with HER2 overexpressing tumour[4]. Such HER2 overexpressing tumour represents around 20% of GCs[5]. German cooperative group developed a taxane based chemotherapy protocol called FLOT which was tested in perioperative setting. Such therapy gave better response rates than platin based chemotherapies and improved relapse free survival[6,7]. In France, an alternative protocol called TeFOX using 5-FU in a 46h continuous infusion at the dose of 2400 mg/m2, instead of 24 h continuous infusion at the dose of 2600 mg/m2 in FLOT regimen, was developed in metastatic disease. TeFOX is safe and give impressive response rates in first line metastatic disease[8]. This regimen in a retrospective study gave disease control rate of 87.6%, respectively. Median PFS and OS observed in this study were 9.7 months and 14.3 months respectively. In addition, 40% of metastatic patients could have secondary resection. The toxic of the TeFOX regimen is modest with less haematological toxicity than observed with the FLOT regimen[8].
Based on these results, neoadjuvant therapy of GC was modified in our centre and patients received TeFOX or TeFOX with trastuzumab if they had a HER2 overexpressing tumour. Our objective is to describe the safety and the efficacy of this protocol.
All consecutive patients treated for histologically confirmed, previously untreated, non-metastatic, operable adenocarcinoma of the stomach or gastroesophageal junction between May 15, 2013 and August 29, 2018 in Centre Georges Francois Leclerc, were included. Follow-up ended in December 2018.
Eligibility criteria for inclusion in the study were: (1) Local gastric or gastroeso-phageal junction (GEJ) adenocarcinoma, without metastases detected following CT-scan and TEP-scan; (2) The possibility of curative resection as assessed by a digestive surgery multidisciplinary staff; (3) WHO performance status of 0 or 1; and (4) Absence of previous cancer therapy. The study was conducted in accordance with the Declaration of Helsinki. All participating patients fully agreed with the use of their medical records in clinical research. The study was performed in agreement with the General Data Protection Regulation European law.
Treatment consisted of an intravenous injection of TeFOX regimen with or without trastuzumab for patients with HER2 overexpressing tumours. TeFOX consisted of docetaxel (50 mg/m2), oxaliplatin (85 mg/m2), and leucovorin (400 mg/m2) F5U bolus (400 mg/m2) on day 1, followed by continuous infusion of 5FU for 46 h (2400 mg/m2) administered every 2 wk. Trastuzumab was given at 4mg/kg every two weeks. Prophylactic treatments included corticosteroids and antiemetic’s given accordingly to standard recommendations. G-CSF, a hematopoietic factor, was systematically given as a prophylactic treatment in all patients (Filgrastin 34 MUI/d during 4 d, starting the day after the end of 5FU infusion). The number of cycles of chemotherapy expected was 6 before and after surgery. Dose reductions and treatment discontinuations were performed according to physician decision, based on toxicity.
Toxicity was evaluated before each cycle according to the NCI-CTC-AE v5.
Efficacy of neoadjuvant chemotherapy was tested using pathological examination. Tumour regression grade was quantified using the Becker classification[9]. This classification gave an estimation of the percentage of vital tumour cells in the tumour core: TRG1a means complete pathological response; TRG1b means subtotal regression with less than 10% of residual tumour cells; TRG2 means partial regression with around to 10% to 50% of viable tumour cells and TRG3 means no or minor regression[10].
Toxicity and response to neoadjuvant chemotherapy were evaluated in the intent-to-treat population, defined as patients who received at least one cycle of TeFOX with or without trastuzumab. Toxicity of neoadjuvant and adjuvant chemotherapy as well as surgery related toxicities were evaluated. Time to relapse was defined as the time between surgery and the discovery of the first metastatic site. All patients alive without disease relapse at the last follow-up date were censored. Overall survival (OS) was defined as the time between the first cycle of chemotherapy and death (all causes). All patients alive at the last follow-up date were censored. Survival curves were estimated using the Kaplan–Meier method. Median follow-up and its 95% confidence interval (CI) were calculated with the reverse Kaplan–Meier method. All statistical analysis were performed using MedCalc Software.
Between May 15, 2013 and August 29, 2018, 33 patients that received at least one cycle of neoadjuvant chemotherapy for a localized GC, were enrolled. The median age was 63 years. The majority of patients had a WHO performance status of 0. Pre-treatment patient characteristics are shown in Table 1. Only 5 patients have signet ring cell carcinoma. Five patients have HER2 overexpressing tumours and received in addition to TeFOX, trastuzumab during neoadjuvant chemotherapy. Median number of neoadjuvant chemotherapy cycles was 5 (range 2-8). Median number of adjuvant chemotherapy cycles was 3 (range 0-6). Eleven patients underwent transthoracic oesophagectomy, 11 total gastrectomy and 11 subtotal gastrectomy. Following surgery, only 28 patients received adjuvant chemotherapy (2 patients refused further therapy and 3 had a poor performance status following surgery and were excluded from further therapy). The median number of therapy cycles was 4 (range 1-7).
| Age (yr) | 63 (41–80) |
| Sex | |
| Male | 28 (84) |
| Female | 5 (16) |
| WHO performance status | |
| 0 | 20 (60) |
| 1 | 13 (40) |
| Denutrition | |
| > 10% weight loss | 10 (30) |
| Localization | |
| Gastric | 15 (45) |
| Gastro-oesophageal junction Siewert I | 12 (35) |
| Gastro-oesophageal junction Siewert II | 3 (10) |
| Gastro-oesophageal junction Siewert III | 3 (10) |
| Surgery | |
| Lewis Santy | 11 (33) |
| Total Gastrectomy | 11 (33) |
| Subtotal Gastrectomy | 11 (33) |
| Clinical tumour stage | |
| cT3/T4 | 7 (81) |
| cT1/T2 | 5 (18) |
| cTx | 21 (1) |
| cN+ | 22 (77) |
| cN– | 8 (23) |
| Histological type | |
| Intestinal | 28 (84) |
| Singet Ring cells | 5 (16) |
| HER2 overexpressing | 5 (16) |
There was no treatment-related death. Toxicities of neoadjuvant chemotherapy are described in Table 2. Only 2 patients did not present side effects during neoadjuvant chemotherapy. Ten patients developed grade 3-4 toxicities. The most common grade 3-4 toxicities were asthenia, and peripheral neuropathy which occurred in 19% and 21% of patients respectively. Febrile neutropenia occurred in one patient (3). Dose reduction occurred in seven patients with elimination of docetaxel in 4 patients and oxaliplatin dose reduction in 3 patients. Discontinuation of therapy occurred in 6 patients due to important side effects. Granulocyte colony-stimulating factor (G-CSF) was prophylactically given to all patients. Perioperative medical or surgical grade 3 and 4 complications According to Clavien-Dindo classification within 90 d of surgery were observed in 6 patients. No death was observed in the 90 d post-surgery. The most frequent serious adverse events were pneumonia, in 7 patients (21), and abdominal infection, in 5 patients (15). Incidence of surgical and perioperative complications were higher in the group of patients that undergone esophagectomy, with 5 patients within 11 (45) with grade 3 or 4 complications versus 1 with 22 (4) in patients that undergone gastrectomy. Nineteen patients had no or a reduced number of adjuvant chemotherapy cycles and 8 of whom have undergone esophagectomy. Seventeen patients within the 28 that received adjuvant chemotherapy had grade 3 or 4 side effects (60). Occurrence of adverse effects was the unique cause of adjuvant therapy ending (Table 3).
| Maximal toxicity | All | Grade 3/4 |
| All | 31 (92) | 10 (30) |
| Neutropenia | 3 (10) | 1(3) |
| Febrile neutropenia | 1 (3) | 1 (3) |
| Anaemia | 2 (6) | 0% |
| Thrombocytopenia | 2 (6) | 0% |
| Neurotoxicity | 21 (63) | 7 (21) |
| Nausea | 7 (21) | 0% |
| Asthenia | 12 (36) | 6 (19) |
| Vomiting | 2 (6) | 0% |
| Mucositis | 4 (12) | 2 (6) |
| Diarrhoea | 12 (36) | 3 (10) |
| Allergic reaction | 1(3) | 1 (3) |
| Patients with at least one grade 3-4 adverse event during perioperative time | 6 (18) |
| Medical complication | 7 (21) |
| Anastomotic leak | 2 (6) |
| Wound healing disorder | 1 (3) |
| Pneumonia | 7 (21) |
| Pleural complication | 1 (3) |
| Sepsis and infection | 5 (15) |
| Intestinal occlusion | 2 (6) |
| Bleeding | 1 |
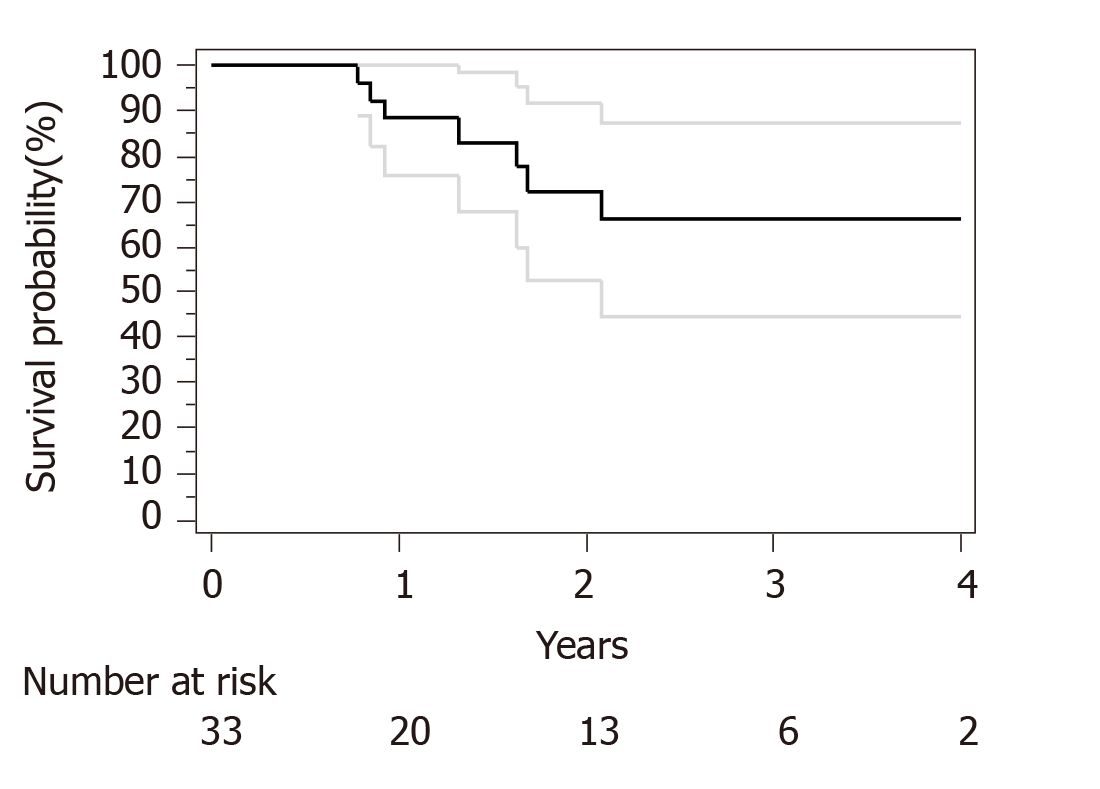
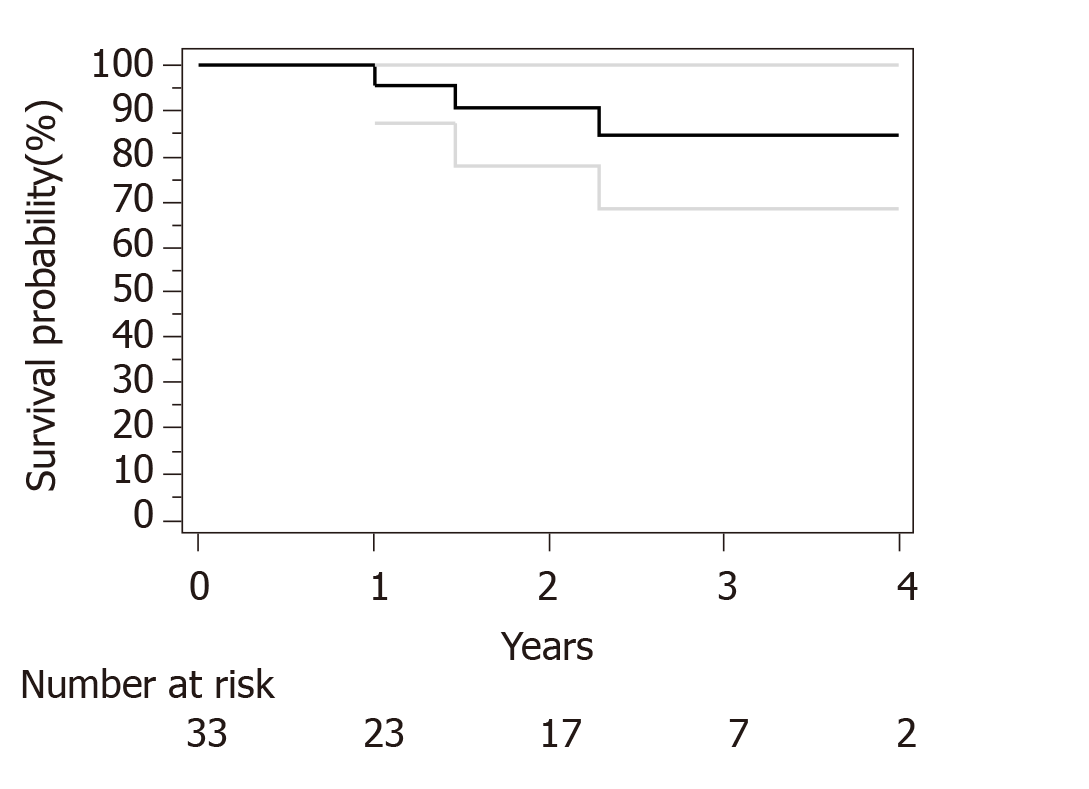
Median follow-up for surviving patients was 32 mo. Surgical and pathological results are presented in Table 4. R0 resection was obtained in 30 out of 33 patients. Only R1 resection was achieved for an esophagectomy and 2 subtotal gastrectomy. We have used Becker regression criteria classification to estimate tumour regression and response rate. We found 12 (36) patients with complete response TRG1a, 8 (24) patients with TRG1b, 4 (13) patients with TRG2 and 9 (27) with TRG3. No particular difference was observed between complete and incomplete responders in term of histological type, tumour stage or number of cycles of neoadjuvant chemotherapies. For HER2 overexpression tumor complete response (TRG1a) was observed in 3 out of five patients. Two-year OS and PFS were respectively 90% and 73%. Median OS and PFS were not reached at data base lock (Figures 1 and 2). We observed 6 metastatic relapses and 1 localized relapse. No relapses were observed in patients with TRG1A histological response.
| Type of surgery | |
| Subtotal gastrectomy | 11 (33) |
| Total gastrectomy | 11 (33) |
| Oesophagectomy | 11 (33) |
| Resection Grade | |
| R0 | 30 (90) |
| R1 | 3 (10) |
| Complete (TRG 1a)† | 12 (36) |
| Subtotal (TRG 1b) | 8 (24) |
| Partial (TRG 2) | 4 (13) |
| Minimal or none (TRG 3) | 9 (27) |
| yN0 | 21 (63) |
| yN 1 | 6 (19) |
| yN2 | 6 (19) |
This study underlines the safety and feasibility of TeFOX or TeFOX plus trastuzumab regimen for patients with localized GC. Neoadjuvant therapy is the standard of care for localized GC. Recently the FLOT4 study demonstrated the superiority of FLOT perioperative regimen in comparison to ECX regimen[6,7]. In particular, while ECX led to 6% TRG1A complete response, the FLOT4 gave rise to 16% of TRG1 (95%CI: 10%-23). These results are comparable with previous studies like OEO5[11] and ST03 trials[12] which showed a TRG1a rate of 7% and 8%, respectively. In most clinical trials testing combination of chemotherapies with taxane, the proportion of patients with complete pathological response are similar to the ones obtained with FLOT regimen, with complete responses ranging from 14% to 20%[13,14]. In our study, we have observed 36% of TRG1a (95%CI: 19-62). Such data compares favourably to previous trials and suggests that the TeFOX regimen might be at least as efficient as other taxane based regimens. Importantly, relapse rate is small, 21% of relapse (95%CI: 8-43) with a median follow up of more than two years. In FLOT4 trial, the relapse rate at 2 years are 57% and 47% for ECX and FLOT4 regimen[6]. Interestingly, no patients with TRG1a had a relapse, suggesting that complete response is a good surrogate to predict absence of recurrence. No clinical variable was associated with complete response or recurrence. Notably, neither singet ring cell presence nor the number of cycles of neoadjuvant chemotherapy were associated with relapse or complete response.
In our study, TeFOX perioperative chemotherapy gives rise to grade 3-4 side effects in 30% of the patients (95%CI: 14-55). The main toxicities observed are asthenia and neuropathy. Such results are very similar to the FLOT regimen which induced 34% of grade 3-4 toxicity. In FLOT4 trial, 52% of patients have grade 3-4 neutropenia but in our series only 10% are observed. The difference probably relies on the systematic and prophylactic use of G-CSF. Higher rate of neuropathy was observed in our study. This difference may rely on a higher number of chemotherapy cycles. Similarly, surgery morbidity is comparable to FLOT prospective randomized trial. Not surprisingly, we have observed a higher incidence of complications with esophagectomy. Interestingly, adjuvant treatment could not be started in 5 patients and had to be stopped in 14 patients because of major side effects. The incidence of grade 3-4 side effects reached 60% and required treatment arrest for most patients. Such data suggest a higher toxicity of adjuvant therapy than neoadjuvant therapy.
Limitations of our study include the retrospective and monocentric design and a selection of patients with good performance status. However, we believe that such data support that TeFOX results might be comparable to FLOT regimen results and could be used in neoadjuvant setting of localized gastric and gastroesophageal junction cancer.
In conclusion, our study gives information on safety and efficacy of TeFOX regimen in perioperative setting of localized gastric and gastroesophageal junction cancer. These data support further development in phase II clinical trials.
Localized oeso-gastric cancer (GC) are treated by perioperative chemotherapy and surgery. The use of taxane seems to improve response rate and outcome.
Only the efficacy of the german regimen FLOT was previously reports.
To determine the efficacy of the Frence TeFOX regimen.
This retrospective study aims to test efficacy and safety of the perioperative TeFOX regimen given alone in patients with localized GC.
Thirty-three consecutive patients were included. Median follow-up of surviving patients was 32 mo. R0 resection was obtained in 30 (91) patients. Twelve patients (36) have a pathological complete response and 8 (24) patients a nearly complete pathological response.
TeFOX regimen could be safely administrated in perioperative treatment of localized GC.
TeFOX and the FLOT regimen have comparable efficacy and safety profiles and could be considered as an alternative regimen.
| 1. | Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3585] [Cited by in RCA: 5047] [Article Influence: 630.9] [Reference Citation Analysis (2)] |
| 2. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ. MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4739] [Article Influence: 237.0] [Reference Citation Analysis (7)] |
| 3. | Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, Genève J, Lasser P, Rougier P. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1551] [Article Influence: 103.4] [Reference Citation Analysis (0)] |
| 4. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5823] [Cited by in RCA: 5532] [Article Influence: 345.8] [Reference Citation Analysis (3)] |
| 5. | Plum PS, Gebauer F, Krämer M, Alakus H, Berlth F, Chon SH, Schiffmann L, Zander T, Büttner R, Hölscher AH, Bruns CJ, Quaas A, Loeser H. HER2/neu (ERBB2) expression and gene amplification correlates with better survival in esophageal adenocarcinoma. BMC Cancer. 2019;19:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 6. | Al-Batran SE, Hofheinz RD, Pauligk C, Kopp HG, Haag GM, Luley KB, Meiler J, Homann N, Lorenzen S, Schmalenberg H, Probst S, Koenigsmann M, Egger M, Prasnikar N, Caca K, Trojan J, Martens UM, Block A, Fischbach W, Mahlberg R, Clemens M, Illerhaus G, Zirlik K, Behringer DM, Schmiegel W, Pohl M, Heike M, Ronellenfitsch U, Schuler M, Bechstein WO, Königsrainer A, Gaiser T, Schirmacher P, Hozaeel W, Reichart A, Goetze TO, Sievert M, Jäger E, Mönig S, Tannapfel A. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016;17:1697-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 542] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 7. | Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, Kopp HG, Mayer F, Haag GM, Luley K, Lindig U, Schmiegel W, Pohl M, Stoehlmacher J, Folprecht G, Probst S, Prasnikar N, Fischbach W, Mahlberg R, Trojan J, Koenigsmann M, Martens UM, Thuss-Patience P, Egger M, Block A, Heinemann V, Illerhaus G, Moehler M, Schenk M, Kullmann F, Behringer DM, Heike M, Pink D, Teschendorf C, Löhr C, Bernhard H, Schuch G, Rethwisch V, von Weikersthal LF, Hartmann JT, Kneba M, Daum S, Schulmann K, Weniger J, Belle S, Gaiser T, Oduncu FS, Güntner M, Hozaeel W, Reichart A, Jäger E, Kraus T, Mönig S, Bechstein WO, Schuler M, Schmalenberg H, Hofheinz RD; FLOT4-AIO Investigators. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2086] [Cited by in RCA: 1820] [Article Influence: 260.0] [Reference Citation Analysis (0)] |
| 8. | Pernot S, Dubreuil O, Aparicio T, Le Malicot K, Tougeron D, Lepère C, Lecaille C, Marthey L, Palle J, Bachet JB, Zaanan A, Taieb J. Efficacy of a docetaxel-5FU-oxaliplatin regimen (TEFOX) in first-line treatment of advanced gastric signet ring cell carcinoma: an AGEO multicentre study. Br J Cancer. 2018;119:424-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Becker K, Mueller JD, Schulmacher C, Ott K, Fink U, Busch R, Böttcher K, Siewert JR, Höfler H. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer. 2003;98:1521-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 628] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 10. | Becker K, Langer R, Reim D, Novotny A, Meyer zum Buschenfelde C, Engel J, Friess H, Hofler H. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg. 2011;253:934-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 296] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 11. | Alderson D, Cunningham D, Nankivell M, Blazeby JM, Griffin SM, Crellin A, Grabsch HI, Langer R, Pritchard S, Okines A, Krysztopik R, Coxon F, Thompson J, Falk S, Robb C, Stenning S, Langley RE. Neoadjuvant cisplatin and fluorouracil versus epirubicin, cisplatin, and capecitabine followed by resection in patients with oesophageal adenocarcinoma (UK MRC OE05): an open-label, randomised phase 3 trial. Lancet Oncol. 2017;18:1249-1260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 181] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 12. | Cunningham D, Stenning SP, Smyth EC, Okines AF, Allum WH, Rowley S, Stevenson L, Grabsch HI, Alderson D, Crosby T, Griffin SM, Mansoor W, Coxon FY, Falk SJ, Darby S, Sumpter KA, Blazeby JM, Langley RE. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): primary analysis results of a multicentre, open-label, randomised phase 2-3 trial. Lancet Oncol. 2017;18:357-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 219] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 13. | Homann N, Pauligk C, Luley K, Werner Kraus T, Bruch HP, Atmaca A, Noack F, Altmannsberger HM, Jäger E, Al-Batran SE. Pathological complete remission in patients with oesophagogastric cancer receiving preoperative 5-fluorouracil, oxaliplatin and docetaxel. Int J Cancer. 2012;130:1706-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Schulz C, Kullmann F, Kunzmann V, Fuchs M, Geissler M, Vehling-Kaiser U, Stauder H, Wein A, Al-Batran SE, Kubin T, Schäfer C, Stintzing S, Giessen C, Modest DP, Ridwelski K, Heinemann V. NeoFLOT: Multicenter phase II study of perioperative chemotherapy in resectable adenocarcinoma of the gastroesophageal junction or gastric adenocarcinoma-Very good response predominantly in patients with intestinal type tumors. Int J Cancer. 2015;137:678-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alkan A, Quero L S-Editor: Ma YJ L-Editor: Filipodia E-Editor: Qi LL